Supplemental Digital Content is available in the text.
Keywords: control groups; informed consent; intracranial hemorrhages; magnetic resonance imaging; stroke, acute; tissue-type plasminogen activator
Background and Purpose—
We assessed whether lower-dose alteplase at 0.6 mg/kg is efficacious and safe for acute fluid-attenuated inversion recovery-negative stroke with unknown time of onset.
Methods—
This was an investigator-initiated, multicenter, randomized, open-label, blinded-end point trial. Patients met the standard indication criteria for intravenous thrombolysis other than a time last-known-well >4.5 hours (eg, wake-up stroke). Patients were randomly assigned (1:1) to receive alteplase at 0.6 mg/kg or standard medical treatment if magnetic resonance imaging showed acute ischemic lesion on diffusion-weighted imaging and no marked corresponding hyperintensity on fluid-attenuated inversion recovery. The primary outcome was a favorable outcome (90-day modified Rankin Scale score of 0–1).
Results—
Following the early stop and positive results of the WAKE-UP trial (Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke), this trial was prematurely terminated with 131 of the anticipated 300 patients (55 women; mean age, 74.4±12.2 years). Favorable outcome was comparable between the alteplase group (32/68, 47.1%) and the control group (28/58, 48.3%; relative risk [RR], 0.97 [95% CI, 0.68–1.41]; P=0.892). Symptomatic intracranial hemorrhage within 22 to 36 hours occurred in 1/71 and 0/60 (RR, infinity [95% CI, 0.06 to infinity]; P>0.999), respectively. Death at 90 days occurred in 2/71 and 2/60 (RR, 0.85 [95% CI, 0.06–12.58]; P>0.999), respectively.
Conclusions—
No difference in favorable outcome was seen between alteplase and control groups among patients with ischemic stroke with unknown time of onset. The safety of alteplase at 0.6 mg/kg was comparable to that of standard treatment. Early study termination precludes any definitive conclusions.
Registration—
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02002325.
Recently, the WAKE-UP trial (Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke) revealed the efficacy and safety of alteplase (a recombinant plasminogen activator) at 0.9 mg/kg guided by a mismatch between diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR; negative FLAIR pattern or DWI-FLAIR mismatch) in patients with acute ischemic stroke with an unknown time of onset.1 In Japan, a different alteplase dose of 0.6 mg/kg is only available for patients with acute ischemic stroke based on a single-arm trial, observational studies, and guidelines.2–5 The ENCHANTED (Enhanced Control of Hypertension and Thrombolysis Stroke Study) revealed that small difference of efficacy and overall similarity regarding the balance between efficacy and safety between 0.9 and 0.6 mg/kg of alteplase though this trial did not reach its primary end point of noninferiority.6 Herein, we describe the THAWS trial (Thrombolysis for Acute Wake-Up and Unclear-Onset Strokes With Alteplase at 0.6 mg/kg; www.ClinicalTrials.gov, Identifier: NCT02002325; UMIN clinical trial ID: UMIN000011630), which was conducted to test whether alteplase at 0.6 mg/kg is effective and safe in patients with acute ischemic stroke with an unknown time of onset who had acute ischemic lesions on DWI and no marked corresponding hyperintensity on FLAIR.7
Methods
THAWS was an investigator-initiated, multicenter, randomized, open-label, blinded-end point evaluation, controlled trial involving patients with an unknown time of stroke onset. The design was similar to that of the WAKE-UP trial. We did not use placebo mainly due to the financial limitation. All patients met the clinical criteria for intravenous thrombolysis in Japan,5 other than a time last-known-well >4.5 hours. Patients could undergo randomization if MRI showed a negative FLAIR pattern (Figure I in the Data Supplement) with standard settings on FLAIR based on the imaging guidelines provided by the WAKE-UP group.
The trial was approved by the local ethics committee or institutional review board at each participating center. Patients or their relatives provided written informed consent according to ethical regulations.
Investigators are listed in the Data Supplement. A Steering Committee was responsible for the design, interpretation, and supervision of the trial.
The trial was overseen by an independent data and safety monitoring board. No efficacy interim analysis was conducted, but one safety interim analysis with an initial 150 patients was planned. The Research Electronic Data Capture system was used to collect trial data. The authors confirmed the accuracy and completeness of the data and adverse event reporting and the fidelity of the trial to the protocol.
De-identified individual participant data may be available upon request, only if the request is intended to contribute to the improvement of people’s health and welfare.
Additional information is described in the Data Supplement.
