Abstract
Purpose:
This study compares the efficacy of a behavioral skills mentoring program (Mentored Planning to Be Active [MBA]) to a teacher-led program (Planning to Be Active [PBA]) for increasing physical activity in Appalachian teens on health outcomes (weight loss, body mass index (BMI), and body fat).
Methods:
Secondary analysis of a larger group-randomized controlled trial was conducted in 20 rural Appalachian schools. Descriptive Pearson correlations and multivariate analyses with between-subject effects were conducted. Effect sizes (ES) using Cohen’s d and odds ratios (OR) with 95% confidence intervals were calculated.
Findings:
The obese MBA group lost 77.5% more weight by T3 compared to the PBA group; T2 was (F = 8.51, P = .000) and T3 was (F = 7.62, P = .000). ES was 0.34. OR = 1.45 (95% CI: 0.558–3.792) at T2 and OR = 3.32 (95% CI: 1.103–9.978) at T3. Extremely obese in the MBA group lost 80.0% more weight compared to the PBA group; T2 was (F = 5.23, P = .025) and at T3 (F = 6.33, P = .015) ES was 0.58. OR = 4.36 (95% CI: 0.981–19.34). Extremely obese females lost more weight compared to males (F = 4.75, P = .034). BMI and body fat had similar results; youth in the MBA group had the most improvement.
Conclusions:
Rural Appalachian youth are disproportionately extremely obese. BMI does not capture adiposity or cardiovascular risk. BMI, BMI percentile, raw weight, fat mass, and percent body fat are more complete analyses of adiposity and cardiovascular risk.
Keywords: body mass index, extremely obese, health promotion, obesity, weight loss
The majority (72%) of the United States’ land area is rural.1 Residing in a rural area, coupled with poverty, is associated with disparate suffering from high rates of chronic disease and disability related to the early onset of obesity.2 Compared to other sub-populations, rural Appalachian youth are disproportionately obese or extremely obese. In a sample of 333 ninth-grade rural Appalachian students, Smith and associates found obesity rates were 16.5% and 13.2% of youth were extremely obese.3 According to National Health and Nutrition Examination Survey [NHANES] data, national rates of extreme obesity range from 5.9% to 7.8%, whereas the obesity rate of high school youth is approximately 20%.4 Children and youth residing in rural areas are 25% more likely to be overweight or obese, inclusive of extremely obese, compared to those residing in metropolitan areas.5,6 Obesity-related medical costs are estimated to cost the United States up to $210 billion annually.6,7
The high prevalence of extreme obesity combined with high rates of sedentary behaviors and poor nutritional quality place Appalachian teens at increased risk for diabetes and cardiovascular disease earlier in life, resulting in a poorer quality of life and earlier mortality.2,3 A body mass index (BMI) over 40 kg/m2 reduces life expectancy by 6.5–13.7 years in adults.8 Heart disease, diabetes mellitus type II, and certain cancers increase as BMI increases.8,9 Extremely obese youth have earlier onset of illness and earlier death due to early acquisition of comorbidities such as nonalcoholic fatty liver disease, musculoskeletal problems, and long-term risk of disease.4–6,10 Cardiovascular and metabolic profiles of youth suffering from extreme obesity are poorer compared to even less obese counterparts.11–14 Extremely obese youth have early onset of hypertension, dyslipidemia, inflammation, and hyperinsulinemia.12–14 Ramifications of teen extreme obesity are a serious concern in rural Appalachia and pose important clinical and public health challenges.
Appalachian resident’s personal health behaviors are related to the region’s poor health outcomes.3 Appalachians have poorer health and fewer positive health-related behaviors compared to other US populations.4–6 Compared to other Americans, Appalachians are less physically active in their leisure time.15,16 Appalachian youth also consume up to 4 times the amount of sugared-sweetened beverages (SSBs) compared to non-Appalachian youth.16–18 Cultural norms found within Appalachia fuel this disproportionate consumption of SSBs. These norms include “sipping soda or pop throughout the day,” a preference for acidic beverages, and skepticism of outsiders delivering health-related programming.5,6,17 Youth residing in high-poverty areas such as rural Appalachia are affected more frequently by poor dietary behaviors such as SSBs.6,18
Effective interventions to improve overall health are needed in rural Appalachia because overcoming health disparities for low-income Appalachian teens has not been adequately addressed.6,19 In rural, under-resourced areas such as Appalachia, physical inactivity is significantly higher than national levels.2,15,16 Appalachian youth are among the most sedentary.3,16,20 One explanation may be circumstances in Appalachia. For example, relying on organized sports to promote physical activity (PA) behaviors has not been effective for Appalachian youth.1 Opportunities to participate in sports are limited due to inadequate school resources, lack of transportation, and limited availability of school teams.3,6,15,16 School-sponsored programs enroll a small percentage of students. For Appalachian youth, the motivation to be physically active must shift from competitive sports to personal fitness grounded in personal motivation. Using trained peer mentors can reach more youth and is more likely to carry over into adulthood. Our experience of working in rural Appalachia suggests that many students do not have access to exercise and fitness facilities characteristic of urban and suburban settings. Lack of transportation and distance to facilities are barriers for many Appalachian youth. In Appalachia, interventions should emphasize activity done in rural neighborhoods and at home. These skills are useful for sustaining goal-directed behavior change.
