Abstract
Background
Central compartment atopic disease (CCAD) is a recently described variant of chronic rhinosinusitis with nasal polyp (CRSwNP) associated with inhalant allergy. An association with asthma was noted to be uncommon within our clinical practice. The purpose of this study was to determine allergy and asthma prevalence in CCAD and other CRSwNP subtypes.
Methods
A retrospective analysis at a tertiary care institution was performed over the period from 2015 to 2019. CRSwNP was grouped into the following subtypes: allergic fungal rhinosinusitis (AFRS); aspirin-exacerbated respiratory disease (AERD); CCAD; and CRSwNP not otherwise specified (CRSwNP NOS). Patients with sinonasal polyps and concomitant polypoid disease in the central compartment (CRSwNP/CC) were analyzed as a separate cohort for the purpose of this study. Prevalence of allergy and asthma was compared between groups.
Results
Three hundred fiy-six patients were included. CRSwNP NOS was the most common subtype (37.1%) and CRSwNP/CC was the least common (3.7%), with other CRS subtypes ranging between 11.5% and 24.2%. Asthma prevalence was highest in AERD (100%) and CRSwNP NOS (37.1%), but substantially lower in AFRS (19.0%) and CCAD (17.1%). Asthma was significantly more common in AERD and CRSwNP NOS when compared with CCAD (p < 0.001 and p = 0.039, respectively). Prevalence of allergy was significantly higher in AFRS (100%), CCAD (97.6%), CRSwNP/CC (84.6%), and AERD (82.6%) when compared with CRSwNP NOS (56.1%) (p < 0.001).
Conclusion
CCAD represents a clinically distinct phenotype of CRSwNP with a high prevalence of allergy and low prevalence of asthma. Patients with both CCAD and diffuse sinonasal polyps had an allergy prevalence approaching that of CCAD and an asthma prevalence approaching CRSwNP NOS.
Keywords: allergy, asthma, central compartment atopic disease, chronic rhinosinusitis, nasal polyposis
Chronic rhinosinusitis (CRS) is an inflammatory condition that affects 4% to 16% of the United States population.1 It is commonly divided into 2 major entities based on the presence or absence of nasal polyposis, chronic rhinosinusitis with nasal polyposis (CRSwNP), and chronic rhinosinusitis without nasal polyposis (CRSsNP). CRSwNP is of particular interest because within this broad category there are several different variants or clinically relevant phenotypes,2 including allergic fungal rhinosinusitis (AFRS), aspirin-exacerbated respiratory disease (AERD), cystic fibrosis (CF), eosinophilic granulomatosis with polyangitis (formerly Churg-Strauss syndrome), CRSwNP not otherwise specified (CRSwNP NOS), and the more recently described central compartment atopic disease (CCAD).3
An association between edematous/polypoid changes of the middle turbinate (MT) and positive allergy status was first described in 2014.4 This association was further supported by 2 additional studies.5,6 CCAD, described in 2017, is a nasal inflammatory polypoid condition strongly associated with inhalant allergy, involving the superior nasal septum (NS) with or without the MT and/or superior turbinate (ST).7,8 Inhalant allergen deposition in these central compartment structures is related to the course of normal nasal airflow. Our group and others have previously reported a strong association between CCAD and allergy.7,8 In our clinical practice, we have also noted a low association of CCAD with asthma.
Knowledge of associated comorbidities, including allergy and asthma, aid in distinguishing between CRSwNP subtypes. For example, diagnostic criteria for AFRS and AERD include allergy and asthma, respectively. Studies have evaluated allergy and asthma prevalence in CRSwNP patients. However, most of those studies included all CRSwNP subtypes within a single “RSwNP cohort,” thus limiting conclusions.9,10 This has been particularly true when attempting to elucidate allergy prevalence, which has been estimated to range from 50% to 84%.11–13 A recent prospective, cohort study reported on asthma prevalence within specific CRSwNP subtypes, but CCAD was not included in the analysis.14
As CCAD is increasingly recognized as a distinct sinonasal inflammatory subtype, further knowledge regarding its associated comorbidities will aid in the understanding, diagnosis, and management of this entity. The objective of this study was to determine the prevalence of allergy and asthma in CCAD, as compared with other CRSwNP subtypes.
