Abstract
Adolescent risky behavior is related to developmental changes in decision-making processes and their neural correlates. Yet, research investigating how the family environment relates to risk processing in the adolescent brain is limited. In the present study, longitudinal data were collected from 167 adolescents (13–15 years, 53% male) who self-reported household chaos and their parent’s monitoring practices, and completed a decision-making task during fMRI at Time 1 and Time 2 (one year apart). Parental knowledge was positively related to insular risk processing only among adolescents in low-chaos environments at both time points. Results highlight environmental correlates of insular risk processing in the developing brain.
Keywords: Adolescence, Risk Processing, Parenting, Chaos, Risky Decision-Making, fMRI
Adolescence is a critical maturational period involving greater exposure to risky situations in which outcomes of choices are uncertain (Steinberg, 2008; Casey, Getz, & Galvan, 2008). Some adolescents engage in problematic risky behaviors (e.g., substance use, reckless driving) that have deleterious health consequences, which may persist into adulthood (Kann et al., 2016). In an effort to identify why some adolescents engage in risky behaviors, past research has focused on how the adolescent brain processes and responds to valued rewards, and the environmental factors that shape such responses (e.g., Telzer, Fuligni, Lieberman, & Galvan, 2013). For instance, recent work has shown that reward-related circuitry is influenced by quality of parent-child relationships, which may contribute to risk-taking behavior (Qu, Fuligni, Galvan, & Telzer, 2015). While literature to date focuses on neural responses to rewards (see Silverman, Jedd, & Luciana, 2015 for meta-analysis), prior work on decision-making shows that risky decisions involve both the evaluation of reward values and the risk or likelihood of receiving such rewards (d’Acremont & Bossaerts, 2008; Mohr, Biele, & Heekeren, 2010). Furthermore, the evaluation of risk may be influenced by socioecological factors including proximal processes such as parent-child interactions and environmental context such as the household environment (Bronfenbrenner, 1999). Here, we examine how environmental variables, namely parental monitoring and household chaos, are related to adolescent neural risk processing.
Leading theories have explained adolescent risky behavior to be the result of a developmental imbalance between a more mature motivational or incentive processing system (e.g., ventral striatum, orbitofrontal cortex) and a less mature cognitive control system (e.g., dorsolateral prefrontal cortex, anterior cingulate cortex; Casey, Getz, & Galvan, 2008). While this motivational system is often reflected by hyper-responsivity of reward-related circuitry and used to explain adolescent risky behavior (Silverman et al., 2015), it is likely a more complex system that involves multiple processes. One such process found to contribute to risky decision-making in both adults and adolescents is the evaluation of risk associated with outcomes or the likelihood of receiving potential rewards (Clark et al., 2008; Mohr et al., 2010; van Duijvenvoorde et al., 2015). That is, one makes risky choices because one values the outcome of a risky option or the valued outcome has a high probability of occurring.
Value-based decision-making research has shown that a key region consistently associated with the processing of risk information and guiding risky choices is the insular cortex (Clark et al., 2008; Mohr et al., 2010). Activation of the insular cortex has been shown to precede risk-averse choices (Kuhnen & Knutson, 2005) and relate to behavioral risk aversion (van Duijvenvoorde et al., 2015). Moreover, lesions to this area lead to risky choices that result from a failure to adjust behavior in the face of unfavorable odds, implying that the insular cortex may be a neurobiological mechanism of risky choice (Clark et al., 2008). In line with these findings, van Duijvenvoorde and colleagues (2015) showed that adolescents versus children and adults exhibit heightened insular cortex reactivity to options of greater risk (i.e., increases in variance), and that this signal was related to increased risk aversion. Kim-Spoon and colleagues (2016) reported that risk-related insular cortex responses interact with neural cognitive control to predict risky behavior in adolescents. Thus, previous research suggests that heightened insular cortex activity during risky choice may represent a neural signal reflecting expectancy of unfavorable outcomes guiding individuals toward safer choices. However, it remains unclear how socioecological factors contribute to the development of insular risk-related processing during adolescence.
Problematic risk-taking behaviors—including delinquency and substance use—have been related to two important features that shape an adolescent’s environment: (i) deficiencies in parental monitoring (Stattin & Kerr, 2000); and (ii) household chaos (Wachs & Evans, 2010). First, healthy development during adolescence often necessitates parental monitoring involving both knowledge of teen activities as well as behavioral control (Smetana, 2008; Stattin & Kerr, 2000). Having parents who are aware of and attempt to control their children’s behaviors, whereabouts, and with whom they associate with, acts as a protective factor leading to decreased misconduct, substance use, and risky sexual behavior (Crouter, Bumpus, Davis & McHale, 2005; Farley & Kim-Spoon et al., 2017; Stattin & Kerr, 2000). In addition, monitoring strategies, which promote child disclosure of activities and preferences, facilitate positive self-regulation strategies and independence (Smetana, 2008). Positive parental monitoring strategies encompassing both knowledge and behavioral control may influence how the adolescent brain evaluates options and processes risk information. Specifically, parents may serve as exemplars for signaling which situations are particularly risky or act as models for selecting appropriate behaviors in risky situations. Importantly, parental monitoring, especially parental knowledge, typically declines during adolescence due to increases in autonomous behavior (Laird, Pettit, Bates, & Dodge, 2003). If parental monitoring declines to a greater extent compared to what would be expected based on average developmental change, then this could negatively impact adolescents’ adjustment. Indeed, adolescents who experienced greater declines in parental knowledge reported steeper increases in delinquency (Laird et al., 2003).
Second, a household environment that balances stimulation and enrichment with stability and structure is known to support optimal development (Wachs & Evans, 2010). Chaotic household environments with high levels of noise, a lack of family routines, and crowded spaces predict deficits in adolescents’ social-cognitive development (Wachs & Evans, 2010). According to Bronfenbrenner’s (1999) bioecological theory of human development, human development is the product of the interaction between proximal processes, such as parent-child relationships and context, which represent the social and physical environment, respectively. In chaotic home environments, lack of home structure may inhibit the formation of parent-child interactions that promote children’s social-cognitive skills. More recent viewpoints emphasize household chaos as a context in which parenting effects may vary (Bronfenbrenner & Evans, 2000). Prior work on children has shown that the association between poor quality parent-child relationships (e.g., low positivity, harsh discipline) and problematic behaviors is exacerbated by high household chaos (Asbury, Dunn, Pike, & Plomin, 2003; Coldwell, Pike, & Dunn, 2006). In addition, during adolescence, reactive and harsh parenting styles predicted more callous-unemotional traits in high but not low chaos environments (Kahn, Deater-Deckard, King-Casas & Kim-Spoon, 2016). Such findings suggest that household chaos could moderate the association between parental monitoring and adolescent risk taking.
