Abstract
Knee osteoarthritis (KOA) is a prevalent disease, especially in the elderly. The present study examined the expression of matrix metalloproteinase-13 (MMP-13), NF-κBp65 and interleukin (IL)-lβ in the synovial tissues of KOA patients and the role of MMP-13 and the NF-κBp65 signalling pathway in KOA pathogenesis. A total of 100 KOA patients were enrolled in our hospital from December 2015 to December 2017 and were classified into either a mild KOA group (Outerbridge grade 1 and 2) or a severe KOA group (Outerbridge grade 3 and 4). Non-OA patients were included as controls. Synovial tissues from patients in both groups were collected for detection of the mRNA and protein expression of MMP-13, NF-κBp65 and IL-lβ. Synovial tissue slices were subjected to haematoxylin and eosin staining and immunohistochemistry (SP method). Cartilage tissues were observed under a light microscope after Safranin O-fast green staining. Reverse transcription-quantitative PCR and western blot analyses demonstrated that the expression of MMP-13, NF-κBp65 and IL-lβ in the mild and severe groups were substantially upregulated compared with the control group (all P<0.05). A positive correlation between MMP-13 and NF-κBp65 expression in the KOA synovial tissues was identified (P<0.05). Immunohistochemistry revealed that the expression of MMP-13 and NF-κBp65 was related to the severity of KOA (MMP-13: severe, 92.54%; moderate, 76.52%; control: 32.14%; and NF-κBp65: severe, 85.56%; moderate, 48.12%; control: 28.32%). This evidence indicated that the severity of KOA was related to MMP-13 and NF-κBp65 expression. The NF-κB signalling pathway may be activated during OA progression, which could upregulate the expression of MMP-13 and IL-1β and accelerate the deterioration of articular cartilage.
Keywords: knee osteoarthritis, matrix metalloproteinase-13, NF-κB p65, interleukin-1β, NF-κB signalling pathway
Introduction
Osteoarthritis (OA) is the most prevalent type of arthritis, and it is characterized by a progressive degradation of articular cartilage, new bone formation and acute inflammation (1). Knee osteoarthritis (KOA) with hip osteoarthritis is primarily responsible for the pain and locomotor disability worldwide (2,3). With the increasing trend of ageing and obesity of the population of the world, health services for KOA with hip OA will be in higher demand (4). OA is characterized by chronic pain and tissue destruction, which is concomitant with inflammation in some cases, and patients with OA suffer from constant chronic pain, consequently resulting in disabilities and a heavy social burden (5). The risk factors for KOA include knee extensor muscle weakness, lower extremity muscle strength, afferent sensory dysfunction and genetic issues (6,7). Joint swelling is one clinical feature of OA that is attributed to inflammation (8). Data in a previous study supported the crucial role of inflammation in determining the severity, progression risk, and clinical symptoms of OA (9). Evidence also indicated that inflammation, as evidenced by synovitis or effusion, is a main driver for pain sensitization in KOA (10). Therefore, a better understanding of the inflammatory mechanisms in KOA is required to better manage KOA pain.
In addition to its role in cancer initiation and progression, the NF-κB family of transcription factors also serves an essential role in inflammation and innate immunity (11). Notably, NF-κB is a pivotal regulator of inflammation in rheumatoid arthritis because, in rheumatoid arthritis, persistent NF-κB activation mediates the over-expression of inflammatory cytokines and tissue injury (12,13). Chondrocytes are the primary cell type in articular cartilage, and the loss of these cells induces fatigue of the extracellular matrix (ECM), which results in failure of the cartilage and impairment of the entire joint (14). The pathogenesis of OA is closely related with pro-inflammatory cytokines production by chondrocytes, such as interleukin (IL)-1, which leads to the activation of matrix metalloproteinases (MMPs) and deterioration of OA (15). Mechanical and inflammatory stresses to articular cartilage may disturb the chondrocyte energy balance during the progression of OA, which may deteriorate the pathogenesis of OA (16). However, the exact mechanism of these pro-inflammatory cytokines in OA progression remains an area of active research. Furthermore, no clinical data regarding the expression levels of IL-1β, MMP-13 and NF-κB in KOA has been reported. In this regard, the present study, from a perspective view of a clinical study, was conducted to investigate the expression of pro-inflammation cytokines, MMP-13, NF-κBp65 and IL-1β, in synovial tissue to determine their function in the progression of KOA. A possible mechanism is proposed based on the results raised in this study with the hope of shedding light for better understanding of KOA inflammation.