Patients
Patients with stroke symptoms on awaking or with unknown time of onset were eligible if they presented >4.5 hours since last-known-well and within 4.5 hours after symptom recognition were 20 years or older and had a premorbid modified Rankin Scale (mRS) (Table I in the Data Supplement). We initially set an upper time limit of 12 hours after last-known-well in May 2014 and revised this to no time limit in August 2015 to match this eligibility criterion with that of the WAKE-UP trial. Patients were excluded if they had mild stroke with National Institutes of Health Stroke Scale (NIHSS; ranging from 0 to 42, with higher score indicating more severe stroke) <5 (revised to <2 in August 2015 due to the same reasons given above) or severe stroke with NIHSS >25 in contrast to no lower and upper limit of NIHSS in the WAKE-UP trial. Otherwise, patients fulfilled the standard eligibility criteria for the use of alteplase (Table I in the Data Supplement).5 Patients underwent MRI examination with DWI, FLAIR, T2*, and time-of-flight magnetic resonance angiography of the circle of Willis. The details of DWI and FLAIR parameters, which have been described previously,7 were matched to those used in the WAKE-UP trial. Patients underwent randomization if they showed mismatch between the presence of an abnormal signal on DWI and no marked signal change on FLAIR (negative FLAIR pattern) in the corresponding region of the acute stroke. Patients with clinically acute ischemic stroke and a negative FLAIR pattern who did not display an abnormal signal on DWI were also enrolled. A detailed imaging guidebook provided by the WAKE-UP steering committee included extensive examples illustrating the inclusion and exclusion criteria for MRI. Patients were excluded if they had any contraindications for MRI (eg, cardiac pacemaker), were planned or anticipated to undergo treatment with surgery or endovascular reperfusion strategies, were pregnant, lactating, or potentially pregnant, or had a life expectancy of 6 months or less according to the judgment of an investigator. As MRI criteria, patients were excluded with intracranial hemorrhage or large infarct with Alberta Stroke Program Early CT Score of 4 or less in the territory of the middle cerebral artery, or with visual lesion volume over 50% of the anterior cerebral artery or posterior cerebral artery, more than half of the brain stem or more than half of the unilateral cerebellar hemisphere.
Randomization and Treatment
Eligible patients were randomized in a 1:1 ratio to receive either intravenous alteplase (alteplase group) or standard treatment (control group), based on a minimization scheme with stratification by severity of symptoms as assessed using the NIHSS score (≤11 or >11) though those in the WAKE-UP trial were done by the NIHSS (≤10 or >10) and by age (≤60/>60 years). Treatment was allocated by an in-house, validated, interactive email-based system, right after a patient was enrolled by an investigator from the study site. Both patients and investigators were aware of treatment allocation. Primary and secondary outcomes were assessed without information regarding treatment allocation by independent and certified neurologists, neurosurgeons, nurses, or clinical research coordinators. Just after randomization, patients randomized to the alteplase group were treated with intravenous alteplase at 0.6 mg/kg (with 10% bolus administration and 90% by 60-minute infusion) within 4.5 hours of waking up or discovery, and those randomized to the control group were treated with the standard treatment using 1 to 3 antithrombotic drugs, including oral aspirin (160–300 mg/day), oral clopidogrel (75 mg/day), intravenous argatroban, or intravenous unfractionated heparin, but excluding the combination of argatroban and heparin, according to decisions of the attending physician. All antithrombotics were basically prohibited for use in the alteplase group within the initial 25 hours. Treatment was initiated as soon as possible within 60 minutes after MRI examination. Thrombolytic agents such as urokinase, monteplase, and tenecteplase (unavailable in Japan) were prohibited during the 90-day study period in both groups.
Clinical and Imaging Assessment
Certified neurologists, neurosurgeons, nurses, or clinical research coordinators performed clinical assessments at baseline, at 22 to 36 hours, at 7 to 14 days, or at hospital discharge (if earlier), and at 90 days after randomization. At 90 days, mRS and adverse events were blindly assessed without information on treatment assignment by an independent physician, nurse, or clinical research coordinator. Brain MRI was performed at baseline, at 22 to 36 hours to identify intracranial hemorrhage, and at 7 to 14 days to delineate final infarct volume. The details of clinical and imaging assessments and major protocol violation are described in the Data Supplement.
Outcome Measures
The primary efficacy end point was favorable outcome, defined as mRS score of 0 to 1 at 90 days after stroke onset. Secondary efficacy end points were category shift in mRS score at 90 days after stroke onset, mRS score 0 to 2 at 90 days after stroke onset, category shift in NIHSS score at 24 hours after initiation of treatment, and category shift in NIHSS score at 7 days after initiation of treatment. Imaging outcomes (exploratory end points) included recanalization of the culprit artery on magnetic resonance angiography at 22 to 36 hours after initiation of treatment, infarct volume on FLAIR at 7 days and infarct growth as defined by infarct volume on FLAIR at 7 days minus infarct volume on DWI at baseline. Recanalization was defined by modified Mori grade 3.8
Safety end points were symptomatic intracranial hemorrhage (sICH) at 22 to 36 hours and major extracranial bleeding9 and death due to any cause at 90 days. The definition of sICH was an increase in NIHSS score by ≥4 from baseline and parenchymal hematoma type II on MRI at 22 to 36 hours after initiation of treatment.
Statistical Analysis
We determined the sample size on the basis of the primary end point. A total of 278 patients was required to ensure 1−β=90% probability to demonstrate that the relative effect of intravenous alteplase compared with standard treatment for ischemic stroke patients is more than a fraction of 0.5 of the combined relative effect of intravenous alteplase across the stroke thrombolysis studies,10–13 by using a 1-sided χ2 test of significance level of 2.5%, where the effects of intravenous alteplase and standard treatment are assumed to be 30% and 20% commonly for Japanese patients and comparable combined studies. We planned to recruit a total of 300 patients, accounting for possible treatment failures, protocol violations, and dropouts.
Statistical analyses are elaborated in a prespecified statistical analysis plan. Primary analyses were undertaken according to an intention-to-treat principle. A secondary per-protocol analysis excluded patients with major protocol violations. We also performed an as-treated analysis to assess whether the intention-to-treat basis analysis resulted in any underestimation of the treatment effect. The details of missing data management and primary, secondary, safety data, and subgroup analyses are described in the Data Supplement.