In the present longitudinal study, we examined how familial environmental factors—parental monitoring and household chaos—relate to the development of insular risk-related processing, a candidate neural mechanism of adolescent risk-taking behavior. We hypothesized that higher levels of parental monitoring (i.e., knowledge and behavioral control) are associated with higher levels of insular cortex processing during risky decision-making. We further hypothesized that the effect of parental monitoring on insular risk processing may depend on household chaos. Specifically, we expected the beneficial effects of high parental monitoring to be more prominent amidst low household chaos or the negative effects of low parental monitoring to be exacerbated in high household chaos. Apart from looking at the cross-sectional associations between absolute levels of parental monitoring and insular cortex activation at different ages, we also examined whether changes in parental monitoring were related to changes in insular-related risk processing. Given the literature suggesting that adolescent brain development is influenced by both puberty (Crone & Dahl, 2012) and experience-dependent plasticity, we also explored the effects of pubertal development on insular risk processing.
Method
Participants
The present study used data from 167 adolescents (53% male) that participated in an ongoing longitudinal study spanning across middle adolescence, the period during development when risk-taking behavior increases (Steinberg, 2008). The current analyses used available data from the first two waves of this longitudinal study (data collected between January 2014 and February 2016). Adolescents were on average 14.13 years old (SD = 0.54) at Time 1 and 15.05 years old (SD = 0.54) at Time 2; 80% were White, 13% African American, and 7% of other racial groups. Mean family annual income ranged from $25,000 and $34,999 at Time 1 and Time 2. The sample was representative of the region of the state for household income and race/ethnicity. At Time 1, 157 adolescents participated in the study. At Time 2, 17 adolescents did not return for reasons including: ineligibility for tasks (n = 2), declined participation (n = 7), and lost contact (n = 8). Ten additional adolescents were invited to participate at Time 2, yielding a final sample of 167 adolescents. Multiple logistic regression analyses indicated that attrition was not significantly predicted by demographic (age, income, race, sex) and study variables at Time 1 (household chaos, parental control, parental knowledge, pubertal status, insula activation, risky choices, all ps > .09). Exclusion criteria were claustrophobia, history of head injury resulting in loss of consciousness for >10 minutes, orthodontia impairing image acquisition, and contraindications to magnetic resonance imaging.
Procedure
Participants were recruited by advertisement methods including flyers, recruitment letters, and e-mail. Data collection took place at university offices where adolescents were interviewed by trained research assistants. All adolescent participants provided written assent and their parents provided written permission for a protocol approved by university’s institutional review board.
Measures
Parental Knowledge and Behavioral Control
Adolescents reported on their parents’ monitoring practices on the 8-item parental knowledge and 6-item parental behavioral control subscales of the Parental Monitoring Scale (Stattin & Kerr, 2000) at Time 1 and Time 2. Example items are “Do your parents know what you do during your free time (knowledge)?” and “Do you need to have your parent’s permission to stay out late on a weekday evening (control)?” Response options range from “1 = yes, always” to “5 = no, never.” Items were recoded so higher scores indicated higher parental knowledge and behavioral control, and averaged separately for parental knowledge and behavioral control. Both parental knowledge (α = .81 at Time 1, α = .76 at Time 2) and behavioral control (α = .83 at Time 1, α = .86 at Time 2) showed good reliability.
Household Chaos
Adolescents reported on the level of household chaos using the 6-item Confusion, Hubbub, and Order Scale (CHAOS; Matheny, Wachs, Ludwig, & Phillips, 1995) at Time 1 and Time 2. An example item is “You can’t hear yourself think in our home.” Response options range from “1 = definitely untrue” to “5 = definitely true”. Mean scores were calculated with higher scores indicating higher levels of household chaos. The reliability of the scale was relatively low in the present sample (α = .59 at Time 1, α = .64 at Time 2), which is consistent with prior research (e.g., Asbury et al., 2003; Coldwell et al., 2006).
Pubertal Development
Adolescents completed the 5-item Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988) at Time 1 and Time 2. Adolescents answered questions about their growth spurt, body hair, and skin changes. Additionally, female adolescents reported their breast development and their menarche and male adolescents on vocal and facial hair changes. Responses were given on a scale ranging from “1 = no changes” to “4 = changes completed.” An overall index of pubertal development status was calculated with a mean score, with higher scores indicating more advanced pubertal development.
