MMP-10 cleaves myo-fibroblast PD-L1 to enhance Th1/Th17 responses in CD
Keywords: Crohn’s disease, matrix metalloproteinases, membrane-bound and soluble PD-L1, myo-/fibroblasts
Abstract
Increased T helper (Th)1/Th17 immune responses are a hallmark of Crohn’s disease (CD) immunopathogenesis. CD90+ (myo-)fibroblasts (MFs) are abundant cells in the normal (N) intestinal mucosa contributing to mucosal tolerance via suppression of Th1 cell activity through cell surface membrane-bound PD-L1 (mPD-L1). CD-MFs have a decreased level of mPD-L1. Consequently, mPD-L1-mediated suppression of Th1 cells by CD-MFs is decreased, yet the mechanism responsible for the reduction in mPDL-1 is unknown. Increased expression of matrix metalloproteinases (MMPs) has been reported in CD. Herein we observed that when compared to N- and ulcerative colitis (UC)-MFs, CD-MFs increase in LPS-inducible levels of MMP-7 and -9 with a significant increase in both basal and inducible MMP-10. A similar pattern of MMP expression was observed in the CD-inflamed mucosa. Treatment of N-MFs with a combination of recombinant human MMP-7, -9 and -10 significantly decreased mPD-L1. In contrast, inhibition of MMP activity with MMP inhibitors or anti-MMP-10 neutralizing antibodies restores mPD-L1 on CD-MFs. CD-MFs demonstrated reduced capacity to suppress Th1 and Th17 responses from activated CD4+ T cells. By contrast, supplementation of the CD-MF:T-cell co-cultures with MMP inhibitors or anti-MMP neutralizing antibodies restored the CD-MF-mediated suppression. Our data suggest that (i) increased MMP-10 expression by CD-MFs and concomitant cleavage of PD-L1 from the surface of CD-MFs are likely to be one of the factors contributing to the decrease of mPD-L1-mediated suppression of Th1/Th17 cells in CD; and (ii) MMPs are likely to have a significant role in the intestinal mucosal immune responses.
Introduction
Crohn’s disease (CD) is one of the major forms of inflammatory bowel disease (IBD) for which currently there is no cure. The hallmark of CD immunopathogenesis is increased T helper (Th)1 (1) and Th17 type (2) immune responses (3). However, a clear delineation of the mechanisms by which these increases in Th1/Th17 cell responses occur in CD is not yet complete.
Innate immune cells are major regulators of the Th1/Th17 cell activity. CD90+ mesenchymal stromal cells, also known as (myo-)fibroblasts (MFs), are an important subset of intestinal mucosal innate immune cells that suppress proliferation of effector T cells via signaling through the two PD-1 ligands, PD-L1 and PD-L2 (4, 5). PD-L1 has been found to have two forms: membrane bound (mPD-L1) and soluble (sPD-L1) (6). We recently demonstrated that MFs in the normal colonic mucosa contribute to the suppression of Th1 cell responses via signaling through mPD-L1 (7). While the role of PD-1 ligands in the regulation of Th17 responses within intestinal mucosa is less well explored, we previously reported mPD-L1 mediated suppression of Th17 cell activity by gastric epithelial cells during chronic Helicobacter infection (8).
The delineation of the role of PD-1 ligands in IBD is far from complete. A decrease in the mPD-L1 was recently observed by us and others in CD intestinal lamina propria (9, 10). Robertson et al. showed that professional antigen-presenting cells in CD intestinal lymphoid patches failed to up-regulate PD-L1 in response to the uptake of nanomineral-antigen-peptidoglycan (10). Further, we observed a decrease in the expression of mPD-L1 by CD-derived colonic MFs when compared to normal (N)- and ulcerative colitis (UC)-derived MFs (9) and that CD-MFs with lower mPD-L1 levels had a reduced capacity to suppress Th1 responses.
While the exact mechanism of the dysregulation of the PD-L1 expression in IBD remains unknown, it is well established that regulation of this protein can occur on multiple levels including transcriptional, translational and post-translational (11). Current knowledge about the regulation of PD-L1 mostly comes from tumor immunology, since abnormal increases in this ligand were observed in several solid tumors, contributing to the escape from anti-tumor immunity (6, 11). Proteolytic cleavage by proteases can contribute to the protein post-translational modifications. Among the most studied human proteases are the class of molecules known as matrix metalloproteinases (MMPs). Most recent studies show that in head and neck squamous cell carcinoma MMP-7 and -13 regulate the PD-L1 protein level (12). Similar observations have been made for cancer- and foreskin-derived fibroblasts; on these cells surface PD-L1 was cleaved by MMP-13 (13). Finally, it has been recently published that in melanoma, fibroblasts can facilitate tumor evasion of immunotherapy via MMP-9-mediated cleavage of PD-L1 (14).
There is compelling evidence that the activity of MMP-2, -7 and -9 is increased in CD, specifically in fibro-stenotic disease (15). Over the last decades, several studies have documented the prominent role of the MMPs in modification of extracellular matrix (ECM) composition during CD development. Intestinal MFs are among the major producers of MMPs (16, 17). However, the immune functions of MMPs in IBD have not been well studied despite their previously reported critical role in the release of the IBD key cytokine, TNF-α (18). It is also unclear how the increase in the MMP activity impacts the immunosuppressive/tolerogenic function in CD.
Therefore, the objective of this study was to evaluate if increased MMP proteolytic activity within CD colonic mucosa cleaves PD-1 ligands from the surface of MFs resulting in a decrease of MF-mediated suppression of Th1/Th17 type responses. Herein, we report that decreased expression of mPD-L1 in CD-MFs is a result of increased MMP-7, -9 and, in particular, MMP-10 secretion and its auto-proteolytic activity on these cells. Differential expression of MMPs was observed in CD-inflamed mucosa when compared to non-inflamed adjacent tissue. This reduction of mPD-L1 level on CD-MFs results in impairment of their ability to suppress Th1 and Th17 type responses and, thus, is likely contributing to heightened Th1 and Th17 responses during the immunopathogenesis of CD.
Methods
Antibodies, recombinant protein and chemicals
Fluorochrome-conjugated forms of murine IgG1κ, isotype control and monoclonal antibodies (mAbs) directed against human CD90 (clone 5E10) were purchased from BD PharMingen (San Diego, CA, USA). Fluorochrome-conjugated mAbs against human PD-L1 (clone MIH1), PD-L2 (clone MIH18), human Fc receptor blocker and functional grade mouse IgG1κ isotype control were purchased from Thermo Fisher-eBioscience (San Diego, CA, USA). Anti-human PE-conjugated MMP-7 (clone 111433) and FITC-conjugated MMP-9 (clone 56129) mAbs were purchased from R&D Systems (Minneapolis, MN, USA). Anti-human EpCAM mAbs, clone 1B7, were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Functional grade anti-human MMP-10 mAbs, clone LA-12 and clone 117239 were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA) and R&D Systems, respectively. Lipopolysaccharide (LPS) was purchased from InvivoGen (San Diego, CA, USA) and used at a final concentration of 1 μg ml−1. Recombinant human MMP-1, -7, -9 and MMP-10 were obtained from R&D Systems. MMP inhibitor II (CAS 203915-59-7) was purchased from and used at a final concentration of 30 nM.