A value of P<0.05 based on a 2-sided test was considered significant. Primary and secondary end points and predefined subgroup analyses mentioned above were performed using SAS/STAT software, version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Patients’ Characteristics
Following the early stop and positive results of the WAKE-UP trial, the THAWS steering committee suspended further enrollments on July 10, 2018. From May 1, 2014 to July 10, 2018, a total of 465 patients underwent screening at 40 centers. Of these, 352 were excluded, including 207 who had no mismatch between findings on DWI and FLAIR or had intracerebral hemorrhage and 45 for whom thrombectomy was planned (Figure 1). A total of 131 patients underwent randomization, as compared with the targeted enrollment of 300 patients.
Figure 1.
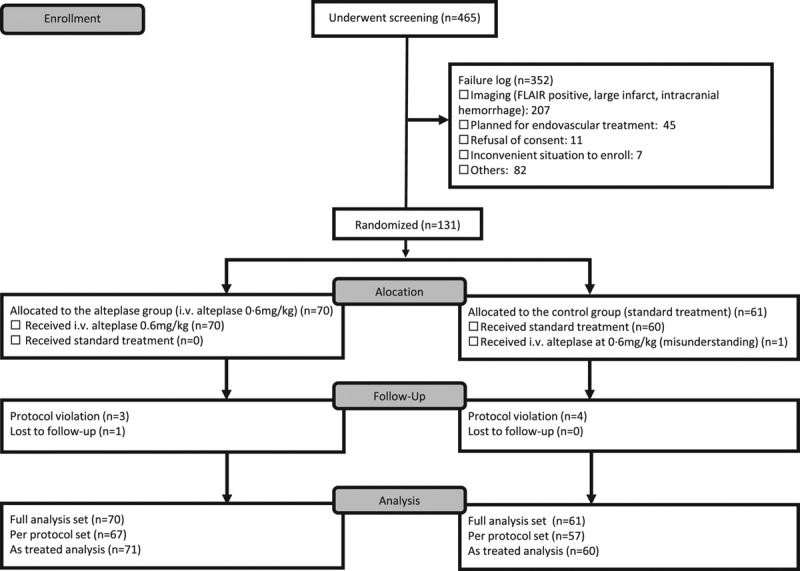
Screening and randomization of patients. FLAIR indicates fluid-attenuated inversion recovery.
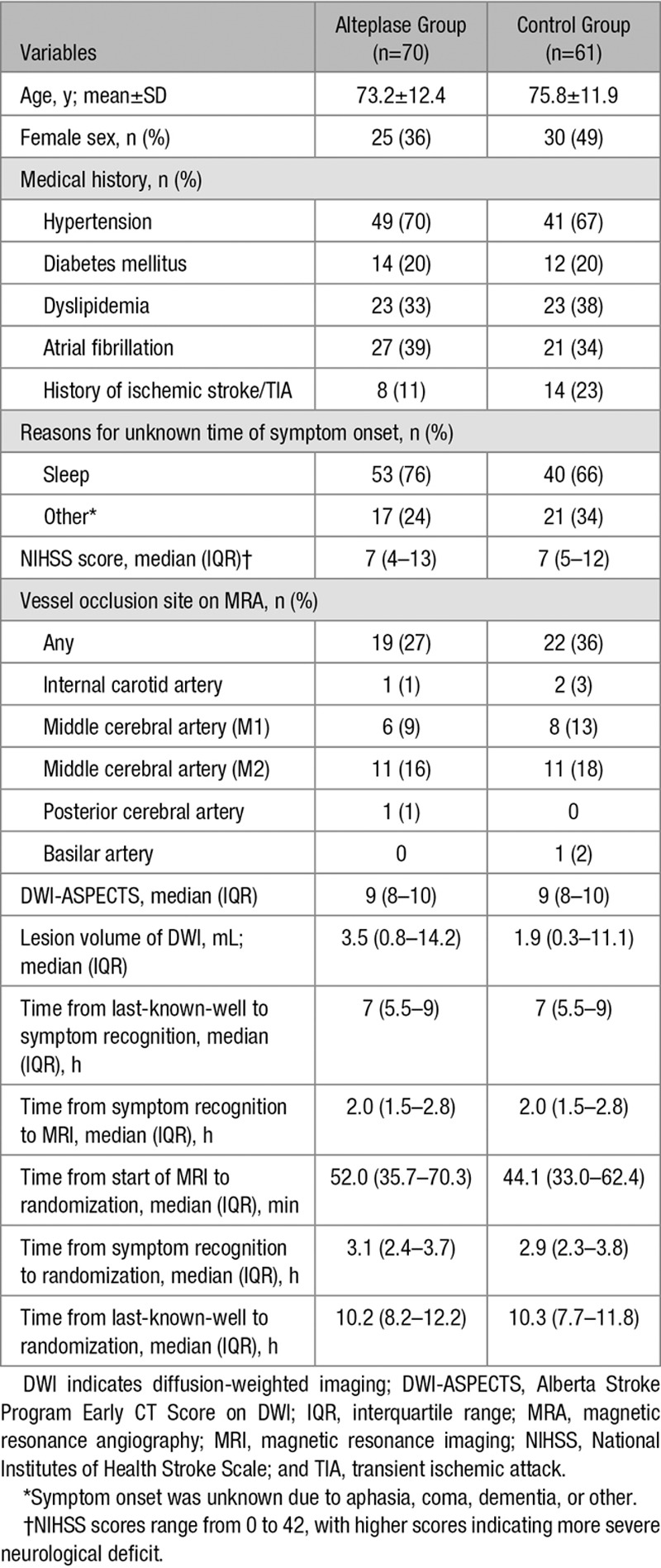
Of the 131 enrolled patients, 70 were assigned to the alteplase group and 61 to the control group (Figure 1). Baseline demographic and clinical characteristics in both groups are shown in Table 1. Median NIHSS score at the time of baseline examination was 7 for each group. Wake-up stroke was found in 53 patients (75.7%) in the alteplase group and 40 patients (65.6%) in the control group. Median time between last-known-well and symptom recognition was 7.0 hours for each group. Median interval between last-known-well and randomization was 10.0 hours for each group.
