Abstract
In total, 30 cases of patients undergoing health check-ups with the diagnostic criteria of sepsis were included in the present study. The clinical data of each patient with sepsis were recorded at admission. In the present study, the association between the proportion of T cells in patients with sepsis and those in a healthy condition were observed. The expression of immunosuppressive molecules on the surface of Vδ1 T cells were examined, as well as studying the secretion of inflammatory cytokines in Vδ2 T cells, and the ability of the Vδ1 T cells to inhibit the secretory level of interferon-γ (IFN-γ) and the inflammatory function of Vδ2 T cells were monitored. The inhibition of proliferation of naïve CD4 T cells by Vδ1 T cells and inflammatory function of Vδ2 T cells were examined. The number of Vδ1 T cells in the peripheral blood of patients with sepsis was significantly increased compared with healthy controls (P<0.01); the proportion of Vδ2 T cells was opposite to that of Vδ1 T cells. The Sequential Organ Failure Assessment score, survival and survival time were positively associated with Vδ1 T cell ratio (P<0.05) and negatively correlated with Vδ2 T cells. The expression of cytotoxic T-lymphocyte protein 4 and T cell immunoglobulin and mucin domain-containing protein 3 on the surface of Vδ1 T cells in the peripheral blood of patients with sepsis was significantly increased compared with the healthy controls (P<0.01), and the levels of IFN-γ and tumor necrosis factor-α secreted by Vδ2 T cells were significantly decreased (P<0.01). The immunosuppressive function of Vδ1 T cells was significantly higher, and the function of Vδ2 T cells was significantly reduced (P<0.01). The phosphorylation level of Erk1/2 in Vδ2 T cells was significantly lower (P<0.01). The present results suggested that the imbalance and functional changes of different γδ T cell subtypes in the peripheral blood of patients with sepsis are associated with sepsis, and may be involved in sepsis progression.
Keywords: sepsis, T cells, proportion, function, peripheral blood
Introduction
Sepsis is a systemic inflammatory response syndrome caused by infection with clinical confirmation or a suspected bacterial infection reaching the blood (1). Sepsis is a serious social problem threatening human health and life. The global incidence of sepsis is 1.5-8.0% per year, and the mortality rate can reach 30-70% (2,3). At present, to the best of the authors' knowledge, there has been no fundamental breakthrough in the treatment of sepsis because the underlying mechanisms of sepsis are still unclear. Evidence has shown that immunosuppression plays an important role in sepsis (4). The initial immune response of patients with sepsis is triggered by a high inflammatory state (a large number of cytokines are produced), but the high inflammatory state soon changes to an immunosuppressive state (4). The immunosuppressive state can increase the likelihood of a secondary opportunistic infection or reactivate the latent infection (5,6). Therefore, restoring the immunosuppressive state of patients plays an important role in the treatment of sepsis.
The maintenance of immune suppression involves the participation of various immunoregulatory cells. In many cells with an immunomodulatory function, regulatory T cells (Tregs) have been shown to be crucial in maintaining immune balance and tolerance. Tregs are a relatively early cell to appear with immunosuppressive function (7,8). Tregs have been demonstrated to be associated with the pathogenesis, prognosis and drug treatment of patients with sepsis (9,10). Wan (9) have demonstrated that in the early stages of sepsis, there is a significant proportion of abnormal Treg cells, which are mainly manifested as an increase in proportion and enhancement of immunosuppressive function. Huang et al (11) identified a significant increase in the proportion of CD39+ Tregs in the peripheral blood of patients with sepsis. The increase in the proportion of CD39+ Tregs in the peripheral blood of patients with sepsis was closely related to prognosis (11). Shao et al (12) demonstrated that drug therapy can play a therapeutic role by inhibiting the function of CD4+ CD25+ Tregs. In addition to Tregs, regulatory B cells have also been demonstrated to play an important role in the pathogenesis of neonatal sepsis (10).
γδ T cells are the main effector cells involved in the innate immune response of the host, and are the bridge connecting innate immunity and adaptive immunity. γδ T cells appear early in the immune response and efficiently produce inflammatory cytokines, such as interferon-γ (IFN-γ) and tumor necrosis factor (TNF) (13). It has been observed in literature that γδ T cells can inhibit the differentiation of Tregs by secreting the soluble cytokine IFN-γ and increase the transformation of antigen-specific Treg cells (14). γδ T cells have been documented to be associated with disease activity and survival in patients with sepsis (15). γδ T cells can be further divided into two types of cell subtypes, in vivo Vδ1 T cells and Vδ2 T cells. These two cell subtypes have different functions; specifically, Vδ1 T cells have an immunosuppressive function and participate in the immune escape process of tumors; while Vδ2 T cells are inflammatory cells and inhibit tumor occurrence (16-19). Therefore, the functional changes of Vδ1 T cells in patients with sepsis may be consistent with Tregs, but further data are required to verify the changes in Vδ2 T cells in patients with sepsis. The changes in total γδ T cells in patients with sepsis, and the changes in Vδ1 and Vδ2 T cells were observed to provide new insight for the study of sepsis.
Patients and methods
Patients
Between December 2016 and December 2017, 30 patients with sepsis (14 patients with sepsis, 9 patients with severe sepsis and 7 patients with septic shock) and 30 healthy control (HC) patients at the same time were enrolled from the intensive care unit of Yueqing People's Hospital.