MF isolation from human tissue
Full-thickness fresh human mucosal samples were obtained from biopsies or discarded surgical resection material of colon and ileum in compliance with protocols approved by the University of Texas Medical Branch, University of New Mexico and PennState Health Milton S. Hershey Medical Center Institutional Review Board. Vulnerable populations (e.g. age <18) were not included in study. MFs were isolated according to the protocol of Mahida et al. (19, 20), which is routinely used in our laboratory (21). The purity of isolated CD90+ MFs (98–99%) was confirmed by flow cytometry, as previously described (21). Studies were performed with primary MFs isolated from normal, CD and UC mucosa at passages 3–9. Cells were cultured at 37°C in a 5% CO2 atmosphere in minimum essential medium supplemented with non-essential amino acids, 2 mM l-glutamine, 1 mM sodium pyruvate, 100 U ml−1 penicillin/streptomycin (Cellgro) and 10% heat-inactivated fetal bovine serum (Sigma-Aldrich).
MMP activation and inhibition
Recombinant human MMP-1, -7, -9 and MMP-10 (R&D Systems) stock preparation was performed per manufacturer’s instructions in 50 mM Tris,10 mM CaCl2, 150 mM NaCl, 0.05% Brij-35 (w/v), pH 8.0 (TCNB) and filtered through a 0.2-μM filter. MMPs, at a concentration of 100 μg ml−1, were activated in TCNB containing 1 mM 4-aminophenylmercuric acetate at 37°C for 2 h. Activated MMPs were added to Hanks’ buffer containing Ca++/Mg++ to a final concentration of 200 ng ml−1 and this MMP solution was added to the fully confluent monolayer of MFs for 3 h at 37°C. In some experiments MMP inhibitor II (EMD Millipore) or anti-human MMP-10 mAbs were used to inhibit MMP activity on CD-derived MFs (CD-MFs) at final concentrations of 30 nM and 1 μg ml−1, respectively. The placebo or murine IgG1k isotype control, respectively, was included in these experiments.
Co-stimulation of T-cell responses
Assays were performed as previously described using allogeneic MF:T-cell co-cultures (7). Briefly, human CD4+ CD45RA+ T cells were purified from peripheral blood mononuclear cells of healthy donors by negative selection using a CD4+ T Cell Isolation magnetic bead Kit II (Miltenyi Biotec, Auburn, CA, USA). The T cells were pre-activated with anti-human CD3 and CD28 microbeads (Thermo Fisher Scientific, per manufacturer’s instructions) for 1 h prior to co-culture initiation. Then, CD4+ T cells were plated in 24-well plates in the presence or absence of MFs at a ratio of 2.5:1. In some experiments, MFs were pre-treated with MMP inhibitors or anti-MMP-10 mAbs for 24 h as described above and then used in the co-cultures with pre-activated T cells. T cells were recovered from the co-cultures on day 5 and used for the analysis of the Th1/Th17 gene expression. Conditioned media from mono- and co-cultures of T cells and MFs were collected on the day 5 and analyzed for cytokine production using a multiplex cytokine array (EMD Millipore) per the manufacturer’s instructions.
Real-time RT–PCR and RNA microarray
Analysis was performed according to the two-step RT real-time PCR protocol. Briefly, cDNA preparation was prepared using iScript™ cDNA synthesis kit (Bio-Rad) according to the manufacturer’s instruction. The appropriate assays-on-demand™ gene expression FAM™ labeled primer/probe mix was purchased from Thermo Fisher Scientific. Human β-actin gene was used as a housekeeping gene. FastStart Universal Probe Master mix (Roche Diagnostic USA, Indianapolis, IN, USA) was used to prepare a PCR mix according to the manufacturer’s instruction. The reactions were carried out in a 20-μl final volume using a Bio-Rad Q5 real-time PCR machine according to the following PCR protocol: 2 min at 50°C, 10 min at 95°C (1 cycle) and 15 s at 95°C and 1 min at 60°C (40 cycles).
Prime PCR Extracellular Matrix and Adhesion Molecules miniMicroarray was purchased from Bio-Rad and was used to analyze expression of the MMPs. Experiments were performed according to the manufacturer’s protocol. Gene expression clustering was done using the software Gene Cluster 3.0 and Java TreeView according to the software guidelines.
MMPs, cytokine and PD-L1 secretion analysis
Cytokine, MMP and PD-L1 secretion was measured using Multiplex Th1/Th2 cytokine, MMPs multiplex array kits (Bio-Rad, R&D Systems, Millipore) and PD-L1 Human ProcartaPlex™ Simplex Kit (Invitrogen) according to the manufacturers’ instructions. For the analysis of the production of these molecules in tissue, surgical resections from matched CD involved and uninvolved areas of intestinal mucosa were divided into 8 mg pieces and incubated for 16 h in complete Roswell Park Memorial Institute 1640 Medium media. Supernatants were collected and analyzed using multi-/singleplex arrays as described above.
Immunofluorescence and confocal microscopy
Frozen human colonic step sections were fixed and immunostained as described previously (4). Samples were then mounted in SlowFade® Gold antifade reagent with DAPI (Thermo Fisher Scientific, Waltham, MA, USA). Immunofluorescent microscopy was performed using an Echo Hybrid microscope Revolve4 Inverted (VWR). Confocal microscopy was performed with a Zeiss LSM880 Inverted laser scanning confocal/widefield microscope (Carl Zeiss, Thornwood, NY, USA) (4).
Flow cytometry
Following treatment of fully confluent monolayers, MFs were detached from the culture flasks by treatment with non-enzymatic cell dissociation buffer (Sigma) at 37°C for 15–30 min, followed by two washes with cold PBS. Immunostaining for human PD-L1 and PD-L2 or isotype controls was performed according to a standard eBioscience surface immunostaining flow cytometry protocol. Cells were analyzed by flow cytometry using a BD LSRFortressa™ cytometer (BD Biosciences) per the manufacturer’s procedures. Area, height and width parameters for forward and side scatters (FSC and SSC, respectively) were used to discriminate single live cells. An additional gate was set based on the negativity for the fixable viability dye eFluor® 780 (eBioscience), which was added during the surface marker staining to exclude dead cells from the analysis. Flow cytometry data were analyzed using FACSDiva 6.3 (BD Biosciences) software.
Statistical analysis
Unless otherwise indicated, the results were expressed as the mean ± SE of data obtained from at least three independent experiments, each performed in triplicate. Differences between means were evaluated by one-way ANOVA for multiple comparisons and Student’s t-test for the analysis of the significance between two groups. Values of P < 0.05 were considered statistically significant. Associations between MMPs and PD-L1 production were examined using Pearson correlation and linear regression analysis.