School-based interventions have also been limited in their scope and effect on obesity prevention especially targeting teens.6,20 Low efficacy of these programs may be due to unique cultural challenges such as a preference for informal sharing of information among local residents rather than health content delivered by professionals.6 Though school-based interventions increase health knowledge, there is less evidence of effectiveness for health behavior changes and improving health outcomes.19–24 School-based interventions typically deliver programs as part of a course such as health or physical education via teachers or adults. Low efficacy of these programs may also be due to cultural challenges in Appalachia.6,25 Other factors include varying fidelity of program delivery by classroom teachers, school environments not consistent with program goals, and a lack of theoretical programing targeting health behaviors.20,21,26 In rural Appalachia, resources for preventive care are lacking. Resources to engage in healthful behaviors such as recreational facilities, organized sports, or neighborhood parks or facilities for PA are inadequate in rural Appalachia.5 To better understand the unique challenges to healthy lifestyle behaviors in rural Appalachia, community-based studies are needed.20
Behavioral interventions are usually the first line of treatment for obese adolescents.27 Few intervention studies with behavioral and lifestyle interventions have focused on extremely obese youth.27–29 For youth suffering from obesity and extreme obesity, interventions have mostly resulted in modest reductions in weight and BMI because baseline BMI values are so high.29
A promising new approach for the delivery of school-based interventions may be to use trained peer mentors. Rarely have school-based interventions used peer mentors to deliver content and offer support for building skills to change behavior. Mentoring has been effective at addressing health risk behaviors among Appalachian youth,30 including overweight and obesity.23,24 Smith and Holloman found that Appalachian peer mentors helped elementary-aged mentees improve short-term dietary behaviors. Appalachian youth assigned to peer mentors (versus adult leaders) demonstrated improved BMI and increased PA on a short-term basis.24
Mentored Planning to Be Active [MBA] was adapted from Planning to Be Active [PBA] for delivery by trained peer mentors. MBA emphasizes the social determinants of health by using a social networking approach that trains peer mentors to support teens. PBA is a 10-lesson unit delivered over 10 weeks and designed to teach self-regulation of PA in the home and neighborhood among teens. PBA’s overall objective is to help youth develop and implement a personalized PA program at home with no exercise equipment. The personalization occurs with weekly goal setting and tracking of behaviors. A subsequent review of weekly goals and activities are reviewed the following week with feedback and revision, if needed. Expanding PBA for delivery by trained peer mentors via MBA may better promote and sustain adoption of daily regular PA through self-regulation of PA in discretionary time. By addressing personal concerns as well as using existing social networks or not using health professionals to deliver the message, MBA may strengthen the tailoring of PA to personal interests, talents, and neighborhood.
Social Cognitive Theory (SCT) is extensively used for determinants of PA31–33 and is the framework for the PBA and MBA curriculum. PBA was developed and tested over the course of prior studies.31–33 Studies revealed that SCT variables were strongly related to moderate and vigorous PA; PBA increased self-regulation of PA; and a 10-lesson dose led to the greatest improvement in PA. The PBA curriculum addresses psychosocial determinants, self-regulation, and environment affecting PA adherence (Figure 1). Psychosocial determinants include outcome expectancies and exercise self-efficacy. Self-regulation includes goal setting, self-monitoring, overcoming barriers, time management, self-reward, and social support. Environmental determinants are home, neighborhood, and school opportunities for PA.
Figure 1.
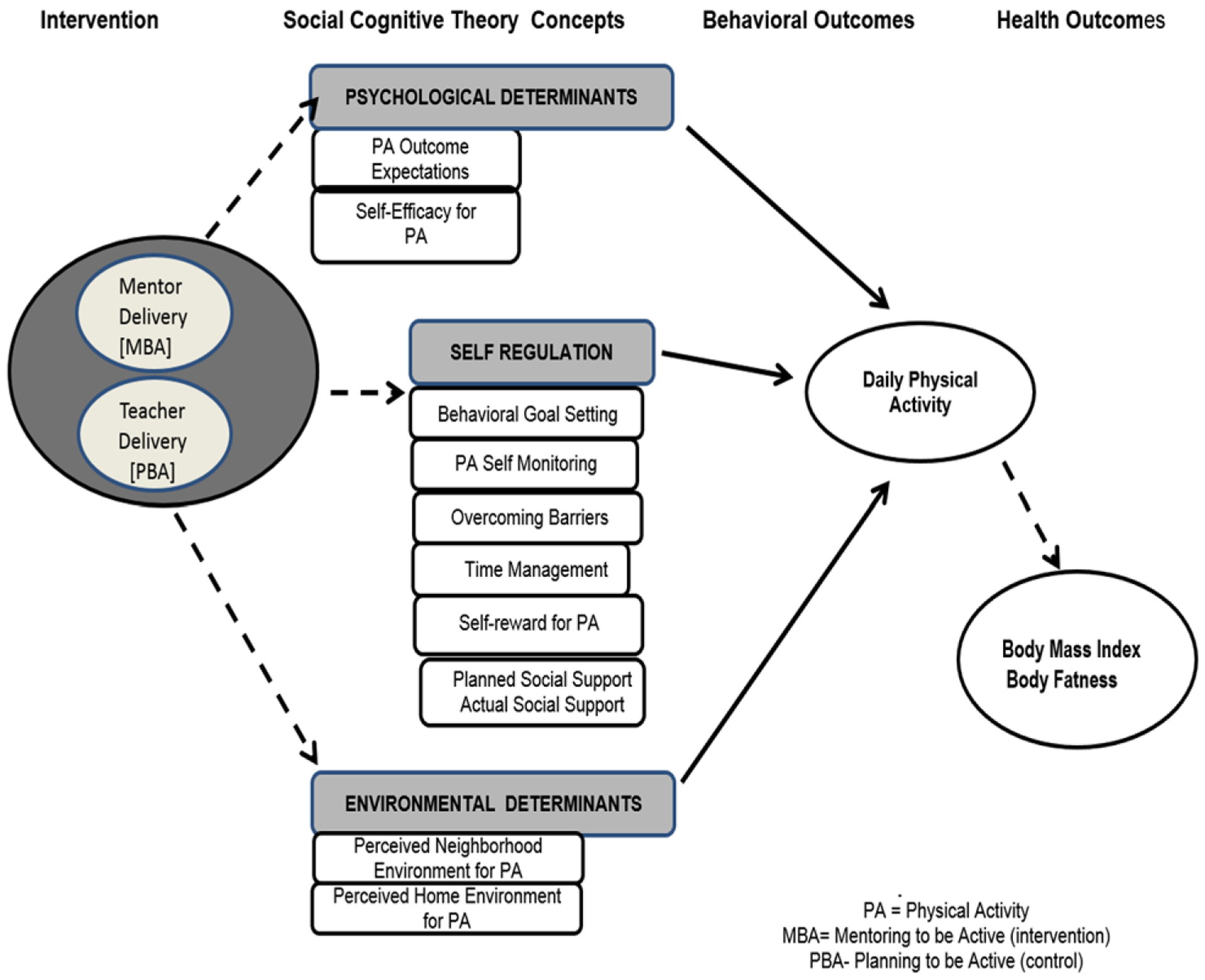
Curricular Components with Social Cognitive Theory Concepts and Outcomes.