The inclusion criteria were as follows: Patients aged >18 years and met the sepsis diagnostic criteria established by The International Conference on Sepsis in Washington, DC in December 2001(20). The following were exclusion criteria: Autoimmune diseases, acute stroke, myocardial infarction, viral hepatitis, HIV infection and use of hormone or immunosuppressive agents in March before admission. The age and sex of patients with sepsis matched the data of the HCs (P>0.05). The Sequential Organ Failure Assessment (SOFA) score, which can dynamically reflect changes in organ function, was assessed for the patients (20). The daily difference score was taken daily; the higher the score, the worse the prognosis. The SOFA score is based on each indicator in the SOFA score table. The detailed clinical data of patients with sepsis are presented in Table I. All patients signed informed consent. The present study was approved by The Ethics Committee of Yueqing People's Hospital.
Table I.
Clinical characteristics of patients.
| Characteristic | Healthy controls | Patients with sepsis |
|---|---|---|
| Number | 30 | 30 |
| Age, year | 39.4.2±13.7 | 38.9±14.6 |
| Sex, male/female | 15/15 | 16/14 |
| SOFA score | - | 11.5±4.0 |
| Source of infection | ||
| Lung | - | 15 (50.0%) |
| Abdomen | - | 6 (20%) |
| Urinary tract infection | - | 1 (3.3%) |
| Pathogen | - | 8 (26.7%) |
| Gram-negative bacillus | - | 16.1±6.7 |
| Gram-positive bacillus | - | 5.5±4.8 |
| Fungus | - | 10 (33.3%) |
| Negative | - | 28.2±9.1 |
| White blood cell count, x109/l | - | 56.7% (17/13) |
| PCT, ng/ml | 30 | 30 |
| Mechanical ventilation | 39.4.2±13.7 | 38.9±14.6 |
| Renal transplantation | 15/15 | 16/14 |
| ICU hospitalization, days | - | 11.5±4.0 |
| Mortality, survival/death | - | - |
SOFA, Sequential Organ Failure Assessment; PCT, procalcitonin; ICU, intensive care unit.
Main reagents
The reagents and sources used were as follows: RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) and FBS (Gibco; Thermo Fisher Scientific, Inc.); FITC-anti human T cell receptor (TCR) Vδ1 antibody (1:1.000; cat. no. 331208; BioLegend, Inc.), phycoerythrin (PE)-anti-human-CD3 antibody (1:1,000; cat. no. 300408; BioLegend, Inc.), FITC-anti human TCR Vδ2 antibody (1:1,000; cat. no. 331410; BioLegend, Inc.), allophycocyanin (APC)-anti-human cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody (1:1,000; cat. no. 369612; BioLegend, Inc.), Pecy5-anti-human T cell immunoglobulin and mucin-domain containing-3 (TIM-3) antibody (1:1,000; cat. no. 318308; BioLegend, Inc.); CellTrace™ carboxyfluorescein succinimidyl ester (CFSE) cell proliferation kit (Invitrogen; Thermo Fisher Scientific, Inc.); amplification of purified anti-human CD3 antibody (1:1,000; cat. no. 561806; BD Biosciences); and purified anti-human CD28 antibody (1:1,000; cat. no. 553295; BD Biosciences); purified anti-human Vδ1 antibody (1:1,000; cat. no. B49309; Beckman Coulter, Inc.); anti-human phosphorylated (p)-Erk antibody (1:1,000; cat. no. 4370; Cell Signaling Technology, Inc.) and HRP goat anti-rat secondary antibody (1:1,000; cat. no. 7077; Cell Signaling Technology, Inc.); and Supersignal West Femto/Pico HRP sensitive chemiluminescence substrate (Saiser World Science and Technology Co., Ltd.).
Peripheral blood mononuclear cell acquisition and flow cytometry staining
Peripheral blood mononuclear cells (PBMCs) were extracted from the patients and healthy subjects as previously described (21). The concentration of PBMCs in the RPMI-1640 medium containing 10% FBS was 1x107 (10 µl PBMC in an Eppendorf tube, 3 µl PE-anti-CD3 antibody, 3 µl FITC-anti-TCR Vδ1 antibody/3 µl FITC-anti-TCR Vδ2 antibody, and 3 µl APC-anti-human CTLA-4 antibody/3 µl Pecy5-Pecy5-anti-human TIM-3 antibody). The solution was incubated at 4˚C for 30 min. After two passages of RPMI-1640 culture with 10% FBS, the cells were suspended in 0.1 ml 4% polyformaldehyde fixing solution at room temperature for 10 min and examined by flow cytometry. Cell surface antigens were evaluated by flow cytometry with a FACSCalibur flow cytometer or BD LSRFortessa (BD Biosciences). Data were analyzed using FlowJo 7.6.1 (Tree Star, Inc).
Peripheral blood Vδ1T cell surface immunosuppressive molecules (CTLA-4 and TIM-3) expression and Vδ2T cell inflammatory factors (IFN-γ and TNF-α) secretion detection
The concentration of PBMCs in RPMI-1640 medium containing 10% FBS was 1x107 (10 µl PBMC was obtained from an Eppendorf tube and 1 ml RPMI-1640 medium containing 10% FBS was added). In the Eppendorf tube, Cell Activation Cocktail (cat. no. 423304; BioLegend, Inc,) was added for incubation for 6 h at 37˚C with 5% CO2. The cells were collected, and the advanced cell surface molecules were stained with Vδ2 TCR at 4˚C for 30 min, then 0.5 ml cell membrane was added to immobilize the permeation fluid to suspend the cells, while avoiding light for 30 min at the room temperature. The cells were twice-washed with permeation liquid, and the cells were suspended in 0.1 ml 4% paraformaldehyde fixative at room temperature for 10 min and detected by flow cytometry, then 5 µl IFN-γ antibody/TNF-α antibody was added while avoiding light for 30 min at room temperature. The cells were twice-washed in 0.1 ml 1% paraformaldehyde fixative, then detected by flow cytometry.