Materials and methods
Subjects
A total of 100 KOA patients admitted to Nanjing Luhe People's Hospital to receive knee replacements from December 2015 to December 2017 were included in the case group, which had an average age of 60.5 years old (range, 56-81 years old). The body mass index (BMI) for the included subjects was 22.58±3.27 kg/m2. There were 49 males and 51 females. There were 57 subjects with lesions in their left knee, and 43 subjects with lesions in their right knee. The following inclusion criteria were used for the case group: i) meeting the diagnostic basis for OA (17); ii) disease progression for more than 6 months; iii) in cases of bilateral KOA, selecting the more severe side for inclusion; iv) lacking lesions in the heart, blood vessels, liver or kidney; and v) discontinuing medication use, such pain killers, corticosteroids, or any other Chinese herbal preparation 4 weeks before surgery. The criteria for exclusion were as follows: i) patients with active gastroenteric disease or with a history of peptic ulcer or duodenal bleeding; and ii) patients with joint diseases, such as rheumatoid arthritis or infectious arthritis. Patients in case group were classified according to the Outerbridge grade (18) into a mild KOA group (n=59; Outerbridge grades 1 and 2) or a severe KOA group (n=41; Outerbridge grades 3 and 4). A total of 72 patients with injury of the cruciate ligament of the knee joint or meniscus injury in our hospital were included as the control group; the control group had an average age of 54 years (range, 41-81 years old). The time from injury to enrollment of patients in the control group was within three weeks. The control group consisted of 31 males and 41 females; 39 patients had lesions on their left knee, and 33 patients had lesions on their right knee. The living style of patients in the case and control groups, such as smoking history and exercise habits, were recorded for comparison. Sex, age, BMI, smoking history and exercise habits between the two groups were not significantly different, which indicated the comparability of the two groups. A 1.0x1.0x0.5 cm synovial sample of the anterior cruciate of the knee was collected, sealed in double plastic bags and immediately stored in a freezer at -70˚C. Cartilage tissues from the unloaded surface of the condyle were collected in the case and control groups and stored in a freezer at -70˚C until further usage. The present study was performed based on protocols proposed by the Ethics Committee of Nanjing Luhe People's Hospital. All patients signed written informed consent prior to participation in the study.
Haematoxylin & eosin staining
Tissue samples were fixed in 4% paraformaldehyde at 20˚C for 24 h and embedded in paraffin. Paraffin-embedded tissues were sliced into 4-µm thick sections. Some of the sections were subsequently dewaxed twice in xylene at 20˚C for 10 min and rehydrated in a descending alcohol series (90, 80 and 70%), prior to being washed with distilled water for 2 min. The slices were stained using haematoxylin for 5 min before rinsing and re-staining in 5% eosin for 2 min, both at 20˚C. Sections were sealed by using neutral resins for observation under a light microscope (magnification, x200; Olympus Corporation). The remaining tissue sections were stored at -20˚C until further use for Safranin O-Fast Green staining and immunohistochemistry.
Safranin O-Fast Green staining
Safranin O-Fast Green staining (Sigma-Aldrich; Merck KGaA) was performed to observe the morphology of cartilage tissues under a light microscope. Paraffin-embedded slices were dewaxed in xylene at 20˚C for 10 min and rehydrated with absolute alcohol for 5 min, 95% alcohol for 5 min and 80% alcohol for 5 min, prior to being washed with distilled water for 2 min. Sections were subsequently stained with haematoxylin at 20˚C for 3 min, before being washed three times with distilled water. Hydrochloric acid (1%) and ethanol were used for 15 sec at 20˚C to differentiate the slices. The slices were washed three times with distilled water and immersed in a 0.02% Fast Green solution at 20˚C for 3 min. The stained slices were washed in 1% glacial acetic acid and stained in 0.1% Safranin O at 20˚C for 3 min. The slices were sealed to observe the cellular matrix staining, tidemark and calcification of cartilage tissues under a light microscope (magnification, x100).