Results
CD-MFs express higher basal and inducible level of MMP-7, -9 and -10 when compared to N-MFs
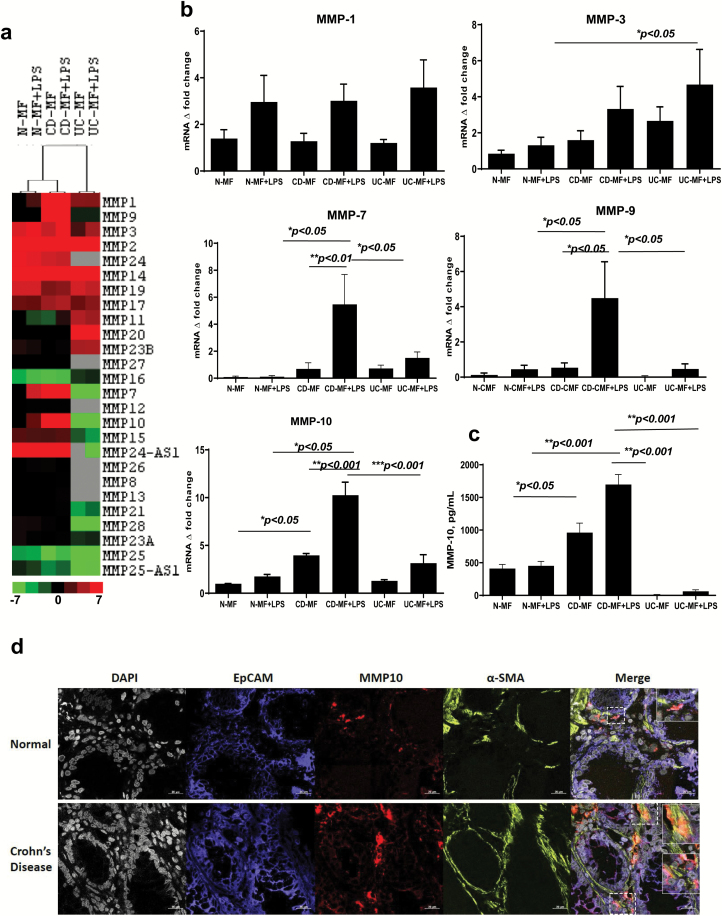
Expression of several MMPs is increased in the intestinal mucosa of CD patients and CD-derived MFs are believed to be among the major contributors to this process although there is uncertainty about the patterns of specific MMP expression (22, 23). Thus, we first analyzed the changes in the basal and inducible MMP expression by N- and CD-MFs using a Prime PCR Extracellular Matrix and Adhesion Molecules miniMicroarray. UC-MFs and N-CMFs were also included in this study. Among the 25 MMPs analyzed, basal levels of MMP-1, -7, -9 and -10 mRNA expression were increased (up to 7.0-fold) in CD-MFs when compared to N-MFs and/or UC-MFs (Fig. 1a, Supplementary Table 1). By contrast, we observed a strong decrease (up to −3-fold) in the expression of MMP-16 and -25 (Fig. 1a, Supplementary Table 1). Because signaling through Toll-like receptor (TLR) 4 is implicated in the immunopathogenesis of CD (24–27), we analyzed the MF-MMP profile upon exposure to the TLR4 agonist LPS. LPS stimulation of CD-MFs resulted in an increased expression of MMP-1, -3, -7, -9 and -10 when compared to the untreated CD-MFs, or LPS-stimulated N- and UC-MFs (Fig. 1a, Supplementary Table 1). Thus, in the next set of experiments we focused on these MMPs, since they were differentially up-regulated in CD-, but not N- or UC-MFs.
Fig. 1.
Expression of basal and LPS-inducible patterns of MMPs differs in CD-MFs when compared to N- and UC-MFs. (a) mRNA microarray analysis shows differential MMP expression in CD-MFs compared to N-MFs and UC-MFs at basal and TLR4-stimulated conditions. Sample-based hierarchical clustering was carried out as described in Methods. The color chart at the bottom of the figure shows levels of fold decrease and increase, log2 fold changes are shown. (b) Basal and LPS-inducible differential expression of MMP-7, -9 and -10 by MFs isolated from normal, CD and UC mucosa was analyzed on mRNA level using real-time PCR. (c) Basal and LPS-inducible secretion of MMP-10 by MFs isolated from CD mucosa is increased when compared to normal, and UC mucosa-derived MFs, multiplex MMP array analysis. LPS was used at concentration 1 μg ml−1, 72-h exposure. Data are shown as means ± SEM, n = 5, *P < 0.05; **P < 0.01; ***P < 0.001. (d) MMP-10 expression is increased within α-SMA+ MFs in CD intestinal mucosa when compared to the healthy controls. Confocal microscopy images of representative cross-sections from CD and normal human intestinal mucosa (n = 4 per group) are shown. MFs were detected by anti-α-SMA mAb (in green), and epithelial cells were identified with anti-EpCAM mAb (in blue). Co-localization of MMP-10 (in red) with α-SMA+ MFs results in formation of yellow-orange color on merged images. High resolution areas in the merged images are outlined in the boxes.
Since increased MMP proteolytic activity has been suggested to regulate PD-L1 expression in CAFs (12, 13), next we proceed to the validation of the microarray data for MMPs that were increased by using real-time RT–PCR. While we observed a trend to an increase in the basal and inducible MMP-1 and MMP-3 mRNA expression in CD-MF, when compared to normal it did not reach significance and did not differ from the pattern observed in UC-MFs (Fig. 1b). However, an increase in expression of an inducible MMP-3 by UC-MF, when compared to N-MFs was observed (Fig. 1b). By contrast, we observed that when compared to N- and UC-MFs, CD-MFs showed a trend toward a higher basal and a significant increase in LPS-inducible levels of MMP-7 and -9 mRNA. (Fig. 1b). By contrast, mRNA expression of basal MMP-10 was significantly increased in CD-MFs when compared to N-MFs and UC-MFs, an effect which was further enhanced following LPS stimulation (Fig. 1b).
CD-MFs produce higher levels of soluble MMP-10 when compared to N-MFs
Several MMPs can be found in two forms: one form associated with the outer cell membrane and the other within the extracellular space in culture medium (28). Therefore, we used a MMP multiplex array to analyze MMP-1, -3, -7, -9 and -10 secretion by MFs. As was previously observed on the mRNA level, CD-MFs showed no significant differential secretion of MMP-1 and -3 (Supplementary Figure 1a) when compared to N-MFs. Among the other tested MMPs only MMP-10 was detected in conditioned media of MFs and it was significantly increased on both the basal and inducible levels in CD-MFs when compared to N-MFs and UC-MFs (Fig. 1c). The last observation suggests that MMP-7 and -9 on CD-MFs are likely to be membrane bound as previously reported in colon cancer cells (29) and polymorphonuclear neutrophils (30). In fact, using flow cytometry analysis we were able to detect both MMP-7 and MMP-9 on the surface of CD-MFs (Supplementary Figure 1b). In situ analysis of the normal and CD-inflamed colonic mucosa also demonstrated an increase in MMP-9, and to a lesser extent MMP-7. CD-MFs were among cells associated with the increase of both MMPs. This was particularly true for MMP-9, while expression of MMP-7 was more prominent within the apical compartment of the epithelium (Supplementary Figure 2). Thus, taken together our data suggest that other mucosal cells in CD contribute to the increase in MMP-7 and -9. In summary, while our data show that there was more variation between different CD-MF isolates in the expression of MMP-7 and -9, we observed a trend that CD-MFs produce higher levels of basal and a significant increase in inducible levels of these MMPs. Importantly, all tested CD-derived MFs consistently expressed and produced statistically significant increased basal and inducible levels of MMP-10 when compared to N-MFs. Another interesting observation was that this increase in MMP-10 was unique to CD; we found no increase in the MMP-10 expression and production by UC-MFs (Fig. 1b and c). Additionally, to confirm that the increase in MMP-10 is not an artifact of the ‘in culture’ experiment we used immunostaining followed by confocal microscopy to analyze expression of the MMP-10 by MFs in intestinal mucosa. In situ analysis demonstrated that MMP-10 expression was strongly increased on MFs in CD intestinal mucosa (formation of orange-yellow color on merged images), when compared to healthy controls (Fig. 1d).