When Vδ1T cells were used to inhibit the secretion of cytokines in Vδ2T cells, the two cell subtypes were co-cultured for 72 h at a 1:5 ratio, then the aforementioned steps were repeated.
Detection of CFSE cell proliferation
The cells were washed once with 10 ml serum-free RPMI-1640 medium, followed by CFSE dye at a final concentration of 5 mmol/l, and placed in an incubator containing 5% CO2 at 37˚C for 10 min. Then, 5 ml pre-cooled CFSE staining terminator was immediately added to the cells, including 5% FBS RPMI-1640, and placed on ice for 5 min to stop the dyeing. The cells were then centrifuged at 400 x g for 8 min at room temperature, and washed with 10 ml RPMI-1640 medium. After the cells were suspended with RPMI-1640 complete medium, the Vδ1 and naïve CD4 T cells were added at a 1:5 ratio into a 48-well plate containing 1 µg/ml CD3 antibody and 2 µg/ml CD28 antibody. After 5 days of incubation, the cells were harvested, and the cell suspension was added to a 5 ml flow tube, 100 µl per tube, blocked with 5% BSA at room temperature for 1 h, and the dead cells were removed, and 1:500 dilutions of FITC-anti human TCR Vδ1 antibody and FITC-anti human TCR Vδ2 antibody was added, gently mixed, and incubated at 4˚C for 30 min in the dark. The cells were then centrifuged (350 x g, 4˚C, 5 min) and washed twice with PBS. The supernatant was discarded; the cells were resuspended and tested by flow cytometry.
Western blotting detection of p-Erk expression in Vδ2 T cells
Vδ2 T cells with purity >90% were obtained by sorting, and after 0, 5, 10 and 15 min stimulation, they were placed in an Eppendorf tube containing RIPA buffer (cat. no. 9806; Cell Signaling Technology, Inc.), 1% PMSF solution was added to prevent protein degradation, homogenized, left to stand for 3 h, centrifuged (400 x g; 5 min; 4˚C) and then the supernatant was removed for dispensing. The protein concentration of the extracted samples was determined using a BCA kit. A total of 30 µg protein/lane was separated by SDS-PAGE on 8% gels. The separated proteins were subsequently transferred to PDVF membranes and incubated for 2 h at room temperature in 5% BSA. Erk antibody (1:500; cat. no. 9102; Cell Signaling Technology, Inc) and anti-p-Erk antibody (1:500; cat. no. 4370; Cell Signaling Technology, Inc) was added and incubated overnight at 4˚C. The next day, the membrane was washed three times with TBS with 0.1% Tween-20 (TBST; 5 min each time) and the corresponding goat anti-mouse HRP-labeled secondary antibody (1:1,000; cat. no. 7077; Cell Signaling Technology, Inc.) was added and incubated at room temperature for 1 h. After washing in 0.1% TBST, the Supersignal West Femto/Pico HRP-sensitive chemiluminescent substrate was used to color the bands. Actin was used as an internal reference. ImageJ software (v2.1.4.7; National Institutes of Health) scans the image to yield gray values that reflect the intensity of protein expression.
Statistical analysis
SPSS 16.0 (SPSS, Inc.) was used to analyze the data. Counting data are presented as percentages and measurement data are presented as the mean ± SD. Experiments were repeated five times. A t-test was used to compare the measurement data between the two groups. Comparisons of experimental groups were evaluated by one-way ANOVA, followed by Bonferroni analysis. Spearman rank correlation analysis was used to determine the correlation between the ratio of Vδ1 and Vδ2 T cells in the peripheral blood of patients with sepsis and patient condition. P<0.05 was considered to indicate a statistically significant difference.
Results
Detection of peripheral blood γδ T cells and the different subtypes
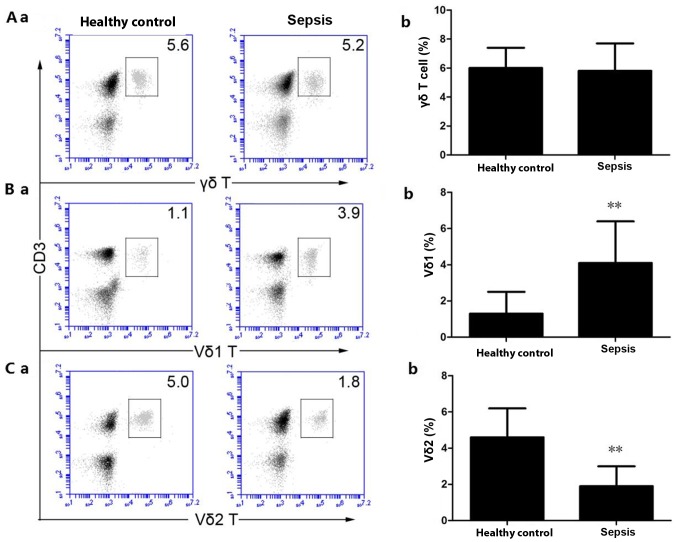
The proportion of γδ T cells (Fig. 1A-a and b) in the peripheral blood of the HCs was 6.01±1.42% compared with 5.81±1.94% in patients with sepsis. Compared with the HCs, the proportion of peripheral blood γδ T cells in patients with sepsis did not significantly change (P>0.05). The proportion of Vδ1 T cells (Fig. 1B-a and b) in the peripheral blood of the HC group was 1.33±1.19% and the proportion of Vδ1 T cells in the peripheral blood of patients with sepsis was 4.22±2.38%. Compared with the HCs, the proportion of Vδ1 T cells in the peripheral blood of the patients with sepsis was significantly increased (P<0.01). The proportion of Vδ2 T cells (Fig. 1C-a and b) in the peripheral blood of the HC group was 4.65±1.67%, and the proportion of Vδ2 T cells in the peripheral blood of the patients with sepsis was 1.94±1.15%. Compared with the HCs, the proportion of Vδ2 T cells in the peripheral blood of the patients with sepsis was significantly decreased (P<0.01).
