Abstract
Bone marrow mesenchymal stem cells (BMSCs) on the repair of spinal cord injury (SCI) in rats as well as the role of transforming growth factor-β (TGF-β)/Smads signaling pathway in the repair were investigated. Rat BMSCs and astrocyte-spinal cords (ASCs) were isolated and cultured in vitro, and the cell purity was detected by flow cytometry. ASCs were co-cultured with TGF-β1, BMSCs and BMSCs + TGF-β1, respectively, and grouped accordingly, and ASCs cultured conventionally were included into control group. 3-(4,5)-Dimethylthiahiazo(-z-y1)-3,5-diphenyltetrazoliumbromide (MTT) assay was conducted to detect the proliferation ability of ASCs in each group. Western blotting (WB) was utilized to examine the expression of TGF-β/Smads signaling pathway-related proteins [TGF-β1, Smad2 and phosphorylated (p)-Smad2] in ASCs and ASCs co-cultured with BMSCs. A rat model of SCI was established, and BMSCs were injected locally. Then (BBB) score was used to evaluate spinal cord repair, and WB was adopted to detect the expression of TGF-β1, Smad2 and p-Smad2 at the injured site. BMSCs and ASCs isolated in vitro grew well. According to MTT assay results, TGF-β1 significantly promoted the proliferation of ASCs (P<0.05), and co-culture of ASCs and BMSCs remarkably reduced the proliferation of ASCs (P<0.05). The detection of protein expression at the SCI site via WB demonstrated that the expression of TGF-β1, Smad2 and p-Smad2 in SCI group were obviously upregulated compared with those in Sham group at 1 week (P<0.05), and the injection of BMSCs could markedly downregulate the expression (P<0.05). After 3 week, there were no significant differences in the expression of TGF-β1, Smad2 and p-Smad2 among groups (P>0.05). The transplantation of BMSCs can improve the spinal function of SCI rats probably by inhibiting the TGF-β/Smads signaling pathway and reducing the proliferation of ASCs.
Keywords: spinal cord injury, bone marrow mesenchymal stem cells, astrocytes-spinal cord, TGF-β/Smads signaling pathway, rat
Introduction
Spinal cord injury (SCI) is traumatic with extremely high mortality and disability rates. The course of the trauma is complex and variable. Besides, SCI can develop continuously in different time stages, often resulting in the expansion of initial injured area, and it has almost no self-repair ability and leads to motor function loss (1). SCI can lead to permanent paralysis, complete loss of sensation in affected limbs and trunks, and loss of bowel, bladder and sexual function (2). Besides, SCI also exerts far-reaching impacts on individuals, families and the whole society. High treatment costs and nursing expenditures have brought huge economic burden to individuals, families and the society (3). However, up to now, there is no definite treatment method that is able to remarkably restore neurological function after SCI (4).
After SCI, changes in local environment cause changes in the physiological function of cells, such as neurons, oligodendrocytes and astrocyte-spinal cords (ASCs) (5). These changes begin with the entry of inflammatory cytokines into the injured spinal cord, releasing a large number of cytokines and chemokines and thereby leading to cell injury. Moreover, SCI triggers the reaction of ASCs and causes their proliferation and hypertrophy (6,7), thus facilitating the expression of proteins, such as glial fibrillary acidic protein (GFAP), nestin and vimentin (8,9) and finally causing scar formation by activating signaling pathways such as STAT3 and transforming growth factor-β (TGF-β)/Smads pathways (7,10,11). Besides, ASCs also regulate their own activities in autocrine manner (7,9).
Mesenchymal stem cells (MSCs) first found in bone marrow can differentiate into osteoblasts, chondrocytes, adipocytes, myoblasts and other functional cells in vitro (12). According to related studies, Basso, Beattie and Bresnahan (BBB) score system confirms that the injection of bone marrow MSCs (BMSCs) in SCI rats can improve their motor function and accelerate motor recovery during spinal transplantation in mice with moderate SCI (13). Furthermore, BMSCs can also migrate to the injured sites of spinal cord tissues to replace injured and lost cells, thus enhancing motor function (14). Ankeny et al (15) found that BMSCs can stimulate the regeneration of injured axons in the whole spinal cord and restore motor control.
This study explored the effect of BMSCs on the motor function repair of SCI rats and the role of the TGF-β/Smads signaling pathway in the repair process, so as to provide theoretical bases and potential therapeutic methods for SCI repair-related research and clinical treatment of SCI.
