Abstract
Background.
Osteomyelitis is common in diabetic foot infections and medical management can lead to poor outcomes. Surgical management involves sending histopathologic and microbiologic specimens which guides future intervention. We examined the effect of obtainment of surgical margins in patients undergoing forefoot amputations to identify patient characteristics associated with outcomes. Secondary aims included evaluating interobserver reliability of histopathologic data at both the distal-to and proximal-to surgical bone margin.
Methods.
Data were prospectively collected on 72 individuals and was pooled for analysis. Standardized method to retrieve intraoperative bone margins was established. A univariate analysis was performed. Negative outcomes, including major lower extremity amputation, wound dehiscence, reulceration, reamputation, or death were recorded.
Results:
Viable proximal margins were obtained in 63 out of 72 cases (87.5%). Strong interobserver reliability of histopathology was recorded. Univariate analysis demonstrated preoperative platelets, albumin, probe-to-bone testing, absolute toe pressures, smaller wound surface area were associated with obtaining viable margins. Residual osteomyelitis resulted in readmission 2.6 times more often and more postoperative complications.
Conclusions:
Certain patients were significantly different in the viable margin group versus dirty margin group. High interobserver reliability was demonstrated. Obtainment of viable margins resulted in reduced rates of readmission and negative outcomes.
Levels of Evidence:
Prognostic, Level I: Prospective
Keywords: osteomyelitis, amputation, limb salvage, diabetes, forefoot, diabetic foot
Osteomyelitis is common in diabetic foot infection and complicates outcomes in mild to severe infection.1,2 Traditionally, bone has been obtained for histopathologic and microbiologic examination to determine the presence of osteomyelitis and to determine further treatment intervention. Accurate diagnosis of osteomyelitis in the diabetic foot and ankle remains a clinical challenge. Clinical tools including thorough inspection along with probe-to-bone testing are largely dependent on the setting and may be overinterpreted.3 In fact, positive probe-to-bone testing does not make the diagnosis of diabetic foot osteomyelitis (DFO) and has varying positive predictive values, which range from 53% in an outpatient setting4 to 97% in an inpatient setting.5 Clinical practice guidelines suggest that DFO can only be confirmed based on (a) a positive histopathologic and microbiologic testing of a bone sample, (b) a positive magnetic resonance imaging (MRI), or (c) intraoperative findings of the presence of pus into the bone(s).3,6 However, bone biopsy is not without its own controversies. It can be debated as a diagnosis tool because conflicting studies show various interobserver reliability issues at any given institution depending on how the osteomyelitis characteristics are defined histologically.7,8 Since there are challenges in making an accurate diagnosis of osteomyelitis, these can affect treatment outcome.
Therefore, we sought to examine the effect of obtainment of proximal surgical margins on patients undergoing ablative diabetic foot surgery for DFO and which patient characteristics are associated with the ‘clean,” or noninfected margin, if any. We introduced a set classification of histopathologic definitions for osteomyelitis to our board-certified pathologists.7,8 We thought that if there was high interobserver reliability in histopathologic data at both the distal-to surgical bone margin and proximal-to surgical bone margin, then basing management on histopathology would improve patient outcomes. We also sought to characterize each patient population and determine differences amongst patient who had residual osteomyelitis at the surgical margin versus those who were free from osteomyelitis. We prospectively collected data on 72 consecutive patients who underwent ablative forefoot amputation (distal to tarsometatarsal joint) over two years and followed them for at a minimum of 12 months postoperatively. All patients were admitted to the hospital prior to receiving medical and surgical care from the podiatry service.
Methods
We conducted an observational prospective study of patients with either type 1 or type 2 diabetes mellitus who underwent forefoot ablative surgery for suspected bone infection as an inpatient at University Hospital (University of Michigan–Ann Arbor) by a faculty podiatrist between October 2015 and October 2017. The definition of a diabetic patient was according to criteria established by the World Health Organization.9 Ablative forefoot surgery was defined as removal of part or whole ray or toe, or combination thereof, distal to the tarsometatarsal (LisFranc) joint.
