We have made enormous strides in atherosclerosis research over the last century. The story of the cholesterol hypothesis provides a model whereby decades of fundamental research bore fruit when translated to the clinic.1 This victory of basic science intertwined with clinical development merits revisiting as it furnishes a pathway which can inspire us and prod us to achieve a similar goal with remaining risk factors beyond cholesterol (see cover figure), but more quickly and adding to the effectiveness of treatment of cholesterol.
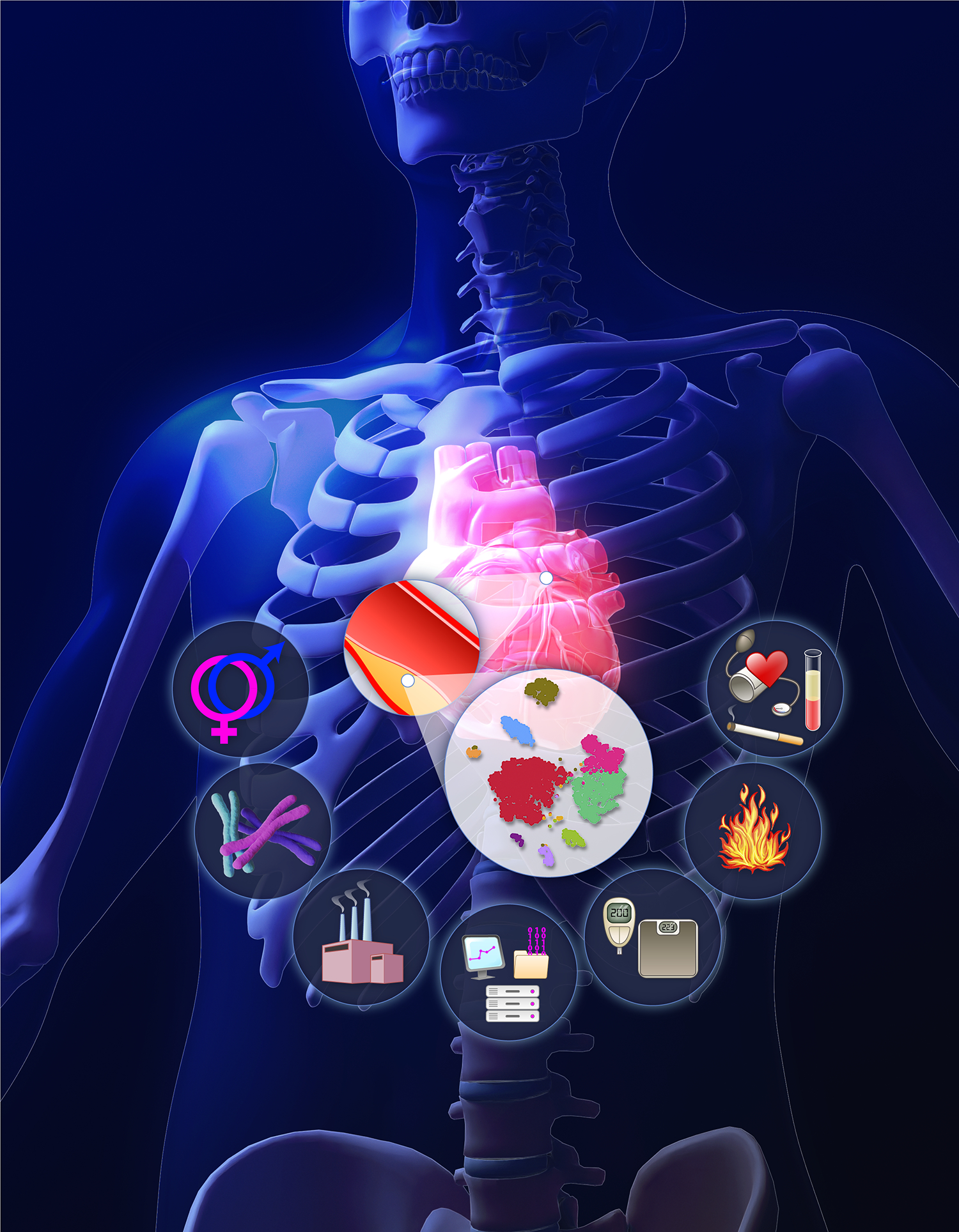
This drawing depicts an artistic version of the results of a single-cell RNA-sequencing experiment showing different clusters of cells isolated from a mouse atherosclerotic plaque. This rendition evokes the application of emerging technologies to the study of atherosclerosis. Surrounding the cell clusters, the icons represent various risk factors, traditional and emerging. Traditional risk factors are represented by a blood pressure cuff and a test tube containing blood from a person with hyperlipidemia. The computer workstation at the bottom of this diagram represents the potential of big data, artificial intelligence, and advanced computational analytic approaches to deal with the massive amounts of data generated by contemporary molecular technologies, and provide the tools for a more precise identification of patients at risk, and the potential of targeting therapies in a more personalized fashion based on application of these informatic technologies.