Figure 1.
Flow cytometric staining was used to detect the percentage of γδ T, γδ1 T and γδ2 T cells. (A-a) Detection of peripheral blood γδ T cells by flow cytometry. (A-b) Quantitative analysis of peripheral blood γδ T cells. (B-a) Detection of peripheral blood Vδ1 T cells by flow cytometry. (B-b) Quantitative analysis of peripheral blood Vδ1 T cells. (C-a) Detection of peripheral blood Vδ2 T cells by flow cytometry, (C-b) Quantitative analysis of peripheral blood Vδ2 T cells. **P<0.01 vs. the healthy control group.
Proportion of Vδ1 and Vδ2 T cells in different types of patients with sepsis
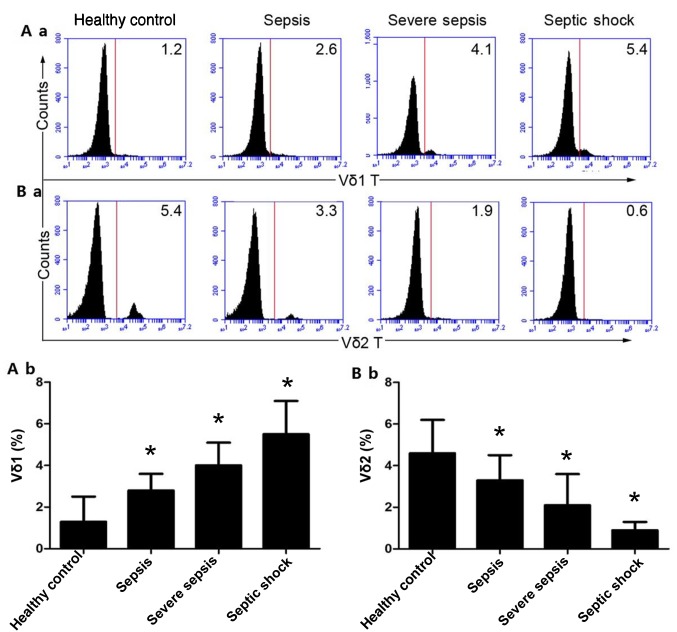
The proportion of Vδ1 T cells in the peripheral blood of the HCs was 1.33±1.19%. The proportion of Vδ1 T cells in the peripheral blood of patients with mild sepsis was 2.84±0.81%, and the proportion of Vδ1 T cells in the peripheral blood of severe sepsis patients was 4.04±1.19%, while the percentage of Vδ1 T cells in the peripheral blood of the septic shock group was 5.51±1.65%. In addition, with the exacerbation of sepsis, the proportion of Vδ1 T cells in the peripheral blood increased gradually (P<0.01; Fig. 2A-a and b). The proportion of Vδ2 T cells in the peripheral blood of the HCs was 4.65±1.67%, and the proportion of Vδ2 T cells in the peripheral blood of patients with mild sepsis was 3.34±1.28%. The proportion of Vδ2 T cells in the peripheral blood of patients with severe sepsis was 2.09±1.54%, and the proportion of Vδ2 T cells in the peripheral blood of patients in the septic shock group was 0.92±1.38%. Moreover, with the exacerbation of sepsis, the proportion of Vδ2T cells in the peripheral blood decreased gradually (P<0.01; Fig. 2B-a and b).
Figure 2.
Ratios of Vδ1 and Vδ2 T cells in patients with different types of sepsis. (A-a) Percentage of Vδ1 T cells was measured by flow cytometry. (A-b) Quantitative analysis of Vδ1 T cells. (B-a) Percentage of Vδ2 T cells was measured by flow cytometry. (B-b) Quantitative analysis of Vδ2 T cells. The histograms are representative examples of the data (14 patients with sepsis, 9 patients with severe sepsis, 7 patients with septic shock and 30 healthy controls). *P<0.01 vs. healthy control.