Table 1.
Baseline Characteristics of Patients
All patients in the alteplase group and 1 patient in the control group received alteplase (Figure 1). Alteplase was discontinued just after bolus infusion due to subdural hematoma on baseline FLAIR in one patient from the alteplase group. Thus, 71 patients who received alteplase and 60 patients who received standard treatment were included in the safety analysis set. Three patients in the alteplase group and 4 patients in the control group showed protocol violation (alteplase group: one patient with alteplase infusion before randomization, one with subdural hematoma, and one with endovascular treatment; control group: one patient with elevated level of activated partial thromboplastin time, one with acute antiplatelet therapy with cilostazol, one with hypoglycemia and one treated with alteplase) and one patient in the alteplase group was lost to follow-up. Four patients in the alteplase group and 4 in the control group underwent 3-month follow-up assessment outside of the allowance schedule or with an unblinded assessor. Details of acute treatments from randomization to 24 hours are summarized in Table II in the Data Supplement. Any antithrombotic therapy, oral antiplatelet, and intravenous anticoagulant therapies were performed in 85%, 49%, and 66%, respectively, in the control group, compared with 13%, 9%, and 6%, respectively, in the alteplase group according to the protocol.
Efficacy Outcomes
Favorable outcomes at 90 days after stroke onset were comparable between the alteplase group (47.1%) and control group (48.3%; RR, 0.97 [95% CI, 0.68–1.41]; P=0.892; Table 2; Figure 2). Sensitivity analyses using the full analysis data set, per-protocol data analysis, and as-treated analysis showed similar results (Table 2).
Table 2.
Primary and Secondary Efficacy and Imaging Outcomes
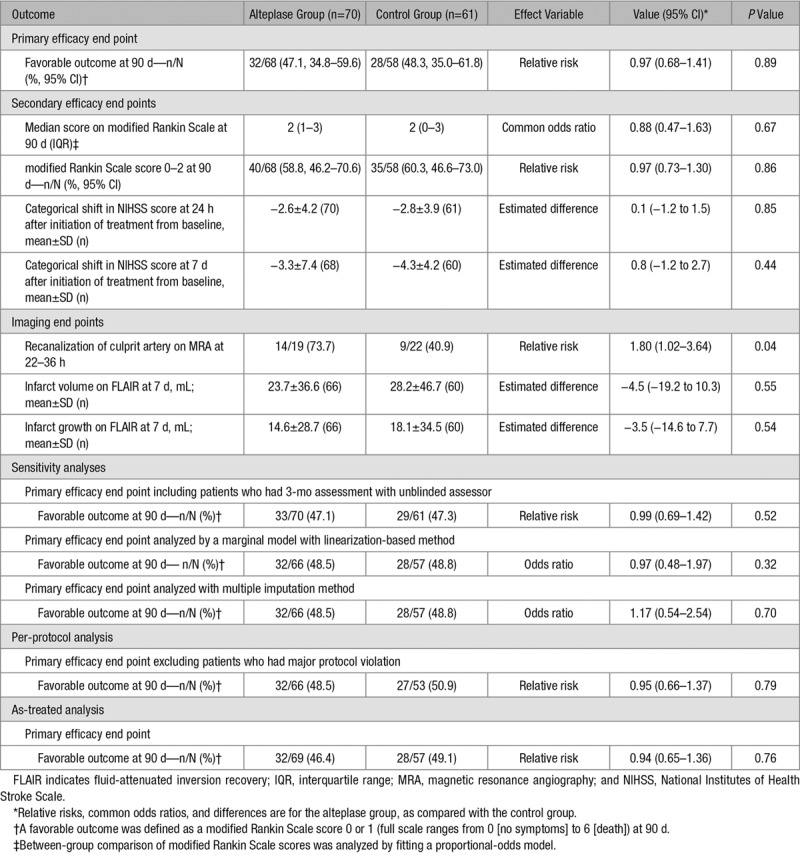
Figure 2.
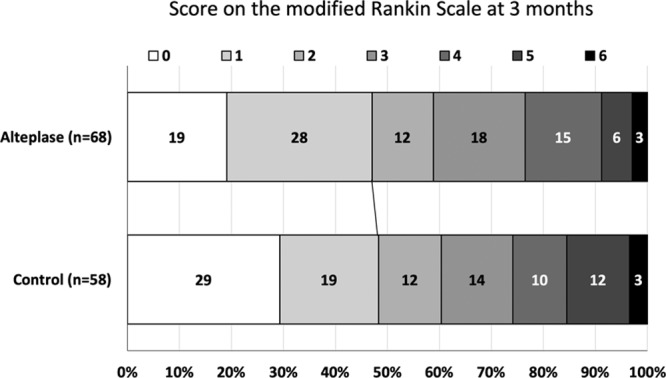
Distribution of scores on 90-d modified Rankin Scale (intention-to-treat population).