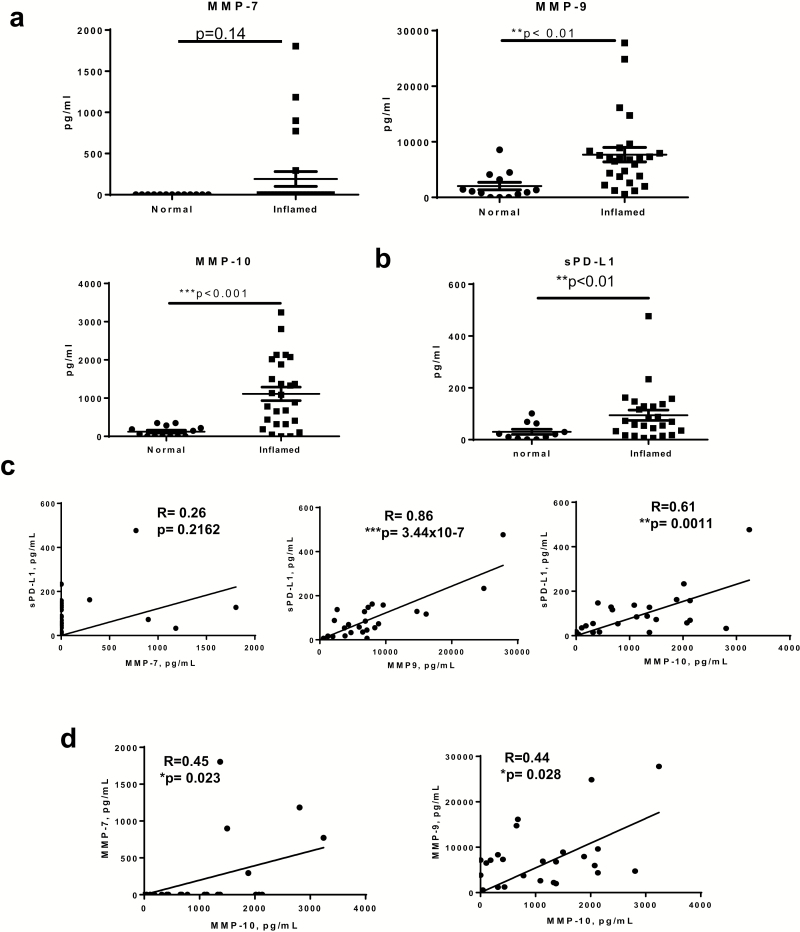
Soluble MMP-9 and -10 are significantly increased within inflamed CD mucosa and correlate with the release of sPD-L1 in CD tissue
Given the significant increase in MMP transcription and/or production of MMP-9, and -10 by MFs, we sought to delineate the production of these proteases in CD mucosa and determine their association with PD-L1. Mucosa of surgical specimens from CD patients were used to compare inflamed versus non-inflamed adjacent tissue. The information on the tissue samples used for the analysis in this study is provided in Table 1. An MMP secretion array kit was used to determine total tissue MMP secretion. In contrast to our findings with primary isolates of MFs, we detected a statistically significant increase in secreted MMP-9, but not MMP-7 secretion in the inflamed CD mucosa (Fig. 2a). These observations are in agreement with our in situ analysis of the MMP-7 and -9 pattern of expression (Supplementary Figure 2) and suggest that other mucosal cells are likely to contribute to the increase in secreted levels of these MMPs within the CD-inflamed mucosa. Consistent with our observations for CD-MFs, a significant increase in secretion of total mucosal MMP-10 was observed in CD (Figs 1d and 2a).
Table 1.
Characteristics of patients with CD
| Characteristics | N a (Percentage) |
|---|---|
| Age (18–40, ≥40) | |
| 18–37 | 10 (40%) |
| 38–57 | 15 (60%) |
| Gender | |
| M | 16 (64%) |
| F | 9 (36%) |
| Location (ileal, colonic, ileocolic, upper intestine) | |
| Duodenum/jejunum | 2 (8%) |
| Ileum/ileostomy | 12 (48%) |
| Right/transverse colon | 3 (12%) |
| Left colon | 2 (8%) |
| Rectum | 4 (16%) |
| Mixed | 2 (8%) |
| Medications | |
| 5-aminosalicylic acid, 5-ASA alone | 1 |
| Budesonide alone | 1 |
| Prednisone alone | 1 |
| Immunomodulators (imm) | 2 |
| Imm + prednisone | 1 |
| Biologics (all anti-TNF except one integrin) | 8 |
| Biologic + 5-ASA | 1 |
| Biologic + budesonide | 1 |
| Biologic + steroids | 2 |
| Biologic + imm + budesonide | 1 |
| Biologic + imm + steroids | 1 |
| Biologic + imm + steroids + 5-ASA | 1 |
| Untreated | 4 |
aAll patients included in this study had active disease, tissue inflammation was scored as moderate/severe.
Fig. 2.
Secreted MMP-9 and -10 are significantly increased within inflamed CD mucosa and associated with an increase in tissue secretion of soluble (s) PD-L1. Mucosa of surgical specimens from CD patients were used to compare inflamed versus non-inflamed normal (normal) adjacent tissue. (a) Multiplexed MMP array assay showed significant increases in the secretion of MMP-9 and -10, but not -7 in the inflamed CD mucosa when compared to adjacent non-inflamed controls. Data are shown as mean ± SEM, n = 11–25 per group. (b) ProcartaPlex™ soluble PD-L1 immunoassay analysis showed increased sPD-L1 in the inflamed CD mucosa when compared to adjacent non-inflamed mucosa. Data are shown as mean ± SEM, n = 11–25 per group. (c) Linear regression and Pearson correlation analysis show that increased sPD-L1 is significantly correlated with secreted MMP-9 and -10 in inflamed CD mucosa, n = 25 per group. (d) Linear regression and Pearson correlation show that increases in MMP-7 and -9 correlate with the increase of MMP-10 in the inflamed CD mucosa, n = 25 per group, **P < 0.01; ***P < 0.001.
We have previously shown (9) that the level of mPD-L1 is decreased in CD mucosa and on CD-MFs. We currently report increased MMP-7, -9 and -10 in CD mucosa. Among those MMPs, MMP-7 and -9 have been implicated in the cleavage of mPD-L1, which may result in an increased level of the sPD-L1 (6, 12, 13). Thus, we analyzed the levels of sPD-L1 in CD colonic mucosa. We observed that the mucosal level of sPD-L1 was increased in inflamed mucosa when compared to non-inflamed adjacent tissue (Fig. 2b). Pearson correlation and linear regression analysis demonstrated that the increases in MMP-9 and -10, but not MMP-7 were significantly associated with the level of sPD-L1 in CD-inflamed mucosa (Fig. 2c). In agreement with these data, we observed that when compared to N-MFs, CD-MFs release higher levels of the basal and LPS-inducible sPD-L1 in culture (Supplementary Figure 3a). It has been previously reported that activation of the MMP-7 and -9 secretion requires cleavage by MMP-10 (31). Our correlation analysis supports this idea in that we demonstrated that levels of MMP-7 and -9 in inflamed CD mucosa were significantly associated with the MMP-10 level, but the r values were relatively modest (Fig. 2d).
