Abstract
The aim of the present study was to evaluate the clinical efficacy and safety of 540 nm-wavelength intense pulsed light (IPL) for the treatment of telangiectasia in late-stage rosacea. Between July 2013 and January 2016, patients with rosacea who tested positive for Demodex folliculorum were recruited. Patients received anti-mite therapy and were then randomly apportioned to receive either three 540 nm-IPL treatments at 4-week intervals (IPL group), or no treatment (control group). Telangiectasia was assessed by the same clinician at baseline and at follow-up intervals over 2 years, where ≥90% clearance of telangiectasia was considered to indicate effective treatment. The rates of effective treatment, improvement (≥30% clearance) and recurrence (original or neo-location) were compared in both groups. After 33 patients were lost during follow-up, the IPL and control groups were comprised of 107 and 120 patients for the final analysis, respectively. The rates of effective treatment and total efficacy in the IPL group (66.36 and 95.33%, respectively) were found to be significantly higher compared with those of the control group (0 and 30.83%, respectively). By contrast, the rates of recurrence were found to be lower in the IPL group (8.41%) compared with the control group (48.33%). Redness-to-blisters associated with IPL treatment (9.7% of analyzed patients) subsided within one week and hyperpigmentation (1.9%) within 3 months. To conclude, treatment with 540 nm-IPL improved facial telangiectasia in late-stage rosacea that remained after sequential anti-mite therapy and effectively reduced the recurrence of rosacea. The present study was registered into the Chinese Clinical Trial Registry under the title ‘Sequential therapy for mites folliculitis’ (Trial registration number: ChiCTR-IPR-15006451; approved May 27, 2015).
Keywords: rosacea, telangiectasia, intense pulsed light
Introduction
Rosacea is a chronic inflammatory skin condition that is characterized by either transient or persistent mid-facial flushing, blushing, erythema, telangiectasia, papules and pustules (1). This condition is associated with blood vessels beneath the skin surface and sebaceous glands, where phymas or rhinophyma may develop in the long term (2,3). Patients with rosacea suffer physical and psychosocial burdens, especially in women and young adults who exhibit high disease incidence rates (4,5). Previous epidemiological studies suggest that patients with rosacea suffer increased risks of depression, anxiety disorders and dementia, especially Alzheimer's disease (6-8).
Despite high morbidity, which is 10% in caucasians (9), the cure for rosacea remains elusive. Although medications, phototherapy and surgery are frequently applied for symptomatic control (7,10,11), these therapies satisfy <50% of the patients according to a recent web-based survey (12). The high recurrence rates and side effects associated with cryotherapy and phototherapy, including scarring and hyperpigmentation, provide motivation for further research into novel treatment methods for rosacea.
It was previously reported that an ornidazole-based (anti-mite) therapy markedly reduced erythema, papules and pustules in patients with rosacea, which was attributed to the suppression of Demodex folliculorum infestation (13,14). However, although this treatment alleviated the development of papules and pustules, erythema and telangiectasia resulting from rosacea persists (13,14).
Brimonidine tartrate gel was the first drug approved by the United States Food and Drug Administration to address facial redness due to rosacea (15). Although this drug temporarily reduces persistent erythema in the middle of the face, it does not affect preexistent dilated capillaries and inflammatory lesions (16-18).
Additional therapeutic strategies for telangiectasia include freezing, intense pulsed light (IPL), lasers and minimally invasive surgeries. Laser treatment options, including pulsed dye, neodymium-doped:yttrium aluminium garnet and IPL, have been previously demonstrated to not only improve erythema but also telangiectasia symptoms (17).
IPL has been applied to treat vascular and pigmented lesions, it was also considered that IPL should be investigated further for the treatment of telangiectasia of late-stage rosacea (17,19). Therefore, the present study evaluated the clinical efficacy and safety of 540 nm-wavelength IPL for the treatment of telangiectasia in late-stage rosacea.
Materials and methods
Ethics
The Ethics Committee of the 940th Hospital of the Joint Logistics Support Force of the Chinese People's Liberation Army approved the present study protocol (Lanzhou, China). Each patient provided signed informed consent prior to enrollment. The clinical trial registration number was ChiCTR-IPR-15006451 (Chinese Clinical Trial Registry).
Patient enrollment and grouping
The study was a prospective randomized controlled trial. The study population (n=260; 214 women and 46 men) was comprised of patients who had received diagnosis of papulopustular rosacea in the dermatological clinics of The 940th Hospital of the Joint Logistics Support Force of the Chinese People's Liberation Army between July 2013 and January 2016. For inclusion, patients conformed to the following criteria: i) Aged 18-60 years; ii) received a clinical diagnosis of rosacea according to classification systems described previously (20,21); iii) underwent examination of facial skin for the presence of live mites by microscopy; and iv) underwent ornidazole-based sequential therapy (12,13) with major resolution of the inflammatory symptoms like papules and pustules, but telangiectasia remained. These patients were considered to have late-stage rosacea and were of types III or IV according to the Fitzpatrick classification (22).