The peer mentoring approach used for MBA builds and strengthens social networks that links people and provides social support.34 One’s social network provides emotional, informational, and appraisal support to create a sense of psychological safety and higher motivation to change behavior. Learning, domain-specific self-efficacy, and behavior change happen when people have a sense of psychological safety or the perception that attempts to change behavior can occur without fear or embarrassment.35 Advantages include enhanced learning and behavioral change support resulting from the perceived social support (emotional, informational, and appraisal) and psychological safety promoted by peer mentoring. Providing intense social support via peer mentoring results in curriculum delivery that promotes better health outcomes compared to classroom teachers delivering the curriculum.
The purpose of this study was to compare the efficacy of a healthy lifestyle skills mentoring program (MBA) to a teacher-led program (PBA) for increasing PA in Appalachian teens on health outcomes (eg, weight loss, BMI, and percentage of body fat). We hypothesized that obese and extremely obese teens in the MBA group would have greater reductions in body weight, BMI, and body fat compared to teens in the PBA group at 3 months (T2) and 6 months (T3) postintervention.
For the main study, we tested the efficacy of a 10-week school-based intervention in promoting self-regulation of PA among youth. A PA program originally designed for classroom delivery (usual care) was adapted to also be delivered via trained peer mentors over 10, 40-minute sessions (intervention).22 Curricular workbooks, manipulation checks (homework), worksheets, reinforcement activities, and weekly goal setting were provided to all participating youth. Each week, participants considered ways to incorporate PA into discretionary time.22 Detailed intervention procedures and data protocols are described elsewhere.22
Methods
This secondary analysis was from a larger group-randomized controlled trial (G-RCT) conducted in 20 rural Appalachian high schools in southern Ohio.25 Rural areas are nonmetropolitan or nonurban areas based on population density, housing, and territory.1,2,9,36,37 Rural counties generally have smaller towns, are outside the boundaries of metropolitan areas, and have lower population density. Descriptive and inferential analyses were completed. Multivariate analyses with tests of between-subject effects using mixed model ANCOVA were conducted. Effect sizes (ES) were calculated using Cohen’s d. When standard deviations were not similar, Glass’ delta technique was performed; if sample sizes were not similar, then Hedges’ g was used. Small ES was defined as <0.2; medium ES = 0.3–0.5; large ES = 0.6 or above. The level of significance was set at P ≤ .05. To further quantify the strength of the association between MBA and PBA on health outcomes, odds ratios with 95% confidence intervals were calculated.
Sample and Procedure
A systematic sample was drawn from schools meeting the following criteria: (1) located in a rural Appalachian county; (2) county-level obesity rates where schools were located were similar to other counties of included schools; and (3) at least half (50%) or more of students were on a free or reduced lunch program.22 From these criteria, a total sample of “obese” adolescents (n = 106) and “extremely obese” teens (n = 84) in grades 9–11 at baseline data collection were recruited. The sample for this analysis was drawn from a larger sample of participants (n = 519). Although the larger study included 329 healthy weight youth, this paper presents results from a subgroup analysis of youth classified as obese or extremely obese. Nearly 37% (36.6%) of the overall sample were obese or extremely obese at baseline. This rate far exceeds the current adolescent obesity rates for 12- to 19-year-olds estimated at 20.6% in the United States.13,38 Baseline (T1), 3-month follow-up (T2), and 6-month follow-up (T3) data were used. Prior to data collection, teen written assent and parental permissions were obtained. The Human Subjects Committee (Protocol # 2014B0094) at the host institution and each school approved the study. The study is registered on ClinicalTrials.gov (ID number: NCT02329262).
Measures
Raw Weight and BMI for Age and Gender (BMI)
Health researchers and health care providers measure BMI as an indicator of obesity.4,6,10 However, BMI does not fully capture adiposity or cardiovascular risk. BMI is highly correlated with many different components of body weight including lean mass, skeletal muscle mass, fat mass, and bone mass, yet BMI cannot distinguish between these different components.10 Studies have found a strong link between BMI and percentage of body fat.9,10,39 In research, BMI remains the most commonly used measure to assess general body composition.10 Using the Tanita DC-430U Body Composition Analyzer,40 Raw Body Weight (RW) and BMI for age and gender were assessed.
The Tanita portable professional grade BIA analyzer has been valid and reliable in estimating body weight, BMI, and the percentage of body fat in adolescents when compared to dual-energy X-ray (DXA).12,41 Procedures in the Tanita User’s Guide were followed.40 Height was measured by having the teen stand without shoes on a portable stadiometer facing forward. Age (in years), gender, and height were entered into the analyzer for calculations. Teens stood on the analyzer without shoes or socks, having their feet on the measuring pads and hands directed down the side of their legs.