Materials and methods
Study design and population
Approval of the institutional review board at Emory University was obtained before initiating this retrospective study. All patients were treated at a single, tertiary academic center between 2015 and 2019. CRSwNP diagnosis was made based on criteria established by the International Consensus Statement in Allergy and Rhinology: Rhinosinusitis15 and the European Position Paper on Rhinosinusitis and Nasal Polyps.16
CRSwNP patients were categorized into the following subtypes: AFRS; AERD; CRSwNP NOS; and CCAD. The patients were diagnosed with AFRS if there was evidence of nasal polyposis, characteristic radiographic findings, hypersensitivity to fungi, and documented history of eosinophilic mucin with noninvasive fungal hyphae.17 AERD was diagnosed based on presence of asthma and nasal polyposis, as well as a history of positive aspirin challenge or respiratory reaction to aspirin or nonsteroidal anti-inflammatory drugs.18 CCAD was diagnosed based on endoscopic findings of inflammation/polypoid changes located within the central compartment (NS, MT, ST).8 For this study, isolated MT polyp patients were included in the CCAD cohort. Patients who met the diagnostic criteria for AERD or AFRS and who also demonstrated central compartment findings on nasal endoscopy were included only in the AERD and AFRS groups, respectively, as this was their primary and predominant pathology. Central compartment involvement in AERD patients is common, but it is uncommon in AFRS. CRSwNP NOS included all other sinonasal polyp patients that did not meet criteria for one of the other phenotypes. Patients within the CRSwNP NOS cohort did not meet criteria for AERD or AFRS and did not exhibit polypoid changes within the central compartment. A small group of patients with both sinonasal polyps and concomitant polypoid disease in the central compartment (here termed CRSwNP/CC) were analyzed as a separate cohort for the purpose of this study (Fig. 1). To ensure that CRSwNP subtypes were classified correctly, extensive review of the electronic medical record (EMR) (including operative reports and logs, office notes, pathology/laboratory reports, computed tomography [CT] imaging data, outside records) was performed. Other CRSwNP subtypes, including CF and eosinophilic granulomatosis with polyangiitis, were not included in this analysis. Patients with comorbid immunodeficiency or systemic autoimmune disease were also excluded.
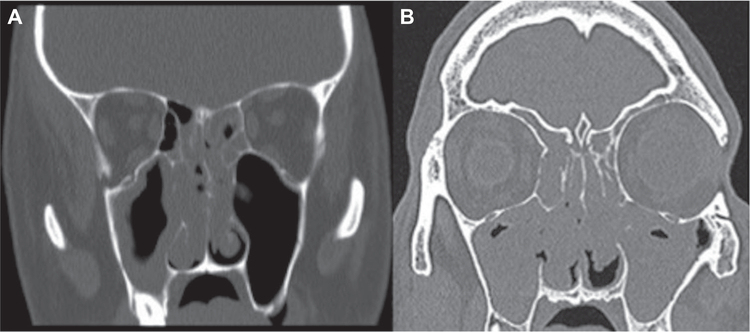
FIGURE 1.
CT scans demonstrating: (A) isolated CCAD and (B) CRSwNP/CC. CCAD = isolated central compartment atopic disease; CRSwNP = chronic rhinosinusitis with nasal polyposis; CRSwNP/CC = chronic rhinosinusitus with sinonasal polyps and central compartment involvement; CT = computed tomography.
The following patient data were collected: (1) demographics (age, sex, race); (2) documented asthma diagnosis by a pulmonologist or allergist (strong clinical history with confirmation by pulmonary function testing [PFT])19; (3) clinical history of allergic rhinitis (AR); and (4) results of allergy testing. Clinical AR was diagnosed based on criteria established by the International Consensus Statement in Allergy and Rhinology: Allergic Rhinitis.20 In patients who underwent allergy testing, aeroallergen sensitization was defined by either serologic assessment or skin testing. Confirmation of allergy testing results was obtained for patients tested before presentation to our institution. For those tested at our institution, the protocol was performed as described elsewhere.8
Statistical analyses
Demographics and clinical characteristics of CRSwNP patients were tabulated. Nonparametric one-way analyses of variance were performed with Bonferroni-corrected post-hoc analyses to examine specific group differences. Statistical tests were performed using SPSS version 24 (IBM, Armonk, NY). Statistical significance was set at p < 0.05.