Correlation between the ratio of V δ1 and Vδ 2 T cells in the peripheral blood of patients with sepsis and patient condition
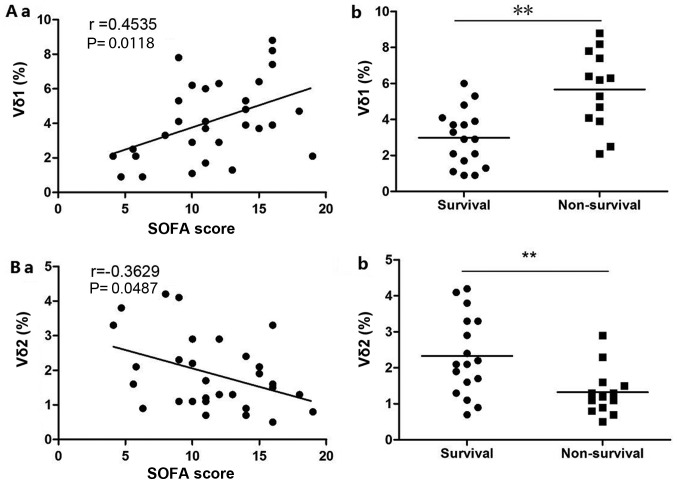
The proportion of Vδ1 T cells (Fig. 3A-a) in patients with sepsis was positively correlated with the SOFA score (r=0.4535; P<0.05) and the proportion of Vδ2 T cells (Fig. 3B-a) was negatively correlated with the SOFA score (r=-0.3629; P<0.05). A higher Vδ1 T cell ratio correlated with lower patient survival rate (Fig. 3A-b), while a lower Vδ2 T cell ratio correlated with lower patient survival rate (Fig. 3B-b).
Figure 3.
Correlation between the ratio of Vδ1 T cells and Vδ2 T cells in the peripheral blood of septic patients and patient condition. (A-a) The higher the Vδ1 T cells ratio, the higher the SOFA score. (A-b) The higher the Vδ1 T cells ratio, the lower the patient survival rate. (B-a) The lower the Vδ2 T cells ratio, the higher the SOFA score. (B-b) The higher the Vδ1 T cells ratio the lower the patient survival rate. **P<0.01. SOFA, Sequential Organ Failure Assessment.
Expression of immunosuppressive molecules on Vδ1 T cells and secretion of inflammatory cytokines from Vδ2 T cells
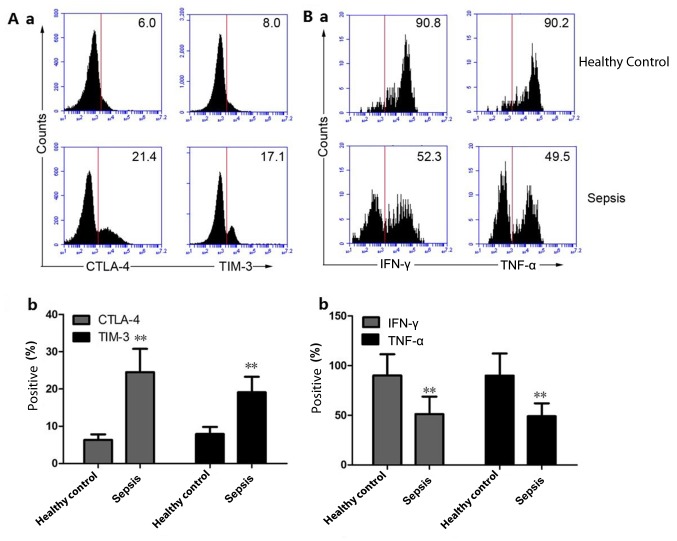
The percentage of CTLA-4- and TIM-3-positive Vδ1 T cells in the peripheral blood of the HC group was 6.32±1.52 and 7.78±1.91%, respectively, while the percentage of CTLA-4- and TIM-3-positive Vδ1 T cells in the peripheral blood of patients with sepsis was 24.8±6.31 and 19.1±4.19%, respectively. Compared with the HCs, the percentage of CTLA-4- and TIM-3-positive Vδ1 T cells in the peripheral blood of patients with sepsis increased significantly (P<0.01; Fig. 4A-a and b). The percentage of IFN-γ and TNF-α positive Vδ2 T cells in the peripheral blood of the HC group was 90.1±21.3 and 89.9±22.3%, respectively, and the percentage of IFN-γ and TNF-α Vδ2 T cells in the peripheral blood of patients with sepsis was 51.2±17.6 and 49.1±12.9%, respectively. Compared with the HC group, the percentage of IFN-γ and TNF-α positive Vδ2 T cells in the peripheral blood of patients with sepsis was significantly decreased (P<0.01; Fig. 4B-a and b).
Figure 4.
Expression of immunosuppressive molecules on Vδ1 T cells and secretion of inflammatory cytokines by Vδ2 T cells. (A-a) The expression of CTLA-4 and TIM-3 on the surface of Vδ1 T cells was detected by flow cytometry. (A-b) Quantitative analysis of the expression of CTLA-4 and TIM-3 on the surface of Vδ1 T cells in healthy controls and sepsis patients. (B-a) The secretion of IFN-γ and TNF-α from Vδ2 T cells was detected by flow cytometry. (B-b) Quantitative analysis of the expression of IFN-γ and TNF-α from Vδ2 T cells in healthy controls and sepsis patients. The histograms are representative examples of the data (30 patients with sepsis and 30 healthy controls). **P<0.01 vs. the healthy control group. CTLA-4, cytotoxic T lymphocyte-associated antigen-4; TIM-3, T cell immunoglobulin and mucin domain 3; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α.