A preoperative diagnosis of DFO was deduced from a combination of clinical and constitutional symptoms, probe-to-bone testing, available laboratory and inflammatory markers, and imaging studies using published criteria.10
Patient comorbid disease states, including diabetes and its control, as represented by hemoglobin A1c (HbA1c) 3 months prior to and during admission, was recorded. Previous antibiotic drug therapy before, during, and after hospital discharge were recorded until study period ended. An institutional algorithm for diabetic foot infection was followed and antibiotic therapy was empiric initially and narrowed once cultures were finalized. Patients without palpable pedal pulses had noninvasive vascular studies prior to surgery using Doppler ultrasound to determine ankle-brachial and toe-brachial indices (ABI/TBIs). Bone resection was based on several criteria, including (a) soft tissue coverage, (b) foot mechanics and function, (c) definitive level of healing, and (d) preoperative radiographs or MRI. MRI was ordered based on admitting team protocol.
All patients underwent surgery under local anesthetic blockade and monitored anesthesia care sedation by 1 of 5 podiatric physicians at our institution. Surgical bone margins were obtained intraoperatively and sent for microbiologic and histopathologic analysis in a standardized fashion. Standardized intraoperative proximal-to surgical margin obtainment occurred as follows: amputation was performed, and all nonviable soft tissue and bone was debrided. Next, the amputation site was irrigated with copious amounts of sterile normal saline (approximately 3 L). The surgery team then exchanged gloves and instruments to reduce contamination. Finally, the margin proximal to the amputation site was transected with power instrumentation to obtain a sample of cortical and cancellous bone, which was split into 2 specimens and labeled “clean bone margin” and sent for aerobic, anaerobic, acid-fast, and fungal culture and to histopathology for microscopic evaluation.11
Primary and secondary outcomes were then analyzed. The primary outcome was to the reliability of agreement among board-certified pathologist diagnosis using the kappa coefficient for both distal-to and proximal-to surgical bone margins. Each pathologist was asked to choose between 1 of 5 primary diagnoses for the proximal surgical margin: (a) acute osteomyelitis, (b) chronic osteomyelitis, (c) subacute osteomyelitis, (d) fibrosis, and (e) no osteomyelitis. Patient characteristics of each histology group was described. Secondary outcome measurements included patient outcomes (wound dehiscence, reulceration, reamputation, death) following surgical intervention for 1 year.
Statistical analysis was performed. A univariate analysis was performed using STATA software (2014). Paired Student’s t tests were performed to compare equal sample sizes between eras. When unequal variances and unequal sample sizes were demonstrated, a Welch’s t test was performed. When contingency tables were used, Fischer’s exact tests were performed and Bartlett’s test for equal variances applied. Statistical significance difference of less than 5% (P ≤ .05) was assumed to be significant for type I errors.
Results
Descriptive Statistics
A total of 802 inpatient consults were made to the podiatry service at our institution during the study period. Seventy-two patients met inclusion criteria. Fifty-nine patients identified as white individuals, 11 identified as black or African American, while 1 identified as Asian and 1 identified as other. The average age of the clean margin group was 56.84 years (95% CI 53.74–59.94) while the dirty margin group was 57.33 years (95% CI 53.81–60.84). The average body mass index in the clean margin group was 34.62 kg/m2 (95% CI 32.90–36.34) and in the dirty margin group 33.55 kg/m2 (95% CI 29.19–37.91). Overall, 59 were male and 13 were female. Seventy-one individuals were classified as ambulatory prior to surgical intervention, while 1 was nonambulatory prior to surgical intervention. A total of 28 amputations occurred on the left foot and 43 occurred on the right foot. Fourteen subjects were currently employed at time of surgery. Significance was not reached with each of the defined descriptors listed above (P > .10) (Table 1).
Table 1.
Descriptive Characteristics of Study Patients.