The story of cholesterol in atherosclerosis begins experimentally with the work of Anichkov, who demonstrated the creation of fatty lesions in the arteries of rabbits that consumed a diet enriched in cholesterol.2 Adolf Windaus chemically characterized cholesterol in atherosclerotic plaques.3 A technological advance spurred the next chapter in the cholesterol story. The advent of the ultracentrifuge equipped John Gofman and his colleagues in the middle of the 20th century to characterize the lipoproteins that bear cholesterol and other lipids in the bloodstream.4 This work ushered an era of rapid progress in the characterization of different classes of lipoproteins, their correlation with disease states, and their chemical characterization. Gofman and his colleagues recognized low-density lipoproteins (LDL) and high-density lipoproteins (HDL), a classification that permitted them and many others to pinpoint LDL as a risk factor for atherosclerotic cardiovascular disease.
The discovery of the LDL receptor pathway provided the next boost to cholesterol research.5 Akira Endo’s discovery of natural products that inhibit hydroxymethylglutaryl coenzyme A (HMG-CoA), the rate-limiting step in the synthesis of sterols, a pathway elucidated by Konrad Bloch, opened the door to the development of effective new treatments for atherosclerotic cardiovascular disease.6, 7 The discovery by industrial investigators of ezetimibe, which acts in part by inhibiting cholesterol absorption in the small intestine, led to the discovery of the important role of the cholesterol transfer protein (Niemann-Pick C1-like 1 protein, NPC1L1) in enterocytes. Inhibition of NPC1L1 can lower LDL cholesterol independently of statins.8, 9 The observations of Catherine Boileau in kindreds with autosomal dominant hypercholesterolemia, coupled with the biochemical observations of Nabil Seidah, led to the cholesterol-regulating role of proprotein convertase subtilisin/kexin type 9 (PCSK9). This protein became the target of another series of LDL cholesterol therapeutics, both neutralizing antibodies and a small interfering RNA.10 Large-scale clinical trials validated the ability of PCSK9 inhibition to improve cardiovascular outcomes.11, 12 This evolution from basic discovery to clinical translation took all in all more than a century but provided us with the tools to lower LDL cholesterol markedly and enrolled enormous inroads into the prevention and treatment of cardiovascular diseases.
Yet, the battle, although joined, is not yet won. A considerable residual burden of cardiovascular disease persists despite substantial LDL lowering, and other highly effective preventive measures ranging from smoking cessation through treatment of hypertension. Coronary heart disease remains a leading cause of disability and death in the developed world.13 With the epidemiologic transition in the developing world, the epidemic of atherosclerotic disease has spread globally, threatening vast numbers of individuals. The waning conquering of communicable disease permits survival such that atherosclerotic cardiovascular disease can develop later in life. In both the developed world and countries in development the global increase in aging also fuels atherosclerotic cardiovascular disease.14
Perhaps if human populations had lifelong LDL cholesterol concentrations of a newborn or of many non-primate animal species, atherosclerosis would be an orphan disease. Yet, the seed of atherogenesis is already sown for many adults and concerningly, many children as well. Moreover, the burgeoning pandemic of obesity, diabetes, and attendant dysmetabolism provides further alarm that even in an era of conquest of LDL cholesterol, we have not yet vanquished atherosclerosis.