Patients with any of the following were excluded from this analysis: i) Photosensitivity; ii) immune deficiency and the use of immunosuppressants; iii) diabetes; iv) malignant tumor; v) epilepsy; vi) serious liver, kidney or heart disease; vii) infectious or inflammatory diseases at the treatment site; viii) scar diathesis; ix) pregnancy/lactation; x) patients using oral application of tretinoin during the previous 6 months or topical tretinoin application within the previous 3 months; xi) patients with sun overexposure within the past 2 weeks; or xii) patients who had received a diagnosis of rosacea and received any treatment other than ornidazole-based sequential therapy prior to the study period.
Patients who fulfilled the inclusion criteria were randomly allocated into the following two groups: i) IPL treatment (experimental group; n=130); or ii) no treatment (control group; n=130).
IPL treatment in the experimental group
Patients in the experimental group were first cleansed of facial cosmetics and were placed in a supine position. A photograph was then taken for the record before treatment. A layer (1-2 mm) of IPL gel (MIBO) was evenly applied onto the face of the patient.
Both the doctor and patient wore safety goggles throughout the treatment procedure. IPL was applied three times, at 4-week intervals, using an Alma Lasers Lovely II (Alma Lasers Ltd.). The IPL parameters were as follows: i) Wavelength, 540 nm; ii) spot size, 1.5x4 cm2; iii) fluence, 10-16 J/cm2; iv) pulse width, 12 msec and v) pulse interval, 10-15 msec. There was no overlapping in the application of pulses, ensuring that each area received a single pulse. Treatment was initiated by stepping on a pedal switch, at which time the treatment pulse was emitted. A test was first performed on a small area of the skin using the selected parameters, where the subsequent reaction was examined. If the patient showed slight redness of the skin without abnormal stabbing pain, the treatment procedure was commenced. The handheld light-emitting module was held at a 90˚ angle to the skin and in slight contact with the gel. The treatment area overlapped with the normal skin by 1-2 mm. Regardless of the size of the lesion, a full-scale exposure was applied to avoid the uneven distribution of facial pigments. Treatment was ended when the expanded blood vessel disappeared or turned dark purple. IPL treatment promotes the formation of blood clots in dilated blood vessels according to the principle of photocoagulation, leading to the occlusion and disappearance of the expanded capillaries (23). A shading plate was used when the vascular lesion was located and the surrounding skin required protection from the laser. After treatment, the IPL gel was gently wiped off, following which the parameter settings and the immediate skin responses were recorded. The entire process lasted 15-20 min per session.
After treatment, ice-cold compresses were applied for 30-60 min. The patient was advised to avoid hot baths and cosmetics for 1 week after treatment and to avoid sun exposure during the entire treatment regimens duration. A light brown scab appeared 2-3 days after treatment in some patients who experienced small facial blisters, which fell off by itself after 5-7 days.
Control group
Patients in the control group were not treated with IPL after anti-mite therapy. If telangiectasia worsened or did not improve during the follow-up period, the observation was terminated and additional symptomatic treatments, including topical boric acid solution, moisturizing cosmeceutical products and oral hydroxychloroquine sulfate, were administered. All patients in the control group received further treatment after the follow-up period.
Curative effect evaluation
For each patient, the severity of telangiectasia was measured upon enrollment (baseline), subsequently at monthly intervals during the first 6 months of the follow-up, and every 6 months thereafter for 1.5 years. Measurements were made by YL and JZ; for each patient, all measurements were made by the same clinician. All photographs were taken using a digital camera (magnification, x10; 2x107 pixels; Lumix DMC-ZS110GK; Panasonic Corporation), using which the percentage of vascular lesion lightening and reduction in 1 cm2 within the lesions were recorded.
The treatment of telangiectasia was evaluated according to the four grades of clearing percentage, as previously reported (24): i) Effective (≥90%); ii) markedly improved (60-90%); iii) improved (30-60%); or iv) ineffective (<30%). The method of scoring before IPL treatment was based on the National Rosacea Society Expert Committee's guidelines for erythematotelangiectatic rosacea symptoms (1,25), namely: i) Flushing (transient erythema); ii) non-transient erythema; iii) telangiectasia; iv) burning or stinging; v) plaques; and vi) dry appearance and edema, according to the physician's (XL) global assessment and patient's global assessment. The total efficacy rate was calculated as the percentage of all patients with telangiectasia that were defined as ‘effective’, ‘markedly improved’ or ‘improved’. The total efficacy rate and effective sample size were calculated using the following equation: Effectiveness index = (total score before IPL treatment-total score after IPL treatment)/total score before treatment) x100. Recurrence was defined as the emergence of skin lesion(s) with the same symptoms in the original or new location of the face after the patient had been considered as experiencing ‘effective’ treatment.
Follow-up
In all patients with rosacea, skin lesions were observed and assessed at monthly intervals for 6 months beginning from the baseline, and then every 6 months for 18 months to evaluate the recurrence of rosacea.