Materials and methods
Main materials
Sprague Dawley (SD) rats, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), trypsin, phosphate-buffered saline (PBS) and double-antibody (Gibco; Thermo Fisher Scientific, Inc.), cluster of differentiation 34 (CD34)-phycoerythrin (PE), CD90-PE, CD105-PE, GFAP, TGF-β1, Smad2, p-Smad2 and β-actin antibodies (Abcam), TGF-β1 (PeproTech) and 0.22 µm pinhole filter (EMD Millipore). This study was approved by the Animal Ethics Committee of Jining No. 1 People's Hospital Animal Center (Jining, China).
Establishment of rat SCI models
Rat SCI models were established using the Allen method (16): After 10% chloral hydrate (300 mg/kg) was injected intraperitoneally into the rats (180-200 g), the skin was prepared locally, and the rats were fixed on the operating Table in the prone position. No rat exhibited signs of peritonitis after the administration of 10% chloral hydrate. Then a 2-3 cm incision was made in the center of the back with T10 spinous process as the center, so as to expose the T9-T11 spinous processes and vertebral plates, and the interspinous ligament was cut off to expose T10 spinal cord. Subsequently, a 3.5 mm round plastic pad was placed on T10 spinal cord, and a 10 g iron cone was used to hit against the pad in a free fall at a height of 3 cm to cause impact injury to the spinal cord. Thereafter, it was observed that the rapid retraction and springing appeared in the hind legs, and the tail gradually tilted and fell down. The relaxation and collapse of the hind legs indicated successful modeling. Next, the spinal cord was washed with warm normal saline, and the incision was sutured. At 3 days after operation, antibiotics were injected continuously, and the rats were fed regularly. The movement of both hind limbs and tail were observed and recorded daily.
Isolation and culture of BMSCs
Cervical dislocation (after being anesthetized using 10% chloral hydrate at a dose of 250 mg/kg) was used as the method of euthanasia. No rat exhibited signs of peritonitis after the administration of 10% chloral hydrate. We verified that the experimental animals were properly sacrificed by observing the indications of breathing and heartbeat. Then the rat tibia was taken out under aseptic conditions and cleaned with PBS to open the capitulum, and DMEM (with 1% double-antibody) containing 10% FBS was used to wash the bone cavity through a syringe. Then the harvested cell suspension was centrifuged at 4˚C, 1,050 x g for 10 min, and an appropriate amount of DMEM (with 1% double-antibody) containing 10% FBS was added to suspend the precipitate again. After counting, the cells were inoculated into a 100 mm culture dish at the density of 1x105 cells/ml and cultured in an incubator containing 5% CO2 at 37˚C. The solution was replaced every other day until the cells covered 90% of the bottom of the dish. After washing twice with PBS, the cells were digested with 0.25% trypsin and subjected to passage at a density of 1:3.
Isolation, culture and purification of ASCs
The spinal cord of SD rats was taken at 1-3 days under aseptic conditions, the spinal membrane and vascular tissues were removed in pre-cooled DMEM, and the remaining spinal cord tissues were cut into 1 mm3 tissue blocks. After that, an appropriate amount of 0.125% trypsin digestion solution was added for digestion at 37˚C for 5 min, after which the tissues were taken out and blown off. Then DMEM + 10% FBS culture solution was added to terminate digestion, and the harvested cell suspension was centrifuged at 4˚C, 1,050 x g for 10 min to collect precipitates. After resuspension with DMEM + 10% FBS culture solution, the cells were inoculated into a culture dish at 1x105 cells/ml and cultured in the incubator containing 5% CO2 at 37˚C. The solution was replaced every other day until the cells covered 90% of the bottom of the dish. Cell purification: Cells were inoculated into a polylysine-coated culture dish at 1x105 cells/ml. Twenty-four hours later, a culture solution containing 5 µg/ml of Ara-C was added and replaced with DMEM + 10% FBS culture solution after 48 h. The solution was replaced every 3 days, and the cells were continuously cultured for ~10 days.
Cell co-culture experiment
In vitro, ASCs and BMSCs were cultured and proliferated, respectively. P3 ASCs and P4 BMSCs growing well were taken for experiments and divided into 4 groups: ASCs culture group (ASCs group), ASCs + 0.5 µg/l of TGF-β1 culture group (ASCs + TGF-β1 group), ASCs + BMSCs co-culture group (ASCs + BMSCs group), and ASCs + BMSCs + 0.5 µg/l of TGF-β1 culture group (ASCs + BMSCs + TGF-β1 group). ASCs were inoculated into the lower layer of a 96-well Transwell plate at a density of 1x105 cells/ml, while BMSCs were inoculated into the upper layer of the plate at a density of 1x105 cells/ml. The basic culture solution was DMEM + 10% FBS, and the cell proliferation was detected by 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-diphenyltetrazoliumbromide (MTT) (Sigma-Aldrich; Merck KGaA) after 48 h of cell culture.