Immunohistochemistry
A 3% H2O2 solution was incubated with the paraffin-embedded sections at 20˚C for 10 min to terminate the activity of endogenous peroxidase and antigen retrieval buffer was added to the sections prior to boiling for 5-10 min; the sample was cooled for 5 min, and this boiling-cooling cycle was repeated twice. The slices were cooled to room temperature, and 5% BSA (Boster Biological Technology) blocking buffer was added at room temperature for 20 min. Primary mouse anti-human antibodies against MMP-13 (1:500; cat. no. ab219620; Abcam), NF-κBp65 (1:100; cat. no. ab16502; Abcam) and GAPDH (1:1,000; cat. no. ab181602; Abcam) were incubated at 4˚C overnight. Biotinylated goat anti-mouse IgG (1:2,000; cat. no. ab64257; Abcam) was added for incubation at 37˚C for 40 min. The slices were washed, and DAB (Beijing ZSGB-BIO; OriGene Technologies, Inc.) was applied for colour development. The dried slices were observed and photographed under a microscope. Cells with brownish yellow or dark brown particles were positive cells. Five high-powered fields (magnification, x400) were selected to calculate the percentage of positive cells in all cells and the positive cell rate, which was the relative protein expression. Two independent technicians observed and assessed all slices by using a double-blind method.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from frozen tissues using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and preserved at -80˚C. The PrimeScript™ RT reagent kit (Perfect Real-Time; Takara Biotechnology Co., Ltd.) was used to reverse-transcribe the total RNA into cDNA, according to the manufacturer's protocol, which was stored at -20˚C until further use. GAPDH was used as an internal control. An ABI7500 quantitative PCR instrument (ABI; Thermo Fisher Scientific, Inc.) and a SYBR-Green PCR reagent kit (Thermo Fisher Scientific, Inc.) was used to perform the qPCR. The reaction was performed using the following conditions: Initial denaturation at 95˚C for 5 min; followed by 40 cycles of denaturation at 90˚C for 30 sec, annealing at 60˚C for 40 sec and extension at 72˚C for 40 sec. The primer sequences used for RT-qPCR are listed in Table I. Each sample was measured three times, and the 2-ΔΔCq method (19) was used to calculate the relative expression of target genes, and expression levels were normalized to GAPDH.
Table I.
Primer sequences used for reverse transcription-quantitative PCR.
| Gene | Primer sequence (5'→3') |
|---|---|
| MMP-13 | F: AATATCTGAACTGGGTCTTCCAAAA |
| R: CAGACCTGGTTTCCTGAGAACAG | |
| NF-κBp65 | F: TGGAGCAAGCCATTAG |
| R: GGGCACGGTTATCAA | |
| IL-1β | F: AGTGCCTACGCACATGTCTTC |
| R: TGCGTCACACAGAAACTCGTC | |
| GAPDH | F: GCACCGTCAAGGCTGAGAAC |
| R: ATGGTGGTGAAGACGCCAGT |
F, forward; R, reverse; IL-1β, interleukin-1β; MMP-13, matrix metalloproteinase-13;
Western blotting
RIPA protein lysis buffer (Beyotime Institute of Biotechnology) was added to frozen tissues for collection of total protein. The Bradford method (Thermo Fisher Scientific, Inc.) was used for protein quantitation. Then, 50 µg protein was subjected to 12% SDS-PAGE, and the separated proteins were transferred to polyvinylidene fluoride membranes. The membranes were blocked in 5% skimmed milk powder at 37˚C for 1 h. Primary rabbit anti-human antibodies against MMP-13 (1:3,000; cat. no. ab39012; Abcam), NF-κBp65 (1:2,000; cat. no. ab16502; Abcam) and IL-lβ (1:1,000; cat. no. ab9722; Abcam) and a monoclonal antibody against GAPDH (1:1,000; cat. no. ab181602; Abcam) were incubated with the membranes at 4˚C overnight. PBS-0.05% Tween-20 was then used to wash the membrane three times, each for 5 min. Horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:4,000; cat. no. ab6721; Abcam) were added for incubation at room temperature for 2 h. The membranes were washed after incubation with antibodies. Luminol Reagent and Peroxide Solution (EMD Millipore) was prepared at a 1:1 ratio for colour development and imaging. The ratio of the grey values of the target band and control band indicated the relative protein expression. Relative protein levels were semi-quantified using ImageJ 1.40 software (National Institutes of Health). Each measurement was performed three times to calculate an average value.