Statistical analysis
The sample size of this study was calculated according to the following formula (26): N=Z2 x[P x (1-P)]/E2, where N is the sample size, Z represents the level of confidence, E is the error value, P is the probability value and the expected error value was 10%. When the significance level was set to P=0.5 and the confidence interval was set to 95%, the sample size was determined to be 96. All data were analyzed using IBM SPSS Statistics for Windows, Version 19.0. (IBM Corp.). χ2 test was used to compare the indices of efficacy between the IPL and control groups. P<0.05 was considered to indicate a statistically significant difference and data were presented as the mean ± standard deviation where appropriate.
Results
Baseline data
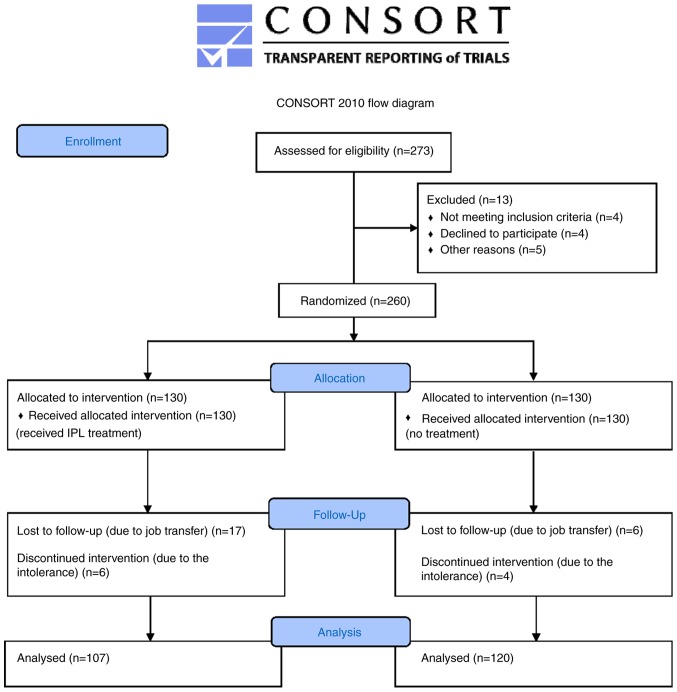
Of the 260 patients who were initially enrolled, 227 patients completed the study and were included in the final analysis. In the IPL group, 23 patients were lost to follow-up, specifically 17 due to job transfer and 6 due to intolerance to IPL treatment, with symptoms including blisters, burning, redness, and edema. In the control group, 10 patients were lost to follow-up, specifically 6 due to job transfer and 4 due to the aggravation of skin lesions. The 10 patients with aggravation in both groups were withdrawn from clinical observation and were offered medication. For the final analysis, there were 107 patients in the IPL experimental group and 120 in the control group (Fig. 1).
Figure 1.
Schematic diagram of the experimental design. IPL, intense pulsed light.
Of the 227 patients included in the final analysis, 202 (88.99%) were women and 25 (11.01%) were men. The overall age ranged from 18to 60 years (mean age, 40.20±10.76 years), and the disease course ranged from 1 month to 20 years (mean disease course, 2.28±3.28 years). Of the 107 patients in the IPL experimental group, 97 (90.65%) were women and 10 (9.35%) were men, with a mean age of 36.2±10.45 years and a mean disease course of 2.19±3.21 years. By contrast, of the 120 patients in the control group, 105(87.50%) were women and 15 (12.50%) were men, with a mean age of 38.5±10.51 years and a mean disease course of 2.27±3.20 years. There were no significant differences in age, sex and disease course between the IPL and the control group (P>0.05), making the two groups comparable in treatment efficacy analyses.
IPL efficacy
All patients were followed-up for ≥2 years. The indications and disease course of representative patients are shown in Fig. 2 for the experimental group and Fig. 3 for the control group. For the IPL group, 1 month after the initial IPL treatment, 19 patients (17.76%) with facial telangiectasia were characterized as improved (Table I; Fig. 2C). At 3 months, the total efficacy rate had increased to 39.25% (Table I; Fig. 2D). At 4 months, 72 patients (67.29%) showed some degree of improvement, where 35 patients (32.71%) showed effective treatment. At 6 months, 102 patients (95.33%) showed improvement, where 71 patients (66.36%) were characterized as effective (Table I).