Subsequently, the cells were divided into two groups, namely, ASCs group and ASCs + BMSCs group. ASCs were inoculated into the lower layer of a 6-well Transwell plate at a density of 1x105 cells/ml, whereas BMSCs were inoculated into the upper layer of the plate at a density of 1x105 cells/ml. The basic culture solution was DMEM + 10% FBS, and the protein expression of TGF-β1, Smad2 and p-Smad2 were examined by western blotting (WB) after cell culture for 3 and 7 days, respectively.
Cell injection
BMSCs were cultured and proliferated in vitro, and P4 BMSCs in good growth status were collected, and cell suspension was prepared at a density of 5x107 cells/ml. Subsequently, the rat models of SCI established above were divided into SCI group and BMSCs transplantation group, and sham operation (Sham) group was used as control. In BMSCs transplantation group, 10 µl of cell suspension was injected into the subarachnoid space at the spinal injury site at 30 min after SCI modeling. In SCI group and Sham group, the same amount of normal saline was injected.
MTT assay
The culture medium was discarded, BMSCs in the upper layer was removed in ASCs + BMSCs group, with only ASCs in the lower layer left. Then the remaining ASCs were washed with PBS 2-3 times, and 100 µl of fresh culture solution was added. Then, 20 µl of MTT solution (5 mg/ml) was added to each well, and the culture solution was removed from the well after continuous culture for 4 h. Thereafter, each well was added with 150 µl of dimethyl sulfoxide (DMSO) and oscillated on a shaker at a low speed for 10 min, and the optical density at 490 nm in each well was measured using enzyme-linked immunosorbent assay after the crystal was fully dissolved.
Analysis via flow cytometry
Well-growing cells were analyzed via flow cytometry. Then the cells were washed twice with PBS, digested with 0.25% trypsin to form single cell suspension, and washed with PBS three times to adjust the cell density to 3x105 cells/ml. Thereafter, BMSCs were incubated with CD34-PE, CD90-PE and CD105-PE flow antibodies at 4˚C in the dark, whereas ASCs were incubated with CD34-PE flow antibody at 4˚C in the dark. After fixation, ASCs were incubated with GFAP antibody and mouse anti-rabbit IgM/PE-Cy3. Next, cell samples incubated with anti-rat IgG1-PE antibody were used as the same type control. After 30 min, PBS was utilized for washing 3 times, and flow cytometry (C6) was employed for detection on the machine, followed by data analysis using CFlow Plus software.
WB
Cells co-cultured in vitro or injured spinal tissues were added with an appropriate amount of cell lysate for lysis overnight at 4˚C, and the total protein was extracted by centrifugation at 4˚C, 10,500 x g for 15 min, the concentration was determined by bicinchoninic acid (BCA) (Pierce; Thermo Fisher Scientific, Inc.). Then the protein was separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene fluoride membrane (Roche Diagnostics). After that, the membrane was sealed in Tris-buffered saline with 0.1% Tween-20 and 5% skim milk powder, and TGF-β1, Smad2, p-Smad2 and β-actin primary antibodies were used to gently shake the membrane overnight at 4˚C. After the reaction with primary antibody, horse radish peroxidase (HRP)-labeled secondary antibody was applied for incubation. Finally, the exposed detection protein was treated with electrochemiluminescence (ECL) reagent.
BBB score
The spinal motor function of rats was scored by BBB behavioral function evaluation method (17) (21 grades in total). The rats were placed in an open basin, and the basin wall was knocked to make the rats crawl. The movement of each joint part was observed and recorded, and the motor function of each rat was evaluated according to scoring rules. The observation time was 5 min each time, and the final score was the average score of bilateral hind limbs.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 20.0 software (IBM, Corp.) was adopted to process the data. The data in each group were expressed as mean ± standard deviation (mean ± SD), and the intergroup comparison was conducted using independent samples t-test. P<0.05 indicates that the difference is statistically significant.
