Abstract
Objective:
To evaluate hearing preservation (HP) outcomes in adult cochlear implant recipients with a mid-scala electrode.
Setting:
Tertiary academic center.
Patients:
Adult patients implanted with a mid-scala electrode between May 2013 and July 2015.
Interventions:
Cochlear implantation.
Main Outcome Measure(s):
Age, sex, surgical approach, residual hearing changes post cochlear implantation, HP rates using different published classifications, and speech perception scores.
Results:
Fifty ears for 47 patients (mean age, 58.2 yr; range, 23–86) were implanted with the electrode. Recognizing that not all patients were true HP candidates and/or underwent generally accepted HP surgical techniques, 39 ears had preoperative low-frequency hearing (audiometric threshold ≤85 dB HL at 250 Hz), 24 preserved acoustic hearing postoperatively (75.0%). Patients who had preserved acoustic hearing were implanted via round window (N = 18), extended round window (N = 4), or via cochleostomy (N = 2) approaches. Mean threshold elevation for low-frequency pure-tone average (125, 250, and 500 Hz) was 20.2 dB after surgery. 43.8% of patients had aidable low-frequency hearing at activation, 30.0% at 6-months postoperatively, and 30.8% 1-year postoperatively. Using a formula outlined by Skarzynski and colleagues, at 6-months postoperatively, 15.0% of patients had complete HP, whereas 40.0% had partial HP. At 1-year, these percentages decreased to 0% and 38.5%, respectively. Age, type of approach, and perioperative steroid use were not correlated with HP outcomes at activation and 6-months postoperatively (p > 0.05).
Conclusion:
The mid-scala electrode evaluated allows preservation of low-frequency hearing in patients undergoing cochlear implantation at rates and degrees of preservation close to other reports in the cochlear implant literature.
Keywords: Cochlear implantation, Hearing preservation
Indications for cochlear implantation are expanding, with many centers offering implantation to patients with residual hearing (1). In those patients, several studies have demonstrated benefit to patients in music appreciation, directional cues, and speech recognition in noise situations (2–8). Given these findings, and considering hearing preservation as a surrogate marker for atraumatic insertion with implantation, surgeons have been challenged to maximize the cochlea’s residual hearing during implantation.
Current cochlear implant electrodes are available in both straight and precurved—the latter of which is also referred to as perimodiolar—which have been shown to be more likely to translocate from the scala tympani to the scala vestibuli, with reports attributing the trans-location to the physical properties of the electrode array (9). As a result, a new mid-scala, precurved electrode array was designed to be less rigid with a less severe wrapping factor so that the electrode would be situated within the middle of the scala tympani, ideally avoiding contact with the modiolus and lateral wall.
Given that the mid-scala electrode received FDA approval relatively recently in May 2013, there are little data in the peer-reviewed literature describing the atraumatic nature of this electrode and associated patient outcomes. This holds significant clinical relevance for individuals pursuing cochlear implantation who have residual low-frequency acoustic hearing, as well as for surgeons aiming for minimally invasive surgery to minimize intracochlear trauma. Thus, the purpose of this exploratory project was to summarize our experience and hearing preservation (HP) outcomes with implantation of the mid-scala electrode in patients over 18 years of age, recognizing not all patients were HP candidates.
MATERIALS AND METHODS
After institutional review board approval (141196), a retrospective chart review was conducted. All patients more than 18 years of age who had been implanted with Advanced Bionics’ (AB, Valencia, CA) mid-scala (HiFocus Mid-Scala) electrode between FDA approval in 2013 and July 2015 were included. Fifty ears were implanted in 47 patients, with three bilateral recipients. Covariate variables assessed in this study included age, sex, surgical approach, and steroid administration (preoperative, intraoperative, and postoperative).
Audiometric data were collected with unaided pure-tone air-conduction thresholds before implantation, as well as at activation, which was generally scheduled approximately 2 to 3 weeks after implantation. If possible, subsequent unaided air-conduction thresholds were obtained at approximately 3, 6, and 12 months after activation. Frequencies tested included 125 to 8000 Hz, in octave steps, as well as occasional interoctave frequencies as dictated by audiometric protocol. The consonant–nucleus–consonant (CNC [10]) words, Arizona Biomedical (AzBio [11]) sentences, and the Bamford-Kowal-Bench Speech-in-Noise (BKB-SIN [12]) speech perception test were administered in the cochlear implant-only condition with an ear plug placed in the nonimplanted ear preoperatively and postoperatively, approximately 3, 6, and 12 months after activation. Given that the electroacoustic processor was not approved for use in the United States during this time period, all testing was completed with the cochlear implant only without acoustic amplification in the implanted ear.