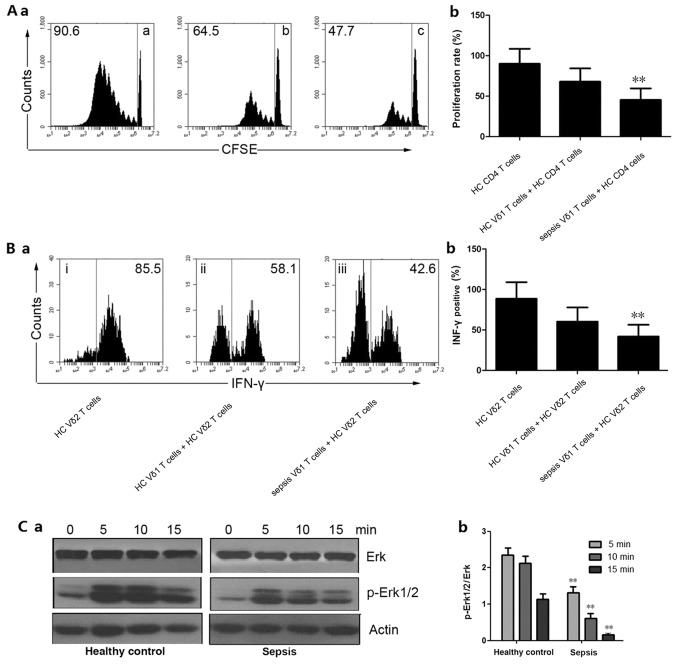
Functional detection of Vδ1 and Vδ2 T cells
The level of CD4 T cell proliferation in the peripheral blood of the healthy control Vδ1 T and Vδ2 T cell co-incubation group was 90.1±18.5%. The level of CD4 T cell proliferation in the peripheral blood of the healthy control Vδ1 T and Vδ2 T cell co-incubation group was 67.9±16.4%, and the level of Vδ1 and CD4 T cell proliferation in the peripheral blood of patients with sepsis was 45.3±14.3% (Fig. 5A-a and b). The IFN-γ secretory capacity of Vδ2 T cells in the peripheral blood of the healthy control Vδ1 T and Vδ2 T cell co-incubation group was 88.5±20.6%, and the IFN-γ secretion ability of Vδ2 T cells was 60.3±17.5% after incubation of Vδ1 T cells and Vδ2 T cells in the healthy control Vδ1 T and Vδ2 T cell co-incubation group. The IFN-γ secretory ability of Vδ1 T and Vδ2 T cells in the peripheral blood of patients with sepsis was 41.8±14.6% (Fig. 5B-a and b). These results suggested that the immunosuppressive function of peripheral blood Vδ1 T cells in patients with sepsis was significantly higher than the HCs (P<0.01). The western blotting results showed that the level of Erk signaling pathway phosphorylation associated with Vδ2 T cells in patients with sepsis was significantly lower than the control group (P<0.01; Fig. 5C-a and b).
Figure 5.
Functional detection of Vδ1 and Vδ2 T cells. (A-a) Peripheral blood CD4 T proliferation was detected using CFSE staining by flow cytometry. (A-b) Quantitative analysis of proliferation rate. **P<0.01 vs. HC Vδ1 T cells + HC CD4 T cells. (B-a) IFN-γ secretion was detected by flow cytometry. (B-b) Quantitative analysis of IFN-γ-positive cells. **P<0.01 vs. HC Vδ1 T cells + HC CD4 T cells. (C-a) Western blotting was used to detect Erk and p-Erk1/2 expression. Actin was used as an internal reference protein. (C-b) Quantitative analysis of p-Erk1/2 expression. **P<0.01 vs. the respective HC group. IFN-γ, interferon-γ; p, phosphorylated; CFSE, carboxyfluorescein succinimidyl ester; HC, healthy control; a, HC CD4 T cells; b, HC Vδ1 T cells + HC CD4 T cells; c, sepsis Vδ1 T cells + HC CD4 cells; i, HC Vδ2 T cells; ii, HC Vδ1 T cells + HC Vδ2 T cells; iii, sepsis Vδ1 T cells + HC Vδ2 T cells.
Discussion
Even with standard treatment, sepsis remains a major cause of death worldwide. One of the reasons for the lack of effective treatment for sepsis is the complexity and incomplete understanding of the underlying mechanism of sepsis. In sepsis, the body destroys the immune homeostasis by inducing an initial strong systemic inflammatory response, followed by a rapid negative feedback of the modern compensatory anti-inflammatory response, and decreased function of T cells (22). γδ T cells are a group of T cells expressing the γδ chain, which accounts for 0.5-5% of T cells in peripheral blood, and play an important role in anti-infection and immune regulation (23). The present study identified that the number of Vδ1 T and Vδ2 T cells in the peripheral blood of patients with sepsis. Vδ1 T cells in patients with sepsis was significantly increased compared with the healthy controls, and the expression rate was highest in the septic shock group. The proportion of Vδ1 T cells was positively correlated with the SOFA score.
Andreu-Ballester et al (24) demonstrated that the percentage of peripheral blood γδ T cells is reduced in patients with sepsis. The present results showed that the proportion of γδ T cells in the peripheral blood of patients with sepsis did not change significantly, which is in contrast from previously reported results (24). Analysis of the difference between the present study and the literature revealed that the average age of the patients in the literature was 66.3 years, while the average age of the patients in the present study was 38.9 years. Another previous study published by Andreu-Ballester et al (25) identified that there is a correlation between the number of γδ T cells in the peripheral blood and age, and the proportion of γδ T cells in the peripheral blood decreased with age. Therefore, it was hypothesized that the age difference is the basis for the differences in the study results, which suggested that the number of γδ T cells in the peripheral blood of patients with sepsis in different ages should also be further investigated in the future. CD3 staining was additionally conducted; the present study first measured γδ T cell levels, followed by measurement of γδ T cell levels. This may also be the reason for the difference between the two studies.