Results
Isolation and culture of rat BMSCs in vitro
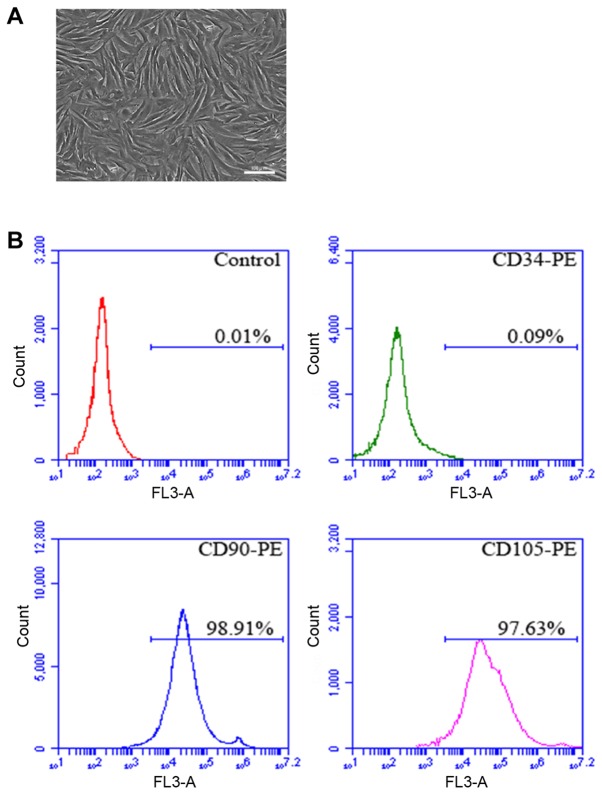
The rat tibia was isolated in sterile environment, the bone marrow was obtained, and BMSCs were isolated and cultured in vitro. After amplification and culture, the cells displayed plump morphology and were spindle-like growing in spiral shape (Fig. 1A). In addition, flow cytometry results manifested that the positive rates of CD90 and CD105 in P4 cells were 98.91 and 97.63%, respectively, and CD45 was not expressed (Fig. 1B).
Figure 1.
Morphology image and flow cytometry analysis of rat BMSCs. (A) Morphology image of P5 BMSCs. (B) Image of flow cytometer analysis: the positive rates of CD90, CD105 and CD45 are 98.91, 97.63 and 0.09%, respectively. Scale, 100 µm. BMSCs, bone marrow mesenchymal stem cells.
Isolation and culture of rat ASCs in vitro
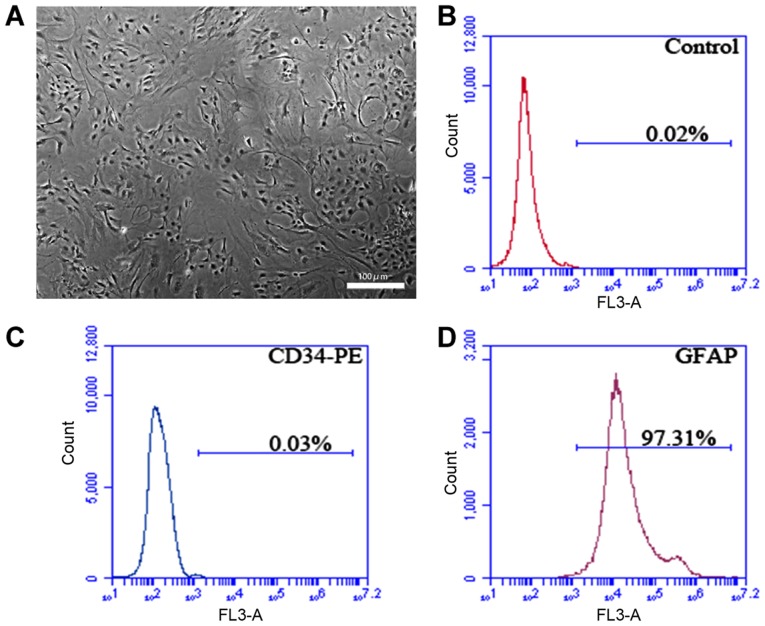
The rat spine was isolated in sterile environment, and ASCs obtained were isolated and cultured in vitro, showing normal morphology (Fig. 2A). According to flow cytometry detection, the positive rate of the specific marker of P3 ASCs GFAP was 97.31%, and CD34 was not expressed (Fig. 2B-D).
Figure 2.