Some patients received a medrol dosepak or a prednisone taper preoperatively. The prednisone taper started 3 days before surgery, initially at 60 mg orally daily for 6 days, and then 50, 40, 30, 20, and 10 mg daily for 2 days each. All patients received intraoperative intravenous steroids for postoperative nausea and vomiting, whereas one surgeon routinely injected and perfused the middle ear with 4 mg/cc of dexamethasone at various time points throughout the procedure.
The round window approach was defined as opening the round window with either a straight pick, Rosen, or 27-gauge needle, with removal of the bony overhang as necessary, but without enlargement of the membrane. An extended round window approach was defined as enlargement of the round window bony opening by drilling in its antero-inferior quadrant, whereas a cochleostomy was defined as drilling a completely new hole in the antero-inferior quadrant.
Postoperative HP rates were calculated using a formula and classification system outlined by Skarzynski et al. for defining HP following CI (13).
PTA, as defined in this equation, is the pure-tone average for all unaided frequencies between 125 and 2000-Hz, in octave steps. PTApost is the postoperative pure-tone average, PTApre is the preoperative pure-tone average, and PTAmax is the maximum pure-tone average measurable by the audiometer. A percentage is then obtained from this formula, which can be converted into a categorical scale, with complete hearing preservation defined as greater than 75%, partial hearing preservation greater than 25 to 75%, minimal hearing preservation greater than 0 to 25%, and complete loss of hearing at 0%.
Data analysis was performed using Microsoft (Redmond, WA) Excel 2011, Version 14.8.8, and StatPlus (AnalystSoft, Vancouver, CA), Version 5. Continuous variables were compared using the Pearson product moment coefficient. One-way ANOVA tests and t tests were used to evaluate categorical and continuous variables. Categorical variables were compared using the χ2 test. All p values were two-tailed, with significance defined as p < 0.05.
RESULTS
A total of 50 ears in 47 patients over the age of 18 years were implanted with a mid-scala electrode between August 2013 and December 2014. Patient demographics and preoperative variables are listed in Table 1. Average follow-up was 10.0 months (range, 1.8–18.6) after activation. We defined HP CI candidates as patients with preoperative audiometric thresholds ≤85 dB HL at 250-Hz given that target gain via acoustic amplification would theoretically be achievable preoperatively figuring a half-gain rule and typical low-frequency gain limits for conventional amplification in the range of 40 to 45 dB (14,15). Given this criterion for HP candidacy retrospectively, 39 ears were candidates for HP CI, with preoperative 250-Hz thresholds ranging from 15 to 85 dB HL. Twenty-six (66.7%) patients underwent round window, 25.6% underwent extended round window, and 7.7% underwent cochleostomy surgical approaches to the cochlea. The mean and median preoperative hearing thresholds at 250-Hz for HP candidates were 65.4 dB HL and 70 dB HL, respectively (Table 2).
TABLE 1.
Summarized patient demographics and perioperative variables
| Demographic | N | % |
|---|---|---|
| Total patients | 47 | |
| Sex (women) | 14 | 29.8 |
| Average age (yr) | 58.1 | |
| Left ear implanted | 30 | 60.0 |
| HP candidates | 39 | |
| Preoperative steroids | 7 | 17.9 |
| Steroid taper | 6 | 15.4 |
| Chronic use | 1 | 2.6 |
| Intraoperative steroids | ||
| 5–12 mg IV Dex | 36 | 92.3 |
| IV + middle perfusion | 7 | 17.9 |
| Postoperative steroids | 13 | 33.3 |
| Continued steroid taper | 7 | 17.9 |
| Medrol dose pak | 6 | 15.4 |
| Surgical approach | ||
| Round window | 26 | 66.7 |
| Extended round window | 10 | 25.6 |
| Cochleostomy | 3 | 7.7 |
TABLE 2.