A review by Wan (9) stated that in the early stages of sepsis, Tregs showed significant abnormal proportions. Specifically, there was an increase in the proportion and enhancement of immunosuppressive function (9). The present study also detected changes in the proportion and function of Vδ1 T cells in patients with sepsis, and showed that the proportion of Vδ1 T cells was increased. The level of Vδ1 T cell expression in patients with sepsis was also higher than the controls, while the immunization inhibition test also confirmed that the function of Vδ1 T cells was enhanced. This finding is consistent with the changes in the number and function of Tregs cells reported in patients with sepsis. Previous studies reported that Treg cells exert an immunosuppressive function via two pathways (26,27): Direct cell contact, mainly through the expression of related immunosuppressive receptors; and secreting cytokines with immunosuppressive functions, such as IL-10 and TGF-β. It has been demonstrated that Vδ1 T cells play an immunosuppressive role mainly through cell contact, and play an important role in the pathogenesis of systemic lupus erythematous (28). In the present study, it was detected that the level of Vδ1 T cell expression was associated with contact immunosuppression, and it was demonstrated that the expression of Vδ1 T cells in the peripheral blood of patients with sepsis was increased. A limitation of the present study is that it was not possible to study the immunosuppressive effect of Vδ1 T cells in patients with sepsis.
CTLA-4 plays an important role in inhibiting T cell activation and maintaining immune tolerance. Moderate regulation of CTLA-4 expression can balance the inhibition signal and the stimulation signal to enhance protective immunity reaction (29). TIM-3 is a key molecule that regulates the T cell immune response and is involved in the induction of immune tolerance, and its sustained expression leads to depletion of T cell function (30). T cells regulate immune responses mainly by secreting INF-γ and TNF-α (30). The results of the present study showed that the expression of CTLA-4 and TIM-3 on the surface of Vδ1 T cells in peripheral blood of patients with sepsis was significantly increased, and the levels of IFN-γ and TNF-α secreted by Vδ2 T cells were significantly decreased. The immunosuppressive function of Vδ1 T cells was significantly enhanced, and the function of Vδ2 T cells was significantly reduced.
Erk1/2 is an important member of the Erk family. When members of the Erk family are activated by signals such as the external environment or cytokines, they can transmit signals to the nucleus to regulate biological behavior, such as cell proliferation and differentiation (31). The Erk1/2 signaling pathway plays a significant regulatory role in the activation of γδ T cells (31). In the present study, western blot analysis showed that the expression of p-Erk1/2 in γδ T cell subset Vδ2 T cells in the peripheral blood of patients with sepsis was significantly downregulated, which further impaired the function of γδ T cells contributing to the development of sepsis. The present study provided novel insight for the mechanism underlying sepsis.
The present study examined the expression level of surface molecules associated with Vδ1 T cells in contact with immunosuppression, suggesting that the expression of immunosuppressive molecules in peripheral blood Vδ1 T cells is elevated in patients with sepsis. However, a limitation of the present study was that it was not possible to study the pathway by which Vδ1 T cells exert immunosuppressive effects in patients with sepsis. The present study also detected changes in Vδ2 T cells in patients with sepsis. The present study first observed changes in the proportion and function of Vδ2 T cells in the peripheral blood of patients with sepsis, which promoted the study of the mechanism underlying sepsis.
In conclusion, the results of the present study suggested that the proportion of Vδ1 and Vδ2 T cells in the peripheral blood of patients with sepsis is out of balance, the immune inhibition function of Vδ1 T cells is significantly enhanced, and the level of inflammatory factors secreted by Vδ2 T cells is significantly reduced. As a result, the immune function of patients with sepsis is inhibited. This change may be closely associated with the prognosis of patients with sepsis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XW and XC conceptualized and designed the study and performed statistical analysis. WL performed literature research. DZ, HZ and PC performed experimental studies and data acquisition. XW, WL, DZ and HZ analyzed the data. XW, WL and XC prepared, edited and reviewed the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by The Ethics Committee of Huangshi Central University/The Affiliated Hospital of Hubei Polytechnic University. All patients signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nam M, Son BH, Seo JE, Kim IR, Park CK, Kim HK. Improved diagnostic and prognostic power of combined delta neutrophil index and mean platelet volume in pediatric sepsis. Ann Clin Lab Sci. 2018;48:223–230. [PubMed] [Google Scholar]