We used actual BMI as the outcome of interest because this was a long-term trial. BMI z-scores have been widely used and are mostly recommended for cross-sectional comparisons. Recently, several authors have cautioned against the use of BMI z-scores for research using longitudinal designs or for teens with high BMI values, citing concerns that their use could result in spurious differences between groups.8,9,11 Children at the extreme ends of BMI distribution require substantially greater changes in weight than their thinner counterparts for the same change in z-score. Because the BMI z-score curves were constructed using only data between the 3rd and 97th percentiles, caution when using growth curves outside this range is recommended.8,11 Berkey and Colditz noted that the difference between z-scores reflects larger differences in BMI in older compared to younger children.9
Body Fat Percentage
Using standard body fat ranges for children,14,39,40 body fat percentage was measured as the amount of body fat as a proportion of body weight by the Tanita DC-430 Body Composition Analyzer. Body fat percentage estimates used the Bioelectrical Impedance Analysis Method (BIA).12 Although DXA is the best individual method to measure body composition, the use of DXA is not feasible in community-based or school-based settings; in these settings, the BIA methods used by the analyzer have shown acceptable reliability and validity when used with adolescents.12,41 In community studies, the Tanita Body Composition Analyzer demonstrated acceptable accuracy for estimating percentage of fat when compared to DXA in adolescent samples.12 Standard measurement modes were applied for the best reliability.
BMI Percentile for Age and Gender
BMI percentile is used in clinical practice and public health research as an indicator of growth trajectory, growth abnormality, and growth variability.10 Percentiles describe growth patterns over time and allow one to rank youth relative to others based on age and gender.10 Using the CDC Calculator, each participant’s date of birth, gender, day of data collection, weight to the nearest one-eighth pound, and height in feet and inches to the nearest one-eighth inch were used. Using sex-specific CDC guidelines by age and gender, we calculated BMI percentiles, with healthy weight status defined as between the 5th and 85th percentile; overweight status was defined as between the 85th and 95th percentile; obese was defined as above the 95th percentile; and “extremely obese” was defined as at or above the sex-specific 120% of the 95th percentile.13,28,42 Using data from 6 countries, these BMI cutoffs have been found to be statistically equivalent to adult cutoffs for classifying weight status.10,14,39 Measuring adiposity via BMI, BMI percentile, changes in weight, fat mass, and body fat percentage provides a more complete analysis.
We conducted a G-RCT to evaluate mentored delivery of a school-based intervention to improve health outcomes in teens from Appalachia Ohio.22 G-RCTs randomly assign identifiable social groups to study conditions. Measures taken on group members assess the impact of the intervention.43,44 G-RCTs are appropriate to evaluate an intervention that manipulates the physical or social environment, involves social processes, or cannot be delivered to individuals without risk of contamination. In our situation, students attending the same school socialize together; a G-RCT was necessary to avoid the risk of cross-contamination. We recruited high schools in 3 waves, with 4 in Wave 1, 8 in Wave 2, and 8 in Wave 3, for a total of 20 schools. For each wave of schools, we randomly assigned half of the schools to each condition—intervention (MBA) and comparison (PBA)—for 10 schools in each of the 2 conditions by study’s end. We collected data at baseline (T2), 3 months follow-up (T2), and 6 months follow-up (T3).22
Sample Power Calculation for Primary Study
We conducted a careful analysis of sample size calculations for primary analysis of BMI in teens at T3. Data from the Ohio Family Health Survey were used to estimate the school-level ICC for BMI among 9th graders in Appalachian Ohio counties at 0.023. We estimated the over-time correlation of BMI measurements at 0.70, and adjusting for age and gender would explain approximately 30% of the variance in BMI. With these assumptions, 10 schools per condition had 82% power to detect a modest intervention effect (0.2 standard deviation difference between groups). This ES would correspond to a difference in mean BMI between groups of 1.04 kg/m2 if the observed variation in BMI is similar to that of all Appalachian ninth graders (mean BMI from = 23.41, SD = 5.2). Teens each of the 20 schools are included in this analysis.
Results
Most participants were Caucasian (87.9%) and male (52.6%). See Table 1 for a demographic description of the sample. Teens ranged in age from 14 to 17 years old with the mean age being 15.03 (SD = 0.836) years. Due to the recruitment criteria of the main RCT study, the majority of the sample were in ninth (56.8%) or 10th (41.1%) grade. Students in either ninth or 10th grade (n = 111) were recruited to participate in the intervention (MBA) arm of the main study. Students enrolled in a health course at PBA schools were recruited for participation (n = 76). Most students in health courses at the comparison schools also were in either ninth or 10th grade.
Table 1.
Demographic Description of the Sample (n = 190)
| Demographic Variables | Number | Percent |
|---|---|---|
| Age in years | ||
| 14 | 55 | 28.9 |
| 15 | 82 | 43.2 |
| 16 | 46 | 24.2 |
| 17 | 7 | 3.7 |
| Mean (SD) 15.03 years (0.826) | ||
| Gender | ||
| Male | 100 | 52.6 |
| Female | 90 | 47.4 |
| Grade in school | ||
| 9 | 108 | 56.8 |
| 10 | 78 | 41.1 |
| 11 | 4 | 2.1 |
| Race/ethnicitya | ||
| White, not of Hispanic origin | 167 | 87.9 |
| American Indian or Native American | 15 | 7.9 |
| Black, not of Hispanic origin | 9 | 4.7 |
| Hispanic or Latino | 2 | 1.1 |
| Asian American, Pacific Islander | 1 | 0.5 |
| Household structureb | ||
| Lives with Mother | 159 | 83.7 |
| Lives with Father | 109 | 57.4 |
| Lives with at least 1 grandmother | 41 | 21.6 |
| Lives with at least 1 grandfather | 29 | 15.3 |
| Lives with foster parent or legal guardian | 1 | 0.5 |
| Baseline BMI classification | ||
| Obese | 106 | 55.8 |
| Extreme obese | 84 | 44.2 |
| Class 2c | 49 | 58.3 |
| Class 3c | 29 | 34.5 |
| Class 4c | 6 | 7.1 |
| Randomized study condition | ||
| Mentoring to Be active (peer mentoring delivery) | 114 | 60.0 |
| Planning to Be active (classroom teacher delivery) | 76 | 40.0 |
Two participants selected “White” and “Hispanic or Latino”; percentages >100 due to rounding.