Lottery Choice Task
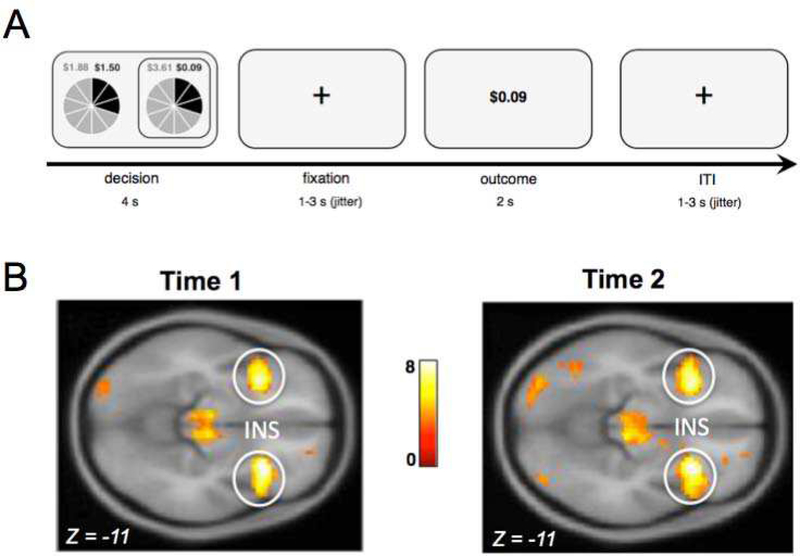
At Time 1 and Time 2, adolescents made choices between pairs of uncertain gambles in a modified economic lottery choice task (Holt & Laury, 2002; for task information, see Supplemental Information Appendices A and B) while their blood-oxygen-level-dependent (BOLD) response was monitored using functional magnetic resonance imaging (fMRI; Figure 1A). Each gamble contained a high and low monetary outcome with a specific probability represented as slices of a pie. Outcomes and probabilities were represented with corresponding colors (grey or black). Probabilities associated with potential monetary outcomes were represented with pie charts to maximize comprehension of numerical information for adolescent participants. Each pie contained 10 slices, in which each slice corresponded to a probability of 10%. Probabilities associated with each outcome were represented with corresponding colors (grey or black). Risk associated with each gamble was quantified as coefficient of variation (CV), a scale-free measure calculated by dividing the standard deviation of potential outcomes by their expected value (Appendix A). Previous research has shown that CV (compared to standard deviation or variance) is a better metric for explaining choice behavior because outcome variability is often encoded relative to the average outcome rather than in an absolute manner (Weber, Shafir & Blais, 2004). Since probabilities were the same for both gambles in a given trial, the difference between low and high monetary amounts differentiated the level of risk between options. That is, the option with the smaller difference in values (e.g., $1.88 - $1.50 = 0.38, Figure 1A) indicates relatively low risk compared to the option with the larger difference in values (e.g., $3.61 - $0.09 = 3.52, Figure 1A). Adolescents were monetarily compensated based on actual outcomes from 5 randomly selected trials in addition to compensation for study completion. Participants practiced on six trials that were excluded from the experiment and were instructed that each trial was equally likely to be selected for compensation. The task consisted of 72 trials and it took participants approximately 30 minutes to complete. Risk taking behavior in the task was quantified as percentage of risky choices made out of 72 trials, where higher percentages reflected greater risk-seeking behavior.
Figure 1.
A) Adolescents were asked to make decisions between pairs of uncertain gambles in an economic lottery choice task. For each gamble, there was a high and low monetary outcome, each associated with a probability. Outcomes and probabilities were represented with corresponding colors (grey and black). The time course of a given trial included a decision phase followed by a jittered fixation interval and an outcome phase, in which participants were shown the results of their choice followed by a jittered inter-trial interval (ITI). B) During the decision phase of the economic lottery choice task, adolescents exhibited increased BOLD responses in the bilateral anterior insular cortex to chosen gambles that were of higher relative to lower levels of risk (i.e., coefficient of variation; CV) at both Time 1 [t (145) = 7.22, p (FWE correction) < .05)] and Time 2 [(t (135) = 7.91, p (FWE correction) < .05.
Imaging acquisition and analysis
Functional neuroimaging data were acquired on a 3T Siemens Tim Trio MRI scanner with a standard 12-channel head matrix coil. Structural images were acquired using a high-resolution magnetization prepared rapid acquisition gradient echo sequence with the following parameters: repetition time (TR) = 1200 ms, echo time (TE) = 2.66 ms, field of view (FoV) = 245×245 mm, and 192 slices with the spatial resolution of 1×1×1 mm. Echo-planar images were collected using the following parameters: slice thickness = 4mm, 34 axial slices, FoV = 220×220mm, TR = 2 s, TE = 30 ms, flip angel = 90 degrees, voxel size = 3.4×3.4×4 mm, 64×64 grid, and slices were hyperangulated at 30 degrees from anterior-posterior commissure. Imaging data were preprocessed and analyzed using SPM8 (Wellcome Trust Neuroimaging Center). For each scan, data were corrected for head motion using a six-parameter rigid body transformation and realigned. The mean functional image was co-registered to the anatomical image, then the anatomical image was segmented and registered to the MNI template and functional volumes were normalized using parameters from the segmented anatomical image, and were smoothed using a 6mm full-width-half-maximum Gaussian filter.
Within a general linear model analysis (GLM), at the subject level, decision period and outcome events were modeled with a duration of 4 and 2 s, respectively (Figure 1A; Appendix A). A parametric regressor of decision phase BOLD activity corresponding to the CV of chosen gambles was included in the model in order to assess neural responses of risk processing. A parametric regressor of the outcome phase was also included corresponding to whether subjects received the high or low value outcome. Additional regressors of no interest included the button press and six motion regressors. At the group level of the GLM, whole brain analysis was conducted to determine how CV for chosen gambles related to BOLD response at Time 1 and Time 2, separately (Figure 1B). For both Time 1 and Time 2, a cluster-level family-wise error (FWE) corrected level of p < .05 and a cluster-defining primary threshold of p < .001 were used to correct for multiple comparisons. Whole-brain analysis revealed that during the decision phase, BOLD responses in bilateral insula, dorsal anterior cingulate cortex, and ventral striatum were associated with magnitude of risk at both Time 1 and Time 2 (Appendices C & D). Given extensive evidence implicating the insular cortex in risk processing (see meta-analysis Mohr et al., 2010), we focused our analyses on the insular cortex. Thus, eigenvariate values were extracted for the peak voxel coordinates of the right and left insular cortex using a 6mm sphere (Time 1: right: x = 30, y = 20, z = −11, left: x = −30, y = 17, z = −14; Time 2: right: x = 30, y = 20, z = −11, left: x = −33, y = 20, z = −11).
Using the extracted ROIs from the whole-brain analysis, we conducted confirmatory factor analyses (CFA). Creating CFA factor scores is well suited for integrating multiple ROIs related to a latent construct (e.g., Kim-Spoon et al., 2016; Nees et al., 2012). Using latent variables gives unbiased parameter estimates in statistical analyses (Little, Card, Preacher, & McConnell, 2009). This is because the manifest variables contain measurement errors, whereas the latent variable is free from such measurement error. Thus, the latent variable represents a construct in its purest form (Bollen, 1989). In the CFA models, standardized left and right anterior insular cortex activation scores loaded on overall insular cortex factor score, separately for Time 1 and Time 2. The models were fully saturated models (χ2 = 0, df = 0), and the two factor loadings (i.e., left and right anterior insula activation) were constrained to be equal for model identification purposes. The factor loadings were significant (.86 for insula activation Time 1, .93 for insula activation Time 2, p < .001). Bilateral insula factor scores were negatively correlated with behavioral risk taking during the lottery choice task (i.e., percentage of risky choices; r = −.39 at Time 1, and r = −.43 at Time 2, ps < .001), indicating that higher BOLD responses in the bilateral insula during the lottery choice task were associated with fewer risky choices (i.e., higher risk sensitivity/aversion).