Preoperative, activation, and 6-month and 1-year postoperative thresholds, pure-tone averages, and median threshold differences
| Preoperative | |
| Mean threshold at 250 Hz | 65.4 dB HL |
| Median threshold at 250 Hz | 70 dB HL |
| Mean PTA at 125, 250, and 500 Hz | 66.3 dB HL |
| Median PTA at 125, 250, and 500 Hz | 70 dB HL |
| Mean PTA at 250, 500, and 1000 Hz | 77.4 dB HL |
| Median PTA at 250, 500, and 1000 Hz | 79.2 dB HL |
| Mean PTA at 250, 500, 1000, and 2000 Hz | 84.6 dB HL |
| Median PTA at 250, 500, 1000, and 2000 Hz | 82.5 dB HL |
| At activation | |
| Mean PTA at 125, 250, and 500 Hz | 86.4 dB HL |
| Median PTA at 125, 250, and 500 Hz | 86.7 dB HL |
| Mean PTA at 250, 500, and 1000 Hz | 99.5 dB HL |
| Median PTA at 250, 500, and 1000 Hz | 100 dB hL |
| Mean PTA at 250, 500, 1000, and 2000 Hz | 102.7 dB HL |
| Median PTA at 250, 500, 1000, and 2000 Hz | 103.8 dB HL |
| Median threshold difference at 125 Hz | 12.5 dB HL |
| Median threshold difference at 250 Hz | 20 dB HL |
| Median threshold difference at 500 Hz | 22.5 dB HL |
| Median threshold difference at 1000 Hz | 20 dB HL |
| At 6 months | |
| Median threshold difference at 125 Hz | 10 dB HL |
| Median threshold difference at 250 Hz | 15 dB HL |
| Median threshold difference at 500 Hz | 20 dB HL |
| Median threshold difference at 1000 Hz | 15 dB HL |
| At 12 months | |
| Median threshold difference at 125 Hz | 10 dB HL |
| Median threshold difference at 250 Hz | 15 dB HL |
| Median threshold difference at 500 Hz | 20 dB HL |
| Median threshold difference at 1000 Hz | 10 dB HL |
PTA indicates pure-tone average.
We chose 250 Hz because it determines whether hearing preservation is functionally useful, as it is generally the lowest frequency for which we are able to reliably amplify to prescriptive targets, such as the National Acoustic Laboratories-Non-Linear 2 (NAL-NL2), a commonly used hearing aid fitting formula (16). In assessing the 39 HP CI candidate ears, 32 had unaided thresholds obtained at activation, 20 had unaided thresholds obtained 6-months postactivation, and 13 ears had unaided thresholds at 1-year postactivation. Median thresholds and threshold differences are shown in Figure 1.
FIG. 1.
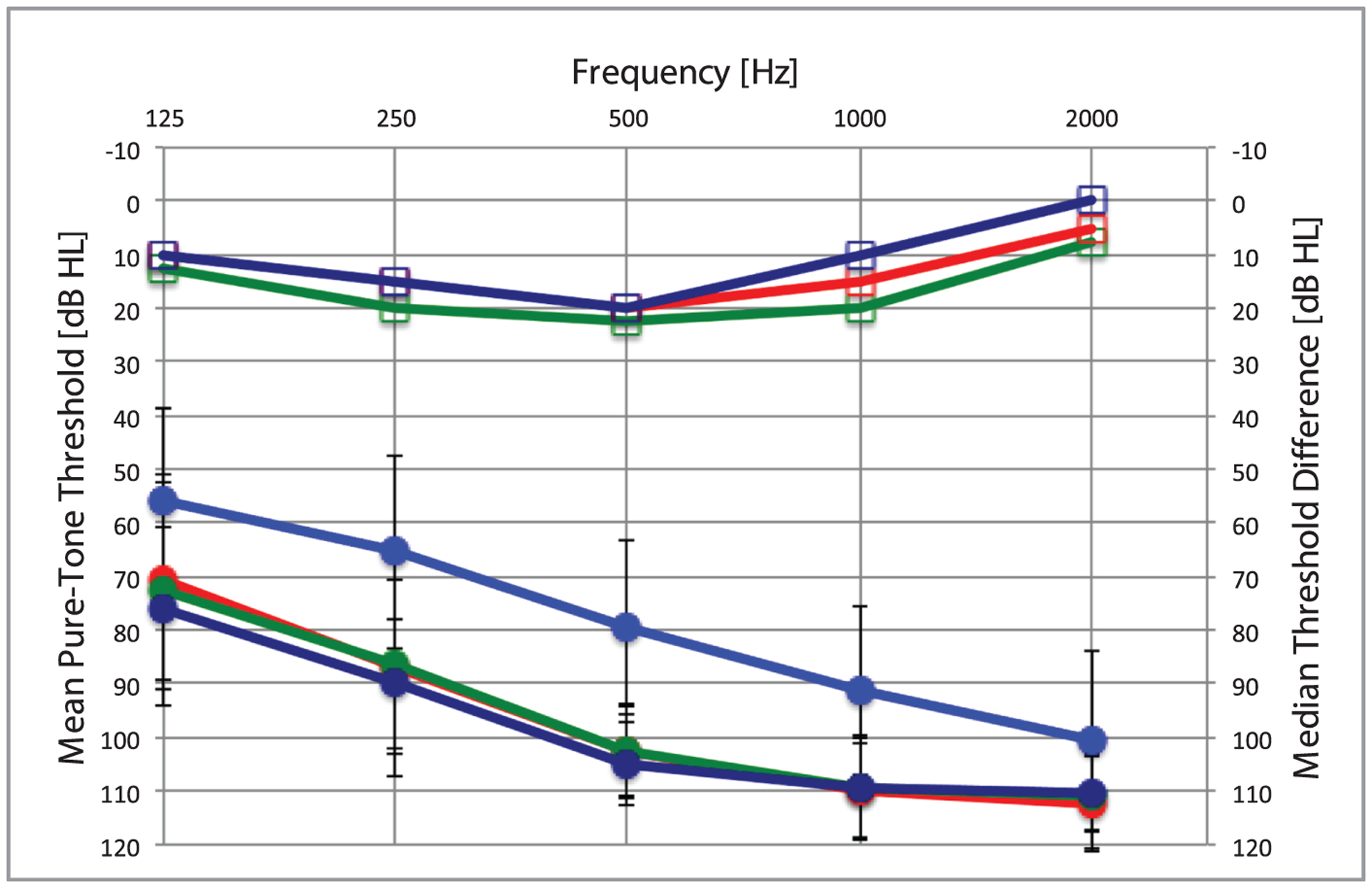