An mRS score of 0 to 2 at 90 days after stroke onset was also comparable between the alteplase group (58.8%) and control group (60.3%; RR, 0.97 [95% CI, 0.73–1.30]; P=0.862; Table 2; Figure 2). No difference in category shift in NIHSS score was seen at 24 hours and at 7 days after the initiation of treatment between groups. Regarding imaging outcomes, early recanalization of the culprit artery was more frequent in the r-tPA group as compared with the control group though the number of patients involved in this analysis (n=41) was relatively small. There was no significant difference in infarct volume and infarct growth between the 2 groups.
In prespecified subgroup analyses of the primary end point, a significant interaction was apparent between treatment groups and premorbid antithrombotic medication (Figure 3). In patients with premorbid antithrombotic medication, favorable outcome was more frequent in the alteplase group though the number of patients (n=40) was small. In additional subgroup analyses of the primary end point, patients with NIHSS ≥5, those who had culprit arterial occlusion, those with baseline Alberta Stroke Program Early CT Score on DWI <10, or those with acute ischemic lesion on 24-hour FLAIR in the alteplase group tended to show a higher percentage of favorable outcome as compared with those in the control group, although no significant differences were evident (Figure 3).
Figure 3.
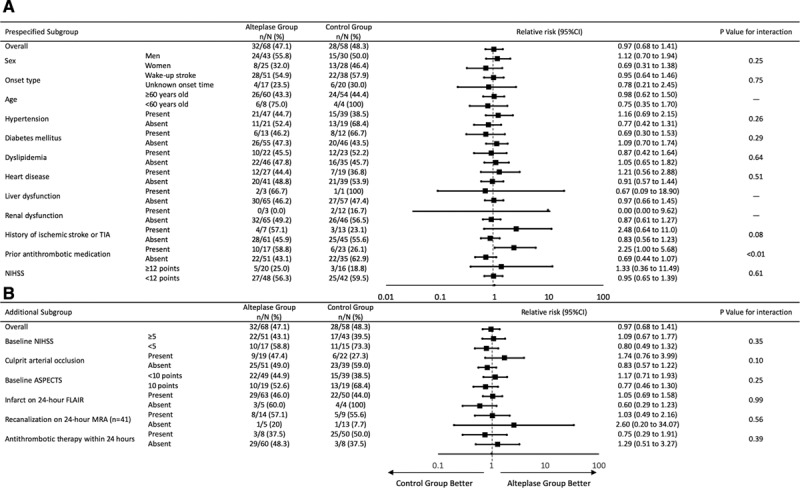
Prespecified (A) and additional (B) subgroup analyses: relative risks and 95% CIs for favorable outcome. ASPECTS indicates Alberta Stroke Program Early CT Score; FLAIR, fluid-attenuated inversion recovery; MRA, magnetic resonance angiography; and NIHSS, National Institutes of Health Stroke Scale.
Safety Outcomes
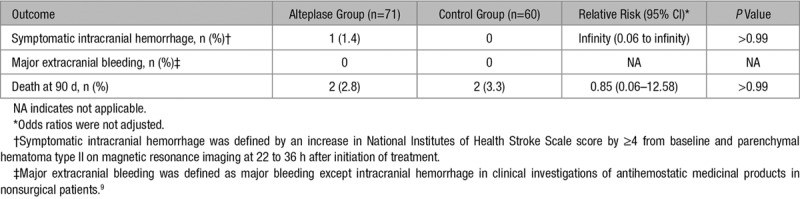
In the safety analysis set, sICH at 22 to 36 hours occurred in one of 71 patients (1.4%) in the alteplase group and none of 60 patients in the control group (RR, infinity [95% CI, 0.06 to infinity]; P>0.999; Table 3). No patients experienced major extracranial bleeding in either group (Table 3). Two of the 70 patients (2.8%) in the alteplase group and 2 of 61 patients (3.3%) in the control group died within 90 days (RR, 0.85 [95% CI, 0.06–12.58]; P>0.999) (Table 3). Causes of death were heart failure in 1 patient each for both groups, gastric cancer in another patient in the control group, and unknown in another patient in the alteplase group who showed sudden death. In the alteplase group, 9 patients (12.7%) experienced a serious adverse event, as compared with 6 patients (10%) in the control group (P=0.632). Detailed lists of any and serious adverse events are provided in Table III in the Data Supplement.
Table 3.
Safety Outcomes
Discussion
We compared intravenous thrombolysis with alteplase at 0.6 mg/kg against standard medical treatment in patients with stroke symptoms on waking or with unknown time of onset who had no clearly visible signal change on FLAIR. No difference in the proportion of favorable outcome as defined by mRS score 0 to 1 was evident between the alteplase and standard treatment groups. Early termination with the small sample size precluded definitive conclusions. Regarding the safety profile, the proportion of sICH and that of death did not differ between groups. Therefore, the safety of intravenous alteplase at 0.6 mg/kg was comparable to standard treatment in acute ischemic stroke on waking or with unknown time of onset who showed a negative FLAIR finding.