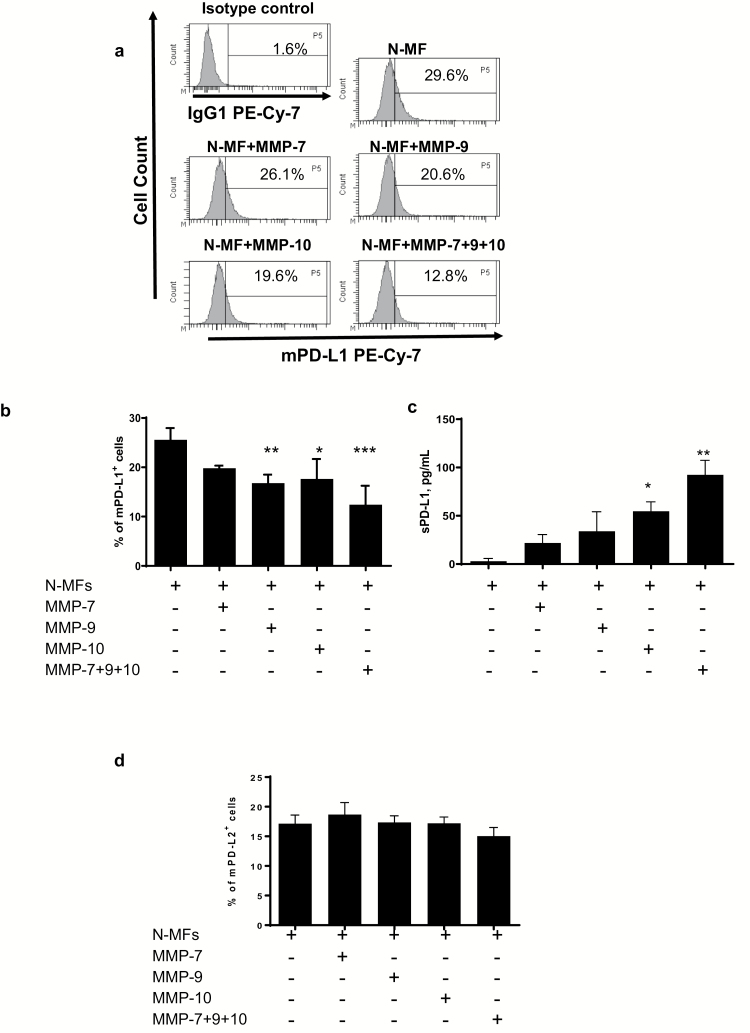
Treatment of N-MFs with recombinant MMP-9 and -10 resulted in the decrease of mPD-L1
Previously we reported that the level of mPD-L1 in tissues and MF is decreased in CD (9). In the data presented above we observed that several tested CD-MFs produce higher levels of MMP-7, -9 and -10, while an increase in sPD-L1 was significantly correlated with MMP-9 and -10 secretion in CD mucosa (Fig. 2). Furthermore, it has also been reported in foreskin fibroblasts that MMPs can cleave mPD-L1 from the surface of these cell (13). Thus, next we analyzed whether MMP up-regulation in CD may affect the cell level of mPD-L1 on N-MFs. Treatment of N-MFs with recombinant human (rh) MMP-9 or -10 significantly reduced mPD-L1 levels, and a combination of MMPs produced an additive effect on cleavage of mPD-L1 from the surface of the N-MFs (Fig. 3a and b). Although not statistically significant, treatment with MMP-7 moderately reduced cell surface levels of PD-L1. Finally, the use of these MMPs in combination showed stronger reduction of the mPD-L1 on the surface of N-MFs. Additionally, in the above experiments we observed that the level of sPD-L1 was increased in the conditioned media of N-MFs treated with the tested rhMMPs (Fig. 3c). This MMP cleavage was specific to PD-L1 as no significant changes were observed in PD-L2 cell surface levels (Fig. 3d).
Fig. 3.
Recombinant human-activated MMP-9 and -10 reduce levels of membrane-bound (m) PD-L1 on N-MFs. Primary human isolates of N-MFs were treated with MMP-7, -9, -10 or combinations of these MMPs for 3 h as described in Methods, then immunostained and used for multi-color flow cytometry. Condition media from these cultures were used to determine sPD-L1 by PD-L1 singleplex assay. Live events were gated based on forward and side scatters, as well as negativity for the incorporation of the viability dye (conjugated to eFluor™ 780) and were analyzed for the surface expression of mPD-L1 and mPD-L2. (a) Representative histograms of mPD-L1 expression on the surface of N-MFs following treatment with activated MMPs are shown. Summary of the (b) mPD-L1 and (c) sPD-L1 expressed/produced by N-MFs following treatment with activated MMPs. Summary of the (d) mPD-L2 protein expression on surface of N-MFs following treatment with activated MMPs. Data are shown as mean ± SEM, n = 5, *P < 0.05; **P < 0.01; ***P < 0.001.
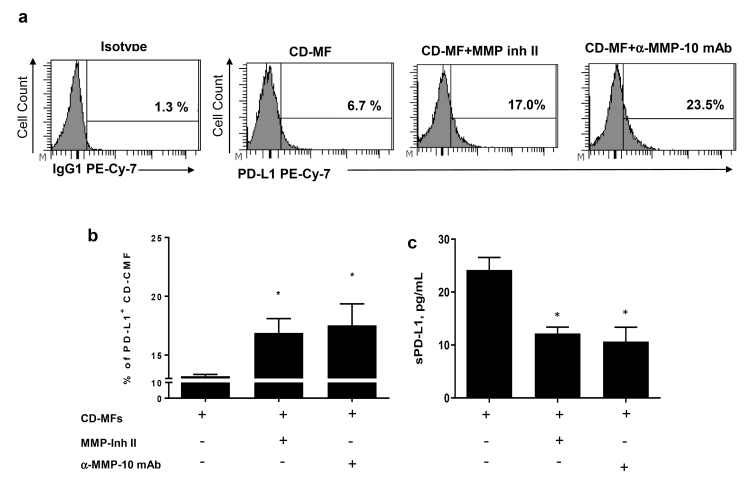
MMP proteolytic activity contributes to the reduced mPD-L1 level on the surface of CD-MFs
We have previously shown that MFs in CD colitis have reduced mPD-L1 when compared to N-MFs (9). In the experiments above we demonstrated that mPD-L1 of N-MFs is susceptible to the cleavage by rhMMPs-9 and -10, and possibly by MMP-7 (Fig. 3). Thus, next we determined if inhibition/neutralization of these MMPs could restore mPD-L1 on cell surface of CD-MFs. Treatment of the CD-MFs with a broad spectrum MMP inhibitor II (CAS 203915-59-7), which is known to inhibit the activity of several MMPs (as per manufacturer’s specification), including MMP-7 and -9, but not MMP-10, resulted in a strong increase of the mPD-L1 on CD-MFs (Fig. 4a and b). Given the increased transcription and secretion of MMP-10 in MFs, and, because there is no currently commercially available small molecule MMP-10 inhibitor, we used anti-MMP-10 mAb to neutralize this MMP in CD-MF cultures. Treatment of CD-MFs with anti-MMP-10 mAb (clone LA-12) restored mPD-L1 on the surface of CD-MFs (Fig. 4a and b). Since recent data indicate that clone LA-12 of anti-MMP-10 mAb may cross-react with MMP-3 (up to 20%), we repeated the experiments described above using clone 117239 of MMP-10 mAb, which is not known to cross-react with other MMPs produced by MFs. A similar pattern of the restoration of the PD-L1 on the surface CD-MFs was observed when clone 117239 was used (Supplementary Figure 3b). Additionally, a decrease in the soluble sPD-L1 was observed in the conditioned media derived from CD-MFs upon neutralization of MMP-10 (Fig. 4c). Taking into consideration that MMP-10 activity is reported to activate pro-MMP-7 and pro-MMP-9 (31), our data suggest that increased expression of MMP-10 in CD-MFs may be among the hey regulators of the MMP-mediated reduction of the mPD-L1 from the cell surface of CD-MFs, and from other cells in the CD-inflamed mucosa.
Fig. 4.
The proteolytic activity of MMPs contributes to the reduction of the mPD-L1 on cell surface of CD-MFs. Primary human isolates of CD-MFs were treated or not with the 30 nM of MMP Inhibitor II (CAS 203915-59-7) or anti-human MMP-10 mAbs (clone LA-12) for 72 h, then immunostained and used for multi-color flow cytometry. Live events were gated based on forward and side scatters, as well negativity for the incorporation of the viability dye (conjugated to eFluor™ 780) and were analyzed for the surface expression of mPD-L1. (a) Representative histograms and (b) summary of the flow cytometry analysis show that expression of mPD-L1 is reduced on cell surface of CD-MFs. (c) Summary of sPD-L1 shows an increase in sPD-L1 in the conditioned media from CD-MFs. Addition of broad spectrum MMP Inhibitor-II, which targets MMP-1, -3, -7, -9 or anti-MMP-10 mAb restores mPD-L1 level on the surface of CD-MFs and decreases sPD-L1 concentration in the condition media derived from these cells. Data are shown as mean ± SEM, n = 4, *P < 0.05.