| Overall | Clean Surgical Margin (n = 63) | Dirty Surgical Margin (n = 9) | P | |
|---|---|---|---|---|
| Age, years, mean ± SD | 56.9 ± 12.8 | 56.8 ± 12.6 | 57.3 ± 15.2 | .915 |
| Gender, % men | 82 | 83 | 78 | .661 |
| Body mass index, kg/m2, mean ± SD | 34.5 ± 6.9 | 34.6 ± 6.9 | 33.6 ± 6.7 | .875 |
| Race, % African American | 12.5 | 12.7 | 11.1 | 1 |
| Employed, % | 21.7 ± 0.4 | 21.6 | 22.2 | 1 |
| Smoking, % active | 45.7 ± 0.5 | 44.2 | 55.5 | .389 |
| Coronary artery disease, % | 51.4 ± 0.5 | 47.6 | 77.8 | .153 |
| Chronic kidney disease, % | 41.7 ± 0.5 | 38.1 | 66.7 | .15 |
| Hypertension, % | 41.7 | 41.3 | 44.4 | 1 |
| Hyperlipidemia, % | 20.8 | 20.6 | 22.2 | 1 |
| Chronic obstructive pulmonary disease, % | 4.2 | 3.2 | 11.1 | .334 |
| Peripheral neuropathy, % | 47.2 | 44.4 | 66.7 | .291 |
| Depression, % | 5.6 | 4.8 | 11.1 | .421 |
| Previous amputation, % | 36.6 ± 0.5 | 37 | 33.3 | 1 |
| Established podiatry care, % | 73.6 ± 0.4 | 77.8 | 44.4 | .048* |
Statistically significant (P < .05).
Comorbid conditions included active smoking history, coronary artery disease, chronic kidney disease, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, peripheral neuropathy, depression, and previous amputation (Table 1). The prevalence of the aforementioned conditions is not different among groups. However, clean surgical margins were more obtained in patients who had previously established care with podiatry (77.8% vs 44.4%; P < .05) (Table 1).
Sixty-seven patients received antibiotics prior to podiatry consult and surgical intervention. On average, patients who underwent surgery and received antibiosis were treated for an average of 5.56 (95% CI 3.39–7.72) days prior to surgery in the clean margin group versus 3.50 days (95% CI 0.96–6.03) in the dirty margin group and was not significant.
Postoperative antibiotic therapy length of treatment for each group was not significantly different. Those patients where clean margins were obtained were treated with antibiotics for 23.69 days (95% CI 19.23–28.14) while those in the dirty margin group were treated for 32 days (95% CI 23.34–40.66, P > .10).
Ablative procedures were performed in 72 out of 72 patients. Clean proximal surgical margins were obtained in 63 of 72 cases (87.5%). Of the 63 patients with bone margins labeled “clean margin,” 49 demonstrated no histopathologic evidence of osteomyelitis (68.5%), and only 19 of the clean margin group demonstrated microbiologic growth (30.2%). In contrast, the dirty margin group demonstrated microbiologic growth in 8 out of 9 samples (88.9%) with all 9 samples demonstrating osteomyelitis histologically. Many microorganisms were cultured. These included: Hemophiulus sp, Enterococcus sp, Pseudomonas aerginousa, Klebsiella pneumoniae, Staphylococcus aureus, Porphyromonas sp, Escherichia coli, coagulase-negative Staphylococcus aureus, Bacteroides sp, Streptococcus anginosus, Corynebacterium sp, Serratia sp, methicillin-resistant Staphylococcus aureus, and Achromobacter sp. Histograms from each cohort demonstrate the majority of cultured organisms were gram-positive bacteria accounting for 76.9% (Figure 1) and 66.7% (Figure 2) in the clean and dirty surgical margin groups, respectively (P = .37). Of all organisms, the most common cultured organisms were methicillin-resistant Staph aureus and methicillin-susceptible Staph aureus.
Figure 1.
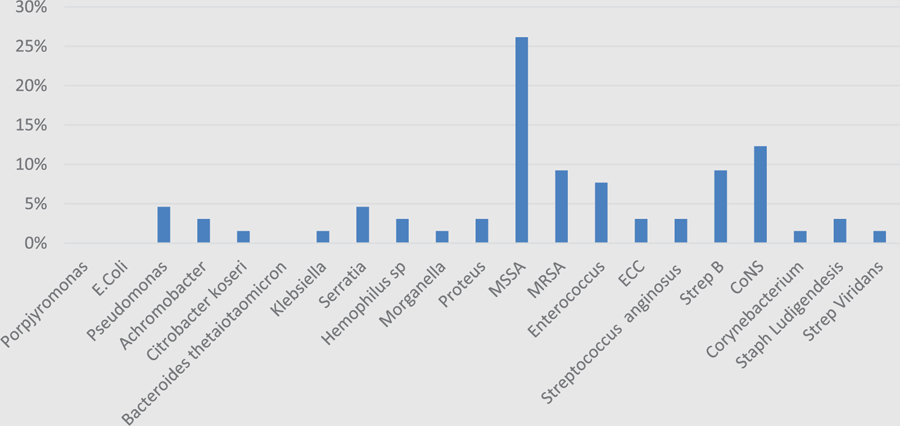
Bacterial histogram in clean surgical margin cohort, organized by gram stain.