A well-designed single-arm trial (MR-WITNESS) and a randomized controlled trial (WAKE-UP) both tested alteplase at 0.9 mg/kg in patients with acute ischemic stroke with unknown time of onset.1,14 The proportion of favorable outcome at 3 months after onset in our alteplase group (47.1%) was comparable to results from MR WITNESS (43.5% in patients with premorbid mRS score 0–1) and WAKE-UP (53.3%). Median NIHSS in the alteplase group was similar among the 3 clinical trials, at 7 (IQR, 4–13) in this trial, 7.5 (IQR, 4.3–13.8) in MR WITNESS, and 6 (IQR, 4–9) in WAKE-UP.1,14 Efficacy of alteplase at 0.6 mg/kg thus may not be inferior to that at 0.9 mg/kg. Regarding the safety profile, the proportion of sICH in the alteplase group was 1.4% (SITS-MOST criteria: parenchymal hematoma type II with deterioration in NIHSS score by ≥4) in this trial, 1.3% (ECASS III criteria: any intracranial hemorrhage with deterioration in NIHSS score by ≥4) in MR WITNESS, and 2.0% (SITS-MOST criteria) in WAKE-UP. Mortality at 3 months in the alteplase group was 2.8% in this trial, 8.8% in MR WITNESS, and 4.1% in WAKE-UP. Because the negative FLAIR pattern indicates that ischemic stroke occurred within the preceding 4.5 hours,15,16 MRI-based patient selection using the FLAIR negative pattern is a reasonable method offering acceptable safety profiles.
On the other hand, the proportion of favorable outcome at 3 months after onset in the control group (48.3%) appeared high as compared with that of WAKE-UP (41.8%). The proportion of sICH in the control group was 0% in this trial and 0.4% in WAKE-UP. The mortality rate in the control group was 3.3% in this trial and 1.2% in WAKE-UP. Somewhat surprisingly, about half of the patients with acute ischemic stroke with unknown time of onset and negative FLAIR finding showed mRS score 0 to 1 at 3 months.
Better than expected outcome for the control group seems to be caused by the open treatment design that allows early initiation of antithrombotic therapy within the initial 24 hours. Antithrombotic therapy was initiated in 13% of alteplase-treated patients versus 85% of control patients within the initial 24 hours (Table II in the Data Supplement). In particular, dual antiplatelet therapy became common among patients with acute noncardioembolic stroke after publication of the CHANCE trial (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) and a meta-analysis;17,18 the therapy was initiated in 3% of alteplase-treated patients versus 23% of control patients within the initial 24 hours, such intergroup changes were not present in the blinded treatment trials though direct evidence of better outcomes with dual antiplatelet therapy was not reported. Another reason for better than expected outcomes in both alteplase and control groups were that the efficacy and safety of acute mechanical thrombectomy were established in 2015.19 During the registration period of the THAWS trial, adding mechanical thrombectomy therapy became standard treatment for all kinds of acute ischemic stroke. Thereafter, patients with major artery occlusion were often excluded at the initial assessment and instead taken to the catheterization laboratory. For this reason, patient enrollment was slower than expected and neurological severity of enrolled patients shifted to milder population. The greater the proportion of patients with mild stroke symptoms involved, the smaller the difference in effect between alteplase and standard treatments that might be achieved, as shown by the recent PRISM trial.20 Patients with mild stroke symptoms were likely to have had lacunar infarcts. In the secondary post hoc analysis of the WAKE-UP trial showed no better effect in outcome with alteplase.21 Finally, three-fifths of enrolled patients did not show magnetic resonance angiography-confirmed arterial occlusion. The frequency of arterial recanalization was somewhat high in the alteplase group (75%) as compared in the control group (58%), this would not have influenced overall outcomes.
Recently, the EXTEND trial (Extending the Time for Thrombolysis in Emergency Neurological Deficits) revealed the efficacy of thrombolysis between 4.5 and 9 hours after onset of stroke with a nonsignificant tendency toward an increase in sICH.22 EXTEND tested thrombolysis with alteplase at 0.9 mg/kg in patients with ischemic stroke, including wake-up stroke with significant penumbral mismatch on computed tomography or MRI. ECASS4, which was a similar trial to EXTEND, did not show the efficacy of thrombolysis, probably due to the small number of patients.23 In contrast to the EXTEND and ECASS4 trials, which involved patients with penumbral imaging mismatch mainly due to major artery occlusion, the THAWS trial involved many patients without major artery occlusion and who were thus likely to have had small-vessel disease. The WAKE-UP and MR-WITNESS trials might have had the same problem, in that patients with moderate to severe stroke may have joined the trial less frequently because of the predominant performance of thrombectomy. However, this problem might have most strongly influenced our trial due to the later registration period.
The strength of this trial was the identical imaging and clinical criteria to the WAKE-UP trial; this would make the comparison of results between the 2 trials straightforward. The baseline DWI lesion volume was similar to that of the WAKE-UP trial. Of the 131 patients, 39 had a baseline DWI Alberta Stroke Program Early CT Score of 10 followed by 9 cases with no lesions seen on FLAIR at 7 days. Although these cases showed certain neurological deficits (median NIHSS 5 for both), and even blurry equivocal lesions did not meet the measures of Alberta Stroke Program Early CT Score, some of these cases might have represented stroke mimics.
Some limitations to this trial need to be considered. First, the premature termination of the trial resulted in a smaller number of patients than planned. The second key limitation was the open treatment design, which might have affected the treatment process during the observational period. Third, the exclusion to perform mechanical thrombectomy may have caused a selection bias. Finally, the present dose of alteplase (0.6 mg/kg) differed from that used in the WAKE-UP and MR-WITNESS trials. This difference might make comparison among trials difficult.
Conclusions
No difference in favorable outcome was seen between alteplase and control groups in patients with unknown time of stroke onset and negative FLAIR findings. In contrast, we confirmed the safety of alteplase at 0.6 mg/kg in these patients. The trial seemed to include many patients with small-vessel disease that had no large artery occlusion, for which early initiation of aggressive antithrombotic treatment might be effective as compared with thrombolysis.