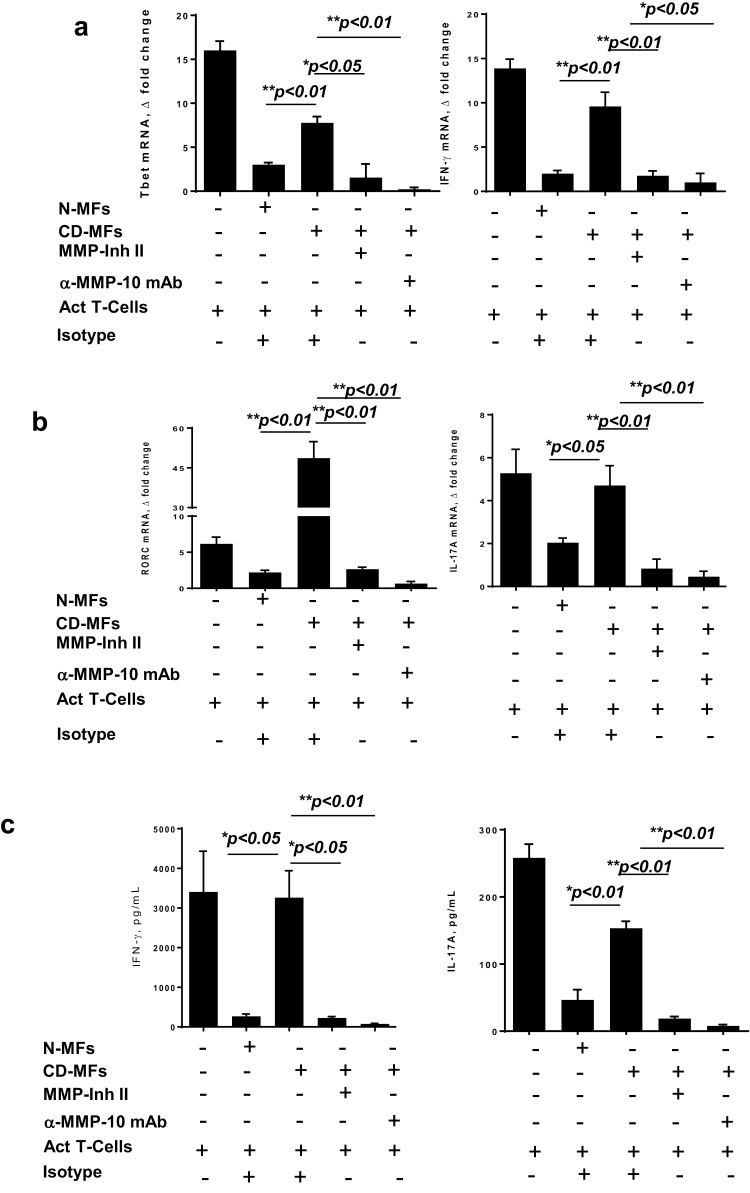
Inhibition of MMP-10 restores colonic MF-mediated suppression of Th1 and Th17 cell responses
Increased Th1 and Th17 cell responses are a hallmark of CD (1, 2). We have previously shown that normal human colonic MFs suppress Th1 cell activity by CD4+ T cells in a PD-L1-dependent manner (4, 7, 9). Up-regulation of PD-L1 was also reported to restrain Th17 cell differentiation (32). To understand how MMP proteolytic activity on PD-L1 is implicated in the impaired T-helper suppressive function, CD-MFs were co-cultured with CD3/CD28 pre-activated CD4+ T cells in the presence of MMP inhibitor II or anti-MMP-10 neutralizing mAb to determine if blocking/neutralization of the MMPs can restore CD-MFs-mediated inhibition of Th1 and Th17 cell activity to the level observed for N-MFs. As shown in Fig. 5(a) and (b), we found that inhibition of T-cell MMP-7 and -9 activity by the MMP inhibitor II significantly increased CD-MF-mediated suppression of Th1 (measured by Th1 transcriptional factor Tbet and key cytokine IFN-γ) and Th17 (measured by Th17 transcriptional factor RORc and key cytokine IL-17A). We confirmed these data by cytokine array showing that levels of secreted IFN-γ, and IL-17A are decreased in the presence of MMP-II inhibitor and these levels were comparable to those observed when pre-activated T cells were co-cultured with N-MFs (Fig. 5c). This effect was even stronger in the presence of the anti-MMP-10 neutralizing mAb (Fig. 5). Importantly, we confirmed that increased MMP-10 secretion is maintained in CD-MFs when co-cultured with activated T cells, and is suppressed with anti-MMP-10 mAb (Supplementary Figure 3c).
Fig. 5.
MMP inhibition restores the capacity of CD-MFs to suppress activated Th1/Th17 cell responses. MFs were co-cultured with CD3/CD28 pre-activated allogeneic CD4+ T cells for 5 days in the presence or absence of the MMP inhibitor II (30 nM) or anti-MMP-10 (1 μg ml−1) blocking mAbs. (a) Tbet and IFN-γ mRNA expression by the T cells was analyzed using real-time RT–PCR analysis. (b) RORC and IL-17A mRNA expression by the T cells was analyzed using real-time RT–PCR analysis. (c) IFN-γ and IL-17A production was analyzed using multiplex cytokine analysis. The means ± SEM are shown, n = 4 allogeneic donor pairs per group, two experimental replicates each, *P < 0.05; **P < 0.01.
Taken together, our data indicate that although inhibition of MMP-7 and -9 only partially restores T-cell suppressive capacity in CD-MFs, inhibition of MMP-10 fully restores mPD-L1 surface levels and results in a restoration of CD-MF-mediated suppression of Th1 and Th17 cell activity. Thus, our data suggest that MMP-mediated reduction (in particularly MMP-10) in mPD-L1 expression by CD-MFs contributes to increases in Th1, and Th17 activity.
Discussion
MMPs in IBD have been shown to regulate epithelial barrier function, angiogenesis, fibrosis and wound healing (33). However, the role of these proteases in the regulation of the immune responses during the immunopathogenesis of IBD has not been completely elucidated. This is despite the fact that an important role of MMPs in the regulation of cytokines and other immune active molecules has been previously demonstrated (33, 34). Herein we reported that increases in MMP-10 within the mucosa and in MFs in particular are likely to contribute to the immunopathogenesis of CD via cleavage of the immune regulatory molecule PD-L1 from the surface of MFs.
Analysis of the pattern of MMP expression in IBD mucosa remains contradictory. MMP-2, -9 and -13 have been reported to be expressed by epithelial, mononuclear and fibroblast-like cells from CD mucosa (35). Increased expression of MMP-2 in epithelium and of MMP-7, but not MMP-9, in the inflammatory infiltration was observed in CD (15). More recently, increased levels of fecal MMP-9 were reported to correlate with the severity of the endoscopic ulceration in CD (15, 36). We demonstrated that expression of MMP-7, -9 and -10 is increased at basal and LPS-inducible levels in CD-MFs, when compared to N-MFs. Importantly, this increase in MMP-7, -9 and -10 was unique to CD and was not observed in UC-MFs. A significant increase in MMP-10 and, to a lesser degree, MMP-9 was also observed by us in CD-inflamed mucosa when compared to non-inflamed adjacent tissue. While our study is among the first to clearly show the specificity of the MMP-10 increase within CD-, and not UC-MFs, earlier work by Makitalo et al. (37) observed an increase in MMP-10 expression in the CD, but not UC lamina propria. Given that MMP-10-mediated proteolytic activity is known as an activator of pro-MMP-7 and -9 (38, 39), our data, taken with already published reports, suggest that increased MMP-10 is likely to be a key protease responsible for the release/increase of functional MMP-7 and MMP-9 in CD intestinal mucosa. Further, our current report suggests that the increase in MMP-10 is critical to the decrease in mPD-L1 on CD-MFs.