Results
Study population and demographics
A total of 356 patients were included for analysis. Mean age was 48.4 (range, 18–85) years. One hundred ninety-five patients (54.7%) were male. One hundred eighty-three patients (51.4%) were Caucasian. CRS subtype classification was as follows: CRSwNP NOS, 132 (37.1%); AERD, 86 (24.2%); AFRS, 84 (23.6%); CCAD, 41 (11.5%); and CRSwNP/CC, 13 (3.7%). CRSwNP subtypes differed significantly with respect to age (p < 0.001). Average age of patients with CRSwNP NOS was greater compared with all other subtypes (p = 0.004). CRSwNP groups did not differ by gender (Table 1).
TABLE 1.
Demographics of patients included in the study
| CRSwNP NOS | AERD | AFRS | CCAD | CRSwNP/CC | p Value | |
|---|---|---|---|---|---|---|
| Patients [n (%)] | 132 (37.1%) | 86 (24.2%) | 84 (23.6%) | 41 (11.5%) | 13 (3.7%) | |
| Age, mean (years) | 56.1 | 48.9 | 36 | 46.5 | 50.9 | p < 0.001a |
| Female [n (%)] | 57 (43.2%) | 48 (55.8%) | 34 (40.5%) | 17 (41.5%) | 5 (38.5%) | p = 0.462 |
| Race [n (%)] | ||||||
| Caucasian | 87 (66%) | 44 (51.2%) | 30 (35.7%) | 17 (41.5%) | 5 (38.5%) | |
| African American | 36 (27.2%) | 34 (40%) | 44 (52.3%) | 20 (48.8%) | 6 (46.1%) | |
| Other | 8 (6%) | 3 (3%) | 1 (1.2%) | 3 (7.3%) | 1 (7.7%) | |
| Unknown | 1 (0.8%) | 5 (5.8%) | 9 (10.8%) | 1 (2.4%) | 1 (7.7%) |
Statistically significant difference between groups.
AERD = aspirin-exacerbated respiratory disease; AFRS = allergic fungal rhinosinusitis; CCAD = isolated central compartment atopic disease; CRSwNP NOS = chronic rhinosinusitis with nasal polyposis, not otherwise specified; CRSwNP/CC = patients with central compartment and sinonasal polyps.
Allergy prevalence
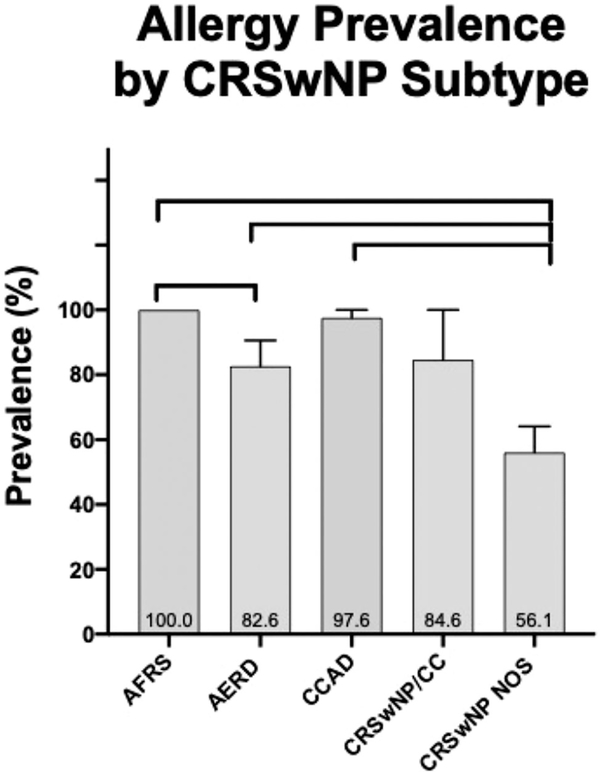
Allergy prevalence, as determined by documented clinical history of AR, was 78.7% across all patients in this series. Allergy breakdown by subtype was 100% in AFRS, 97.6% in CCAD, 84.6% in CRSwNP/CC, 82.6% in AERD, and 56.1% in CRSwNP NOS. Allergy prevalence was significantly higher in CCAD, AERD, and AFRS compared with CRSwNP NOS (p < 0.001). There was also a significant difference in allergy prevalence between AFRS and AERD (p = 0.022) (Fig. 2).