Participants may select more than 1 response.
Calculated for those who were initially classified as “Extreme Obese” (n = 84); Obese is defined as (≥95th sex-specific percentile); Extreme Obese is defined as (≥120th sex-specific percentile). United States Centers for Disease Control and Prevention (CDC) BMI-for-age Growth Charts.
At the beginning of the study, youth were well below the recommendations of 60 minutes of moderate to vigorous physical activity (MVPA) most days of the week. At baseline, both groups’ average time in moderate to vigorous activity was only 20.9 minutes per day. At follow-up, obese mentees increased MVPA slightly by 1.2 minutes per day. Extremely obese mentees had the greatest improvement with an increase of MVPA of 3.35 minutes per day. It is important to note that this increase in activity was during student’s free time after school. Classroom students decreased MVPA at follow-up. Obese classroom students had 1.78 minutes per day decrease and extremely obese classroom students’ MVPA did not change from baseline to follow-up.
Although the focus of PBA and MBA was improving and sustaining MVPA, reduced SSB consumption and increased water consumption were found, especially among the extremely obese students. Extremely obese students decreased SSBs by consuming them fewer days per week and increased water consumption from less than 5 servings per day to nearly 7 daily servings. Given the results for PA rates, the changes in health outcomes may be partially attributed to these and other dietary changes.
Descriptive Results of BMI, BMI Percentile, and Body Fat Percentage
At baseline, RW ranged from 147.4 to 429.4 pounds. Body fat percentage (BF %) ranged from 20.2% to 56.8%. BMI ranged from 26.8 to 62.5. Over 44% of the subsample were extremely obese at baseline. Of the teens classified as extremely obese, most (58.3%) were in the Class 2 Risk category. Over 34% were in the Class 3 Risk category and the remainder (7.1%) were at the highest risk (Class 4). At baseline, no differences by gender were found for the rates of extremely obese (P = .885) or for rates of obesity (P = .211). At baseline, the rate of extremely obese youth did not differ between the MBA group and the PBA group (t = 0.575, df [82], P = .567).
H1: The MBA Group Will Have More Weight Loss at T2 and T3 Compared to the PBA Group
For all youth, an average of 7.3 pounds was lost from T1 to T2 regardless of group assignment. At T3, 10.81 pounds (additional 3.5 pounds) were lost from baseline regardless of group.
Obese (N = 106)
The MBA mentees lost more raw weight at both follow-up time points compared to the PBA group (see Table 2). The MBA group lost 77.5% more total weight compared to the PBA group. These group differences in weight loss were significant. The difference in weight loss between the MBA and PBA groups was clinically meaningful. Obese teens in the MBA group were over 3 times more likely to lose weight relative to the PBA group. Obese mentees lost 4.5% of weight; classroom students lost 2.5% of weight.
Table 2.
Weight Loss, Change in BMI, Fat Loss and Change in Percentage of Body Fat by Group
| Group | Initial Follow-up | Final Follow-up | Group Differences | Effect size | Odds Ratios |
|---|---|---|---|---|---|
| Obese—weight loss | |||||
| Mentees | −6.1 pounds | −8.7 total lost | T2:(F = 8.51,P***) | 3.32* | |
| 0.34 | Cl: 1.10–9.98 | ||||
| Classroom | −4.3 pounds | −4.9 total lost | T3: (F = 7.62, P***) | ||
| Extremely obese—weight Loss | |||||
| Mentees | −10.2 pounds | −13.4 total lost | T2: (F = 5.23, P*) | 4.34* | |
| 0.58 | Cl: 0.98–19.34 | ||||
| Classroom | −4.6 pounds | −7.4 total lost | T3:(F = 6.33,P**) | ||
| Obese—change in BMI | |||||
| Mentees | −0.843 | −1.17 total | T2:(F = 4.81,P**) | 1.29 ns | |
| 0.19 | Cl: 0.48–3.39 | ||||
| Classroom | −0.586 | −0.751 total | T3:(F=1.89, ns) | ||
| Extremely obese—change in BMI | |||||
| Mentees | −1.17 | −1.55 total | T2:(F = 4.81,P**) | 3.09* | |
| 0.26 | Cl: 0.67–10.71 | ||||
| Classroom | −0.73 | 1.11 total | T3: (F=12.5, P***) | ||
| Obese—fat loss | |||||
| Mentees | −2.1 pounds | −3.1 total | T2: (F = 0.92, ns) | .76 ns | |
| 0.11 | Cl: 0.289–2.00 | ||||
| Classroom | −1.9 pounds | 3.0 total | T3:(F = 0.88, ns) | ||
| Extremely obese—fat loss | |||||
| Mentees | −4.74 | −4.93 total | T2:(F = 2.98, ns) | 2.38 ns | |
| 0.20 | Cl: 0.69–8.26 | ||||
| Classroom | −1.60 | −3.68 total | T3:(F = 3.86,P*) | ||
| Obese—body fat percentage | |||||
| Mentees | −0.53% | −0.73% total | T2:(F = 8.51,P***) | 2.19* | |
| 0.12 | Cl: 0.879–5.46 | ||||
| Classroom | −0.37% | −0.43% total | T3:(F = 7.62,P***) | ||
| Extremely obese—body fat percentage | |||||
| Mentees | −0.103% | −0.374% total | T2: (F = 0.583, ns) | .988 ns | |
| 0.12 | Cl: 0.329–2.96 | ||||
| Classroom | +0.127% | −0.118% total | T3: (F = 0.806, ns) |
P = .000;
P ≤ .01;
P ≤ .05
ns = not significant
Extremely Obese (N = 84)
The MBA group lost more weight at both follow-up time points compared to the PBA group (see Table 2). The MBA group lost nearly 80% more total weight compared to the PBA group. Group differences in weight loss were significant. The difference in weight loss between the MBA group and the PBA group was statistically and clinically meaningful. The odds of extremely obese teens in the MBA losing weight relative to the PBA group were over 4 times (OR = 4.34) more likely for mentees. In both groups, females lost more weight at T2 compared to males (F = 4.75, P = .034). At T3, gender differences were not found (P = .72). Youth most at risk for clinical disease, the extremely obese, had the greatest reduction in weight in the MBA group.