Collaborative analyses with similar trials are planned to assess effective treatments in patients with unknown time of stroke onset.
Sources of Funding
This trial was funded mainly by the Japan Agency for Medical Research and Development (AMED; 19ek0210091h0003 and 19lk0201094h0001, and the Ministry of Health, Labour, and Welfare, and partly by the Mihara Cerebrovascular Disorder Research Promotion Fund.
Disclosures
Dr Koga reports honoraria from Bayer, BMS/Pfizer, Otsuka, Daiichi-Sankyo, Nippon Boehringer Ingelheim and Takeda, scientific advisory board from Ono, and research supports from Takeda, Daiichi-Sankyo, Nippon Boehringer Ingelheim, Astellas, Pfizer and Shionogi, outside the submitted work. Dr Itabashi reports personal fees from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Boehringer Ingelheim, Pfizer, Stryker, Medtronic, Otsuka Pharmaceutical, GE, Mitsubishi Tanabe Pharma Corporation, and Johnson and Johnson, grants from Tohoku Fukushi University, outside the submitted work. Dr Iwama reports grants from Ogaki Tokusyukai Hospital, and GlaxoSmithKline, Dr Kamiyama outside the submitted work. Dr Nagakane reports personal fees from Bayer, Bristol-Myers Squibb, Eisai, Daiichi Sankyo, Mochida, Ono, and Takeda, outside the submitted work. Dr Okada reports personal fees from Boehringer Ingelheim, Bayer, Pfizer, Daiichi-Sankyo, Bristol Myer Squibb, and Otsuka, outside the submitted work. Dr Hasegawa reports personal fees from Boehringer Ingelheim Japan, Inc during the conduct of the study. Dr Sakai reports personal fees from Otsuka Pharma and grants and personal fees from Daiichi-Sankyo outside the submitted work. Dr Yoshimura reports personal fees from Boehringer-Ingelheim, Daiichi Sankyo, Bayer, Bristol-Meyers Squibb, Medtronic, and Biomedical Solutions outside the submitted work. Dr Urabe reports personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, AstraZeneca K.K., Bayer Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Takeda Pharmaceutical Co, Ltd, Mitsubishi Tanabe Pharma Corporation, UCB Japan Co, Ltd, Kowa Shinyaku Co, Ltd, Sumitomo Dainippon Pharma Co, Ltd, Astellas Pharma, Inc, AbbVie GK, Medtronic Japan Co, Ltd, Eisai Co, Ltd, Daiichi Sankyo Co, Ltd, CSL Behring, and Japanese Physical Therapy Association, outside the submitted work. Dr Ihara reports grants from Otsuka Pharmaceutical Ltd, Bristol Myers Squibb, and Shimadzu Corporation outside the submitted work. Dr Kitazono reports personal fees from Bayer Yakuhin Ltd, grants and personal fees from Daiichi Sankyo Co Ltd, and Chugai Pharmaceutical Co Ltd, grants from Takeda Pharmaceutical Co Ltd, Eisai Co Ltd, Astellas Pharma, Inc, MSD KK, and Mitsubishi Tanabe Pharma Corporation, outside the submitted work. Dr Sasaki reports personal fees from Mitsubishi Tanabe Pharma Corporation, Bayer, Astellas, Ono Pharma, Mediphysics, Actelion, Ezai, FujiFilm, Daiichi Sankyo, Nippon Boehlinger Ingelheim, Otsuka, and Chugai, grants and personal fees from Hitachi, grants from Actelion, and GE Healthcare, outside the submitted work. KK reports personal fees (honoraria for lecture presentations) from Bayer Yakuhin. Dr Minematsu reports personal fees and other from Bayer Yakuhin, personal fees from Otsuka Pharmaceutical, Boehringer-Ingelhaem, and Mitsubishi Tanabe Pharma Corporation, outside the submitted work; and personal fees (honoraria for seminar presentation) and other (Advisory Board) from AstraZeneca; personal fees (honoraria for seminar presentation) from Pfizer, Japan Stryker, Dai-ichi Sankyo, Astellas Pharma, Nippon Chemiphar, and Fuji Film RI Pharma; and other (Advisory Board) from CSL Behring, Medico’s Hirata, EPS Corporation, HEALIOS K.K., and T-PEC Corporation. Dr Toyoda reports personal fees from Daiichi-Sankyo, Bayer Yakuhin, Brystol-Meyers-Squibb, Nippon Behringer Ingerheim, outside the submitted work. The other authors report no conflicts.
Supplementary Material
Footnotes
Presented in part at the European Stroke Organisation Conference, Milan, Italy, May 22–24, 2019.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.028127.