Statistical analysis
All data were processed by the SPSS version 20.0 software (IBM Corp.). Enumeration data were expressed as a ratio or a percentage. Measurement data were presented as the mean ± SD and the comparisons among groups was conducted using one-way ANOVA and Tukey's multiple comparison test. The correlation analysis between the expression levels of MMP-13 and NF-κBp65 were determined by Pearson's correlation coefficient. The correlation between the expression levels of MMP-13, NF-κBp65 and IL-1β with the clinicopathological factors of patients were determined using Spearman's correlation coefficient. P<0.05 was considered to indicate a statistically significant difference.
Results
Severe KOA tissues exhibit inflammatory cell infiltration and collagen deposition
Synovial tissues of the control group contained few cells in the synovial intima with smooth cell surfaces, and cells in the synovial lining and synovial subintima were evenly distributed without inflammatory cell infiltration (Fig. 1A). Loosening synovial tissues, uneven cell distribution, some proliferative collagenous fibres and inflammatory cell infiltration were observed in the synovial tissues of the mild KOA group (Fig. 1B), and substantial cell infiltration in the synovial lining, irregular cell arrangement, inflammatory cell infiltration and increased collagen disposition in the synovium were observed in the synovial tissues of the severe KOA group (Fig. 1C).
Figure 1.
Haematoxylin and eosin staining of synovial tissues reveals that the severe KOA group has the worst inflammatory cell infiltration and the most collagen deposition. (A) Control group, cells in synovial lining without inflammatory cell infiltration. (B) Mild KOA group, looser synovial tissues, irregular cell distribution, inflammatory cell infiltration. (C) Severe KOA group, substantial cell infiltration, irregular cell distribution, increased collagen deposition. Magnification, x200; scale bars, 50 µm. KOA, knee osteoarthritis.
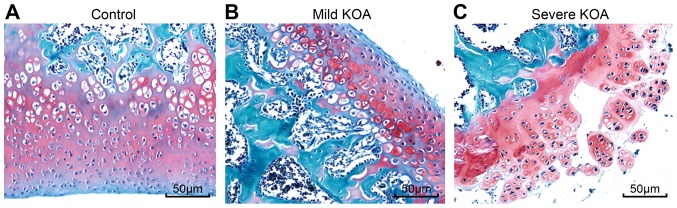
Irregular cell arrangement and thin cartilage in KOA
Safranin O-Fast Green staining was performed to observe chondrocytes under a light microscope. The cartilage and subchondral bone of the control group were stained red and blue, and the cell nuclei were stained dark blue. Based on the boundary of the tidemark along the cartilage and subchondral bone, the control group exhibited many chondrocytes of uniform size that were well arranged and distributed. The tidemark in the mild KOA group was enlarged; the chondrocytes were not evenly stained and exhibited an irregular arrangement. Safranin O-fast green staining also revealed that the chondrocytes in the severe KOA group were markedly disordered compared to the mild KOA group, presenting many enlarged chondrocytes, irregular round and columnar cells, thin trabecular bone, numerous cartilage spaces and a discontinuous tidemark (Fig. 2).
Figure 2.
Safranin O-Fast Green staining of synovial tissues to observe the morphology of chondrocytes. (A) Control group, chondrocytes were well arranged and distributed. (B) Mild KOA group, chondrocytes were not evenly stained and exhibited an irregular arrangement. (C) Severe KOA group, chondrocytes were markedly disordered, presenting many enlarged chondrocytes, irregular round and columnar cells, thin trabecular bone, numerous cartilage spaces and a discontinuous tidemark. Magnification, x100; scale bars, 50 µm. KOA, knee osteoarthritis.