Median unaided air-conduction preoperative (light blue line), activation (green line), 6-months (red line), and 1-year (dark blue line) postoperative thresholds. Median threshold differences (square open markers) at activation (green line), 6-months (red line), and 1-year (dark blue line) postoperative as compared with preoperatively. Error bars represent one standard deviation above and below the mean.
At activation, 43.8% (14/32) ears had preserved aidable hearing, defined as having postoperative audiometric thresholds ≤85 dB HL at 250-Hz. At 6-months postactivation, 30.0% (6/20) ears maintained aidable hearing at 250-Hz. At 1-year postactivation, 30.8% (4/13) ears maintained aidable thresholds at 250-Hz. Age, surgical approach, and preoperative, intraoperative, and postoperative steroid administration were found to have no correlation with audiometric thresholds at activation or at the 6-months postactivation (p > 0.05).
Given that the expected low-frequency threshold elevation after cochlear implantation is on average 20 dB, we specifically investigated a subset of 16 individuals who had preoperative thresholds at 250 Hz at 65dB HL or better—assuming that poorer preoperative thresholds would likely not result in aidable acoustic HP (17–19). Of these 1 recipient with preoperative thresholds 65 dB HL at 250 Hz, 14 had unaided thresholds measured at activation, 7 at 6-months, and 4 at 1-year. Defining aidable hearing as ≤85 dB HL at 250 Hz, within this subgroup, 78.6% at activation, 57.1% at 6-months, and 75% at 1-year had aidable hearing preserved.
Using the Skarzynski et al. formula as a measure of atraumatic insertion, at activation, 53.1% (17/32) of our study population had complete or partial hearing preservation at activation, whereas 55.0% had complete or partial preservation at 6-months postactivation (Table 3) and (Fig. 2) (13).
TABLE 3.
Hearing preservation rates as calculated by the formula outlined in Skarzynski et al. (13)
| Activation | 3 Months | 6 Months | 12 Months | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Complete (>75%) | 2 | 6.3 | 2 | 18.2 | 3 | 15.0 | 0 | 0.0 |
| Partial (25–75%) | 15 | 46.9 | 5 | 45.5 | 8 | 40.0 | 5 | 38.5 |
| Minimal (<25%) | 10 | 31.3 | 3 | 27.3 | 6 | 30.0 | 7 | 53.8 |
| No hearing | 5 | 15.6 | 1 | 9.1 | 3 | 15.0 | 1 | 7.7 |
| Total | 32 | 11 | 20 | 13 | ||||
FIG. 2.