We have developed an impressive armamentarium of high technology approaches to treating established atherosclerosis (e.g., percutaneous or surgical revascularization) and heart failure (e.g., transplantation and circulatory assist devices). Indeed, ischemic cardiomyopathy, a consequence of the ravages of atherosclerotic cardiovascular disease, persists as the most common cause of heart failure, a syndrome that drains human wellbeing as well as healthcare resources. The current treatment of advanced heart failure, vascular and valvular disease, and arrythmias too often requires the use of resource-intensive interventions or devices. Such wonders of modern medicine include heart transplantation, mechanical circulatory assist devices, deployment of stents or artificial valves, or ablation of arrythmias. But these technology-intensive treatments of late-stage disease actually reflect a failure of prevention or lack of deeper understanding of the disease processes that necessitates deployment of these treatments dubbed “halfway technologies” as signaled by the prescient physician-essayist Lewis Thomas. He pointed out that treating advanced cardiovascular disease with such late-stage high technology interventions presents ethically puzzling and impossibly expensive challenges to human health and healthcare systems.15 He called for more basic research to address disease at an earlier stage and more fundamental levels.
Where can we now turn to continue progress in combatting atherosclerotic cardiovascular disease in an era of mastery over LDL cholesterol and in possession of an impressive armamentarium of interventions for late-stage disease? The model of investment in basic research (as in the case of LDL) and an imperative drive to rapid clinical translation provides a promising path toward this goal. Access of therapies and adherence to pharmaceutical therapies and lifestyle measure represents another critical challenge, a pertinent subject for investigation by behavioral economics and social sciences, a keenly important companion to the fruits of basic research.
This compendium presents a report on progress in atherosclerosis research since last summarized in these pages in 2016.16 The advances reported in this current collection of expert articles range from the microscale to the macroscopic view. The technical advances include a rapidly accelerating toolkit for studying non-coding RNAs and single cell RNA expression.17,18 These technical advances promise to identify new targets for therapies beyond the traditional risk factor control measures. Single-cell RNA-sequencing of atherosclerotic lesions have begun to reveal a surprising number of cell clusters representing different cell types and populations within traditionally defined lesional cell types.17 How to harness this expanded catalogue of cell types to increase fundamental understanding of disease and aid the development of new treatments presents a key challenge going forward. The extensive research on non-coding RNAs has advanced considerably, and has led to strategies to target functional atherosclerosis-relevant microRNAs as potential therapies.
On the macroscopic scale, the concept of “phenotype stacks “ and “big data” promises transformation in the way that we identify patients at risk and target therapies in a more personalized manner than “one size fits all.”19 Multigene risk assessment, facilitated by the scientific and technological advances of rapid, affordable, and widespread genotyping, furnishes another opportunity for a more precision approach to risk stratification and targeting of therapeutic interventions.20 Expansion of genetic discovery and prediction efforts that encompass greater representation from populations of non-European genetic ancestries presents an important goal for the future.
In addition to these technical innovations, a more traditional “candidate-based” approach has identified new targets beyond LDL. Those highlighted in contributions to this compendium include CD31, a molecule expressed by leukocytes, and platelets and endothelial cells that have become a potential therapeutic target.21 Recent research has revealed that CD31 agonists promote the healing of injured arteries in animal experiments. The rapidly developing field of epigenetics is also leading to new therapeutic avenues, for example therapies that target the bromodomain and extra-terminal containing protein family (BETs) that regulates gene transcription by ‘reading’ the specific histone marks that allow euchromatin to transition to the open state needed for transcription. BETs serve as epigenetic reader proteins by binding to acetylated lysine residues on histone tails and facilitating the assembly of transcription complexes and the transcriptional machinery.22
We are witnessing a renaissance of intermediary metabolism as it relates to non-lipid aspects of atherosclerosis biology and therapeutics that reach beyond the traditional tools. Very recently, bempedoic acid received approval by the FDA as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional LDL cholesterol lowering. Bempedoic acid, an ATP citrate lyase inhibitor, prevents the tricarboxylic acid cycle intermediate citrate from conversion to acetyl-CoA, a precursor of cholesterol biosynthesis and other cellular processes. These advances prompt us to dust off our college biochemistry texts, as this venerable subject assumes new prominence in an era of metabolomic research and heightened understanding of heterogeneity in microenvironments within tissues such as the atherosclerotic plaque.23 The increasing realization that cellular metabolites act not only intracellularly, but can exchange among lesional cells and alter their functions represents another timely concept covered in this compendium.23
The involvement of inflammation in atherosclerosis has advanced from theory to a proven reality since the publication of the last Circulation Research compendium on atherosclerosis. In light of the CANTOS trial24, 25, neutrophil extracellular traps (NETs), a fairly recently recognized set of structures that link host defenses, innate immunity, cell death, and thrombosis may also provide new therapeutic targets and ways of subtyping of patients susceptible to specific therapeutic interventions.26 Lifestyle furnishes the foundation of atherosclerosis prevention and remains a cornerstone of management of risk of recurrent atherothrombotic events. Recent experimental work in mice has expanded the fundamental understanding of how measures such as sleep, psychosocial stress, and voluntary exercise can modify atherosclerosis. These non-pharmacologic measures to mitigate this disease intersect with inflammatory pathways and innate immunity by muting hematopoiesis.27 Summarizing one family of pharmacologic targets, interleukin-1 and allied cytokines and the NLRP3 inflammasome, a panel of experts provides an update on this family of inflammatory mediators.28 The role of adaptive immunity in atherosclerosis, very well established experimentally, has spurred a number of attempts to advance vaccination strategies to the clinic.29 This compendium provides an update on the status of these translational undertakings and discusses the remaining obstacles to the development of a potentially an effective and safe atherosclerosis vaccination strategy.