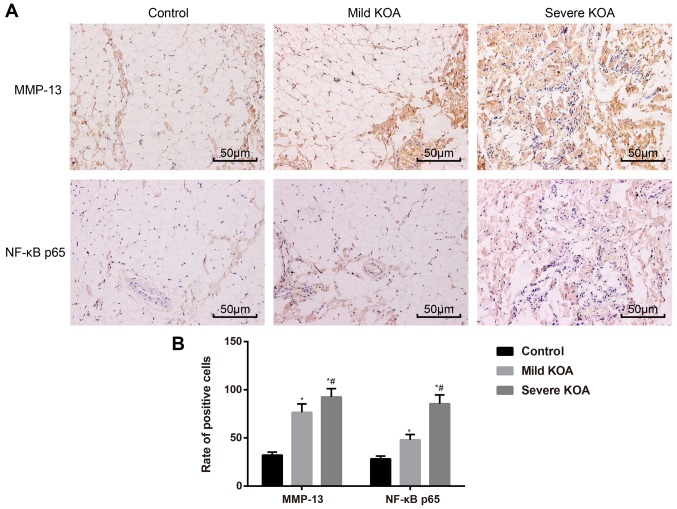
Expression of MMP-13 and NF-κBp65 is associated with KOA severity
Immunohistochemistry was used to evaluate the expression of MMP-13 and NF-κBp65 in synovial tissues. In the synovial tissues of the case group, MMP-13 and NF-κBp65 stained weakly or were stainless, while in the mild KOA group, MMP-13 and NF-κBp65 were stained brown-yellow and were localized intercellularly. In the severe KOA group, MMP-13 and NF-κBp65 were stained brown-yellow or dark brown and localized both intercellularly and in the cytoplasm (Fig. 3A). The rate of MMP-13-positive staining in the three groups was as follows: (76.52±8.76%) in the mild KOA group; (92.54±8.67%) in the severe KOA group; and (32.14±3.23%) in the control group. The rate of NF-κBp65-positive staining in the three groups is as follows: (48.12±5.44%) in the mild KOA group; (85.56±9.16%) in the severe KOA group; and (28.32±2.92%) in the control group. The rates of MMP-13- and NF-κBp65-positive staining in the mild KOA group and the severe KOA group were substantially higher than those of the control group (all P<0.05). A comparison of positive rates of MMP-13 and NF-κBp65 staining between the mild KOA group and severe KOA group also revealed a significant difference (both P<0.05; Fig. 3B). These results support the association of MMP-13 and NF-κBp65 expression with KOA severity.
Figure 3.
Expression of MMP-13 and NF-κBp65 in synovial tissues of the control group, mild KOA group and severe KOA group visualized by using immunohistochemistry. (A) Immunohistochemistry demonstrating MMP-13 and NF-κBp65 expression in synovial tissues. Magnification, x400; scale bars, 50 µm. (B) Statistical analyses of positive rates of MMP-13 and NF-κBp65 in synovial tissues. *P<0.05 vs. Control; #P<0.05 vs. mild KOA. KOA, knee osteoarthritis; MMP-13, matrix metalloproteinase-13.
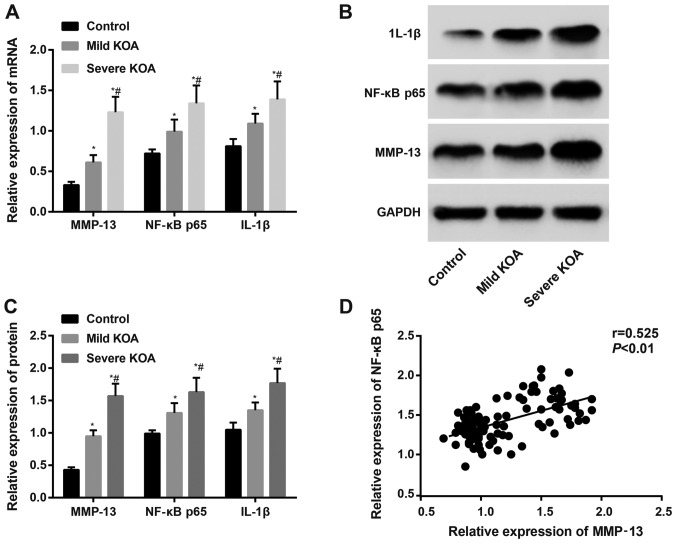
Positive correlation of MMP-13 and NF-κBp65 in KOA
RT-qPCR and western blotting assessed the mRNA and protein expression, respectively, of MMP-13, NF-κBp65 and IL-1β. The results revealed that the mRNA and protein expression of MMP-13, NF-κBp65 and IL-1β in the KOA groups were increased compared to the control group (all P<0.05). The expression of MMP-13, NF-κBp65 and IL-1β in the severe KOA group was higher than the mild KOA group (all P<0.05). Correlation analysis revealed that the expression of MMP-13 in KOA synovial tissues was positively correlated with NF-κBp65 (Fig. 4).