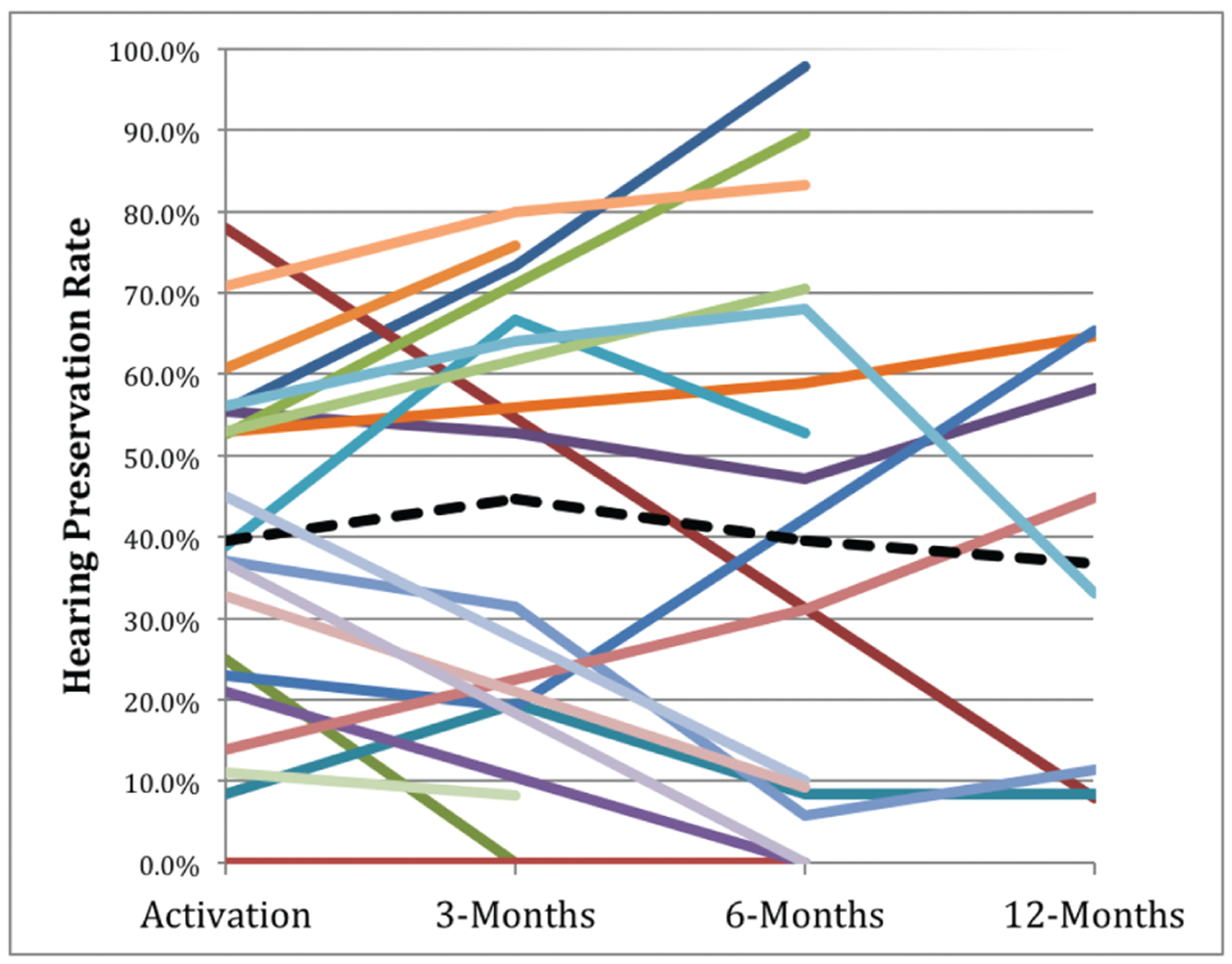
Hearing preservation rates over time as calculated by the formula outlined by Skarzynski et al. (13). Including only patients with at least two data points, the black dashed line represents the average of all data points.
In comparing patients who had complete or partial hearing preservation with those who had minimal preservation or total hearing loss, there were no significant differences in age, surgical approach, and preoperative, intraoperative, and postoperative steroids at activation and 6-months postactivation between groups (p > 0.05).
Average preoperative CNC, AzBio, and BKB-SIN scores in the implanted ear were 13.0%, 16.7%, and 20.2 dB SNR-50, respectively. At 6-months postactivation, in the implanted ear, mean CI only CNC and AzBio scores increased to 44.1% and 54.5%, respectively, whereas the BKB-SIN decreased (i.e., improved) to 14.7 dB SNR-50, all of which represented significant improvements (p < 0.05) (Table 4).
TABLE 4.