H2: The MBA Group Will Have a Greater Reduction of BMI at T2 and T3 Compared to the PBA Group
Regardless of group, the change in BMI was significant (F = 3.65, P = .029) but also quadratic (F = 6.5–7, P = .013). Some teens reduced BMI while others increased BMI.
Obese
The MBA group had greater reductions in BMI at both time points. The odds of the MBA group reducing BMI relative to the PBA group were 29.2% more likely for mentees. At T3, the overall change in BMI for the MBA group was −1.17 compared to the PBA group. A meaningful effect on BMI reduction was not found. At initial follow-up, 72.3% of the MBA group reduced BMI, whereas 19.3% increased BMI. Similarly, 69.2% of PBA teens reduced BMI, whereas 28.2% increased BMI. At final follow-up, 66.7% of the MBA group reduced BMI, compared to 65.5% in the PBA group. Comparatively, 33.4% of the MBA group and 34.5% of PBA group increased BMI. In both groups, males had a greater decrease in BMI at initial follow-up (−0.88) compared to females (−0.70). At final follow-up, females had the greatest reduction in BMI (−1.06) compared to males (−.82), indicating a delayed response by females relative to males (F = 9.68, P = .003).
Extremely Obese
The MBA group had a greater reduction in BMI at both follow-up time points (see Table 2). The odds of the MBA group reducing BMI were over 3 times more likely relative to the PBA group. The difference in BMI reduction between the 2 groups had a clinically meaningful effect. Most importantly, extreme obesity reduced 32.7% in the MBA group compared to 21.9% in the PBA group. Although significantly more in the MBA group began the program as extremely obese (n = 52), compared to the PBA group (n = 32; X2 = 4.22, P = .004), by T2 (P = .06) and T3 (P = .22) no group differences in extremely obese were found.
At initial follow-up, 84.2% of the MBA group reduced BMI, whereas 18.8% increased BMI. Only 63.3% of the PBA group reduced BMI, whereas 33.3% increased BMI. At final follow-up, 87.5% of the MBA group reduced BMI, compared to 72.7% of the PBA group. Comparatively, 12.5% of the MBA group and 27.3% of PBA group increased BMI. The rate of BMI increase was more than twice as high in the PBA group compared to the MBA group. Males had a greater reduction in BMI at T2 (−1.13) compared to females (−0.83). At T3, females had the greatest decrease in BMI (−1.52) compared to males (−1.17), indicating a delayed response from females. However, gendered differences were not significant (P = .63).
H3: The MBA Group Will Have More Fat Loss at T2 and T3 Compared to the PBA Group
Obese
The MBA group lost slightly more body fat compared to the PBA group. This health outcome trended in the hypothesized direction but differences were not significant. Percentage of body fat showed the same trends. The MBA group had a greater reduction of body fat percentage at both T2 and T3 compared to the PBA group. The MBA group who were obese had more than 2 times the odds of reducing their percentage of body fat relative to those who were obese and in the PBA group. See Table 2 for results.
Extremely Obese
The MBA group had more total fat loss compared to the PBA group. The difference between the 2 groups was small but clinically meaningful. At initial follow-up, the MBA group had a slight reduction in percentage of body fat, whereas the PBA group had an increase, but group differences were not significant. The MBA group lost a greater amount of fat pounds at both follow-up time points compared to the PBA group. The odds of the extremely obese assigned to the MBA group losing fat pounds were over twice as likely relative to extremely obese youth in the PBA group (see Table 2).
Discussion
Rural Appalachian teens face several health challenges. Compared to national rates, rural Appalachian adolescents are disproportionately classified as extremely obese. In our primary study, we found that 16.5% of our full sample were extremely obese at baseline. The rate of extreme obesity between males and females was nearly identical. These rates differed from national estimations for teens in 2 important ways: the overall prevalence rates and gender-specific estimations. The overall rates of extreme obesity in teen participants in our primary study exceed the national estimations for Caucasian teens by approximately 3 times the average for males and more than twice the national average for females. According to 2011–2014 NHANES data, the percentage of those extremely obese was 7.8%, with females slightly higher (8%) compared to males (7.7%).38 Other national estimates for teen extreme obesity range from 4% to 6% of all youth.13 Nationally, rates of extreme obesity are lower among non-Hispanic teens compared to other teen populations, with males having the lowest rate (5.9%). Despite our population being predominately Caucasian and non-Hispanic, we found rural Appalachian teen extremely obese rates are disproportionately high regardless of gender.
In many communities such as rural Appalachia, intensive behavioral therapy and or medical management of youth obesity are not available or not feasible.28,45 Barriers to care include the lack of local program availability, difficulty with transportation to receive specialty care, and the lack of insurance. An alternative is tailored school-based interventions targeting behavioral change and health outcomes. Although our larger 20-school, G-RCT did not target youth with obesity and extreme obesity, baseline measurements indicated that a large proportion were either obese (24.2%) or extremely obese (16.5%). The nature of the program being available to all students avoided the stigma of obesity. Program adaptions such as physical activities, goal setting, and social support strategies accommodated the needs of these high-risk youth. Many began with walking goals and light activities rather than more intensive exercises. Our intervention focused on limiting sedentary activity rather than increasing vigorous activities. Peer mentors worked with mentees based on baseline mentee weight classification; obese and extreme obese youth were not co-mentees with those who were of a healthy weight classification.