Figure 4.
Detection of mRNA and protein expression of MMP-13, NF-κBp65 and IL-1β by using RT-qPCR and western blotting. (A) mRNA expression of MMP-13, NF-κBp65 and IL-1β using RT-qPCR. (B) Western blotting of MMP-13, NF-κBp65 and IL-1β protein expression. (C) Protein expression of MMP-13, NF-κBp65 and IL-1β using western blotting. (D) Correlation analyses of MMP-13 with NF-κBp65 in synovial tissues. *P<0.05 vs. Control group; #P<0.05 vs. mild KOA. Correlation analysis was performed using the Spearman's test. RT-qPCR, reverse transcription quantitative-PCR; KOA, knee osteoarthritis; MMP-13, matrix metalloproteainse-13; IL, interleukin.
Expression of MMP-13, NF-κBp65 and IL-1β is positively correlated with KOA grade
Demographic data, such as sex, age, BMI, smoking history, exercise habits and KOA grade, of patients with KOA were recorded. These factors underwent correlation analyses with the expression of MMP-13, NF-κBp65 and IL-1β. The results indicated that the protein expression of MMP-13, NF-κBp65 and IL-lβ was positively associated with the KOA grade (all P<0.05), but no association was revealed for other factors, such as sex, age, BMI, smoking history or exercise habits (Table II).
Table II.
Correlation of the expression of MMP-13, NF-κBp65 and IL-1β with clinicopathological factors of patients with knee osteoarthritis.
| Variables | MMP-13 | NF-κBp65 | IL-lβ | |
|---|---|---|---|---|
| Sex | r | 0.012 | 0.022 | 0.015 |
| P-value | 0.665 | 0.496 | 0.581 | |
| Age | r | 0.019 | 0.058 | 0.059 |
| P-value | 0.897 | 0.312 | 0.692 | |
| Body Mass Index | r | 0.123 | 0.202 | 0.143 |
| P-value | 0.745 | 0.516 | 0.642 | |
| Smoking history | r | 0.043 | 0.039 | 0.049 |
| P-value | 0.543 | 0.475 | 0.611 | |
| Exercise habits | r | 0.145 | 0.179 | 0.212 |
| P-value | 0.321 | 0.411 | 0.549 | |
| KOA grade | r | 0.443 | 0.398 | 0.541 |
| P-value | 0.018 | 0.037 | 0.031 |
MMP-13, matrix metalloproteinase-13; IL, interleukin; KOA, knee osteoarthritis.
Discussion
Numerous studies have investigated the pathology of KOA over the past decades, and a consensus was reached that inflammation plays a central role in the progression of cartilage damage (20). The present study assessed three inflammation-related factors, MMP-13, IL-1β and NF-κBp65, in synovial tissues of patients with KOA. The results of the present study revealed that the severity of cartilage damage was positively correlated with KOA progression. The expression of MMP-13 and NF-κBp65 indicated that the activation of the NF-κB signalling pathway occurs in KOA patients, which upregulates the expression of MMP-13 and its downstream inflammation factor, IL-1β, to accelerate the degradation of articular cartilage.
To support this hypothesis, the morphology of chondrocytes in each group was first observed, and the results revealed that patients with severe KOA had worse inflammatory cell infiltration and collagen deposition. The expression of three cytokines (MMP-13, IL-1β and NF-κBp65) in each group was next assessed, and the expression of MMP-13, IL-1β and NF-κBp65 was revealed to correlated with KOA severity. These results revealed that the expression of these three factors indicated articular cartilage damage. Chondrocytes stimulate the secretion of cytokines, chemokines and adipokines, and numerous cell surface receptors for these molecules exist; further, activation of these receptors stimulates intracellular signalling pathways involved in inflammatory and stress responses in chondrocytes in OA joints (21). Many mediators are implicated in the initiation and development of KOA, including pro-inflammatory cytokines IL-1β and tumour necrosis factor-α (TNF-α), which are synthesized locally by synovial cells and chondrocytes and play a critical role in maintaining cartilage damage in arthritis by creating an imbalance between the degradation and repair processes of cartilage (22). IL-1β could influence the anabolism of chondrocytes by increasing the production of MMPs, which are well known for their role in suppressing cartilage matrix formation (23).