CNC, AzBio, and BKB-SIN speech perception test results
| CI Only | Preoperative | 6 Months | p Value | 12 Months | p Value |
|---|---|---|---|---|---|
| CNC | 13.0% | 44.1% | <0.001 | 55.7% | 0.113 |
| AzBio | 16.7% | 54.5% | <0.001 | 69.7% | 0.093 |
| BKB-SIN (dB SNR-50) | 20.2 | 14.7 | 0.02 | 11.1 | 0.189 |
| Bimodal (CI + HA in contralateral ear) | |||||
| CNC | 23.9% | 53.3% | <0.001 | 62.9% | 0.146 |
| AzBio | 38.6% | 64.0% | 0.005 | 79.1% | 0.143 |
| BKB-SIN (dB SNR-50) | 14.8 | 11 | 0.089 | 8.3 | 0.299 |
One year postactivation, in the implanted ear, mean CI only CNC and AzBio scores increased to 55.7% and 69.7%, respectively, whereas the mean BKB-SIN decreased (i.e., improved) to 11.1 dB SNR-50, which was not significantly improved compared with 6-months postactivation (p > 0.05). In the bimodal (CI contralateral + hearing aid) preoperatively, the mean CNC, AzBio, and BKB-SIN scores were 23.9%, 38.6%, and 14.8 dB SNR-50, respectively. At 6-months postactivation, mean CNC and AzBio scores were 53.3% (p < 0.001) and 64.0% (p = 0.005), respectively, whereas the mean BKB-SIN improved to 11.0 dB SNR-50 (p = 0.089). At 1-year postactivation, the mean CNC and AzBio scores increased to 62.9% and 79.1%, respectively, and the mean BKB-SIN improved to 8.3 dB SNR-50, all of which were not significantly improved compared with 6-months postactivation. Separating patients who had preserved aidable hearing from the non-HP group, there were no significant differences between groups with all three scores in the implanted ear only at 6-months postactivation (p > 0.05). Likewise, no significant differences in scores in the implanted ear only were noted between patients who had complete or partial hearing preservation with those who had minimal preservation or total hearing loss at 6-months postactivation (p > 0.05).
DISCUSSION
In retrospectively reviewing our experience of implanting 50 ears of 47 patients with a mid-scala electrode, 43.8% (14/32) of our study population had 250-Hz thresholds ≤85 dB HL at activation, whereas 30.0% and 30.8% had 250-Hz thresholds 85 dB HL at 6-months and 1-year postactivation, respectively. Assessing median threshold differences (Fig. 1), audiometric thresholds generally improved during the 12-month period after activation.
This improvement in bone conduction thresholds is likely because of a significant amount of blood, fluid, or inflammation present at activation causing a conductive overlay. For this reason, we incorporate bone conduction audiometry into our routine activation and postoperative protocol for all patients who had demonstrated behavioral hearing preoperatively. This way, we are able to describe the time course of HP from the perspective of sensori-neural, conductive, and mixed hearing loss. In reporting CNC, AzBio, and BKB-SIN scores, in the implanted ear only, in the cochlear implant-only condition, all patients significantly improved at 6-months postoperatively as compared with preimplantation, as expected, regardless of the level of HP. However, our finding of no difference in cochlear implant-only scores between HP and non-HP groups is contrary to that reported by Carlson and colleagues, though the current sample size was significantly smaller (20). Further investigation on a larger scale is clearly needed to thoroughly describe the expected cochlear implant-only outcomes for individuals with and without HP.
A strict comparison of the mid-scala electrode to other HP electrodes is difficult given the variety of electrode designs, myriad of HP definitions, and retrospective nature of our study. Many studies report outcomes on the basis of threshold differences following CI. With the FLEX24 electrode, Gstoettner and colleagues defined complete HP when the average low-frequency (125–750 Hz) hearing loss was ≤10 dB as compared with preoperatively. With that classification, they reported complete HP in four out of nine patients (21). Lenarz et al. (19) reported the results of the European clinical trial for the Hybrid-L, with median threshold elevation at activation being 10, 15, and 5 dB at 250, 500, and 1000 Hz, respectively. Similarly, Roland et al. (22) reported the results of the U.S. clinical trial for the Hybrid-L; though they did not report mean or median threshold elevation data, they reported that 66.6% of patients retained aidable hearing postoperatively (low-frequency thresholds ≤85 dB HL). Comparable to our reported outcomes (Table 2), Jurawitz et al. (23) retrospectively looked at 53 patients implanted with the CI422 electrode at 6 months and reported median differences of 15, 25, and 20 dB at 250, 500, and 1000 Hz, respectively.
Recognizing that the mean threshold elevation is between 15 and 20 dB for long electrodes following CI, if patients begin with thresholds around 80 dB HL, we must assume they will not have aidable hearing preserved postoperatively (17–19,23,24). Thus, we analyzed a subgroup of patients who had preoperative thresholds at 250 Hz at 65 dB HL or better. After implantation, 78.6%, 57.1%, and 75% of patients had preserved aidable hearing at activation, 6-months, and 12-months, respectively. These rates are comparable with previously published reports with the FLEXsoft and Freedom CI422 (23).