FIGURE 2.
Prevalence of allergy in the CRSwNP subtypes. AERD = aspirin-exacerbated respiratory disease; AFRS = allergic fungal rhinosinusitis; CCAD = isolated central compartment atopic disease; CRSwNP NOS = chronic rhinosinusitis with nasal polyposis, not otherwise specified; CRSwNP/CC denotes those with sinonasal polyps and central compartment involvement. Connecting lines represent statistically significant differences between groups (p < 0.05).
Two hundred fifty-two patients (70.8%) underwent formal allergy testing, including 75 (87.2%) with AERD, 70 (83.3%) with AFRS, 27 (65.9%) with CCAD, 75 (56.8%) with CRSwNP NOS, and 5 (38.5%) with CRSwNP/CC. Positive allergy testing was present in 100% of AFRS patients, 92.6% of CCAD patients, 88% of CRSwNP NOS patients, 80% of CRSwNP/CC patients, and 77.3% of AERD patients (Table 2).
TABLE 2.
Allergy prevalence and testing results
| CRSwNP subtype | Total patients (n) | Positive allergy history [n (%)] | Allergy testing performed [n (%)] | Positive allergy testing [n (%)] |
|---|---|---|---|---|
| CRSwNP NOS | 132 | 74 (56.1%) | 75 (56.8%) | 66 (88%) |
| CCAD | 41 | 40 (97.6%) | 27 (65.9%) | 25 (92.6%) |
| CRSwNP/CC | 13 | 11 (84.6%) | 5 (38.5%) | 4 (80%) |
| AFRS | 84 | 84 (100%) | 70 (83.3%) | 70 (100%) |
| AERD | 86 | 71 (82.6%) | 75 (87.2%) | 58 (77.3%) |
CRSwNP/CC = chronic rhinosinusitus with sinonasal polyps and central compartment involvement.
AERD = aspirin-exacerbated respiratory disease; AFRS = allergic fungal rhinosinusitis; CCAD = isolated central compartment atopic disease; CRSwNP NOS = chronic rhinosinusitis with nasal polyposis, not otherwise specified.
Asthma prevalence
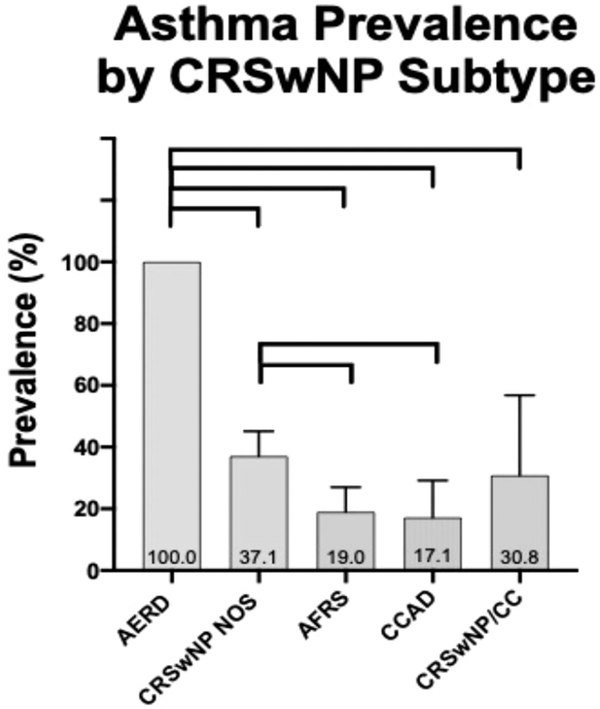
Asthma prevalence was 45.5% among all CRSwNP patients. When analyzed by subtype, prevalence of asthma was as follows: 100% in AERD; 37.1% in CRSwNP NOS; 30.8% in CRSwNP/CC; 19% in AFRS; and 17.1% in CCAD. There was a significant difference in asthma prevalence across subtypes. The AERD group had a significantly higher prevalence of asthma than all other groups (p < 0.001). CCAD and AFRS had a significantly lower prevalence of asthma than CRSwNP NOS (p = 0.039 and p = 0.009, respectively). There were no other significant differences in asthma prevalence across groups (Fig. 3). These associations remained significant even after an adjustment for race (p = 0.016).