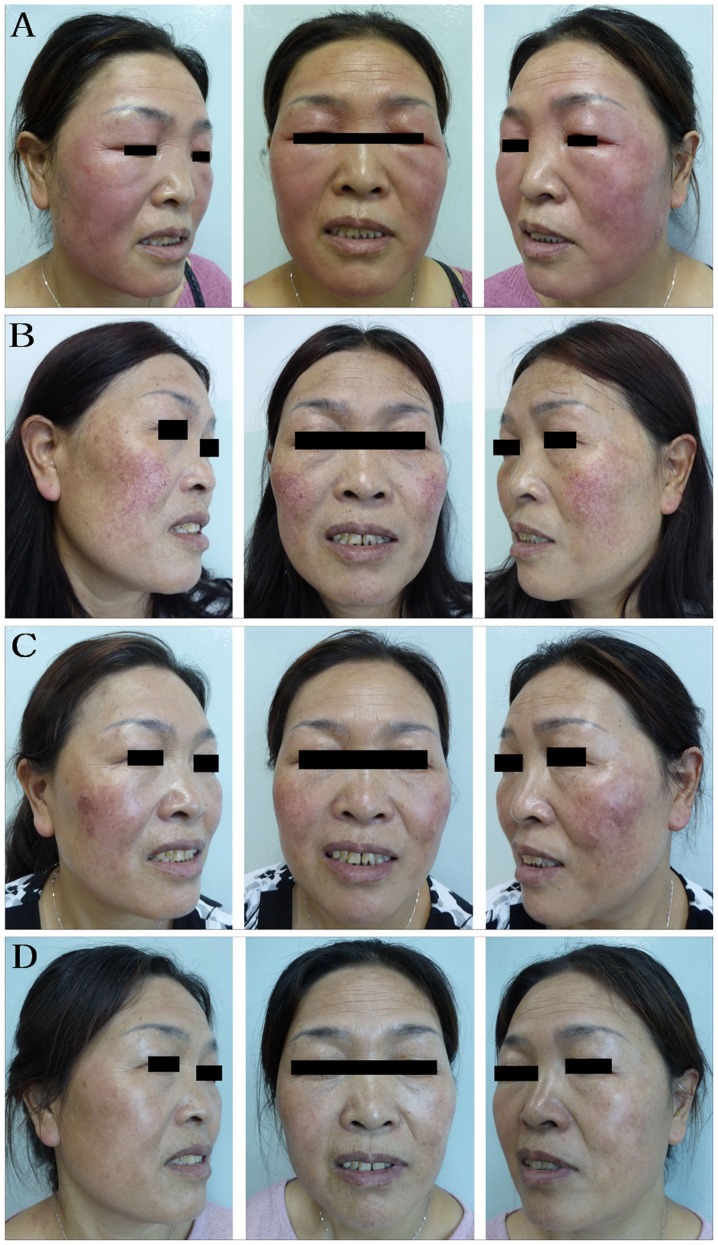
Figure 2.
Representative images from a 47-year-old woman in the IPL group. This woman suffered from rosacea for >1 year prior to presentation and was tested positive for Demodex folliculorum. (A) Before anti-mite treatment. The facial erythema was itchy, hot, dry and tight. (B) After 1 month of treatment with ornidazole, the facial erythema subsided, leaving large areas of vascular lesions. (C) One month after IPL initial treatment, the vascular lesions subsided, leaving a small amount of pigmentation. (D) Three months after initial IPL treatment, the vascular lesion had substantially reduced. IPL, intense pulsed light.
Figure 3.
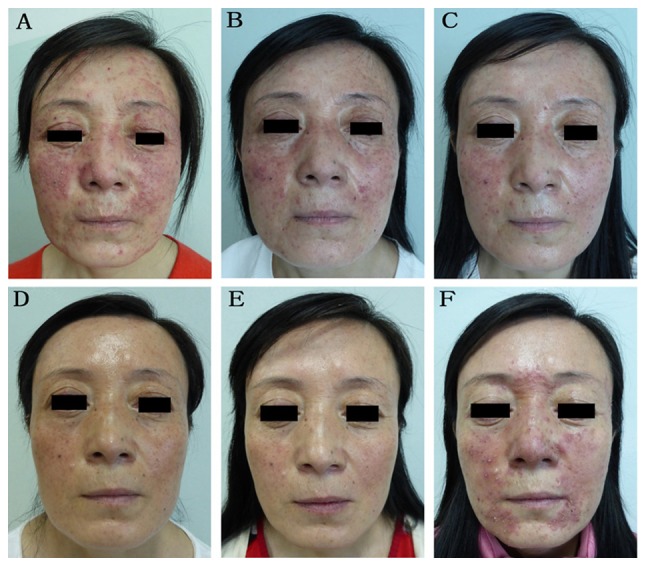
Representative images from a 46-year-old woman in the control group. This woman suffered from repeated erythematous papules with itching of the face for 6 months prior to presentation and was tested positive for Demodex folliculorum. (A) Before anti-mite treatment, a large number of facial erythema papules were present. (B) After 2 weeks of anti-mite treatment, the facial erythema papules were relieved. (C) After 4 weeks of anti-mite treatment, the facial erythema papules were markedly reduced. (D) During the 2-month follow-up, the facial erythema papules had subsided, with the vascular lesions remaining. (E) During the 6-month follow-up, there were no notable reductions in the vascular lesions. (F) During the 7-month follow-up, recurrence of facial erythematous papules was observed.
Table I.
Total efficacy rates of the IPL experimental group at 1-month follow-up intervals.
| Month | Effective | Markedly improved | Improved | Ineffective | Total (%) |
|---|---|---|---|---|---|
| 1 | 0 (0.00) | 0 (0.00) | 19 (17.76) | 88 (82.24) | 17.76 |
| 2 | 0 (0.00) | 12 (11.21) | 12 (11.21) | 83 (77.57) | 22.43 |
| 3 | 17 (15.89) | 13 (12.15) | 12 (11.21) | 65 (66.75) | 39.25 |
| 4 | 35 (32.71) | 21 (19.63) | 16 (14.95) | 35 (32.71) | 67.29 |
| 5 | 48 (44.86) | 27 (25.23) | 18 (16.82) | 14 (13.08) | 86.92 |
| 6 | 71 (66.36) | 22 (20.56) | 9 (8.41) | 5 (4.67) | 95.33 |
Data are presented as n (%) unless stated otherwise. Number of patients at each follow-up, n=107.