We acknowledge that the definition of HP following CI remains unclear. Without a universally adopted method for describing preservation outcomes, confusion will remain a constant in the field. Many studies report HP outcomes on the basis of threshold difference, which is influenced by the patient’s preoperative audiogram (13). For instance, if Patient A has mild hearing loss in the low frequencies, and Patient B has severe to profound hearing loss in the low frequencies, losing 20 dB after implantation affects them differently. Patient A, with mild hearing loss, would arguably now have moderate hearing loss in the low frequencies, still reaping the benefits of maintaining their residual hearing, whereas Patient B may now have lost all residual hearing, preventing them from using any low-frequency benefits. However, on the basis of many reporting standards, both patients would have partial hearing loss since they both lost 20 dB in hearing. Even if threshold differences are ignored, and one assessed means, studies have shown that mean reporting can overestimate HP (25).
We applied the formula and classification proposed by Skarzynski et al. to assess our HP rates. At 6-months postactivation, we found 55.0% of our study population had either complete or partial HP, but only 38.5% at 1-year postactivation. However, given the arbitrarily chosen categories and titles, as well as category modification in previous reports (26), we desired to present the data and outcomes as clearly as possible.
We are aware of two studies using the Skarzynski et al. formula. Mertens et al. reported the HP rates of 11 ears implanted with three different electrodes, with implant experience ranging from 3 to 10 years. They found that 27% patients had complete preservation, 45% had partial preservation, 18% had minimal preservation, and 9% completely lost their hearing (27). Santa Maria et al. (26) also used the Skarzynski et al. formula in evaluating 14 patients implanted with the Flex24 electrode. Though they modified the categories, 22.2% had complete hearing preservation, 66.7% had partial hearing preservation, and 11.1% had minimal hearing preservation 6 to 12 months after surgery (26). Nonetheless, though both studies report slightly higher complete and partial hearing preservation rates as compared with our population, it is difficult to make conclusions with such small study populations.
As such, there are several weaknesses we must acknowledge in the current study. First, as a retrospective review of a small cohort, it carries its own inherent weaknesses. Though patients may have had preoperative low-frequency hearing (≤85 dB HL at 250 Hz), retrospectively it is difficult to truly determine which patients experienced HP techniques, when several patients underwent extended round window or cochleostomy approaches to the cochlea, which runs counter to previous studies demonstrating superior HP outcomes with round window approaches (28). Second, with an average follow-up of 10-months postactivation, no long-term assessments can be made. Third, though more than 90% of our study patients continued to undergo postoperative audiologic testing at our institution, one might presume that those who did not follow up postoperatively had poor audiologic outcomes, which would lower our hearing preservation rates. Lastly, since previous studies have shown that electrode location influences audiologic outcomes, we did not control for electrode location (28,29).
CONCLUSION
To the best of our knowledge, this is the first study assessing hearing preservation rates with a mid-scala electrode array. Depending on the method of assessment of hearing preservation, and the patient group being analyzed, hearing is at least partially preserved in more than half of the cases at 6-months postactivation, though less than half at 1 year. Nonetheless, this study highlights the need for 1) a consensus definition regarding cochlear implant hearing preservation, and 2) long-term, prospective, and larger studies assessing mid-scala electrodes.
Acknowledgments
Internal departmental funding was used without commercial sponsorship or support. D.S.H. is a consultant for Advanced Bionics Corp., Cochlear Corp., MED-EL GmbH, Stryker, Synthes, Grace Medical, and Oticon. G.B.W. is a consultant for Advanced Bionics Corp., Cochlear Corp., and MED-EL GmbH, and Oticon. A.R. is a consultant for Advanced Bionics Corp., Cochlear Corp., MED-EL GmbH, Care-stream, and Grace Medical. R.H.G. is a consultant for Advanced Bionics Corp., Cochlear Corp., and MED-EL GmbH.
Institutional Review Board Approval: Data Integrated Study Console of Vanderbilt’s Research Enterprise (DISCOVR-E) IRB - 141196.