FIGURE 3.
Prevalence of asthma in the CRSwNP subtypes. AERD = aspirin-exacerbated respiratory disease; AFRS = allergic fungal rhinosinusitis; CCAD = isolated central compartment atopic disease; CRSwNP/CC = sinonasal polyps and central compartment involvement. CRSwNP NOS = chronic rhinosinusitis with nasal polyposis, not otherwise specified. Connecting lines represent statistically significant differences between groups (p < 0.05).
Discussion
CCAD represents a distinct nasal inflammatory process related to inhalant allergy. This can be considered a separate CRSwNP subtype, although it primarily involves the central compartment nasal mucosa,with the sinuses becoming involved by obstruction in advanced disease. Although previous studies have demonstrated a strong association with allergy, an association between CCAD and asthma has not been studied but has been noted to be uncommon within our clinical practice. This is the first study to evaluate allergy and asthma prevalence in CCAD with comparison to other CRSwNP subtypes, including AERD, AFRS, and CRSwNP NOS.
Several studies have attempted to better understand the relationship between allergy and CRSwNP. Allergic diseases, especially immunoglobulin E (IgE)-mediated inflammatory processes such as AR, have long been considered to be an inciting factor in the development and/or progression of CRSwNP. However, recent systematic reviews have demonstrated an equivocal relationship between allergy and CRSwNP (aggregate level of evidence D).20,21 Yet, a major limitation in the literature is that all CRSwNP variants were often included within a single “CRSwNP cohort,” despite evidence of a greater association of allergy with specific subtypes, including AFRS and CCAD.9,10 In our analysis, overall allergy prevalence was high (78.7%), as determined by clinical history of AR in CRSwNP patients. However, when separated by subtype, allergy prevalence varied significantly across groups. A higher prevalence of allergy was present in AFRS, CCAD, and AERD when compared with CRSwNP NOS. The high prevalence of allergy in AERD is consistent with a recent report by our group, which also demonstrated a high prevalence of central compartment involvement in AERD patients.22 That study also showed multiple etiologies for polyps can exist in the same patient (ie, sinonasal polyposis from AERD and central compartment polyps from inhalant allergy).
Given the retrospective nature of this study, allergy testing was not available for all patients. However, not all patients with classic AR symptoms and inhalant triggers will have positive allergy testing.23 Patients with CRSwNP NOS were the least likely of the CRSwNP subtypes to have allergy testing performed (75 of 132, 56.8%). Thus, it is likely that only CRSwNP patients with a very strong clinical suspicion for AR underwent allergy testing. Therefore, pretest probability was higher, and testing was more likely to be positive in these select patients, which may account for the higher percentage of allergy test–positive patients (88%) vs those with a clinical history of AR (56.1%). Patients diagnosed with other CRSwNP variants, including AERD and AFRS, were more likely to undergo allergy testing (87.2% and 83.3%, respectively), and thus there was greater agreement between clinical symptoms of AR and positive allergy testing.