Patients with telangiectasia in the control group demonstrated little or no recovery nor significant improvement during the follow-up period (Fig. 3D and E; Tables II and III). At 6 months after the baseline assessments, the condition of 37 patients (30.83%) had improved, whilst 83 patients (69.17%) were characterized as ‘ineffective’ in terms of telangiectasia values from the baseline assessment. The total efficacy rate of the control group was significantly lower compared with that in the IPL group (χ2=99.113, P<0.001).
Table II.
Patients showing improvement in the IPL and control groups at one-month follow-up intervals.
| Month | IPL | Control | χ2 | P-value |
|---|---|---|---|---|
| 1 | 19 (17.76) | 0 (0.00) | N.A. | N.A. |
| 2 | 24 (22.43) | 5 (4.17) | 16.931 | P<0.001 |
| 3 | 42 (39.25) | 16 (13.33) | 19.976 | P<0.001 |
| 4 | 72 (67.29) | 25 (20.83) | 49.885 | P<0.001 |
| 5 | 93 (86.92) | 33 (27.50) | 80.854 | P<0.001 |
| 6 | 102 (95.33) | 37 (30.83) | 99.113 | P<0.001 |
Data are presented as n (%) unless stated otherwise. IPL group, n=107; control group, n=120. At each month, the difference between the IPL and control groups was statistically significant. IPL, intense pulse light.
Table III.
Total efficacy rates of the IPL and control groups at 6 months after baseline assessment.
| Treatment group | Effective | Markedly improved | Improved | Ineffective | Total (%) |
|---|---|---|---|---|---|
| IPL | 71 (66.36) | 22 (20.56) | 9 (8.41) | 5 (4.67) | 95.33 |
| Control | 0 (0.00) | 12 (10.00) | 25 (20.83) | 83 (69.17) | 30.83 |
Data are presented as n (%) unless stated otherwise. IPL group, n=107; control group, n=120. The rates of total efficacy between the two groups were significantly different. χ2=99.113, P<0.001. IPL, intense pulse light.
During the 2-year follow-up period, recurrence occurred in 9 patients (8.41%) in the IPL group and 58 patients (48.33%) in the control group (Fig. 3F; Table IV). Relapses were observed mainly at 3-6 months after anti-mite treatment. The recurrence rate of the IPL group was significantly lower compared with that of the control group (χ2=43.333, P<0.001; Table IV).
Table IV.
Recurrence rates of the IPL and control groups at 3-24 months after baseline assessment.
| Follow-up month | ||||||
|---|---|---|---|---|---|---|
| Treatment group | 3 | 6 | 12 | 18 | 24 | Total |
| IPL | 5 (4.67) | 2 (1.87) | 1 (0.93) | 1 (0.93) | 0 (0) | 9 (8.41) |
| Control | 32 (26.67) | 18 (15.00) | 5 (4.17) | 2 (1.67) | 1 (0.83) | 58 (48.33) |
Data are presented as n (%). IPL group, n=107; control group, n=120. The χ2 test was used to compare the recurrence rate between the IPL and control groups. χ2=43.333, P<0.001. IPL, intense pulse light.
Adverse reactions
Regarding adverse reactions associated with IPL treatment, within 30 min of the IPL treatment and the cold compress, adverse reactions, including mild burning sensation, temporary skin flushing and local skin edema, faded spontaneously. In total, there were 11, 8, 3 and 2 cases of facial burning, facial swelling, hyperpigmentation and facial blisters, respectively. In the 2 patients with facial blisters, they were 0.1-0.3 cm in diameter. All signs of redness, burns and blisters subsided without further treatment within 1 week and hyperpigmentation vanished spontaneously within 3 months of IPL treatment.
Discussion
In the present study, 540 nm-wavelength IPL was administered on patients with late-stage rosacea following sequential ornidazole-based anti-mite treatment. It was concluded that 540 nm-IPL treatment was associated with a significant improvement in telangiectasia and a reduced rate of recurrence by comparing the rates of effective treatment (≥90% clearance of telangiectasia), improvement (≥30% clearance) and recurrence (original or neo-location) of the IPL and control groups. The 227 patients were followed-up for 2 years, where IPL treatment was deemed safe and effective.
Rosacea is a chronic inflammatory cutaneous syndrome characterized by transient facial flushing or persistent erythema, papules, pustules and telangiectasia, with or without burning or itching. According to The National Rosacea Society Expert Committee, there are four subtypes of rosacea: Erythematotelangiectatic, papulopustular (papulae and pustules), phymatous (including enlargement of the nose) and ocular (eye irritation) (9). Among these subtypes, erythematotelangiectatic rosacea is the most prevalent, with symptoms that include flushing, redness and telangiectasia (1). Telangiectasia is a common clinical symptom among a variety of facial skin diseases that is characterized by dotted, patchy, linear or reticular distributions of the capillaries, resulting in reddening of the facial skin. The incidence of secondary telangiectasia has been increasing due to irregular skin care, improper use of skin care products, abuse of acid and alkali chemicals and long-term external use of hormone preparations (19).