Social relationships are a factor related to obesity risk.46 Bruening and associates46 found that BMI is associated with friendship selection. In a sample of emerging adults, rather than having friends with similar BMIs, college students avoided peers with higher BMI levels. To overcome the discrimination of friend selection for obese youth, peer mentoring is one method to strengthen social support and friendship networks. Mentors help youth overcome personal and social barriers, expose them to new relationships and opportunities, and assist in developing decision-making and problem-solving skills in everyday life.35,47 Mentoring relationships have positively influenced behavior change and health outcomes while promoting positive connections to parents and family, including PA23,24,30,35,48,49 and academic achievement.50 Mentoring of teens has resulted in long-term and sustainable behavior change such as reduced substance use and smoking.48,49
Although we assessed family structure and neighborhood characteristics, the social conditions affecting health behaviors of rural Appalachian communities need to be better understood. Rural Appalachia has environmental, economic, and social characteristics that influence health problems.18,19,51 Reduced educational opportunities and high unemployment have led to economic instability and persistent poverty, which is associated with poorer health outcomes.1,2,6,18 Recently, Dietz commented on the results of 2 rigorous trials targeting obesity prevention.52 He noted that social conditions might partially explain the results. Although these trials were conducted with low-income minority populations, similar social conditions exist within Appalachia. Rural Appalachia is plagued by the opioid crisis affecting many families. During our study, youth shared that parents were either incarcerated or deceased because of substance abuse/overdose. Many teens are responsible for the care of younger siblings. Others shared that they did not have a regular home but temporarily stayed with others. A school principal shared that most of her students lived in a small hotel close by. Our study attrition (N = 43) was mostly due to moving from the school prior to T2 data collection. We did not fully explore these broader structural barriers in our study, but they may have affected health behaviors and intervention sustainability. To overcome broader structural barriers to healthy behaviors, policy-based programs to improve economic conditions and community-driven approaches are needed.52 Without research and programing targeting the broader determinants of health-related behaviors, health disparities prevalent in rural Appalachia will persist.
Funding:
Research reported in this study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under Award Number R01HD080866. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.United States Department of Agriculture. Rural America at a glance 2017 edition Washington, DC: USDA, Economic Research Service Economic Bulletin 182; 2017. [Google Scholar]
- 2.Meit M, Knudson A, Gilbert T, et al. The 2014 update of the rural-urban chartbook. Bethesda, MD: Rural Health Reform Policy Research Center; 2014. [Google Scholar]
- 3.Smith LH, Laurent D, Baumker E, Petosa RL. Rates of obesity and obesogenic behaviors or rural Appalachian adolescents: how do they compare to other adolescents or recommendations? J Phys Act Health. 2018;15(11);874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Over-weight and obesity: childhood obesity facts. Available at: http://www.cdc.gov/obesity/data/childhood.html. Accessed on August 26, 2019.
- 5.Lutfiyya M, Lipsky M, Wisdom-Behounek J, Inpanbutr-Martinkus M. Is rural residency a risk factor for overweight and obesity for U.S. children? Obesity. 2007;15(9):2348–2356. [DOI] [PubMed] [Google Scholar]
- 6.Ickes MJ, Slage KM. Targeting obesity in rural and Appalachian children and families: a systematic review of prevention and treatment interventions. Universal J Public Health. 2013;1(3):51–64. [Google Scholar]
- 7.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–230. [DOI] [PubMed] [Google Scholar]
- 8.Freedman DS, Butte N, Taveras EM, Lundeen EA, Blanck HM, Goodman AB, et al. BMI z-scores are a poor indicator of adiposity among 2 to 19-year olds with very high BMIs, NHANES 1999–2000 to 2013–14. Obesity 2017;25(4):739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkey CS, Colditz GA. Adiposity in adolescents, change in actual BMI works better than change in BMI z-score for longitudinal studies. Ann Epidemiol. 2007;17(1);44–50. [DOI] [PubMed] [Google Scholar]
- 10.Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman DS, Berenson GS. Tracking of BMI z scores for severe obesity. Pediatrics. 2017; 140(3):e20171072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabiri LS, Hernandez DC, Mitchell K. Reliability, validity, and diagnostic value of a pediatric bioelectrical impedance analysis scale. Child Obes. 2015;11(5):650–655. Available at: 10.1089/chi.2014.0156. Accessed on April 11, 2019. [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. J Am Med Assoc. 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole TJ, Bellizzi MD, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hortz B, Stevens B, Holden B, Petosa RL. Rates of physical activity among Appalachian adolescents in Ohio. J Rural Health. 2009;25(1):58–61. [DOI] [PubMed] [Google Scholar]
- 16.Iannotti RJ, Wang J. Patterns of physical activity, sedentary behavior and diet in US adolescents. J Adolesc Health. 2013;53(2):280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith LH, Holloman C. Piloting “sodabriety”: a school-based intervention to impact sugar-sweetened beverage consumption in rural Appalachian high schools. J Sch Health. 2014;84:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. 2014 National healthcare quality and disparities report chart book on rural health care. AHRQ Pub. No. 15-0007-9-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015. [Google Scholar]
- 19.National Advisory Council on Rural Health and Human Services. Mortality and life expectancy in rural America: connecting the health and human service safety nets to improve health outcomes over the lifecourse. Rockville, MD: National Advisory Committee on Rural Health and Human Services; 2015. [Google Scholar]