Figure 2.
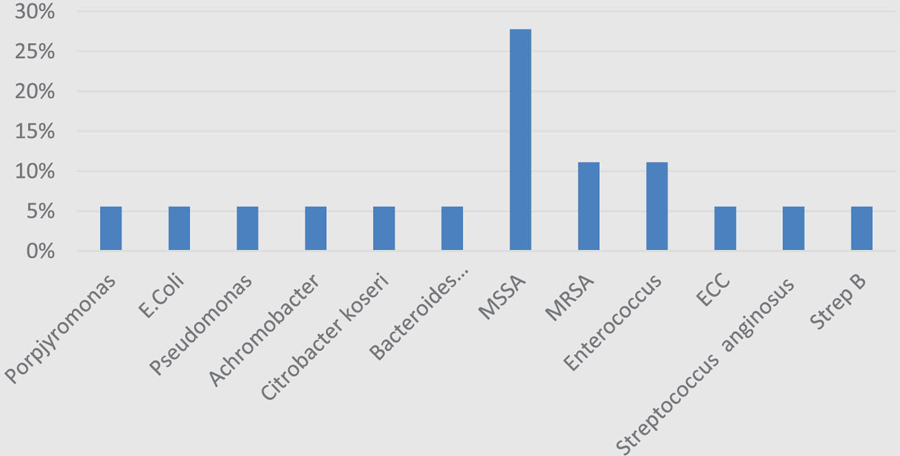
Bacterial histogram in dirty surgical margin cohort, organized by gram stain.
Reliability of Histopathology Data
Two board-certified pathologists with expertise in bone pathology participated in our study to evaluate interrater reliability of histopathology reads for the purposes of this study. Each pathologist was given a strict classification of histopathology definitions to utilize as criteria for making accurate diagnoses of osteomyelitis subcategories with our bone margins.7 We asked each pathologist to evaluate both the distal-to surgical bone margin and the proximal-to surgical bone margin from the amputation site in these patients. The sample size was 56. When asked to identify and differentiate simply between the presence or absence of osteomyelitis based on the distal surgical margin from the surgery, there was a high level of interrater reliability between our pathologists with the kappa coefficient equaling 0.964 (n = 55/56; Table 2). When asked to evaluate and differentiate the proximal-to surgical bone margin based on the above-said classification, interrater reliability dropped to kappa of 0.7142, which remains a good level of agreement (n = 8/56; Table 2).
Table 2.
Reliability of Histopathology Data: Kappa Statistic.
| Outcome | Presence of Osteomyelitis | Overall Agreement | Kappa Statistic |
|---|---|---|---|
| Distal “dirty” margin | 33/56 | 55/56 | 0.964 |
| Proximal “clean” margin | 4/56 | 48/56 | 0.714 |
Univariate Analysis
In the univariate analyses, several factors were associated with increased obtainment of clean proximal surgical bone margins. These included increased platelets (P = .0398, 95% CI 227.122–285.290), albumin (P < .001, 95% CI 3.68–3.96), positive preoperative probe-to-bone testing and toe pressure (P < .05, 95% CI 125.12–155.5986), and decreased diabetic foot ulcer surface area (P < .0001, 95% CI 1.266–2.623), red cell distribution width (P < .05, 95% CI 13.346–15.092), and mean corpuscular volume (P < .02, 95% CI 85.305–88.288) were associated with clean proximal surgical margins. When a patient surgery resulted in dirty proximal-to surgical margins, they were 2.6 times more likely to be readmitted (17% vs 44% and trended toward significance (P = .08) (Table 3).
Table 3.