The causes of rosacea remain controversial, although it has been previously hypothesized to involve the innate immune defense system of the skin, microbial infections, genetic factors, damage to barrier function, vasomotor dysfunction of the facial vessels and dysfunction of neurovascular regulation (27,28). Physiological and pathological expansion of the capillaries result in increased blood flow (flushing) during early stage rosacea, where pathological thickening and expansion of the blood vessels occur over time (27). In addition, Demodex folliculorum mite infestation is considered to be closely associated with the etiology and pathogenesis of rosacea (29,30).
Topical or internal drugs, or combinations, are used routinely to alleviate the clinical symptoms of rosacea and avoid scar formation. These include isotretinoin, antibiotics (mainly tetracyclines), hydroxychloroquine and metronidazole (31). Although they are mostly photosensitive for erythema, papules, and pustules, they remain ineffective for the treatment of telangiectasia (31). In a previous clinical trial, 200 patients with mite-associated folliculitis were sequentially treated with either an ornidazole- or metronidazole-based regimen (13). Skin lesions in ~61%of the patients were transiently aggravated during treatment. Compound betamethasone injection (CBI) significantly alleviated the inflammation and itching of facial lesions that occurred following ornidazole treatment (13). Application of a topical gel containing recombinant bovine basic fibroblast growth factor(rbFGF) significantly improved the repair of skin lesions during both the course of treatment and the follow-up period, as compared with patients without rbFGF treatment (14). Therefore, a treatment regimen that combined ornidazole, CBI and rbFGF gel, which were administered sequentially, effectively killed Demodex folliculorum and alleviated facial inflammation. However, such treatment had no effect on patients with telangiectasia (14).
In the present study, 540 nm-wavelength IPL was used to treat telangiectasia in late-stage rosacea. The 540 nm-wavelength light, excited by the IPL, overlaps with the absorption peak of oxygenated hemoglobin. Therefore, the strong pulse light can penetrate the skin and is absorbed by hemoglobin. The hemoglobin then coagulates, forming blood clots and cause the blood vessels to close, resulting in the dilated capillaries disappearing (32,33). Simultaneously, the thermal effect of IPL can stimulate extracellular matrix protein secretion from fibroblasts, whilst restricting the production of matrix metalloproteinases, increasing the production of collagen (including types I and III) in the dermis (34) and reducing the release of inflammatory mediators (35). In addition, IPL treatment modulates the local immune response, which makes the skin shrink and thicken (32). Therefore, IPL treatment increases skin elasticity, improves skin texture and removes fine wrinkles (32). However, further research is required to elucidate the mechanisms of action by which IPL modulate the immune reactions that are associated with rosacea.
To achieve improved outcomes, the treatment area of IPL was designed to overlap with the observed normal skin by 1-2 mm. Early-stage rosacea is characterized increased capillary responsiveness and temporary expansion of blood vessels, leading to symptoms including burning and tingling (36). The locations of continuously expanding capillaries are difficult to determine precisely, which requires the expansion of treatment coverage to avoid missing telangiectasia that is invisible to the naked eye. To reduce further adverse reactions, a post-treatment cold compress was applied to reduce the skin temperature after IPL treatment. The postoperative cold compress helped to relieve pain and burning sensations, in addition to reducing the occurrence of adverse reactions including redness, blisters and ecchymosis.
Telangiectasia is divided into primary and secondary subtypes, with the latter sometimes attributed to systemic diseases such as rosacea (27). Therefore, the first step of treatment should be to identify and address the underlying causes or contributing factors. It was previously found that anti-mite treatment markedly improved the appearance of papules and pustules caused by rosacea, which indicates the close association between this disease and Demodex folliculorum infestation (13). As the anti-mite treatment dispelled the remote cause of telangiectasia associated with rosacea, IPL was applied in the present study for the coagulation of facial dilated blood vessels, thereby eliminating dysfunctional blood vessels and effectively reducing the recurrence rate of rosacea.
Compared with pulsed dye laser and pulsed laser in rosacea-associated telangiectasia treatment, IPL is advantageous in that it covers large areas of the skin, is more time efficient and more likely to be accepted by patients. Although it may not be as efficient as laser when used to treat certain types of skin lesions including large dilated blood vessels (37). As a composite light with a long pulse width, IPL has been shown to be an improvement in terms of reducing pigmentation and telangiectasia, fine wrinkle elimination and the smoothing and firming of the skin (38,39).