MMP-13 has been revealed to play a role in the physiological turnover of cartilage via cleavage of various ECM molecules, such as type II collagen. Aberrant expression of MMP-13 was revealed to be closely related with numerous diseases, including arthritis, cancer, atherosclerosis and fibrosis (24). One study on rheumatoid arthritis in animal models revealed that rats receiving anti-IL-17 treatment exhibited decreased MMP-13 expression and alleviated inflammation, which supports the role of MMP-13 in the progression of arthritic diseases (25). Notably, an animal study evaluating rodents after hind limb immobilization revealed that a loss of proteoglycan content was associated with increased MMP-3, whereas joint movement prevented protease increase and proteoglycan loss (26). SIRT1 is also a key regulator of inflammation in mammalian cells via its interaction with pro-inflammatory factors (22). A previous study also demonstrated a role for NAD-dependent deacetylase sirtuin-1 (SIRT1) in OA progression, which confirms that SIRT1 inactivation induced the expression of the chondrocyte marker RUNX2 and the secretion of MMP-13 in OA chondrocytes (16). The correlation of MMP-13 and NF-κBp65 was also analysed in patients with KOA. MMP-13 expression was positively correlated with NF-κBp65, which indicates that the activation of the NF-κB signalling pathway induced the production of MMP-13. Once the NF-κB signalling pathway is activated (27), mechanical forces also trigger cell surface mechanoreceptors, which induce mitogen-activated protein kinase (MAPK) signalling (28); MAPK signalling thus mediates the expression levels of downstream target genes, such as MMP-13, NOS2, COX2, ADAMTS and IL-1β genes (21).
The present study however, also has its limitation. As indicated in other studies, females are more prone to suffer from KOA or hip osteoarthritis than males. However, the present study revealed that sex was not a risk factor for KOA. This inconsistency may be explained by the limited sample size of this study or this result may be a random phenomenon that occurred haphazardly. Although the present data failed to demonstrate sex as a risk factor for KOA in this study, more research is required to verify the effect of sex on KOA and attention will be paid on sex distribution in KOA in our future study.
In conclusion, the evidence in the present study indicated that the expression of MMP-13, NF-κBp65 and IL-1β was correlated with the severity of KOA. A positive correlation between MMP-13 and NF-κBp65 and KOA severity was revealed, which indicated that activation of the NF-κB signalling pathway occurs in KOA progression. The activation of this pathway upregulated the expression of MMP-13 and its downstream inflammatory factor IL-1β, which accelerates the degradation of articular cartilage. However, the knowledge gained from studies of cartilage derived clinically and from animal models has elucidated many important biological processes that may be involved in KOA progression. More studies on KOA pathologies are required to further investigate the interactions between inflammation, genetic issues and other factors in this disease.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the Nanjing Medical Science and Technique Development Foundation (grant nos. YKK17242 and YKK18223).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FQL and QHZ designed and performed the experiments; QHZ and RZ analyzed the data and wrote the manuscript; LPL and YXG guided and supervised the experimental process, and interpreted the results; RZ, ZW and ZPS collected the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was performed based on protocols proposed by the Ethics Committee of Nanjing Luhe People's Hospital. All patients signed written informed consent prior to participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Srivastava R, Das SK, Goel G, Asthana A, Agarwal GG. Does long term colchicine prevent degradation of collagen fiber network in osteoarthritis? Int J Rheum Dis. 2018;21:114–117. doi: 10.1111/1756-185X.13022. [DOI] [PubMed] [Google Scholar]
- 2.Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: A systematic literature review. Eur J Pain. 2014;18:1367–1375. doi: 10.1002/j.1532-2149.2014.499.x. [DOI] [PubMed] [Google Scholar]
- 3.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 5.Bayjensen AC, Abramson SB, Samuals J, Byrjalsen I, Krasnokutsky S, Manonjensen T, Karsdal MA, Attur M. SAT0437 osteoarthritis pain is differentially associated with tissue degradation and joint inflammation. Osteoarthritis Cartilage. 2017;25(S353) [Google Scholar]