Statistical Analyses
First, we performed cross-sectional analyses to test the influence of parental knowledge and behavioral control on insula activation and the moderation effects of household chaos on the link between parental knowledge and behavioral control and insula activation, separately for Time 1 and Time 2. Second, we performed longitudinal analyses to test whether changes in parental knowledge and behavioral control were related to changes in insula activation over time, and whether these effects were moderated by household chaos. We calculated residualized change scores for parental knowledge and behavioral control by regressing Time 2 scores on the associated Time 1 scores (e.g., parental control Time 2 was regressed on parental control Time 1). The residualized change scores represent the change from Time 1 to Time 2, adjusting for baseline differences (MacKinnon, Kisbu-Sakarya, & Gottschall, 2013). For insula activation, we performed CFA using residualized change scores for left and right insula activation (i.e., Time 2 left/right insula activation was regressed on Time 1 left/right insula activation and these scores were saved as residualized left and right insula indicators). This CFA model was a fully saturated model (χ2 = 0, df = 0) with the two factor loadings constrained to be equal, and the factor loadings were significant (.90, p < .001). In the longitudinal models, the moderator variable of household chaos was calculated as the mean between Time 1 and Time 2 as it reflected a stable home environmental context (i.e., r = .71, p < .001 between Time 1 and Time 2; paired t(138) = - 0.11, p = .91 for the mean difference between Time 1 and Time 2).
We performed structural equation modeling analyses using Mplus Version 7.4 (Muthén & Muthén, 1998–2015) and Mplus scripts based on the codes developed by Stride, Gardner, Catley, and Thomas (2015). Parental behavioral control and knowledge were simultaneously tested in one model to assess their independent effect on insula activation. In Step 1, we tested for the main effects of parental knowledge and behavioral control and household chaos on insula activation. In Step 2, we added the interaction between parental behavioral control and household chaos and the interaction between parental knowledge and household chaos one at a time (given that these interaction terms were highly correlated; e.g., r = .61 at Time 2, p < .001). For significant interactions, we conducted simple slope analyses to compare adolescents with low and high household chaos (i.e., +/− 1 SD in relation to mean chaos). The predictors were mean-centered to avoid multicollinearity and effects were controlled for pubertal status. Prior to analyses, outliers (> 3 SD) were winsorized by changing the outlier to the next value that was not an outlier (n = 20). Full Information Maximum Likelihood (FIML) estimation was used to account for missing data which resembled a Missing Completely at Random pattern (Little’s MCAR test: χ2 =94.74, df = 84, p = .20). FIML estimation is commonly utilized within structural equation modeling and often outperforms other missing data techniques, such as listwise deletion or imputation (Enders & Bandalos, 2001).
Results
Descriptive statistics and correlations are in Table 1. All variables showed medium to high stability (all ps < .01). Testing within-variable changes using paired t-tests indicated that parental knowledge, but not control, declined between Time 1 and Time 2 and that pubertal status became more advanced. Adolescents on average showed more bilateral insula activation and fewer risky choices at Time 2 compared to Time 1 (Table 1; Appendix F). Change in behavioral risk-taking (i.e., residualized change scores of risky choices between Time 1 and Time 2) and change in insular risk-processing (i.e., residualized change scores of insula activation between Time 1 and Time 2) were negatively correlated, r = −.41, p < .001 (Appendix E), indicating that decreases in risky choices were associated with increases in insula activation during the economic lottery task from Time 1 to Time 2. In other words, adolescents who showed decreases in their risky behavior also showed increases in insular risk processing over time. None of the demographic variables (i.e., race, sex, income, age) were related to insula activation at Time 1 or Time 2 (all ps > .13) and thus were not included as covariates in primary analyses.
Table 1.
Descriptive Statistics of and Correlation Among Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | M | SD | Paired t-test | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Household chaos T1 | 2.44 | 0.67 | ||||||||||||||
| 2 Household chaos T2 | .71*** | 2.45 | 0.65 | −0.11 | ||||||||||||
| 3 Parental control T1 | −.18* | −.11 | 4.34 | 0.67 | ||||||||||||
| 4 Parental control T2 | −.19* | −.23** | .56*** | 4.28 | 0.74 | 0.99 | ||||||||||
| 5 Parental knowledge T1 | −.28*** | −.21* | .34*** | .24** | 4.34 | 0.52 | ||||||||||
| 6 Parental knowledge T2 | −.29*** | −.32*** | .30*** | .60*** | .56*** | 4.23 | 0.55 | 2.88** | ||||||||
| 7 Pubertal status T1 | .22** | .12 | .02 | −.001 | −.04 | −.10 | 2.89 | 0.52 | ||||||||
| 8 Pubertal status T2 | −.03 | −.04 | .08 | .17* | −.02 | .09 | .72*** | 3.09 | 0.46 | −6.16*** | ||||||
| 9 Insula activation left T1 | .10 | .07 | −.01 | .04 | −.04 | .04 | −.17* | −.15 | 0.05 | 0.06 | ||||||
| 10 Insula activation left T2 | −.01 | −.10 | −.11 | .16 | −.06 | .08 | −.08 | −.01 | .27** | 0.61 | 0.83 | −7.76*** | ||||
| 11 Insula activation right T1 | .12 | .04 | −.03 | .06 | .09 | .13 | −.05 | −.05 | .74*** | .35*** | 0.04 | 0.05 | ||||
| 12 Insula activation right T2 | −.02 | −.09 | −.03 | .18* | .01 | .13 | −.08 | −.03 | .28** | .87*** | .31*** | 0.52 | 0.66 | −8.55*** | ||
| 13 Percent risky choices T1 | −.01 | .06 | .03 | −.01 | −.08 | −.08 | .21** | .08 | −.42*** | −.22* | −.32*** | −.26** | 0.47 | 0.16 | ||
| 14 Percent risky choices T2 | .06 | .15 | .01 | −.02 | −.03 | −.07 | .05 | −.05 | −.17 | −.37*** | −.22* | −.46*** | .48*** | 0.41 | 0.18 | 4.50*** |
Note.
p < .05
p < .01
p <.001.