To conclude, the present study found that following treatment with 540 nm IPL, the facial telangiectasia of patients with rosacea was significantly improved, where the rates of effectiveness and total efficacy were higher compared with those of the untreated control group. In addition, IPL treatment was found to be associated with a continuous reduction in the rate of rosacea recurrence during the 2-year follow-up. However, since the present study primarily investigated patients with telangiectasia in late-stage rosacea, further investigation is required on the effects of IPL on the recurrence of other manifestations of rosacea.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YL designed the experiments. XLL collected and interpreted the data. JHZ collected the follow-up data. LXW analyzed the data. NZ collected and analyzed the data, and wrote the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The Ethics Committee of the 940th Hospital of the Joint Logistics Support force of the Chinese People's Liberation Army approved the study protocol (Lanzhou, China). Signed informed consent was obtained from all patients included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wilkin J, Dahl M, Detmar M, Drake L, Liang MH, Odom R, Powell F. Standard grading system for rosacea: Report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907–912. doi: 10.1016/j.jaad.2004.01.048. National Rosacea Society Expert C. [DOI] [PubMed] [Google Scholar]
- 2.Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327–341. doi: 10.1016/j.jaad.2004.03.030. quiz 342-324. [DOI] [PubMed] [Google Scholar]
- 3.Wacker T, Lang GK. Demodex folliculorum: Diagnosis and therapy today. Klin Monbl Augenheilkd (German) 2014;231:241–245. doi: 10.1055/s-0033-1360357. [DOI] [PubMed] [Google Scholar]
- 4.Alinia H, Tuchayi SM, James SM, Cardwell LA, Nanda S, Bahrami N, Awosika O, Richardson I, Huang KE, Feldman SR. Measurement of disease severity in a population of rosacea patients. Dermatol Clin. 2018;36:97–102. doi: 10.1016/j.det.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Oussedik E, Bourcier M, Tan J. Psychosocial burden and other impacts of rosacea on patients' quality of life. Dermatol Clin. 2018;36:103–113. doi: 10.1016/j.det.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Patients with rosacea have increased risk of dementia. Ann Neurol. 2016;79:921–928. doi: 10.1002/ana.24645. [DOI] [PubMed] [Google Scholar]
- 7.Faris C, Manjaly JG, Ismail-Koch H, Caldera S. Rapid treatment of rhinophyma with powered microdebrider. Case Rep Otolaryngol. 2013;2013(621639) doi: 10.1155/2013/621639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Patients with rosacea have increased risk of depression and anxiety disorders: A danish nationwide cohort study. Dermatology. 2016;232:208–213. doi: 10.1159/000444082. [DOI] [PubMed] [Google Scholar]
- 9.Gallo RL, Granstein RD, Kang S, Mannis M, Steinhoff M, Tan J, Thiboutot D. Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148–155. doi: 10.1016/j.jaad.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Micali G, Dall'Oglio F, Verzi AE, Luppino I, Bhatt K, Lacarrubba F. Treatment of erythemato-telangiectatic rosacea with brimonidine alone or combined with vascular laser based on preliminary instrumental evaluation of the vascular component. Lasers Med Sci. 2018;33:1397–1400. doi: 10.1007/s10103-017-2318-3. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy Carney C, Cantrell W, Elewski BE. Rosacea: A review of current topical, systemic and light-based therapies. G Ital Dermatol Venereol. 2009;144:673–688. [PubMed] [Google Scholar]
- 12.Del Rosso JQ, Tanghetti EA, Baldwin HE, Rodriguez DA, Ferrusi IL. The burden of illness of erythematotelangiectatic rosacea and papulopustular rosacea: Findings from a web-based survey. J Clin Aesthet Dermatol. 2017;10:17–31. [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Y, Sun YJ, Zhang L, Luan XL. Treatment of mites folliculitis with an ornidazole-based sequential therapy: A randomized trial. Medicine (Baltimore) 2016;95(e4173) doi: 10.1097/MD.0000000000004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Y, Luan XL, Sun YJ, Zhang L, Zhang JH. Effect of recombinant bovine basic fibroblast growth factor gel on repair of rosacea skin lesions: A randomized, single-blind and vehicle-controlled study. Exp Ther Med. 2019;17:2725–2733. doi: 10.3892/etm.2019.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anzengruber F, Czernielewski J, Conrad C, Feldmeyer L, Yawalkar N, Hausermann P, Cozzio A, Mainetti C, Goldblum D, Goldblum D, et al. Swiss S1 guideline for the treatment of rosacea. J Eur Acad Dermatol Venereol. 2017;31:1775–1791. doi: 10.1111/jdv.14349. [DOI] [PubMed] [Google Scholar]
- 16.Lowe E, Lim S. Paradoxical erythema reaction of long-term topical brimonidine gel for the treatment of facial erythema of rosacea. J Drugs Dermatol. 2016;15:763–765. [PubMed] [Google Scholar]