Candidate Variables for Regression Analysis.
| Overall | Clean Surgical Margin (n = 63) | Dirty Surgical Margin (n = 9) | P | |
|---|---|---|---|---|
| HbA1c 3 months prior | 8.6 ± 2.1 | 8.7 ± 2.2 | 7.9 ± 1.9 | .684 |
| HbA1c at admission | 8.8 + 2.0 | 8.9 + 2.0 | 8.4 ± 1.5 | .494 |
| Ulcer surface area, cm2 | 4.3 ± 9.2 | 1.95 ± 2.1 | 15.3 ± 18.7 | .0001* |
| Probe to bone, % | 52 ± 0.5 | 56.5 | 22.2 | .077 |
| Toe pressure (mm Hg) | 134.9 ± 47.8 | 140.4 ± 46.6 | 86 ± 28.7 | .0291* |
| Toe-brachial index | 0.88 ± 0.3 | 0.896 ± 0.3 | 0.742 ± 0.2 | .318 |
| White blood cell count (×1000) | 10.8 + 5.4 | 10.9 + 5.3 | 10.3 + 6.3 | .758 |
| Erythrocyte sedimentation rate | 73.9 ± 33.5 | 73.3 ± 33.7 | 79.5 ± 34.5 | .668 |
| C reactive protein | 10.5 ± 8.8 | 10.7 ± 8.7 | 9 ± 10.8 | .64 |
| Neutrophil: lymphocyte ratio | 7.0 ± 9.4 | 5.73 ± 4.0 | 26.2 ± 40.1 | .469 |
| Mean corpuscular volume | 87.5 ± 6.3 | 86.8 ± 5.9 | 92.4 ± 6.7 | .011* |
| Red cell distribution width | 14.6 ± 3.9 | 14.2 ± 3.5 | 17.1 ± 5.6 | .035* |
| Platelet count (×1000) | 245.2 ± 117.3 | 256.2 ± 115.9 | 170.5 ± 103.5 | .039* |
| Albumin, mg/dL | 3.7 ± 0.5 | 3.8 ± 0.4 | 3.0 ± 0.3 | .0002* |
| Readmission, % | 20.8 ± 0.4 | 17.5 | 44.4 | .083 |
| IDSA classification | 2.35 ± 0.81 | 2.35 ± 0.79 | 2.36 ± 0.89 | .98 |
Abbreviation: IDSA, Infectious Diseases Society of America.
Statistically significant (P < .05).
Patient Outcomes
Patients were followed for at least 12 months postoperatively. Patients remained hospitalized in the clean margin group for 6.15 days (95% CI 5.22–7.07) versus 7.57 days 95% CI 2.67–12.48) in the dirty margin group and was not significant. In the clean margin group, 22% (n = 14/63) experienced a complication following surgery while in the dirty proximal margin group, the rate was approximately 88.8% (n = 8/9) and was significant (P < .001).
Of the 14 patients who experienced a complication in the clean margin cohort, the complication occurred within 3 months of surgery. Only 14% (n = 2/14) of those who experienced a complication required reoperation. Twelve of the 14 complications in this group were ulcer formation and incision dehiscence. Six- and 12-month follow-up resulted in no additional complications.
Contrarily, in patients who had residual osteomyelitis, the complication rate was higher. Fifty-five percent (n = 5/9) experienced wound dehiscence or ulcer formation within 3 months of the initial surgery. One-third of patients (n = 3/9) required reoperation within 6 months of surgery. No patient from this group underwent reamputation at 12 months. No patient from either cohort died during the 2-year study period.
Discussion
Obtaining clean margins during surgical intervention for management of DFO is important. Several studies have now demonstrated that residual osteomyelitis is associated with poor clinical outcomes.11,12. Using histopathology and/ or microbiologic data to aide in determining length of postoperative antibiotics is common practice. By evaluating the reliability of histopathology at the surgical margin, we feel it can be used to aide in antibiotic length recommendations.
We standardized each aspect of the care process in an attempt to minimize bias in our data. The surgical approach was standardized as were the definitions and interpretation of histopathology. There has been debate about reliability of histopathology to aide in diagnosis and treatment protocols for DFO.7 The initial study that called this practice into question did not use standardized histopathologic definitions.8 When standardized definitions are applied, reliability and agreement by pathologists increases to reliability.7 Our study agreed with the latter as shown by strong kappa coefficient at both the infected and viable/clean margin. Interestingly, the kappa statistic decreased when pathologists were asked to evaluate the proximal bone margin separate from the distal/infected portion. On further evaluation of the difference among the proximal surgical margin, we found that using fibrosis as a histopathological term caused all of the disagreements. The importance of this is not yet fully understood as fibrosis is a nonspecific histologic finding.