Paired t-tests were conducted to examine change in study variables between Time 1 and Time 2. Negative t-values indicate increases, positive t-values indicate decreases across time.
Results of the moderation analyses can be found in Table 2. There were significant interaction effects between parental knowledge and household chaos on insula activation for both Time 1 and Time 2. The interaction effects between parental behavioral control and household chaos were not significant. The main effects of parental knowledge and behavioral control on insula activation were not significant at either Time 1 or Time 2.
Table 2.
Cross-Sectional and Longitudinal Analyses for Effect of Parental Knowledge and Behavioral Control on Insula Activation Moderated by Household Chaos
| Time 1 |
Time 2 |
Change from Time 1 to Time 2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | b* | p | b | SE | b* | p | b | SE | b* | p | |
| Step 1: Main effects | ||||||||||||
| Parental behavioral control | −0.02 | 0.12 | −.01 | .88 | 0.25 | 0.15 | .19 | .09 | 0.30 | 0.16 | .20 | .07 |
| Parental knowledge | 0.13 | 0.17 | .07 | .43 | −0.05 | 0.19 | −.03 | .80 | 0.03 | 0.23 | .01 | .91 |
| Household chaos | 0.24 | 0.12 | .17 | .05 | −0.09 | 0.13 | −.06 | .50 | −0.10 | 0.15 | −.06 | .51 |
| Pubertal status | −0.27 | 0.15 | −.15 | .07 | −0.09 | 0.18 | −.05 | .60 | 0.00 | 0.19 | .00 | 1.00 |
| Step 2: Interaction effects | ||||||||||||
| Parental behavioral control*household chaos | −0.33 | 0.19 | −.15 | .09 | 0.06 | 0.18 | .03 | .75 | −0.21 | 0.25 | −.08 | .41 |
| Parental knowledge*household chaos | −0.52 | 0.26 | −.17 | .04 | −0.51 | 0.25 | −.17 | .04 | −0.34 | 0.36 | −.10 | .34 |
Note. Significant results at p < .05 are presented in bold face. For the longitudinal analyses, we used residualized changes scores for parental behavioral control, parental knowledge, and insula activation and averaged scores between Time 1 and Time 2 for pubertal status and household chaos across . For parental behavioral control, parental knowledge, and insula activation, we calculated residualized change scores, because we were interested in how changes in parental monitoring related to changes in insula activation across time.
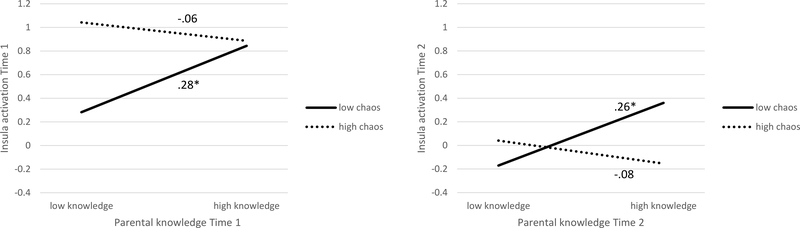
To probe the significant interaction effects between parental knowledge and household chaos, we conducted simple slope analyses. Here, we focused on the effect of parental knowledge that varies depending on the level of household chaos by excluding parental behavioral control given its high correlation with knowledge (particularly at Time 2, r = .60). As depicted in Figure 2, higher parental knowledge was related to greater insula activation in low-chaos households (b = 0.54, SE = 0.26, p = .04 for Time 1; b = 0.49, SE = 0.23, p = .03 for Time 2), but not in high-chaos households (b = −0.15, SE = 0.21, p = .47 for Time 1; b = −0.18, SE = 0.22, p = .43 for Time 2). At both time-points, higher levels of parental knowledge were associated with higher levels of bilateral insula activation during the decision phase of the lottery choice task only in low-chaos households. In contrast, parental knowledge did not predict higher insula activation for adolescents living in high-chaotic households.
Figure 2.
Cross-sectional association between parental knowledge and insula activation for low and high household chaos for Time 1 and Time 2. Note: Low parental knowledge and low chaos are defined as one SD below the mean, high parental knowledge and high chaos are defined as one SD above the mean. Standardized estimates are presented. *p < .05.
In the longitudinal analyses, we tested whether changes in parental monitoring were related to changes in insula activation and whether these effects were moderated by household chaos. As can be seen in Table 2, neither main effects of parental control and knowledge nor their interaction effects with household chaos were significant. Additionally, pubertal status did not significantly predict insula activation at Time 1, Time 2, and change from Time 1 to Time 2.
In supplemental analyses, we performed moderation analyses with behavioral risk taking in the economic lottery choice task (i.e., percentage of risky choices) separately for Time 1, Time 2, and longitudinal change. Results indicated no significant main effects of parental knowledge, parental behavioral control, and household chaos on risky choices (all ps > .10). There was one interaction effect between parental knowledge and household chaos in the longitudinal model that approached significance (B = −0.10, SE = 0.05, b* = −.16, p = .05). Simple slope analysis of this interaction effect, however, showed that the regression paths of changes in knowledge on changes in risky choices at different levels of household chaos were not significant (B = 0.06, SE = 0.05, b* = .17, p = .19 for low chaos and B = −0.06, SE = 0.04, b* = −.16, p = .13 for high chaos). Therefore, we did not interpret this interaction effect further. The other interaction effects between household chaos and either parental knowledge or behavioral control were not significant (all ps > .44). Thus, the beneficial effects of parental monitoring on insular risk processing were only evident at the neural, but not at the behavioral level.