- 6.Øiestad BE, Juhl CB, Eitzen I, Thorlund JB. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:171–177. doi: 10.1016/j.joca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol. 2011;7:57–63. doi: 10.1038/nrrheum.2010.195. [DOI] [PubMed] [Google Scholar]
- 8.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67:956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, Woolf C, Niu J, Bradley LA, Quinn E, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: The multicenter osteoarthritis study. Arthritis Rheumatol. 2016;68:654–661. doi: 10.1002/art.39488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12(86) doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makarov SS. NF-kappa B in rheumatoid arthritis: A pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 2001;3:200–206. doi: 10.1186/ar300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu N, Gu J, Huang T, Zhang C, Shu Z, Li M, Hao Q, Li W, Zhang W, Zhao J, et al. A novel NF-κB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Sci Rep. 2016;6(20059) doi: 10.1038/srep20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia Y, Darling EM, Herzog W. Functional properties of chondrocytes and articular cartilage using optical imaging to scanning probe microscopy. J Orthop Res. 2018;36:620–631. doi: 10.1002/jor.23757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma CH, Wu CH, Jou IM, Tu YK, Hung CH, Hsieh PL, Tsai KL. PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes. Redox Biol. 2018;14:72–81. doi: 10.1016/j.redox.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yui N, Kobayashi H, Terauchi K, Yoshioka H, Fujiya H, Niki H, Musha H, Yudoh K. The role of atp-activated protein kinase(AMPK) in the chondrocyte energy balance and IL-1β-induced production of MMP-13 in osteoarthritis(OA) Osteoarthritis Cartilage. 2016;24:S154–S155. [Google Scholar]
- 17.Kneer W, Rother M, Mazgareanu S, Seidel EJ. A 12-week randomized study of topical therapy with three dosages of ketoprofen in Transfersome® gel (IDEA-033) compared with the ketoprofen-free vehicle (TDT 064), in patients with osteoarthritis of the knee. J Pain Res. 2013;6:743–753. doi: 10.2147/JPR.S51054. European IDEA-033 study group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabriel SM, Clifford AG, Maloney WJ, O'Connell MK, Tornetta P III. Unloading the osteoarthritic knee with a novel implant system. J Appl Biomech. 2013;29:647–654. doi: 10.1123/jab.29.6.647. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Hayer S, Bauer G, Willburger M, Sinn K, Alasti F, Plasenzotti R, Shvets T, Niederreiter B, Aschauer C, Steiner G, et al. Cartilage damage and bone erosion are more prominent determinants of functional impairment in longstanding experimental arthritis than synovial inflammation. Dis Model Mech. 2016;9:1329–1338. doi: 10.1242/dmm.025460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15(375) doi: 10.1007/s11926-013-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon MH, Jeong JK, Lee YJ, Seol JW, Jackson CJ, Park SY. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthritis Cartilage. 2013;21:470–480. doi: 10.1016/j.joca.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Song J, Jin EH, Kim D, Kim KY, Chun CH, Jin EJ. MicroRNA-222 regulates MMP-13 via targeting HDAC-4 during osteoarthritis pathogenesis. BBA Clin. 2014;3:79–89. doi: 10.1016/j.bbacli.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto K, Okano H, Miyagawa W, Visse R, Shitomi Y, Santamaria S, Dudhia J, Troeberg L, Strickland DK, Hirohata S, et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016;56:57–73. doi: 10.1016/j.matbio.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shui XL, Lin W, Mao CW, Feng YZ, Kong JZ, Chen SM. Blockade of IL-17 alleviated inflammation in rat arthritis and MMP-13 expression. Eur Rev Med Pharmacol Sci. 2017;21:2329–2337. [PubMed] [Google Scholar]
- 26.Leong DJ, Gu XI, Li Y, Lee JY, Laudier DM, Majeska RJ, Schaffler MB, Cardoso L, Sun HB. Matrix metalloproteinase-3 in articular cartilage is upregulated by joint immobilization and suppressed by passive joint motion. Matrix Biol. 2010;29:420–426. doi: 10.1016/j.matbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam J, Aguda BD, Rath B, Agarwal S. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: Experiments and modeling. PLoS One. 2009;4(e5262) doi: 10.1371/journal.pone.0005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald JB, Jin M, Chai DH, Siparsky P, Fanning P, Grodzinsky AJ. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem. 2008;283:6735–6743. doi: 10.1074/jbc.M708670200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.