While we previously reported the differential expression of PD-L1 mRNA (transcriptional level) between N-, CD- and UC-derived CD90+ stromal cells (MFs), our current study reveals an additional post-translational mechanism by which the PD-L1 level is regulated on MFs in CD. In fact, while the post-translational mechanism of the PD-L1 modification is observed by us for the first time in CD, it is not without precedent: on various tumor cells and tumor-derived stroma PD-L1 was reported to be regulated on multiple levels: genetic, transcriptional and post-transcriptional (40, 41). Taken together with our previous data (9), our current data suggest that both abnormality in the transcription, and the post-translational regulation are responsible for the decrease of the PD-L1 on MFs in CD. It is likely that mechanisms responsible for the abnormal increase in the basal and, in particular, inducible MMP-7, -9 and in particular MMP-10 by CD-MFs are complex, multifactorial and require further investigation. However, it is not without precedent: alteration in the LPS-mediated activation of MMP-1 and -3 within synovial fibroblasts in rheumatoid arthritis was previously reported and was linked to the enhanced expression of the miR-155 (42). An increase in miRNA 205 was reported to be involved in the up-regulation of the MMP-2 and -10 in ovarian cancer (43).
PD-L1 is reported to exist in two forms, membrane bound and soluble, which may offer varying functions. The mPD-L1 has been widely described as a negative co-stimulatory molecule and is important in activated T-cell suppression and peripheral tolerance (4, 44, 45). While the role of sPD-L1 is still debatable, some studies suggest that it has an opposite function from its membrane-bound counterpart, promoting T-cell proliferation and activity (6, 44). Despite being involved in other autoimmune diseases, sPD-L1 has not been described in IBD. Recently, we have shown MFs to be a major cell expressing PD-L1 in normal and UC colonic mucosa (9). By contrast, mPD-L1 was strongly decreased on MFs in CD colonic mucosa (9). Herein, we report that in contrast to mPD-L1, sPD-L1 is increased in inflamed mucosa of CD patients and the increase is associated with an increase in MMP-9 and MMP-10 production. Furthermore, at least in MFs, these MMPs are likely to be the major cause of the decrease in mPD-L1 and increase in sPD-L1. While our finding is novel with regards to the immunopathogenesis of CD, it has been recently suggested that increases in sPD-L1 in breast carcinoma and foreskin fibroblasts may be a result of increased MMP proteolytic cleavage of mPD-L1 (6, 12, 13).
Interestingly, unlike previous studies that identified MMP-7 capable of regulating PD-L1 in head and neck squamous cell carcinoma (12), changes in CD-MFs basal and inducible MMP-7 mRNA expression did not reach statistical significance. It has also been reported that MMP-7 and -9 may remain membrane associated, which may affect their resistance to natural inhibitors (28). The last observations could explain why we were unable to detect secreted MMP-7 and -9 in MFs. Additionally, since secreted MMP-9, and to a lesser extent MMP-7, was detected by us in the CD mucosa, this suggests that other mucosal cells may also contribute to the increase in these MMPs and consecutively cleave mPD-L1 on MFs. In fact, increased MMP-7 within the epithelium and MMP-9 within polymorphonuclear leukocytes and vascular smooth muscle cells in CD mucosa were previously reported (37, 46, 47).
Over the last decade, individual MMPs have been shown to regulate leukocyte influx and migration, antimicrobial activity, macrophage activation, cytokine production and restoration of barrier function, typically by proteolytic cleavage of a range of non-ECM protein substrates (48–50). However, an understanding of how MMPs regulate overall adaptive immunity and T-cell responses in particular is limited. We have previously shown that N-MFs limit activated T-cell proliferation and Th1 type responses (7, 9). In contrast, CD-MFs lose their capacity to suppress Th1 cell activity (9). Herein, we report that in contrast to N-MFs, CD-MFs also promote Th17 cell responses and offer the novel finding that MMP-10, and to a lesser extent, -9 and -7, contributes to the reduced ability of CD-MFs to inhibit activated Th1 and Th17 cell responses.
In conclusion, considering our previously published and current data, we propose that increased expression/secretion of MMP-7, -9 and, most importantly MMP-10 on MFs contributes to the loss of the MF suppressive activity on Th1/Th17 cell responses via cleavage of mPD-L1. Restoring mPD-L1 to CD-MFs improves their suppressive activity to levels shown by N-MFs. Taking into consideration the central role of MMP-10 in the activation of the MMP-7 and -9 (38), our data suggest that MMP-10 inhibition could offer site-specific control of Th1/Th17 cell activity due to the restoration of mPD-L1 on CD-MFs to normal levels. Finally, advancement of medical therapies including anti-TNF-α and other biologics has greatly improved our ability to control intestinal inflammation; however, these interventions are not curative and many patients may become unresponsive to medical therapy. Additionally, MMPs have been shown to enzymatically inactivate antibodies to TNF-α and such antibodies may be important causes of TNF-α treatment failure (51). These observations in conjunction with our findings support the idea that targeting MMP-10 may be a promising avenue for the development of a novel therapeutic approach for CD.
Funding
NIDDK R01DK103150; NCAT TL1TR001440; NCI R01CA207051; Peter and Marshia Carlino fund for Inflammatory Bowel Disease research.
Conflicts of interest statement: the authors declared no conflicts of interest.