Discussion
The present study investigated the relations among family environmental factors and the development of risk-related processing in the insular cortex during adolescence. In particular, we examined whether the relation of either parental knowledge or parental behavioral control and adolescent insular risk-related processing was moderated by household chaos. Our findings demonstrate that higher levels of parental knowledge were concurrently related to higher levels of insular cortex activation in low-chaos environments, but not for adolescents in high-chaos environments. Although there were no significant longitudinal associations between change in parental knowledge and change in insula activation, our cross-sectional results were consistent across time, suggesting that the statistical moderating effects of household chaos on the link between parental knowledge and adolescent risk taking were robust. Broadly, these results suggest that parental knowledge as perceived by adolescents plays an important role in insular risk-related processing in the adolescent brain, and that the beneficial effects of parental knowledge can be diminished by chaos within home environments.
Understanding how environmental contexts are related to neurobiological processes that guide risky decision-making is key to identifying conditions under which adolescent brain development may become impoverished, and ultimately lead to suboptimal behavior. Using a neuroeconomic approach to objectively measure risk, our study shows that environmental variables impact a key region implicated in risk processing, the insular cortex. Consistent with prior literature (Mohr et al., 2010; van Duijvenvoorde et al., 2015), adolescents at both time points displayed greater bilateral insular cortex activation for increased levels of risk in a lottery choice task, and their elevated insula activation was related to less risky choices. The insular cortex receives inputs from the somatosensory cortex and is hypothesized to act as a hub in which preliminary sensory and affective signals are integrated and projected to brain areas that are action-oriented (e.g., dorsolateral and ventromedial prefrontal cortices; for review, see Smith, Steinberg, & Chein, 2014). Combined with previous literature on risky decision-making, our results suggest that the insular cortex functions as a signal leading adolescents to evaluate potentially risky situations with caution (Mohr et al., 2010).
Parenting practices are key to the socialization of self-control and ability to regulate cognitions, emotions, and behavior, and predictive of health-related outcomes (Gottfredson & Hirschi, 1990). When socialization does not lead to adequate self-control, adolescents may develop aberrant preferences for risky choices. In particular, past research suggests that higher levels of parental monitoring are related to low levels of risk-taking behaviors such as substance use among adolescents (Crouter et al., 2005; Farley & Kim-Spoon, 2017). In the present study, parental knowledge, not behavioral control, was related to insular risk processing. Parental knowledge has been operationalized as an indicator of a well-functioning parent-adolescent relationship with an “effective autonomy balance, as parents grant adolescents autonomy and adolescents respond by keeping parents informed” (Lansford, Laird, Pettit, Bates, & Dodge, 2014, p. 1877). Aligned with this reasoning, prior research identifies parental knowledge (i.e., more child-driven aspects of monitoring) as important for the reduction of adolescent problem behaviors, whereas parental behavioral control (i.e., more parent-driven aspects of monitoring) less consistently relates to adolescent problem behaviors (Racz & McMahon, 2011 for review).
Our data suggest that risk processing in the insular cortex may be a potential mechanism that explains why adolescents with higher parental knowledge are less likely to engage in risky behavior. High parental knowledge about adolescents’ behaviors outside the home often involves parent-child interactions where parents draw the adolescent’s attention to potentially risky outcomes. These interactions may lead adolescents to consider the likelihood of risky outcomes as encoded by the insular cortex. In fact, adolescents who engage in health risk behaviors exhibit diminished hemodynamic activity in the insular cortex during decision-making (Crowley et al., 2015; Kim-Spoon et al., 2016). Using longitudinal data from a relatively large sample, we demonstrated here that parenting monitoring, which is known to promote adolescent selfregulation, is associated with adolescent risk-processing in insular cortex, but such beneficial effects of parental monitoring seem to be compromised in highly chaotic home environments.
Based on the bioecological theory of human development (Bronfenbrenner, 1999; Bronfenbrenner & Evans, 2000), we proposed that the contribution of positive proximal processes, such as parental monitoring, to brain development may be disrupted in chaotic contexts. Our cross-sectional results showed that adolescents living in low chaos environments whose parents are highly knowledgeable regarding their daily behaviors exhibit a greater risk processing signal in the insular cortex. Furthermore, our results indicated that the protective benefits of parental monitoring, namely parental knowledge, on insular risk processing may be suppressed for adolescents living in unstructured environments. The significant moderation effects of household chaos are consistent with previous studies on negative parenting practices, which suggest that household chaos may modify the association between harsh parenting behaviors and child adjustment problems such that harsh parenting behaviors show moderate to substantial correlation with children’s adjustment in high chaos homes, but these correlations are negligible in low chaos homes (Asbury et al., 2003; Coldwell et al., 2006). Our findings further dovetail with recent research demonstrating that higher parental behavioral control predicted better neural cognitive control for adolescents living in low-chaos households but not for their counterparts living in high-chaos households (Kim-Spoon et al., 2017). Taken together, these findings support the bioecological theory of human development by illustrating that the effects of positive parenting qualities, such as parental monitoring, may be compromised within a highly chaotic home environment with respect to the development of neural processes germane to adolescent risk taking.
The interaction finding between parental knowledge and household chaos was robust as repeatedly shown by cross-sectional analysis at Time 1 and at Time 2, however, no such interaction effects were found for the longitudinal association between change in parental knowledge and change in adolescent neural risk processing. Thoughtful consideration for the sampling of measurement occasions (the number of measurement occasions and the timing or spacing of measurement occasions) is key to gaining the most information from longitudinal studies (Kim-Spoon & Grimm, 2016). The non-significant main and interaction effects in longitudinal models may in part reflect that our measurement instruments were not sufficiently sensitive to change over the annual data collection period so that they were unable to track the growth and changes taking place within the individual. It is also important to note that the moderating effect of household chaos was only observed for insular risk-related processing, but not for the behavioral measure of risky decision-making. Despite the phenotypic correlation between insular risk processing and percentage of risky choices in our task, this discrepancy highlights that our findings may be specific to the neural computations involved with evaluating risk information during decision-making rather than risky choice behavior. Another explanation is that laboratory tasks may be limited in capturing real-world behavioral responses, but are able to more accurately represent individual differences in neurobiological processes (Richards, Plate, & Ernst, 2013). Lastly, while the current study focused on the insular cortex, which was grounded in theoretical and empirical work, future research should investigate how environmental variables relate to other brain regions involved in risk-related decision making processes (e.g., anterior cingulate cortex) as well as connectivity within and across different brain networks. These future investigations may provide critical insight into the neural mechanisms through which typical and atypical functioning occurs.