Since histopathology was reliable in our study, patient characteristics were analyzed. Not surprisingly several common predictors of diabetic foot ulcer healing were associated with obtainment of clean margins. These included preoperative albumin13–17 and probe-to-bone testing.4,5,18–21 Meanwhile, higher absolute toe pressures (P < .05) and a smaller surface area of diabetic foot ulcers (P < .0001) were also found to predict obtainment of clean bone margins.
Finally, much like other studies have demonstrated, obtainment of clean margins was associated with reduced postoperative complications and improved outcomes.11,12 Rates of wound dehiscence (%), reulceration (%), and reamputations (%) were reduced in the viable margin group versus the dirty proximal margin group. Fortunately, no major lower extremity amputations or deaths were recorded during our study period. Patients with residual osteomyelitis were 2.6 times more likely to be readmitted (17% vs 44%) and this trended toward significance (P = .08).
This study is not without limitation. First, the authors admit that our study was not fully powered given our event rate ratio (8:1) to perform multivariate analysis for each predictor variable candidate. Our calculations determined that at least 240 samples would be necessary to account for differences using this ratio. However, we have included 95% confidence intervals, which are more telling than an appropriately powered study.22 Confidence intervals give information about both the sample size and variance within the sample.22,23 Second, while use of MRI was not statistically significant between groups (60% vs 33%; P = .145), it may have a been a source of residual confounding and selection bias as MRI positive for osteomyelitis between groups approached significance (83% vs 33%; P = .096). Thus, higher use of MRI may have been useful in preoperative planning and obtaining clean bone margins. The decision to obtain an MRI was guided by the diabetic foot infection algorithm created by our hospital for best practice and was not fully implemented until after the study’s conclusion.
In conclusion, obtaining clean surgical margins from a patient diagnosed with DFO is important for improving clinical outcomes in this patient cohort. The surgeon should attempt to obtain clean surgical margins whenever possible as residual osteomyelitis is a predictor for clinical failure and postoperative complications. Differentiating between “clean” and “dirty” surgical margins is a reliable tool that may assist in therapy guidance. We evaluated a large prospectively collected DFO cohort and discovered that previously undocumented patient characteristics, including decreased wound surface area (cm2), red cell distribution width, and mean corpuscular volume as well as increased absolute toe pressures (mm Hg), platelets, and albumin were associated with clean surgical margins. We believe that this can guide the surgeon in their treatment algorithms for patients with diabetic foot osteomyelitis.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Trial Registration
Not applicable.
Contributor Information
Brian M. Schmidt, Division of Metabolism, Endrocrinology, and Diabetes, Department of Internal Medicine.
Jonathan B. McHugh, Department of Pathology, Michigan Medicine, Ann Arbor, Michigan.
Rajiv M. Patel, Department of Pathology, Michigan Medicine, Ann Arbor, Michigan.
James S. Wrobel, Division of Metabolism, Endrocrinology, and Diabetes, Department of Internal Medicine.