- 20.Sobol-Goldberg S, Rabinowitz J, Gross R. School-based obesity prevention programs: a meta-analysis of randomized controlled trials. Obesity. 2013;21:2422–2428. [DOI] [PubMed] [Google Scholar]
- 21.Militello LK, Kelly S, Melnyk B, Smith LH, Petosa RL. A review of systematic reviews targeting the prevention and treatment of overweight and obesity in adolescent populations. J Adolesc Health. 2018:63;675–687. [DOI] [PubMed] [Google Scholar]
- 22.Smith LH, Petosa RL, Shoben A. Peer mentor versus teacher delivery of a physical activity program on the effects of BMI and daily activity: protocol of a school-based group randomized controlled trial in Appalachia. BMC Public Health. 2018;18:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith LH. Piloting the use of teen mentors to promote a healthy diet and physical activity among children in Appalachia. J Spec in Pediatr Nurs. 2011;16:16–26. [DOI] [PubMed] [Google Scholar]
- 24.Smith LH, Holloman C. Comparing the effects of teen mentors to adult teachers on child lifestyle behaviors and health outcomes in Appalachia, J Sch Nurs. 2013;29:386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith LH, Valenzuela J, Ludke RL. Engaging rural and urban Appalachians in research using a community-based participatory research approach. PRISM: J Rural Engagement. 2012;1(1):3–17. Available at: https://encompass.eku.edu/cgi/viewcontent.cgi?article=1001&context=prism. Accessed on August 23, 2019. [Google Scholar]
- 26.Savoye M, Nowicka P, Shaw M, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics. 2011;127(3):402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.August GP, Caprio S, Fennoy I, et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93:4576–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches. Circulation. 2013;128:1689–1712. [DOI] [PubMed] [Google Scholar]
- 29.Johnson CA, Tyler C, Palcic JL, Stansberry SA, Gallagher MR, Foreyt JP. Smaller weight changes in standardized body mass index in response to treatment as weight classification increases. J Pediatric. 2011;158:624–627. [DOI] [PubMed] [Google Scholar]
- 30.Petosa RL, Smith LH. Peer mentoring for health behavior change: a systematic review. Am J Health Educ. 2014;45:351–357. [Google Scholar]
- 31.Plotnikoff RC, Costigan SA, Karunamuni N, Lubans DR. Social cognitive theories used to explain physical activity behaviors in adolescents: a systematic review and meta-analysis. Prev Med. 2013;56(5):245–253. [DOI] [PubMed] [Google Scholar]
- 32.Hortz BV, Petosa RL. Impact of the “Planning to be Active” leisure-time physical exercise program on rural high school students. J Adoles Health. 2006;39(4):530–535. [DOI] [PubMed] [Google Scholar]
- 33.Winters ER, Petosa RL, Charlton TE. Using social cognitive theory to explain discretionary, “leisure-time” physical exercise among high school students. J Adolesc Health. 2003;32(6):436–442. [DOI] [PubMed] [Google Scholar]
- 34.Keller TE, Blakeslee JE. Social networks and mentoring In: Dubois DL, Karcher MJ, eds. Handbook of Youth Mentoring. 2nd ed Thousand Oaks, CA: Sage; 2014:129–142. [Google Scholar]
- 35.Noam GG, Malti T, Karcher MJ. Mentoring relationships in developmental perspective In: Dubois DL, Karcher MJ, eds. Handbook of Youth Mentoring. 2nd ed Thousand Oaks, CA: Sage; 2014:99–115. [Google Scholar]
- 36.United States Department of Health and Human Services. NCHS urban-rural classification scheme for counties. Vital and Health Statistics. 2012;2(154):1–66. [PubMed] [Google Scholar]
- 37.Health Resources and Services Administration. Defining rural population. 2018. Available at: https://www.hrsa.gov/rural-health/about-us/definition/index.html. Accessed on August 21, 2019.
- 38.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief No. 288 Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 39.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132:204–210. [DOI] [PubMed] [Google Scholar]
- 40.Tanita Corporation of America. Body Composition Analyzer DC-430U Instruction Manual. Arlington Heights, IL: Tanita Corp; 2014. [Google Scholar]
- 41.Barreira TV, Staiano AE, Katzmarzyk PT. Validity assessment of a portable bioimpedance scale to estimate body fat percentage in white and African American children and adolescents. Pediatr Obes. 2013;8(2):e29–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells JC. Toward body composition reference data for infants, children, and adolescents. Adv Nutr. 2014;5(3):320S–329S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray DM. Design and Analysis of Group-Randomized Trials. New York: Oxford University Press; 1998. [Google Scholar]
- 44.Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: a review of recent methodological developments. Am J Public Health. 2004;94(3):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGarvey EL, Leon-Verdin M, Killos LF, Guterbock T, Cohn WF. Health disparities between Appalachian and non-Appalachian counties in Virginia USA. J Community Health. 2011;36:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruening M, van Woerden I, Schaefer DR, et al. Friendship as a social mechanism influencing body mass index (BMI) among emerging adults. PloS One. 2018;13(12):e0208894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karcher MJ. The effects of developmental mentoring and high school mentors’ attendance on their younger mentees’ self-esteem, social skills, and connectedness. Psychol Sch. 2005;42(1):65–77. [Google Scholar]
- 48.Thomas RE, Lorenzetti DL, Spragins W. Systematic review of mentoring to prevent or reduce tobacco use by adolescents. Acad Pediatr. 2013;13:300–307. [DOI] [PubMed] [Google Scholar]
- 49.Rhodes JE, Reddy R, Grossman JB. The protective influence of mentoring on adolescents’ substance use: direct and indirect pathways. Appl Develop Sci. 2005;9(1):31–47. [Google Scholar]
- 50.Larose S, Tarabulsy GM. Academically at-risk students In: Dubois DL, Karcher MJ, eds. Handbook of Youth Mentoring. 2nd ed Thousand Oaks, CA: Sage; 2014:440–453. [Google Scholar]
- 51.Moy E, Garcia MC, Bastian B, et al. Leading causes of death in nonmetropolitan and metropolitan areas – United States, 1999–2014. MMWR Surveillance Summaries, 2017;66(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dietz WH. We need a new approach to prevent obesity in low-income minority populations. Pediatrics. 2019;143(6):e20190839. [DOI] [PubMed] [Google Scholar]