In general, accurate diagnosis of allergy remains a challenge. Not all patients with positive allergy testing demonstrate clinical symptoms consistent with AR,24 which is consistent with allergen sensitivity being higher in the general population than clinical AR. Furthermore, some patients with classic AR symptoms and inhalant triggers will have negative allergy testing, but may have IgE present in the sinonasal cavity–a condition referred to as local allergic rhinitis (LAR).25 LAR can be diagnosed using nasal allergen provocation testing (NAPT). Ideally, combining a clinical history of AR symptoms, both skin- and serum-specific IgE testing as well as NAPT, would provide the most comprehensive assessment of allergy prevalence. However, four methods of allergy evaluation are not typically performed in clinical practice, and therefore, were not available for this retrospective review.
The overall prevalence of asthma in CRSwNP patients was 45.5%, but also differed when separated by CRSwNP subtype. The prevalence of asthma in AERD was 100%, as expected by diagnostic criteria, and was significantly higher than in AFRS, CCAD, and CRSwNP NOS. The prevalence of asthma in isolated CCAD was low (17.1%), similar to asthma prevalence seen in patients with AFRS (19%). Of note, 13 patients with diffuse sinonasal polyposis also had central compartment polypoid findings (CRSwNP/CC). Because it would be arbitrary to determine which disease process was primary, we chose to report this cohort separately. Some of these patients may have primary CCAD with secondary sinus involvement, and others may have primary sinonasal polyposis and secondary involvement of one or more of the central compartment structures, or they may have 2 equal coexisting processes. These patients had a higher prevalence of asthma (30.8%) when compared to patients with isolated CCAD (17.1%). This higher asthma prevalence of 30.8%, which more closely approximates the 37.1% seen in the CRSwNP NOS group, may be associated with greater sinonasal inflammatory disease burden. The CRSwNP/CC group also had a clinical AR prevalence of 84.6%, which is much closer to the 97.6% seen in isolated CCAD than the 56.1% in CRSwNP NOS. This group of patients shows that multiple diagnoses can be present in the same patient, which may help to explain why the allergy and asthma prevalence in this hybrid group is between the CCAD and CRSwNP NOS groups.
As demonstrated by our analysis, CCAD represents a distinct CRSwNP subtype associated with a high prevalence of allergy and low prevalence of asthma. The prevalence of allergy in CCAD patients was high but did not reach 100% in our study population. As inhalant allergens are thought to contribute to the inflammatory/polypoid changes seen in CCAD, we expected all CCAD patients to demonstrate a clinical history of AR. However, even patients without overt clinical allergy symptoms may demonstrate subclinical sinonasal inflammation. In subjects with seasonal and perennial allergic rhinitis, subthreshold doses of allergen have been shown to cause inflammatory cell infiltration in the nasal mucosa, but this did not result in overt allergy symptoms.26 Long-term exposure to inhaled allergens, even at low or subclinical levels, is likely adequate to cause central compartment inflammation.
This study is the first to evaluate the prevalence of asthma in CCAD. The low prevalence of asthma in CCAD patients was expected based on our clinical experience, but this is difficult to explain. Asthma is often sustained by allergic sensitization, and allergic asthma is the most frequently diagnosed asthma endotype, representing >60% of cases.27 Both allergy and allergic asthma are characterized by type 2 inflammation and may be driven by IgE-mediated triggers.3 Thus, it may be expected that CCAD, given its strong association with allergy, would have a higher prevalence of asthma. However, this was not seen in our analysis. Of note, our study population consisted entirely of adults. Although allergic sensitization is considered a strong risk factor in the development of childhood-onset asthma, adult-onset asthma is often nonallergic.28 Given the retrospective nature of this study, the average age of asthma onset in our patient population could not be determined.
Our theory for the combination of findings identified in this analysis is that the central compartment of the nasal cavity acts as a filter for inhalant allergens, thus reducing contamination of the lower airways. The central compartment receives the greatest airflow, and therefore the most allergen deposition. This is the reason for the involvement of the superior NS, MT, and ST in CCAD, a manifestation of longstanding allergic nasal disease. Therefore, the low asthma prevalence seen in CCAD patients may result from the body’s attempt to filter inhalant allergens to minimize their access to the lower airway. When the filtering capacity of the central compartment structures is overloaded, the protective mechanism is overcome and the lower airway receives more allergen exposure. This could account for the higher asthma prevalence in patients with CRSwNP/CC compared with CCAD. Exceeding the filtering threshold of the central compartment could also help explain the progression of the sinonasal polyposis in this group, possibly due to altered airflow and increased exposure of the middle and superior meatus to inhaled allergens. It remains unclear why some patients with AR develop CCAD and others do not. Possible genetic variations could contribute to the differences in manifestation of inflammatory changes.