- 2.Jones BL, Smith SM. Choice of crystalloid and mortality in sepsis-all in the timing? Crit Care Med. 2014;42(e796) doi: 10.1097/CCM.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 3.Barochia AV, Cui X, Eichacker PQ. The surviving sepsis campaign's revised sepsis bundles. Curr Infect Dis Rep. 2013;15:385–393. doi: 10.1007/s11908-013-0351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otto GP, Sossdorf M, Claus RA, Rödel J, Menge K, Reinhart K, Bauer M, Riedemann NC. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15(R183) doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 9.Wan YY. Regulatory T cells: Immune suppression and beyond. Cell Mol Immunol. 2010;7:204–210. doi: 10.1038/cmi.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan X, Ji Z, Xue J. Percentage of peripheral CD19+CD24hiCD38hi regulatory B cells in neonatal sepsis patients and its functional implication. Med Sci Monit. 2016;22:2374–2378. doi: 10.12659/msm.895421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Xu R, Lin F, Bao C, Wang S, Ji C, Li K, Jin L, Mu J, Wang , et al. High circulating CD39(+) regulatory T cells predict poor survival for sepsis patients. Int J Infect Dis. 2015;30:57–63. doi: 10.1016/j.ijid.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Shao M, Liu B, Wang JQ, Tao XG, Zhou SS, Jin K, Zhang CP. Clinical significance of CD4+CD25+ T cell examination in sepsis patients. J Hunan Chin Med Univ. 2011;31:8–10. [Google Scholar]
- 13.Raga S, Julià MR, Crespí C, Figuerola J, Martínez N, Milà J, Matamoros N. Gammadelta T lymphocytes from cystic fibrosis patients and healthy donors are high TNF-alpha and IFN-gamma-producers in response to Pseudomonas aeruginosa. Respir Res. 2003;4(9) doi: 10.1186/1465-9921-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao L, Lu Q, Huang LJ, Ruan LH, Yang JJ, Huang WL, ZhuGe WS, Zhang YL, Fu B, Jin KL, ZhuGe QC. Transplanted neural stem cells modulate regulatory T, γδ T cells and corresponding cytokines after intracerebral hemorrhage in rats. Int J Mol Sci. 2014;15:4431–4441. doi: 10.3390/ijms15034431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreu-Ballester JC, Zamora V, Garcia-Ballesteros C, Benet-Campos C, Lopez-Chuliá F, Tormo-Calandín C, Cuéllar C. Anti-Anisakis sp. antibodies in serum of patients with sepsis and their relationship with γδ T cells and disease severity. Int J Parasitol. 2018;48:483–491. doi: 10.1016/j.ijpara.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Hayday AC. [gamma][delta] cells: A right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 17.Triebel F, Hercend T. Subpopulations of human peripheral T gamma delta lymphocytes. Immunol Today. 1989;10:186–188. doi: 10.1016/0167-5699(89)90321-6. [DOI] [PubMed] [Google Scholar]
- 18.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 19.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. SCCM/ESICM/ACCP/ATS/SIS: 2001 SCCM /ESICM /ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 21.Soyocak A, Kurt H, Ozgen M, Turgut Cosan D, Colak E, Gunes HV. MiRNA-146a, miRNA-155 and JNK expression levels in peripheral blood mononuclear cells according to grade of knee osteoarthritis. Gene. 2017;627:207–211. doi: 10.1016/j.gene.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Kumar V. T cells and their immunometabolism: A novel way to understanding sepsis immunopathogenesis and future therapeutics. Eur J Cell Biol. 2018;97:379–392. doi: 10.1016/j.ejcb.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Ichinohe T, Ichimiya S, Kishi A, Tamura Y, Kondo N, Ueda G, Torigoe T, Yamaguchi A, Hiratsuka H, Hirai I, et al. T-cell receptor variable gamma chain gene expression in the interaction between rat gammadelta-type T cells and heat-shock protein 70-like molecule. Microbiol Immunol. 2003;47:351–357. doi: 10.1111/j.1348-0421.2003.tb03406.x. [DOI] [PubMed] [Google Scholar]
- 24.Andreu-Ballester JC, Tormo-Calandín C, Garcia-Ballesteros C, Pérez-Griera J, Amigó V, Almela-Quilis A, Ruiz del Castillo J, Peñarroja-Otero C, Ballester F. Association of γδ T cells with disease severity and mortality in septic patients. Clin Vaccine Immunol. 2013;20:738–746. doi: 10.1128/CVI.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreu-Ballester JC, García-Ballesteros C, Benet-Campos C, Amigó V, Almela-Quilis A, Mayans J, Ballester F. Values for αβ and γδ T-lymphocytes and CD4+, CD8+, and CD56+ subsets in healthy adult subjects: Assessment by age and gender. Cytometry B Clin Cytom. 2012;82:238–244. doi: 10.1002/cyto.b.21020. [DOI] [PubMed] [Google Scholar]
- 26.Sabbagh P, Karkhah A, Nouri HR, Javanian M, Ebrahimpour S. The significance role of regulatory T cells in the persistence of infections by intracellular bacteria. Infect Genet Evol. 2018;62:270–274. doi: 10.1016/j.meegid.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Cuende J, Liénart S, Dedobbeleer O, van der Woning B, De Boeck G, Stockis J, Huygens C, Colau D, Somja J, Delvenne P, et al. Monoclonal antibodies against GARP/TGF-β1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Sci Transl Med. 2015;7(284ra56) doi: 10.1126/scitranslmed.aaa1983. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Kang N, Zhang X, Dong X, Wei W, Cui L, Ba D, He W. Generation of human regulatory gammadelta T cells by TCRgammadelta stimulation in the presence of TGF-beta and their involvement in the pathogenesis of systemic lupus erythematosus. J Immunol. 2011;186:6693–6700. doi: 10.4049/jimmunol.1002776. [DOI] [PubMed] [Google Scholar]
- 29.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci USA. 2010;107:1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Hou H, Xu L, Jane M, Peng J, Lu Y, Zhu Y, Sun Z. Tim-3 signaling pathway as a novel negative mediator in lipopolysaccharide-induced endotoxic shock. Hum Immunol. 2014;75:470–478. doi: 10.1016/j.humimm.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Lafont V, Liautard J, Sable-Teychene M, Sainte-Marie Y, Favero J. Isopentenyl pyrophosphate, a mycobacterial non-peptidic antigen, triggers delayed and highly sustained signaling in human gamma delta T lymphocytes without inducing eown-modulation of T cell antigen receptor. J Biol Chem. 2001;276:15961–15967. doi: 10.1074/jbc.M008684200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.