Morphology image and flow cytometry analysis of rat ASCs. (A) Morphology image of P3 ASCs. (B-D) Image of flow cytometer analysis: the positive rates of GFAP and CD34 are 97.31 and 0.09%. Scale, 100 µm. ASCs, astrocyte-spinal cords; GFAP, glial fibrillary acidic protein.
Co-culture experiment of ASCs
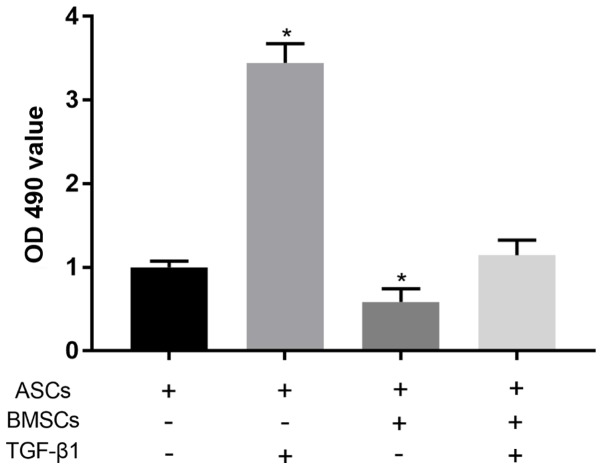
MTT assay showed that compared with normally cultured ASCs, 0.5 µg/l TGF-β1 could significantly promote the proliferation of ASCs (P<0.05). Co-culture of ASCs and BMSCs was able to significantly reduce the proliferation level of ASCs (P<0.05). After the co-culture of ASCs and BMSCs under the effect of TGF-β1, there was no significant difference in the proliferation level of ASCs compared with that in control group (P>0.05) (Fig. 3).
Figure 3.
Proliferation ability of ASCs in each group detected via MTT assay. Compared with normally cultured ASCs, the proliferation ability of ASCs in the presence of 0.5 µg/l TGF-β1 is significantly increased (P<0.05). The co-culture of BMSCs and ASCs markedly reduces the proliferation level of ASCs (P<0.05), but the proliferation ability of ASCs co-cultured with BMSCs under the effect of TGF-β1 displays no obvious difference compared with normally cultured ASCs (P>0.05). ASCs, astrocyte-spinal cords; BMSCs, bone marrow mesenchymal stem cells; TGF-β1, transforming growth factor-β.
Effect of co-culture of ASCs and BMSCs on the TGF-β/Smads pathway
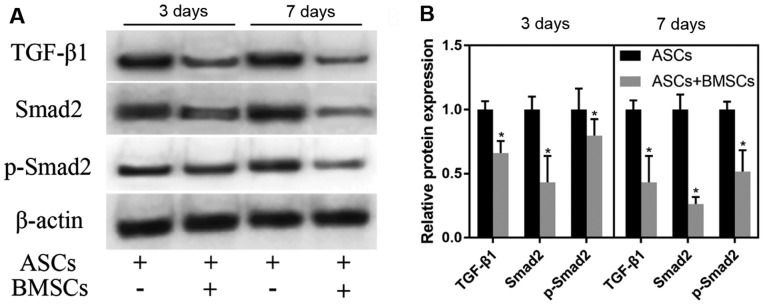
ASCs were co-cultured with BMSCs in vitro. With normally cultured ASCs as control, ASCs were cultured for 3 and 7 days. WB test results demonstrated that the co-culture notably reduced the expression of TGF-β1, Smad2 and p-Smad2 in ASCs (P<0.05). With the extension of co-culture, the degree of the decrease in the expression of TGF-β1, Smad2 and p-Smad2 in ASCs after 3 days of co-culture with BMSCs was lower than that in ASCs after 7 days of co-culture with BMSCs (Fig. 4).
Figure 4.
Effect of co-culture of ASCs and BMSCs on the TGF-β/Smads pathway. (A) Expression of TGF-β1, Smad2 and p-Smad2 detected via WB after BMSCs and ASCs were co-cultured for 3 and 7 days. (B) Relative quantitative expression histograms of TGF-β1, Smad2 and p-Smad2. Co-culture with BMSCs remarkably reduces the expression of TGF-β1, Smad2 and p-Smad2 in ASCs (P<0.05). *P<0.05, there is an obvious difference between ASCs + BMSCs group and ASCs group. ASCs, astrocyte-spinal cords; BMSCs, bone marrow mesenchymal stem cells; TGF-β1, transforming growth factor-β.