- 17.Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmetic and Invest Dermatol. 2016;9:29–40. doi: 10.2147/CCID.S69106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanghetti EA, Jackson JM, Belasco KT, Friedrichs A, Hougier F, Johnson SM, Kerdel FA, Palceski D, Hong HC, Hinek A, Cadena MJ. Optimizing the use of topical brimonidine in rosacea management: Panel recommendations. J Drugs Dermatol. 2015;14:33–40. [PubMed] [Google Scholar]
- 19.Gao L, Gao N, Song W, Dang E, Yin R, Wang L, Wang G. A retrospective study on efficacy of pulsed dye laser and intense pulsed light for the treatment of facial telangiectasia. J Drugs Dermatol. 2017;16:1112–1116. [PubMed] [Google Scholar]
- 20.Jansen T. Clinical presentations and classification of rosacea. Ann Dermatol Venereol. 2011;138:S192–S200. doi: 10.1016/S0151-9638(11)70089-8. [DOI] [PubMed] [Google Scholar]
- 21.Jansen T. Clinical presentations and classification of rosacea. Ann Dermatol Venereol. 2011;138 (Suppl):S138–S147. doi: 10.1016/S0151-9638(11)70089-8. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg DJ. Current trends in intense pulsed light. J Clin Aesthet Dermatol. 2012;5:45–53. [PMC free article] [PubMed] [Google Scholar]
- 24.Dummer R, Graf P, Greif C, Burg G. Treatment of vascular lesions using the VersaPulse variable pulse width frequency doubled neodymium: YAG laser. Dermatology. 1998;197:158–161. doi: 10.1159/000017989. [DOI] [PubMed] [Google Scholar]
- 25.Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, Powell F. Standard classification of rosacea. National Rosacea Society Expert Committee: Report of the National Rosacea Society Expert Committee on the classification and staging of Rosacea. J Am Acad Dermatol. 2002;46:584–587. doi: 10.1067/mjd.2002.120625. [DOI] [PubMed] [Google Scholar]
- 26.Xu Z, Tan X, Ye J, Zhang C, Zhao M, Zhan F. Evaluation of measles catch-up immunization campaign in Hubei, 2010. Chin J Viral Dis. 2011;1:208–211. [Google Scholar]
- 27.Cribier B. Pathophysiology of rosacea: Redness, telangiectasia, and rosacea. Ann Dermatol Venereol. 2011;138 (Suppl):S184–S191. doi: 10.1016/S0151-9638(11)70088-6. [DOI] [PubMed] [Google Scholar]
- 28.Agnoletti AF, DEC E, Parodi A, Schiavetti I, Savarino V, Rebora A, Paolino S, Cozzani E, Drago F. Etiopathogenesis of rosacea: A prospective study with a three-year follow-up. G Ital Dermatol Venereol. 2017;152:418–423. doi: 10.23736/S0392-0488.16.05315-3. [DOI] [PubMed] [Google Scholar]
- 29.Forton FM. Papulopustular rosacea, skin immunity and Demodex: Pityriasis folliculorum as a missing link. J Eur Acad Dermatol Venereol. 2012;26:19–28. doi: 10.1111/j.1468-3083.2011.04310.x. [DOI] [PubMed] [Google Scholar]
- 30.Turgut Erdemir A, Gurel MS, Koku Aksu AE, Falay T, Inan Yuksel E, Sarikaya E. Demodex mites in acne rosacea: Reflectance confocal microscopic study. Australas J Dermatol. 2017;58:e26–e30. doi: 10.1111/ajd.12452. [DOI] [PubMed] [Google Scholar]
- 31.Abokwidir M, Feldman SR. Rosacea management. Skin Appendage Disord. 2016;2:26–34. doi: 10.1159/000446215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campolmi P, Bonan P, Cannarozzo G, Bruscino N, Troiano M, Prignano F, Lotti T. Intense pulsed light in the treatment of non-aesthetic facial and neck vascular lesions: Report of 85 cases. J Eur Acad Dermatol Venereol. 2011;25:68–73. doi: 10.1111/j.1468-3083.2010.03700.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Liu J, Ren Y, Li B, Lu S. Comparative efficacy of intense pulsed light for different erythema associated with rosacea. J Cosmet Laser Ther. 2014;16:324–327. doi: 10.3109/14764172.2014.957218. [DOI] [PubMed] [Google Scholar]
- 34.Wong WR, Shyu WL, Tsai JW, Hsu KH, Lee HY, Pang JHS. Intense pulsed light modulates the expressions of MMP-2, MMP-14 and TIMP-2 in skin dermal fibroblasts cultured within contracted collagen lattices. J Dermatol Sci. 2008;51:70–73. doi: 10.1016/j.jdermsci.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Vigo L, Giannaccare G, Sebastiani S, Pellegrini M, Carones F. Intense pulsed light for the treatment of dry eye owing to meibomian gland dysfunction. J Vis Exp. 2019;146 doi: 10.3791/57811. [DOI] [PubMed] [Google Scholar]
- 36.Rainer BM, Kang S, Chien AL. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9(e1361574) doi: 10.1080/19381980.2017.1361574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim HS, Lee SC, Won YH, Lee JB. The efficacy of intense pulsed light for treating erythematotelangiectatic rosacea is related to severity and age. Ann Dermatol. 2014;26:491–495. doi: 10.5021/ad.2014.26.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babilas P, Schreml S, Szeimies RM, Landthaler M. Intense pulsed light (IPL): A review. Lasers Surg Med. 2010;42:93–104. doi: 10.1002/lsm.20877. [DOI] [PubMed] [Google Scholar]
- 39.Kawana S, Ochiai H, Tachihara R. Objective evaluation of the effect of intense pulsed light on rosacea and solar lentigines by spectrophotometric analysis of skin color. Dermatol Surg. 2007;33:449–454. doi: 10.1111/j.1524-4725.2007.33092.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.