As noted above, atherosclerosis has extended its reach beyond the middle aged Caucasian male that was the typical patient with coronary heart disease in the middle of the last century. The Coronary Drug Project launched by the United States federal government in the mid-1960s studied over 8,000 individuals, all male, in middle age (30–64 years). Studies of atherosclerosis have seldom enrolled substantial numbers of participants of women or of ethnic minorities. In the half century since design of the Coronary Drug Project, the demographics of atherosclerosis have shifted dramatically, as has our profession’s commitment to inclusion and diversity. More recent federal projects such as the Multi-Ethnic Study of Atherosclerosis (MESA) and the Atherosclerosis Risk in Communities Study (ARIC) have striven to enroll a more diverse population. Yet, we lag in experimental studies to provide sufficient attention to sex as a biological variable. This compendium includes a paper that addresses this important gap in both clinical and basic studies and aims to stimulate a more balanced and informative approach to experimental designs in the atherosclerosis field going forward.30 Future personalized medicine approaches depend critically on increased understanding of sex as a biological variable in atherosclerosis and cardiovascular disease.
This compendium purposefully has avoided an encyclopedic approach. We have emphasized areas of atherosclerosis research that have progressed substantially and matured since the 2016 compendium, published just a few years ago. It is a credit to our enterprise that we have made substantial progress worthy of report in so few intervening few years. We have deliberately omitted some topics which have received high-level attention in recent authoritative reviews, for example the microbiome31, 32 or environmental risk factors such as air pollution33.
While we have made much progress and should take just pride in the advances made so far, we must not relent or shrink from the task ahead. We must harness the power of the new technologies including those highlighted in the series, to identify and exploit new targets. We must strive to move beyond catalogues to elucidate novel mechanisms. We must not allow ourselves to be complacent and satisfied with laboratory work alone. We must hasten to bring the fruits of cell culture studies and animal experiments to the clinic and to use human data to guide further mechanistic studies. We should emulate the success of the conquest of LDL as a risk factor for atherosclerosis, but we owe it to the public and our patients to take less than another century to do so.
Acknowledgment:
We thank Drs. Amélie Vromman, Testsushi Nakao, Rajat Gupta and Mr. Shamsudheen Karuthedath Vellarikkal for their contribution to the cover of the Compendium.
Sources of Funding: PL is funded by the National Heart, Lung, and Blood Institute (R01HL080472 and 1R01HL13489); the American Heart Association (18CSA34080399); and the RRM Charitable Fund. KB reports NIH grants P01HL092969, R01HL126028, R01HL127694, R21AI135447, R01HL149685, and P30DK017047.
Nonstandard Abbreviations and Acronyms:
- LDL
low-density lipoproteins
- HDL
high-density lipoproteins
- HMG-CoA
hydroxymethylglutaryl coenzyme A
- NPC1L1
Niemann-Pick C1-like 1 protein
- PCSK9
proprotein convertase subtilisin/kexin type 9
- NETs
neutrophil extracellular traps
- MESA
Multi-Ethnic Study of Atherosclerosis
- ARIC
Atherosclerosis Risk in Communities Study
Footnotes
Disclosures: PL is an unpaid consultant to, or involved in clinical trials for Amgen, Kowa Pharmaceuticals, Novartis, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Merck, Pfizer, and Sanofi-Regeneron. PL is also a member of a scientific advisory board for Amgen, Kowa Pharmaceuticals, Novartis, Corvidia Therapeutics, DalCor Pharmaceuticals, Olatec Therapeutics, Medimmune, and XBiotech, Inc. PL is on the Board of Directors for XBiotech, Inc. and receives laboratory funding from Novartis. KB has no disclosures.