Effect of transplantation of BMSCs on SCI in rats
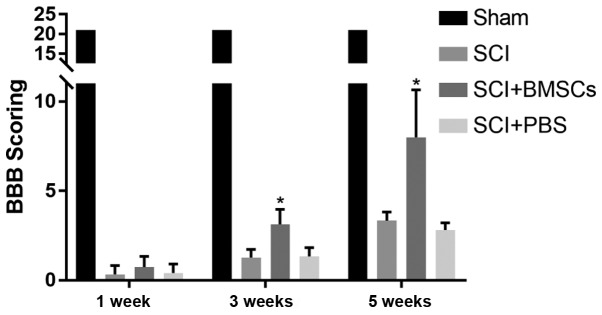
Transplanted BMSCs were injected into the subarachnoid space at the spinal injury site of SCI model rats. The results manifested that BBB score in Sham group was 21 points, and there were no differences at 1, 3 and 5 weeks. BBB score at 1, 3 and 5 weeks in SCI group was 0.33±0.47 points, 1.27±0.44 points and 3.33±0.47 points, respectively, that in SCI + BMSCs group was 0.73±0.57 points, 3.13±0.81 points and 8.00±2.56 points, respectively, and that in SCI + PBS group were 0.40±0.49 points, 1.33±0.47 points and 2.80±0.40 points, respectively. It was found that the BBB score in SCI + BMSCs group was evidently higher than that in SCI group and SCI + PBS group at 3 and 5 weeks (P<0.05), but no obvious difference was found in BBB score between SCI group and SCI + PBS group (P>0.05) (Fig. 5).
Figure 5.
BBB score. At 3 and 5 weeks in SCI + BMSCs group, BBB score is predominantly higher than that in SCI group and SCI + PBS group (P<0.05), but no obvious difference is found in BBB score between SCI group and SCI + PBS group (P>0.05). *P<0.05, obvious difference is detected in comparison among SCI + BMSCs group, SCI group and SCI + PBS group (P<0.05). Sham: sham operation group, SCI: SCI model group, SCI + BMSCs: SCI + BMSCs injection group, SCI + PBS: SCI + PBS injection group. BMSCs, bone marrow mesenchymal stem cells; BBB, Basso, Beattie and Bresnahan; SCI, spinal cord injury; PBS, phosphate-buffered saline.
Influence of the transplantation of BMSCs on the TGF-β/Smads pathway at the spinal injury site of rats
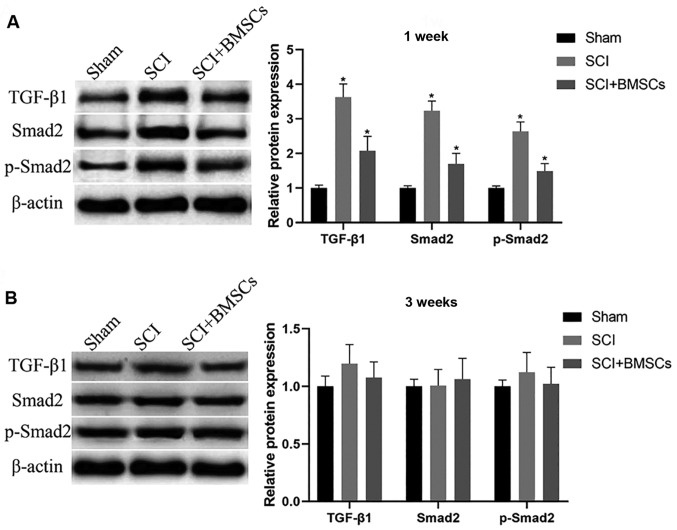
Transplanted BMSCs were injected into the subarachnoid space at the spinal injury site of SCI model rats. WB was used to detect protein expression at the spinal injury site after 1 and 3 weeks, and the results demonstrated that the expression of TGF-β1, Smad2 and phosphorylated (p)-Smad2 in SCI group were obviously upregulated compared with those in Sham group at 1 weeks (P<0.05), and injection of BMSCs markedly downregulated the expression (P<0.05). After 3 weeks, there was no significant difference in the expression of TGF-β1, Smad2 and p-Smad2 among the groups (P>0.05) (Fig. 6).
Figure 6.
Influence of the transplantation of BMSCs on the TGF-β/Smads pathway at the spinal injury site of rats. (A) After 1 week, the expression of TGF-β1, Smad2 and p-Smad2 in each group is remarkably upregulated in SCI group compared with those in Sham group (P<0.05), and injection of BMSCs can downregulate the above expression (P<0.05). (B) After 3 weeks, the expression of TGF-β1, Smad2 and p-Smad2 in each group exhibits no notable differences among groups (P>0.05). *P<0.05, there are significant differences between the groups (P<0.05). BMSCs, bone marrow mesenchymal stem cells; TGF-β1, transforming growth factor-β.