REFERENCES
- 1.Carlson ML, Sladen DP, Haynes DS, et al. Evidence for the expansion of pediatric cochlear implant candidacy. Otol Neurotol 2015;36:43–50. [DOI] [PubMed] [Google Scholar]
- 2.Buchner A, Schussler M, Battmer RD, et al. Impact of low-frequency hearing. Audiol Neurootol 2009;14(Suppl 1):8–13. [DOI] [PubMed] [Google Scholar]
- 3.Turner C, Gantz BJ, Reiss L. Integration of acoustic and electrical hearing. J Rehabil Res Dev 2008;45:769–78. [DOI] [PubMed] [Google Scholar]
- 4.Skarzynski H, Lorens A, Matusiak M, et al. Cochlear implantation with the nucleus slim straight electrode in subjects with residual low-frequency hearing. Ear Hear 2014;35:e33–e43. [DOI] [PubMed] [Google Scholar]
- 5.Gifford RH, Dorman MF, Brown CA. Psychophysical properties of low-frequency hearing: Implications for perceiving speech and music via electric and acoustic stimulation. Adv Otorhinolaryngol 2010;67:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gifford RH, Dorman MF, Skarzynski H, et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear 2013;34:413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gifford RH, Grantham DW, Sheffield SW, et al. Localization and interaural time difference (ITD) thresholds for cochlear implant recipients with preserved acoustic hearing in the implanted ear. Hear Res 2014;312:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn CC, Perreau A, Gantz BJ, et al. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol 2010;21: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassepass F, Bulla S, Maier W, et al. The new mid-scala electrode array: A radiologic and histologic study in human temporal bones. Otol Neurotol 2014;35:1415–20. [DOI] [PubMed] [Google Scholar]
- 10.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord 1962;27:62–70. [DOI] [PubMed] [Google Scholar]
- 11.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear 2012;33:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bamford-Kowal-Bench Speech-in-Noise Test (Version 1.03) [Audio CD]. Elk Grove Village, IL, 2005. [Google Scholar]
- 13.Skarzynski H, van de Heyning P, Agrawal S, et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol Suppl 2013;133:3–13. [DOI] [PubMed] [Google Scholar]
- 14.Byrne D Theoretical prescriptive approaches to selecting gain and frequency response of a hearing aid. Monogr Contemp Audiol 1983;4:1–40. [Google Scholar]
- 15.Lybarger Inventor. U.S. Patent Application SN 543.278 July 3, 1944. [Google Scholar]
- 16.Keidser G, Dillon H, Flax M, et al. The NAL-NL2 prescription procedure. Audiol Res 2011;1:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gstoettner WK, Van de Heyning P, O’Connor AF, et al. Assessment of the subjective benefit of electric acoustic stimulation with the abbreviated profile of hearing aid benefit. ORL J Otorhinolaryngol Relat Spec 2011;73:321–9. [DOI] [PubMed] [Google Scholar]
- 18.Lenarz T, James C, Cuda D, et al. European multi-centre study of the Nucleus Hybrid L24 cochlear implant. Int J Audiol 2013;52:838–48. [DOI] [PubMed] [Google Scholar]
- 19.Lenarz T, Stover T, Buechner A, et al. Hearing conservation surgery using the Hybrid-L electrode: Results from the first clinical trial at the Medical University of Hannover. Audiol Neurootol 2009;14 (Suppl 1):22–31. [DOI] [PubMed] [Google Scholar]
- 20.Carlson ML, Driscoll CL, Gifford RH, et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol 2011;32:962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gstoettner W, Helbig S, Settevendemie C, et al. A new electrode for residual hearing preservation in cochlear implantation: First clinical results. Acta Otolaryngol 2009;129:372–9. [DOI] [PubMed] [Google Scholar]
- 22.Roland JTJ, Gantz BJ, Waltzman SB, et al. United States multi-center clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope 2016;126:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurawitz MC, Buchner A, Harpel T, et al. Hearing preservation outcomes with different cochlear implant electrodes: Nucleus(R) Hybrid-L24 and Nucleus Freedom CI422. Audiol Neurootol 2014;19:293–309. [DOI] [PubMed] [Google Scholar]
- 24.Mick P, Amoodi H, Shipp D, et al. Hearing preservation with full insertion of the FLEXsoft electrode. Otol Neurotol 2014;35:e40–4. [DOI] [PubMed] [Google Scholar]
- 25.James C, Albegger K, Battmer R, et al. Preservation of residual hearing with cochlear implantation: How and why. Acta Otolaryngol 2005;125:481–91. [DOI] [PubMed] [Google Scholar]
- 26.Santa Maria PL, Domville-Lewis C, Sucher CM, et al. Hearing preservation surgery for cochlear implantation: Hearing and quality of life after 2 years. Otol Neurotol 2013;34:526–31. [DOI] [PubMed] [Google Scholar]
- 27.Mertens G, Punte AK, Cochet E, et al. Long-term follow-up of hearing preservation in electric-acoustic stimulation patients. Otol Neurotol 2014;35:1765–72. [DOI] [PubMed] [Google Scholar]
- 28.Wanna GB, Noble JH, Carlson ML, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope 2014;124:S1–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wanna GB, Noble JH, Gifford RH, et al. Impact of intrascalar electrode location, electrode type, and angular insertion depth on residual hearing in cochlear implant patients: Preliminary results. Otol Neurotol 2015;36:1343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