Knowledge of associated comorbidities, including allergy and asthma, are helpful to distinguish between different CRSwNP subtypes. For example, AERD is a challenging diagnosis often made at a late stage, and patients may have undergone several sinonasal surgeries before the diagnosis is considered. Although asthma in AERD patients tends to segregate in the severe persistent category, patients with AERD can have mild or moderate persistent asthma or even intermittent asthma,29 which may be missed if not adequately assessed. Furthermore, comorbid AR and asthma may predict severity of disease and increased symptom burden.30 A recent prospective case-control study demonstrated that CRSwNP with comorbid AR and asthma was associated with increased eosinophil ratios and a higher risk of polyp recurrence.31 Therefore, it is necessary to obtain a thorough allergy and asthma history when CRSwNP is identified.
Limitations of our current study include its retrospective design, with the collected data being restricted to what had previously been captured in the EMR. Also, as previously noted, formal allergy testing was not available for all patients, and was likely only performed for those with a strong clinical history for AR or a CRSwNP subtype typically associated with allergy. Future studies may include combinations of clinical history, allergen-specific IgE skin and/or serum testing, as well as NAPT to provide a more complete assessment of allergy prevalence. In addition, there were only 13 patients within the CRSwNP/CC cohort, which limits our conclusions. Finally, results from this single-institution study may not reflect regional influences. Multi-institutional studies that take into account different regions and populations may help to augment these results.
Conclusion
CCAD represents a clinically distinct nasal phenotype of CRSwNP, involving inflammatory changes in the upper nasal cavity, associated with a high prevalence of allergy and low prevalence of asthma. CCAD is similar to AFRS in this respect, although it differs in the location of its sinonasal manifestation. Further characterization of CRSwNP subtypes and their associated comorbidities may improve the diagnosis, management, and understanding of these varying entities.
Acknowledgments
Potential conflict of interest: S.K.W.: OptiNose, scientific advisory board; SinopSys Surgical, scientific advisory board; Stryker, consultant; NeurENT, consultant. J.M.D.: Medtronic, consultant; Intersect, stockholder; Funding sources for the study: National Institutes of Health (Triological Society Research Career Development Award and National Center for Advancing Translational Sciences grants UL1TR002378 and KL2TR002381 to J.M.L.); Spirox (to J.M.D.).
References
- 1.Anand V Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol Suppl. 2004;113:3–5. [DOI] [PubMed] [Google Scholar]
- 2.Succar EF, Turner JH. Recent advances in understanding chronic rhinosinusitis endotypes. F1000Res. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grayson JW, Cavada M, Harvey RJ. Clinically relevant phenotypes in chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2019;48:23:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White LJ, Rotella MR, DelGaudio JM. Polypoid changes of the middle turbinate as an indicator of atopic disease. Int Forum Allergy Rhinol. 2014;4:376–380. [DOI] [PubMed] [Google Scholar]
- 5.Hamizan AW, Christensen JW, Ebenzer J, et al. Middle turbinate edema as a diagnostic marker of inhalant allergy. Int Forum Allergy Rhinol. 2016;7:37–42. [DOI] [PubMed] [Google Scholar]
- 6.Brunner JP, Jawad BA, McCoul ED. Polypoid change of the middle turbinate and paranasal sinus polyposis are distinct entities. Otolaryngol Head Neck Surg. 2017;157:519–523. [DOI] [PubMed] [Google Scholar]
- 7.DelGaudio JM, Loftus PA, Hamizan AW, Harvey RJ, Wise SK. Central compartment atopic disease. Am J Rhinol Allergy. 2017;31:228–234. [DOI] [PubMed] [Google Scholar]