References
- 1.Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. WAKE-UP Investigators. MRI-Guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611–622. doi: 10.1056/NEJMoa1804355. doi: 10.1056/NEJMoa1804355. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, et al. Japan Alteplase Clinical Trial (J-ACT) Group. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke. 2006;37:1810–1815. doi: 10.1161/01.STR.0000227191.01792.e3. doi: 10.1161/01.STR.0000227191.01792.e3. [DOI] [PubMed] [Google Scholar]
- 3.Nezu T, Koga M, Kimura K, Shiokawa Y, Nakagawara J, Furui E, et al. Pretreatment ASPECTS on DWI predicts 3-month outcome following rt-PA: SAMURAI rt-PA Registry. Neurology. 2010;75:555–561. doi: 10.1212/WNL.0b013e3181eccf78. doi: 10.1212/WNL.0b013e3181eccf78. [DOI] [PubMed] [Google Scholar]
- 4.Toyoda K, Koga M, Naganuma M, Shiokawa Y, Nakagawara J, Furui E, et al. Stroke Acute Management With Urgent Risk-Factor Assessment and Improvement Study Investigators. Routine use of intravenous low-dose recombinant tissue plasminogen activator in Japanese patients: general outcomes and prognostic factors from the SAMURAI register. Stroke. 2009;40:3591–3595. doi: 10.1161/STROKEAHA.109.562991. doi: 10.1161/STROKEAHA.109.562991. [DOI] [PubMed] [Google Scholar]
- 5.Minematsu K, Toyoda K, Hirano T, Kimura K, Kondo R, Mori E, et al. Japan Stroke Society. Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc Dis. 2013;22:571–600. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.001. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, et al. ENCHANTED Investigators and Coordinators. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016;374:2313–2323. doi: 10.1056/NEJMoa1515510. doi: 10.1056/NEJMoa1515510. [DOI] [PubMed] [Google Scholar]
- 7.Koga M, Toyoda K, Kimura K, Yamamoto H, Sasaki M, Hamasaki T, et al. THAWS Investigators. THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0·6 mg/kg (THAWS) Trial. Int J Stroke. 2014;9:1117–1124. doi: 10.1111/ijs.12360. doi: 10.1111/ijs.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori E, Minematsu K, Nakagawara J, Yamaguchi T, Sasaki M, Hirano T Japan Alteplase Clinical Trial II Group. Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke. 2010;41:461–465. doi: 10.1161/STROKEAHA.109.573477. doi: 10.1161/STROKEAHA.109.573477. [DOI] [PubMed] [Google Scholar]
- 9.Schulman S, Kearon C Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 10.Aoki J, Kimura K, Shibazaki K, Sakamoto Y. Negative fluid-attenuated inversion recovery-based intravenous thrombolysis using recombinant tissue plasminogen activator in acute stroke patients with unknown onset time. Cerebrovasc Dis Extra. 2013;3:35–45. doi: 10.1159/000348552. doi: 10.1159/000348552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreto AD, Martin-Schild S, Hallevi H, Morales MM, Abraham AT, Gonzales NR, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40:827–832. doi: 10.1161/STROKEAHA.108.528034. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho AH, Sohn SI, Han MK, Lee DH, Kim JS, Choi CG, et al. Safety and efficacy of MRI-based thrombolysis in unclear-onset stroke. A preliminary report. Cerebrovasc Dis. 2008;25:572–579. doi: 10.1159/000132204. doi: 10.1159/000132204. [DOI] [PubMed] [Google Scholar]
- 13.Kang DW, Sohn SI, Hong KS, Yu KH, Hwang YH, Han MK, et al. Reperfusion therapy in unclear-onset stroke based on MRI evaluation (RESTORE): a prospective multicenter study. Stroke. 2012;43:3278–3283. doi: 10.1161/STROKEAHA.112.675926. doi: 10.1161/STROKEAHA.112.675926. [DOI] [PubMed] [Google Scholar]
- 14.Schwamm LH, Wu O, Song SS, Latour LL, Ford AL, Hsia AW, et al. MR WITNESS Investigators. Intravenous thrombolysis in unwitnessed stroke onset: MR WITNESS trial results. Ann Neurol. 2018;83:980–993. doi: 10.1002/ana.25235. doi: 10.1002/ana.25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoki J, Kimura K, Iguchi Y, Shibazaki K, Sakai K, Iwanaga T. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39–44. doi: 10.1016/j.jns.2010.03.011. doi: 10.1016/j.jns.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Thomalla G, Rossbach P, Rosenkranz M, Siemonsen S, Krützelmann A, Fiehler J, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65:724–732. doi: 10.1002/ana.21651. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. doi: 10.1056/NEJMoa1215340. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 18.Wong KS, Wang Y, Leng X, Mao C, Tang J, Bath PM, et al. Early dual versus mono antiplatelet therapy for acute non-cardioembolic ischemic stroke or transient ischemic attack: an updated systematic review and meta-analysis. Circulation. 2013;128:1656–1666. doi: 10.1161/CIRCULATIONAHA.113.003187. doi: 10.1161/CIRCULATIONAHA.113.003187. [DOI] [PubMed] [Google Scholar]
- 19.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. HERMES Collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 20.Khatri P, Kleindorfer DO, Devlin T, Sawyer RN, Jr, Starr M, Mejilla J, et al. PRISMS Investigators. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA. 2018;320:156–166. doi: 10.1001/jama.2018.8496. doi: 10.1001/jama.2018.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barow E, Boutitie F, Cheng B, Cho TH, Ebinger M, Endres M, et al. WAKE-UP Investigators. Functional outcome of intravenous thrombolysis in patients with lacunar infarcts in the WAKE-UP trial. JAMA Neurol. 2019;76:641–649. doi: 10.1001/jamaneurol.2019.0351. doi: 10.1001/jamaneurol.2019.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. EXTEND Investigators. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380:1795–1803. doi: 10.1056/NEJMoa1813046. doi: 10.1056/NEJMoa1813046. [DOI] [PubMed] [Google Scholar]
- 23.Ringleb P, Bendszus M, Bluhmki E, Donnan G, Eschenfelder C, Fatar M, et al. ECASS-4 Study Group. Extending the time window for intravenous thrombolysis in acute ischemic stroke using magnetic resonance imaging-based patient selection. Int J Stroke. 2019;14:483–490. doi: 10.1177/1747493019840938. doi: 10.1177/1747493019840938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