Contributor Information
Peter Libby, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston MA.
Karin E. Bornfeldt, Department of Medicine, Division of Metabolism, Endocrinology and Nutrition, Department of Pathology, University of Washington Medicine Diabetes Institute, University of Washington, Seattle WA.
References:
- 1.Steinberg D The Cholesterol Wars: The Skeptics vs. the Preponderance of Evidence. 1 ed. San Diego, CA: Elsevier Academic Press; 2007. [Google Scholar]
- 2.Anitchkov N and Chalatow S. On experimental cholesterin steatosis and its significance in the origin of some pathological processes (1913). Reprinted in Arteriosclerosis. 1983;3:178–182. [PubMed] [Google Scholar]
- 3.Windaus A Über den Gehalt normaler und atheromatöser Aorten an Cholesterin und Cholesterinestern. Hoppe-Seyleŕs Zeitschrift für physiologische Chemie. 1910;67:174. [Google Scholar]
- 4.Lindgren FT, Elliott HA and Gofman JW. The ultracentrifugal characterization and isolation of human blood lipids and lipoproteins, with applications to the study of atherosclerosis. J Phys Colloid Chem. 1951;55:80–93. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JL and Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endo A, Kuroda M and Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. Febs Letters. 1976;72:323–6. [DOI] [PubMed] [Google Scholar]
- 7.Bloch K The biological synthesis of cholesterol Nobel Lectures, Physiology or Medicine 1963–1970 Amsterdam: Elsevier; 1972: 78–100. [Google Scholar]
- 8.Altmann SW, Davis HR Jr., Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR Jr., Dean DC, Detmers PA, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci U S A. 2005;102:8132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. New England Journal of Medicine. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. New England Journal of Medicine. 2018. [Google Scholar]
- 13.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020:CIR0000000000000757. [DOI] [PubMed] [Google Scholar]
- 14.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas L Late night thoughts on listening to Mahler’s Ninth Symphony. 3 ed. New York: The Viking Press; 1984. [Google Scholar]
- 16.Libby P, Bornfeldt KE and Tall AR. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ Res. 2016;118:531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams JW, Winkels H, Durant CP, Zaitsev K, Ghosheh Y and Ley K. Single Cell RNA Sequencing in Atherosclerosis Research. Circ Res. 2020;126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jae N and Dimmeler S. Non-coding RNAs in vascular diseases. Circ Res. 2020;126. [DOI] [PubMed] [Google Scholar]
- 19.MacRae CA and Califf RM. Reimagining what we measure in atherosclerosis-a “Phenotype Stack: Questions for Calum MacRae from Robert Califf. CIrc Res. 2020;126. [DOI] [PubMed] [Google Scholar]
- 20.Natarajan P and Aragam K. Multigene assessment of atherosclerotic risk: clinical perspectives and basic implications. Circ Res. 2020;126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caligiuri G CD31 as a therapeutic target in atherosclerosis. Circ Res. 2020;126. [DOI] [PubMed] [Google Scholar]
- 22.Borck P and Plutzky J. Bromo Domains in the regulation of genes implicated in atherosclerosis. Circ Res. 2020;126. [Google Scholar]
- 23.Tabas I and Bornfeldt KE. Intracellular and Intercellular Aspects of Macrophage Immunometabolism in Atherosclerosis. Circ Res. 2020;126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New England Journal of Medicine. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 25.Libby P Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J Am Coll Cardiol. 2017;70:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Döring Y, Libby P and Soehnlein O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases – Recent Experimental and Clinical Insights. Circ Res. 2020;126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schloss MJ, Swirski FK and Nahrendorf M. Modifiable cardiovascular risk, hematopoiesis and innate immunity. Circ Res. 2020;126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW and Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson J and Hansson GK. Vaccination Strategies Targeting Atherosclerosis. Circ Res. 2020;126. [DOI] [PubMed] [Google Scholar]
- 30.Jaffe I and Beckman J. Sex as a Biological Variable in Atherosclerosis. Circ Res. 2020;126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aron-Wisnewsky J and Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12:169–81. [DOI] [PubMed] [Google Scholar]
- 32.Tang WHW, Backhed F, Landmesser U and Hazen SL. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2089–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brook RD, Newby DE and Rajagopalan S. Air Pollution and Cardiometabolic Disease: An Update and Call for Clinical Trials. Am J Hypertens. 2017;31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