The findings of the present study encourage future longitudinal investigation over multiple time points to evaluate the following: (i) whether the plausible beneficial effects of parental monitoring change throughout the adolescence; and (ii) whether the interaction finding in the present study reflects cross-sectional snap-shots of positive functioning shown in those families with low risk-taking adolescents, highly involved parents, and a well-organized home environment. With data covering a longer period of time, future work can examine the developmental trajectory of how parental monitoring and household chaos affects insular risk-related processing and how these relations change throughout adolescence. In the current study, parental monitoring and household chaos were measured based solely upon adolescents’ self-reports. Thus, our findings are specific to adolescents’ perceptions of parental monitoring and household chaos, which may not accurately represent actual parental monitoring strategies or actual home environment, respectively. However, there is evidence that mothers’, fathers’, and adolescents’ reports showed consistent patterns for the associations between parental knowledge and behavioral control with adolescent antisocial behaviors (Wertz et al., 2016). Future studies may benefit from collecting data from multiple informants (e.g., parents, peers, and teachers) and with multiple methods (e.g., observation, interview, and house visits) to reduce possible method variance due to single informant or mono-method bias. These efforts could provide a more comprehensive representation of parenting strategies and household chaos that may not be fully captured by adolescents’ self reports.
In conclusion, utilizing a multilevel approach consistent with Bronfenbrenner’s bioecological theory of human development (Bronfenbrenner, 1999), we demonstrated the significant roles that parental monitoring and household chaos play in explaining individual differences in adolescent risky decision-making. The results of this study elucidate processes through which family environments may shape neurodevelopment during adolescence, which has implications for explaining why a surge in risk taking occurs among some teenagers. The ability to develop sensitivity to potentially harmful stimuli (i.e., risky options) is a potential mechanism at the core of risky decision-making that may lead to more serious health risk behaviors (Ernst, Pine, & Hardin, 2006). The positive association of parental knowledge with neural correlates of risk processing among adolescents living in low chaos environments implies that prevention and intervention efforts to reduce adolescent risky behaviors may benefit from encouraging positive parenting practices and creating environments with limited chaos.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institute of Drug Abuse (DA036017 to Jungmeen Kim-Spoon and Brooks King-Casas and DA042594 to Nina Lauharatanahirun). We thank Alexis Brieant, Jacob Elder, Katherine Faris, Julee Farley, Toria Herd, Anna Hochgraf, Kristin Peviani, and Jeannette Walters for help with data collection. We are grateful to adolescents and parents who participated in our study.
Footnotes
Conflict of Interest: All authors have no conflicts of interest to report.
References
- Asbury K, Dunn JF, Pike A, & Plomin R (2003). Nonshared environmental influences on individual differences in early behavioral development: a monozygotic twin differences study. Child Development, 74, 933–943. doi: 10.1111/1467-8624.00577 [DOI] [PubMed] [Google Scholar]
- Bollen KA (1989). Structural equations with latent variables. Oxford, England: John Wiley & Sons. [Google Scholar]
- Bronfenbrenner U (1999). Environments in developmental perspective: Theoretical and operational models In Friedman SL & Wachs TD (Eds.), Measuring environment across the life span: Emerging methods and concepts (pp. 3–28). Washington, DC: American Psychological Association. [Google Scholar]
- Bronfenbrenner U, & Evans GW (2000). Developmental Science in the 21st Century: Emerging Questions, Theoretical Models, Research Designs and Empirical Findings. Social Development, 9, 115–125. doi: 10.1111/1467-9507.00114 [DOI] [Google Scholar]
- Casey BJ, Getz S, & Galvan A (2008). The adolescent brain. Developmental Review: DR, 28, 62–77. doi: 10.1016/j.dr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MRF, Sahakian BJ, & Robbins TW (2008). Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain: A Journal of Neurology, 131, 1311–1322. doi: 10.1093/brain/awn066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldwell J, Pike A, & Dunn J (2006). Household chaos--links with parenting and child behaviour. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47, 1116–1122. doi: 10.1111/j.1469-7610.2006.01655.x [DOI] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews. Neuroscience, 13, 636–650. Doi: 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Crouter AC, Bumpus MF, Davis KD, & McHale SM (2005). How do parents learn about adolescents’ experiences? Implications for parental knowledge and adolescent risky behavior. Child Development, 76, 869–882. doi: 10.1111/j.1467-8624.2005.00883.x [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Young SE, Sakai JT, Raymond KM, … Banich MT (2015). Adolescents’ Neural Processing of Risky Decisions: Effects of Sex and Behavioral Disinhibition. PloS One, 10, e0132322.doi: 10.1371/journal.pone.0132322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Acremont M, & Bossaerts P (2008). Neurobiological studies of risk assessment: a comparison of expected utility and mean-variance approaches. Cognitive, Affective & Behavioral Neuroscience, 8, 363–374. doi: 10.3758/CABN.8.4.363 [DOI] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural equation modeling, 8, 430–457. doi: 10.1207/S15328007SEM0803_5 [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, & Hardin M (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36, 299–312. doi: 10.1017/S0033291705005891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley JP, & Kim-Spoon J (2017). Parenting and Adolescent Self-Regulation Mediate between Family Socioeconomic Status and Adolescent Adjustment. The Journal of Early Adolescence, 37, 502–524. doi: 10.1177/0272431615611253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson MR, & Hirschi T (1990). A general theory of crime. Stanford University Press. [Google Scholar]
- Holt CA, & Laury S (2002). Risk Aversion and Incentive Effects. doi: 10.2139/ssrn.893797 [DOI] [Google Scholar]