Discussion
SCI mainly leads to nerve conduction interruption, spinal cord structure injury and neuron necrosis, thus resulting in tissue function loss. ASCs are the main glial cell population in the mammalian nervous system, playing an important role. When injuries or diseases such as trauma, neurodegeneration or ischemia occur, ASCs are in a reactive state, manifested as cell hypertrophy, gene expression changes, cell proliferation and other characteristics (9,10). The proliferation of ASCs triggers increased production of extracellular matrix, such as chondroitin sulfate proteoglycan (CSPG) and class I ethylene glycol conjugate (18). CSPG overexpression is closely associated with the formation of glial scars, thus hindering the regeneration and growth of axons (6). After culture in vitro, the proliferation ability of ASCs was evidently enhanced in the presence of TGF-β1, suggesting that ASCs can be stimulated by TGF-β accordingly. Studies have shown that TGF-β increases the expression of calcium ion channel of ASCs as well as the expression of GFAP and CSPGs, thus facilitating the increased number of proliferative ASCs (19).
The TGF-β/Smads signaling pathway mainly consists of TGF-β, TGF-β receptors, Smads and various proteins in the nucleus. TGF-β activates TGF-β receptors, and the activated receptor further phosphorylates Smad2 and other proteins. Then the p-Smad2 combines with other proteins into a complex and enters the nucleus to further function (20). The TGF-β/Smads signaling pathway exerts crucial effects in the process of fibrosis of tissues and organs. When SCI occurs, microglia activation and macrophage invasion are the first reactions. These activated cells release large amounts of cytokines and growth factors, including TGF-β, basic fibroblast growth factor, interleukin-6 and interleukin-1(21). The produced TGF-β further modulates smooth muscle cells, fibroblasts and reactive ASCs (22-24). Moreover, the proliferation ability of ASCs was notably enhanced through the culture of ASCs with TGF-β1.
The TGF-β/Smads signaling pathway plays a vital role in the development process of hypertrophic scars, in which it promotes tissue scar formation and fibrosis. The main physiological mechanism is the high expression of various cytokines in this pathway and the inhibition on the expression of anti-Smads (25). A study of Chin et al (25) revealed that the expression levels of TGF-β1 and TGF-β2 ligands in scar fibroblasts are higher, the expression of TGF-β receptors (type I and II) is increased, and the phosphorylation of Smad3 is raised. O'Brien et al (26) found that ASCs produce complex reactions within 24 h after SCI, and extracellular TGF-β with high concentration is produced immediately after injury. Subsequently, intracellular TGF-β production is observed in the nucleus and cytoplasm of ASCs, intramedullary and extramedullary capillary endothelial cells, and motor neurons. In this study, the upregulated protein expression of TGF-β1, Smad2 and p-Smad2 were detected at the SCI site of SCI rats, consistent with previous research results. It can be concluded that a large quantity of extracellular TGF-β is produced after SCI, which further activates the TGF-β/Smads signaling pathway of ASCs, thus causing massive proliferation of ASCs and generation of hyperplastic scars and hindering axonal regeneration and growth of injured spinal cords (6).
BMSCs have been applied to the treatment of various diseases. After transplanting BMSCs into the injured spinal cord of rats, the function of BMSCs can be improved moderately (27). According to studies, BMSCs can release anti-inflammatory cytokines and neurotrophic factors to protect neurons (28,29). In this study, it was discovered from co-culture of BMSCs and ASCs that BMSCs weakened the proliferation ability of ASCs and reduced the expression levels of TGF-β1, Smad2 and p-Smad2, possible because BMSCs attenuate the effect of TGF-β1 on ASCs through interaction with TGF-β1. Furthermore, SCI rats were treated by transplantation of BMSCs. BBB score displayed that the transplantation improved the recovery of injured spinal cords in animal models, and reduced the protein expression levels of TGF-β1, Smad2 and p-Smad2 in the TGF-β/Smads signaling pathway.
In conclusion, the results of this study verify that the transplantation of BMSCs is able to improve the spinal function of ι SCI rats probably by inhibiting the TGF-β/Smads signaling pathway and reducing the proliferation of ASCs.
Acknowledgements
Not applicable.