References
- 1.Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis 2004;39(suppl 2):S115–S122. [DOI] [PubMed] [Google Scholar]
- 2.Wrobel JS, Schmidt B. Probe-to-bone testing for osteomyelitis in the diabetic foot: a literature review. Diabetic Foot J 2016;19:64–68. [Google Scholar]
- 3.Lipsky BA, Berendt AR, Cornia PB, et al. ; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132–e173. [DOI] [PubMed] [Google Scholar]
- 4.Shone A, Burnside J, Chipchase S, Game F, Jeffcoate W. Probing the validity of the probe-to-bone test in the diagnosis of osteomyelitis of the foot in diabetes. Diabetes Care 2006;29:945. [DOI] [PubMed] [Google Scholar]
- 5.Aragon-Sanchez J, Lipsky BA, Lazaro-Martinez JL. Diagnosing diabetic foot osteomyelitis: is the combination of probe-to-bone test and plain radiography sufficient for high-risk inpatients? Diabet Med 2011;28:191–194. [DOI] [PubMed] [Google Scholar]
- 6.Senneville E. Editorial commentary: probe-to-bone test for detecting diabetic foot osteomyelitis: rapid, safe, and accurate-but for which patients? Clin Infect Dis 2016;63:949–950. [DOI] [PubMed] [Google Scholar]
- 7.Cecilia-Matilla A, Lazaro-Martinez JL, Aragon-Sanchez J. Statistical reliability of bone biopsy for the diagnosis of diabetic foot osteomyelitis. J Foot Ankle Surg 2013;52:692. [DOI] [PubMed] [Google Scholar]
- 8.Meyr AJ, Singh S, Zhang X, et al. Statistical reliability of bone biopsy for the diagnosis of diabetic foot osteomyelitis. J Foot Ankle Surg 2011;50:663–667. [DOI] [PubMed] [Google Scholar]
- 9.Puavilai G, Chanprasertyotin S, Sriphrapradaeng A. Diagnostic criteria for diabetes mellitus and other categories of glucose intolerance: 1997 criteria by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (ADA), 1998 WHO consultation criteria and 1985 WHO criteria. World Health Organization. Diabetes Res Clin Prac 1999;44:21–26. [DOI] [PubMed] [Google Scholar]
- 10.Allahabadi S, Haroun KB, Musher DM, Lipsky BA, Barshes NR. Consensus on surgical aspects of managing osteomyelitis in the diabetic foot. Diabet Foot Ankle 2016;7:30079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atway S, Nerone VS, Springer KD, Woodruff DM. Rate of residual osteomyelitis after partial foot amputation in diabetic patients: a standardized method for evaluating bone margins with intraoperative culture. J Foot Ankle Surg 2012;51:749–752. [DOI] [PubMed] [Google Scholar]
- 12.Kowalski TJ, Matsuda M, Sorenson MD, Gundrum JD, Agger WA. The effect of residual osteomyelitis at the resection margin in patients with surgically treated diabetic foot infection. J Foot Ankle Surg 2011;50:171–175. [DOI] [PubMed] [Google Scholar]
- 13.Akinci B, Yener S, Yesil S, Yapar N, Kucukyavas Y, Bayraktar F. Acute phase reactants predict the risk of amputation in diabetic foot infection. J Am Podiatr Med Assoc 2011;101:1–6. [DOI] [PubMed] [Google Scholar]
- 14.Beck FK, Rosenthal TC. Prealbumin: a marker for nutritional evaluation. Am Fam Physician 2002;65:1575–1578. [PubMed] [Google Scholar]
- 15.Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am 1984;66:71–75. [PubMed] [Google Scholar]
- 16.Lipsky BA, Armstrong DG, Citron DM, Tice AD, Morgenstern DE, Abramson MA. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded, multicentre trial. Lancet 2005;366:1695–1703. [DOI] [PubMed] [Google Scholar]
- 17.Litchford MD. Nutritional issues in the patient with diabetes and foot ulcers In: Bowker JH, Pfeifer MA, eds. Levin and O’Neals’s the Diabetic Foot 7th ed. Philadelphia, PA: Mosby Elsevier; 2008:199–217. [Google Scholar]
- 18.Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995;273:721–723. [PubMed] [Google Scholar]
- 19.Lavery LA, Armstrong DG, Peters EJ, Lipsky BA. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care 2007;30:270–274. [DOI] [PubMed] [Google Scholar]
- 20.Lozano MR, Fernandez GML, Hernandez MD, Montesinos BJV, Jimenez GS, Jurado GMA. Validating the probe-to-bone test and other tests for diagnosing chronic osteomyelitis in the diabetic foot. Diabetes Care 2010;33:2140–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutluoglu M, Uzun G, Sildiroglu O, Turhan V, Mutlu H, Yildiz S. Performance of the probe-to-bone test in a population suspected of having osteomyelitis of the foot in diabetes. J Am Podiatr Med Assoc 2012;102:369–373. [DOI] [PubMed] [Google Scholar]
- 22.Levine M, Ensom MH. Post hoc power analysis: an idea whose time has passed? Pharmacotherapy 2001;21:405–409. [DOI] [PubMed] [Google Scholar]
- 23.Fogel J. Post hoc power analysis—another view. Pharmacotherapy 2001;21:1150. [DOI] [PubMed] [Google Scholar]