- Kahn RE, Deater-Deckard K, King-Casas B, & Kim-Spoon J (2016). Intergenerational similarity in callous-unemotional traits: Contributions of hostile parenting and household chaos during adolescence. Psychiatry Research, 246, 815–820. doi: 10.1016/j.psychres.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Hawkins J, …, Zaza S (2016). Youth risk behavior surveillance—United States, 2015. Morbidity and Mortality Weekly Report. Surveillance Summaries, 65, 1–174. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Lauhartanahirun N, Farley JP, Chiu PH, Bickel WK, & King-Casas B (2016). Neural interaction between risk sensitivity and cognitive control predicting health risk behaviors among late adolescents. Journal of Research on Adolescence. doi: 10.1111/jora.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, & Grimm KJ (2016). Latent Growth Modeling and Developmental Psychopathology In Developmental Psychopathology. John Wiley & Sons, Inc. doi: 10.1002/9781119125556.devpsy122 [DOI] [Google Scholar]
- Kim-Spoon J, Maciejewski D, Lee J, Deater-Deckard K, & King-Casas (2017). Longitudinal associations among family environment, neural cognitive control, and social competence among adolescents. Developmental Cognitive Neuroscience, 26, 69–76. doi: 10.1016/j.dcn.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, & Knutson B (2005). The neural basis of financial risk taking. Neuron, 47, 763–770. doi: 10.1016/j.neuron.2005.08.008 [DOI] [PubMed] [Google Scholar]
- Laird RD, Pettit GS, Bates JE, & Dodge KA (2003). Parents’ monitoring-relevant knowledge and adolescents’ delinquent behavior: evidence of correlated developmental changes and reciprocal influences. Child Development, 74, 752–768. doi: 10.1111/1467-8624.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford JE, Laird RD, Pettit GS, Bates JE, & Dodge KA (2014). Mothers’ and fathers’ autonomy-relevant parenting: Longitudinal links with adolescents’ externalizing and internalizing behavior. Journal of youth and adolescence, 43, 1877–1889. doi: 10.1007/s10964-013-0079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TD, Card NA, Preacher KJ, & McConnell E (2009). Modeling longitudinal data from research on adolescence In Lerner RM & S. L. (Eds.), Handbook of adolescent psychology (3rd ed., pp. 15–54). New York, NJ: Wiley. doi: 10.1002/9780470479193.adlpsy001003 [DOI] [Google Scholar]
- MacKinnon DP, Kisbu-Sakarya Y, & Gottschall AC (2013). Developments in Mediation Analysis. The Oxford Handbook of Quantitative Methods in Psychology: Vol. 2: Statistical Analysis, 2, 338. doi: 10.1093/oxfordhb/9780199934898.013.0016 [DOI] [Google Scholar]
- Matheny AP, Wachs TD, Ludwig JL, & Phillips K (1995). Bringing order out of chaos: Psychometric characteristics of the confusion, hubbub, and order scale. Journal of Applied Developmental Psychology, 16, 429–444. doi: 10.1016/0193-3973(95)90028-4 [DOI] [Google Scholar]
- Mohr PNC, Biele G, & Heekeren HR (2010). Neural processing of risk. The Journal of Neuroscience, 30, 6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, Poustka L, … Consortium, t. I. (2012). Determinants of early alcohol use in healthy adolescents: The differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology, 37, 986–995. doi: 10.1038/npp.2011.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17, 117–133. doi: 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Qu Y, Galvan A, Fuligni AJ, Lieberman MD, & Telzer EH (2015). Longitudinal Changes in Prefrontal Cortex Activation Underlie Declines in Adolescent Risk Taking. The Journal of Neuroscience, 35, 11308–11314. doi: 10.1523/JNEUROSCI.1553-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz SJ, & McMahon RJ (2011). The relationship between parental knowledge and monitoring and child and adolescent conduct problems: A 10-year update. Clinical child and family psychology review, 14, 377–398. doi: 10.1007/s10567-011-0099-y [DOI] [PubMed] [Google Scholar]
- Richards JM, Plate RC, & Ernst M (2013). A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neuroscience & Biobehavioral Reviews, 37, 976–991. doi: 10.1016/j.neubiorev.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, & Luciana M (2015). Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage, 122, 427–439. doi: 10.1016/j.neuroimage.2015.07.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetana JG (2008). “It’s 10 o’clock: Do you know where your children are?” Recent advances in understanding parental monitoring and adolescents’ information management. Child Development Perspectives, 2, 19–25. doi: 10.1111/j.1750-8606.2008.00036.x [DOI] [Google Scholar]
- Smith AR, Steinberg L, & Chein J (2014). The role of the anterior insula in adolescent decision making. Developmental Neuroscience, 36, 196–209. doi: 10.1159/000358918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stattin H, & Kerr M (2000). Parental monitoring: a reinterpretation. Child Development, 71, 1072–1085.doi: 10.1111/1467-8624.00210 [DOI] [PubMed] [Google Scholar]
- Steinberg L (2008). A Social Neuroscience Perspective on Adolescent Risk-Taking. Developmental Review: DR, 28, 78–106. doi: 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stride CB, Gardner S, Catley N, & Thomas F (2015). Mplus code for the mediation, moderation, and moderated mediation model templates from Andrew Hayes’ PROCESS analysis examples. Retrieved from http://www.offbeat.group.shef.ac.uk/FIO/mplusmedmod.htm.
- Telzer EH, Fuligni AJ, Lieberman MD, & Galván A (2013). Meaningful family relationships: Neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience, 25, 374–387. doi: 10.1162/jocn_a_00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Huizenga HM, Somerville LH, Delgado MR, Powers A, Weeda WD, … Figner B (2015). Neural correlates of expected risks and returns in risky choice across development. The Journal of Neuroscience, 35, 1549–1560. doi: 10.1523/JNEUROSCI.1924-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs TD, & Evans GW (2010). Chaos in context In Evans GW, & Wachs TD (Eds.), Chaos and its influence on children’s development: An ecological perspective, (pp. 3–13). Washington, DC: American Psychological Association. [Google Scholar]
- Weber EU, Shafir S, & Blais A-R (2004). Predicting risk sensitivity in humans and lower animals: risk as variance or coefficient of variation. Psychological Review, 111, 430–445.doi: 10.1037/0033-295X.111.2.430 [DOI] [PubMed] [Google Scholar]
- Wertz J, Nottingham K, Agnew-Blais J, Matthews T, Pariante CM, Moffitt TE, & Arseneault L (2016). Parental monitoring and knowledge: Testing bidirectional associations with youths’ antisocial behavior. Development and Psychopathology, 28, 623–638. doi: 10.1017/S0954579416000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.