Funding
National Natural Science Foundation of China (NSFC) (no. 81801906); Natural Science Foundation of Shandong Province (no. ZR2015YL034).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
CL and KG designed the study and performed the experiments, CL and TZ collected the data, KG and KL analyzed the data, CL and KG prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Jining No. 1 People's Hospital Animal Center (Jining, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, Wagner J, Shumsky JS, Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20:278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 2.Li JW, Kuang Y, Chen L, Wang JF. LncRNA ZNF667-AS1 inhibits inflammatory response and promotes recovery of spinal cord injury via suppressing JAK-STAT pathway. Eur Rev Med Pharmacol Sci. 2018;22:7614–7620. doi: 10.26355/eurrev_201811_16375. [DOI] [PubMed] [Google Scholar]
- 3.Priebe MM, Chiodo AE, Scelza WM, Kirshblum SC, Wuermser LA, Ho CH. Spinal cord injury medicine. Economic and societal issues in spinal cord injury. Arch Phys Med Rehabil. 2007;88 (Suppl 1):S84–S88. doi: 10.1016/j.apmr.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Chhabra HS, Arora M. Demographic profile of traumatic spinal cord injuries admitted at Indian Spinal Injuries Centre with special emphasis on mode of injury: A retrospective study. Spinal Cord. 2012;50:745–754. doi: 10.1038/sc.2012.45. [DOI] [PubMed] [Google Scholar]
- 5.Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012;46:251–264. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- 6.Fitch MT, Silver J. Glial cell extracellular matrix: Boundaries for axon growth in development and regeneration. Cell Tissue Res. 1997;290:379–384. doi: 10.1007/s004410050944. [DOI] [PubMed] [Google Scholar]
- 7.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sofroniew MV, Vinters HV. Astrocytes: Biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji M, Bai C, Li L, Fan Y, Ma C, Li X, Guan W. Biological characterization of sheep kidney-derived mesenchymal stem cells. Exp Ther Med. 2016;12:3963–3971. doi: 10.3892/etm.2016.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin GL, Wang H, Dai J, Li X, Guan M, Ding Q, Wang HX, Fang H. Upregulation of UBAP2L in bone marrow mesenchymal stem cells promotes functional recovery in rats with spinal cord injury. Curr Med Sci. 2018;38:1081–1089. doi: 10.1007/s11596-018-1987-x. [DOI] [PubMed] [Google Scholar]
- 14.Syková E, Jendelová P, Urdzíková L, Lesný P, Hejcl A. Bone marrow stem cells and polymer hydrogel - two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26:1113–1129. doi: 10.1007/s10571-006-9007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol. 2004;190:17–31. doi: 10.1016/j.expneurol.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 16.Namjoo Z, Mortezaee K, Joghataei MT, Moradi F, Piryaei A, Abbasi Y, Hosseini A, Majidpoor J. Targeting axonal degeneration and demyelination using combination administration of 17β-estradiol and Schwann cells in the rat model of spinal cord injury. J Cell Biochem. 2018;119:10195–10203. doi: 10.1002/jcb.27361. [DOI] [PubMed] [Google Scholar]
- 17.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 18.McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Z, Yu P, Chen H, Geller HM. Targeted inhibition of KCa3.1 attenuates TGF-β-induced reactive astrogliosis through the Smad2/3 signaling pathway. J Neurochem. 2014;130:41–49. doi: 10.1111/jnc.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 21.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, et al. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schönherr E, Järveläinen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-beta 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- 24.Heimer R, Bashey RI, Kyle J, Jimenez SA. TGF-beta modulates the synthesis of proteoglycans by myocardial fibroblasts in culture. J Mol Cell Cardiol. 1995;27:2191–2198. doi: 10.1016/s0022-2828(95)91479-x. [DOI] [PubMed] [Google Scholar]
- 25.Chin GS, Liu W, Peled Z, Lee TY, Steinbrech DS, Hsu M, Longaker MT. Differential expression of transforming growth factor-beta receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 2001;108:423–429. doi: 10.1097/00006534-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien MF, Lenke LG, Lou J, Bridwell KH, Joyce ME. Astrocyte response and transforming growth factor-beta localization in acute spinal cord injury. Spine. 1994;19:2321–2330. doi: 10.1097/00007632-199410150-00012. [DOI] [PubMed] [Google Scholar]
- 27.Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K, Chou H, Ishikawa N, Matsumoto N, Iwashita Y, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 29.Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. doi: 10.1016/j.brainres.2013.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.