- 8.Hamizan AW, Loftus PA, Alvarado R, et al. Allergic phenotype of chronic rhinosinusitis based on radiologic pattern of disease. Laryngoscope. 2018; 128:2015–2021. [DOI] [PubMed] [Google Scholar]
- 9.Marcus S, Roland L, DelGaudio JM, Wise SK. Relationship between allergy and chronic rhinosinusitis. Laryngoscope Investig Otolaryngol. 2018;4:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus S, DelGaudio JM, Roland LT, Wise SK. Chronic rhinosinusitis: does allergy play a role? Med Sci (Basel). 2019;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedaghat AR, Phipatanakul W, Cunningham MJ. Characterization of aeroallergen sensitivities in children with allergic rhinitis and chronic rhinosinusitis. Allergy Rhinol (Providence). 2014;5:143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutman M, Torres A, Keen KJ, Houser SM. Prevalence of allergy in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130:545–552. [DOI] [PubMed] [Google Scholar]
- 13.Tan BK, Zirkle W, Chandra RK, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Promsopa C, Kansara S, Citardi MJ, Fakhri S, Porter P, Luong A. Prevalence of confirmed asthma varies in chronic rhinosinusitis subtypes. Int Forum Allergy Rhinol. 2016;6:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–S209. [DOI] [PubMed] [Google Scholar]
- 16.Fokkens WJ, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;3:1–298. 3 p preceding table of contents. [PubMed] [Google Scholar]
- 17.Bent JP 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111:580–588. [DOI] [PubMed] [Google Scholar]
- 18.White AA, Stevenson DD. Aspirin-exacerbated respiratory disease. N Engl J Med. 2018;379:1060–1070. [DOI] [PubMed] [Google Scholar]
- 19.Global Initiative for Asthma (GINA). From the global strategy for asthma management and prevention. 2015. https://ginasthma.org/. Accessed XX.
- 20.Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8:108–352. [DOI] [PubMed] [Google Scholar]
- 21.Wilson KF, McMains C, Orlandi RF. The association between allergy and chronic rhinosinusitis with and without nasal polyposis: an evidence based review with recommendations. Int Forum Allergy Rhinol. 2014;4:93–103. [DOI] [PubMed] [Google Scholar]
- 22.DelGaudio JM, Levy JM, Wise SK. Central compartment involvement in aspirin-exacerbated respiratory disease: the role of allergy and previous sinus surgery. Int Forum Allergy Rhinol. 2019:9:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamizan AW, Rimmer J, Alvarado R, et al. Positive allergen reaction in allergic and nonallergic rhinitis: a systematic review. Int Forum Allergy Rhinol. 2017;7:868–877. [DOI] [PubMed] [Google Scholar]
- 24.Arbes SJ, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. [DOI] [PubMed] [Google Scholar]
- 25.Rondon C, Campo P, Togias A. Local allergic rhinitis: concept, pathophysiology and management. J Allergy Clin Immunol. 2012;129:1460–1467. [DOI] [PubMed] [Google Scholar]
- 26.Canonica GW, Compalati E. Minimal persistent inflammation in allergic rhinitis: implications for current treatment strategies. Cl–in Exp Immunol. 2008; 158:260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matucci A, Vultaggio A, Maggi E, Kasujee I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir Res. 2018;19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nijs SB, Venekemp LN, Bel EH. Adult-onset asthma: is it really different? Eur Respir Rev. 2013;22:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevenson DD, Simon RA. Selection of patients for aspirin desensitization treatment. J Allergy Clin Immunol. 2006;118:801–804. [DOI] [PubMed] [Google Scholar]
- 30.Ho J, Alvarado R, Rimmer J, Sewell WA, Harvey RJ. Atopy in chronic rhinosinusitis: impact on quality of life outcomes. Int Forum Allergy Rhinol. 2019;9:501–507. [DOI] [PubMed] [Google Scholar]
- 31.Radabaugh JP, Han JK, Moebus RG, Somers E, Lam K. Analysis of histopatholgical endotyping for chronic rhinosinusitis phenotypes based on comorbid asthma and allergic rhinitis. Am J Rhinol Allergy. 2019;33:507–512. [DOI] [PubMed] [Google Scholar]