Supplementary Material
References
- 1. Fuss, I. J., Neurath, M., Boirivant, M.et al. 1996. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 157:1261. [PubMed] [Google Scholar]
- 2. Strober, W. and Fuss, I. J. 2011. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140:1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heller, F., Florian, P., Bojarski, C.et al. 2005. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129:550. [DOI] [PubMed] [Google Scholar]
- 4. Pinchuk, I. V., Saada, J. I., Beswick, E. J.et al. 2008. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology 135:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Owens, B. M., Steevels, T. A., Dudek, M.et al. 2013. CD90(+) stromal cells are non-professional innate immune effectors of the human colonic mucosa. Front. Immunol. 4:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu, X. and Lang, J. 2017. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget 8:97671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beswick, E. J., Johnson, J. R., Saada, J. I.et al. 2014. TLR4 activation enhances the PD-L1-mediated tolerogenic capacity of colonic CD90+ stromal cells. J. Immunol. 193:2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lina, T. T., Pinchuk, I. V., House, J.et al. 2013. CagA-dependent downregulation of B7-H2 expression on gastric mucosa and inhibition of Th17 responses during Helicobacter pylori infection. J. Immunol. 191:3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beswick, E. J., Grim, C., Singh, A.et al. 2018. Expression of programmed death-ligand 1 by human colonic CD90+ stromal cells differs between ulcerative colitis and Crohn’s disease and determines their capacity to suppress Th1 Cells. Front. Immunol. 9:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robertson, J., Haas, C. T., Pele, L. C.et al. 2016. Intestinal APCs of the endogenous nanomineral pathway fail to express PD-L1 in Crohn’s disease. Sci. Rep. 6:26747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun, C., Mezzadra, R. and Schumacher, T. N. 2018. Regulation and function of the PD-L1 checkpoint. Immunity 48:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hira-Miyazawa, M., Nakamura, H., Hirai, M.et al. 2018. Regulation of programmed-death ligand in the human head and neck squamous cell carcinoma microenvironment is mediated through matrix metalloproteinase-mediated proteolytic cleavage. Int. J. Oncol. 52:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dezutter-Dambuyant, C., Durand, I., Alberti, L.et al. 2016. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. Oncoimmunology 5:e1091146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao, F., Evans, K., Xiao, C.et al. 2018. Stromal fibroblasts mediate anti-PD-1 resistance via MMP-9 and dictate TGFβ inhibitor sequencing in melanoma. Cancer Immunol. Res. 6:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jakubowska, K., Pryczynicz, A., Iwanowicz, P.et al. 2016. Expressions of matrix metalloproteinases (MMP-2, MMP-7, and MMP-9) and their inhibitors (TIMP-1, TIMP-2) in inflammatory bowel diseases. Gastroenterol. Res. Pract. 2016:2456179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Truffi, M., Sorrentino, L., Monieri, M.et al. 2018. Inhibition of fibroblast activation protein restores a balanced extracellular matrix and reduces fibrosis in Crohn’s disease strictures ex vivo. Inflamm. Bowel Dis. 24:332. [DOI] [PubMed] [Google Scholar]
- 17. Monteleone, G., Caruso, R., Fina, D.et al. 2006. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut 55:1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meijer, M. J., Mieremet-Ooms, M. A., van Duijn, W.et al. 2007. Effect of the anti-tumor necrosis factor-alpha antibody infliximab on the ex vivo mucosal matrix metalloproteinase-proteolytic phenotype in inflammatory bowel disease. Inflamm. Bowel Dis. 13:200. [DOI] [PubMed] [Google Scholar]
- 19. Mahida, Y. R., Beltinger, J., Makh, S.et al. 1997. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am. J. Physiol. 273:G1341. [DOI] [PubMed] [Google Scholar]
- 20. Mahida, Y. R., Galvin, A. M., Gray, T.et al. 1997. Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin. Exp. Immunol. 109:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saada, J. I., Pinchuk, I. V., Barrera, C. A.et al. 2006. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J. Immunol. 177:5968. [DOI] [PubMed] [Google Scholar]
- 22. Latella, G., Rogler, G., Bamias, G.et al. 2014. Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J. Crohns Colitis 8:1147. [DOI] [PubMed] [Google Scholar]
- 23. Lakatos, G., Hritz, I., Varga, M. Z.et al. 2012. The impact of matrix metalloproteinases and their tissue inhibitors in inflammatory bowel diseases. Dig. Dis. 30:289. [DOI] [PubMed] [Google Scholar]
- 24. Cario, E. and Podolsky, D. K. 2000. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68:7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oostenbrug, L. E., Drenth, J. P., de Jong, D. J.et al. 2005. Association between Toll-like receptor 4 and inflammatory bowel disease. Inflamm. Bowel Dis. 11:567. [DOI] [PubMed] [Google Scholar]
- 26. Martinez-Chamorro, A., Moreno, A., Gómez-García, M., Cabello, M. J., Martin, J. and Lopez-Nevot, M. Á. 2016. Epistatic interaction between TLR4 and NOD2 in patients with Crohn’s disease: relation with risk and phenotype in a Spanish cohort. Immunobiology 221:927. [DOI] [PubMed] [Google Scholar]
- 27. Schmid, W., Novacek, G., Vogelsang, H.et al. 2017. Platelets Toll-like receptor-4 in Crohns disease. Eur. J. Clin. Invest. 47:109. [DOI] [PubMed] [Google Scholar]
- 28. Van Doren, S. R., Marcink, T. C., Koppisetti, R. K., Jurkevich, A. and Fulcher, Y. G. 2017. Peripheral membrane associations of matrix metalloproteinases. Biochim. Biophys. Acta Mol. Cell Res. 1864(11 Pt A):1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kioi, M., Yamamoto, K., Higashi, S., Koshikawa, N., Fujita, K. and Miyazaki, K. 2003. Matrilysin (MMP-7) induces homotypic adhesion of human colon cancer cells and enhances their metastatic potential in nude mouse model. Oncogene 22:8662. [DOI] [PubMed] [Google Scholar]
- 30. Owen, C. A., Hu, Z., Barrick, B. and Shapiro, S. D. 2003. Inducible expression of tissue inhibitor of metalloproteinases-resistant matrix metalloproteinase-9 on the cell surface of neutrophils. Am. J. Respir. Cell Mol. Biol. 29(3 Pt 1):283. [DOI] [PubMed] [Google Scholar]
- 31. Nakamura, H., Fujii, Y., Ohuchi, E., Yamamoto, E. and Okada, Y. 1998. Activation of the precursor of human stromelysin 2 and its interactions with other matrix metalloproteinases. Eur. J. Biochem. 253:67. [DOI] [PubMed] [Google Scholar]
- 32. Zhang, Y., Ma, C. A., Lawrence, M. G.et al. 2017. PD-L1 up-regulation restrains Th17 cell differentiation in STAT3 loss- and STAT1 gain-of-function patients. J. Exp. Med. 214:2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O’Sullivan, S., Gilmer, J. F. and Medina, C. 2015. Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators Inflamm. 2015:964131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vanheule, V., Metzemaekers, M., Janssens, R., Struyf, S. and Proost, P. 2018. How post-translational modifications influence the biological activity of chemokines. Cytokine 109:29. [DOI] [PubMed] [Google Scholar]
- 35. Altadill, A., Eiró, N., González, L. O.et al. 2012. Comparative analysis of the expression of metalloproteases and their inhibitors in resected Crohn’s disease and complicated diverticular disease. Inflamm. Bowel Dis. 18:120. [DOI] [PubMed] [Google Scholar]
- 36. Buisson, A., Vazeille, E., Minet-Quinard, R.et al. 2018. Fecal matrix metalloprotease-9 and lipocalin-2 as biomarkers in detecting endoscopic activity in patients with inflammatory bowel diseases. J. Clin. Gastroenterol. 52:e53–e62. [DOI] [PubMed] [Google Scholar]
- 37. Makitalo, L., Kolho, K. L., Karikoski, R., Anthoni, H. and Saarialho-Kere, U. 2010. Expression profiles of matrix metalloproteinases and their inhibitors in colonic inflammation related to pediatric inflammatory bowel disease. Scand. J. Gastroenterol. 45:862. [DOI] [PubMed] [Google Scholar]
- 38. Chakraborti, S., Mandal, M., Das, S., Mandal, A. and Chakraborti, T. 2003. Regulation of matrix metalloproteinases: an overview. Mol. Cell. Biochem. 253:269. [DOI] [PubMed] [Google Scholar]
- 39. Zhang, G., Miyake, M., Lawton, A., Goodison, S. and Rosser, C. J. 2014. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer 14:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zerdes, I., Matikas, A., Bergh, J., Rassidakis, G. Z. and Foukakis, T. 2018. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: biology and clinical correlations. Oncogene 37:4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shen, X., Zhang, L., Li, J., Li, Y., Wang, Y. and Xu, Z. X. 2019. Recent findings in the regulation of programmed death ligand 1 expression. Front. Immunol. 10:1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stanczyk, J., Pedrioli, D. M., Brentano, F.et al. 2008. Altered expression of microRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 58:1001. [DOI] [PubMed] [Google Scholar]
- 43. Wei, J., Zhang, L., Li, J.et al. 2017. MicroRNA-205 promotes cell invasion by repressing TCF21 in human ovarian cancer. J. Ovarian Res. 10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dai, S., Jia, R., Zhang, X., Fang, Q. and Huang, L. 2014. The PD-1/PD-Ls pathway and autoimmune diseases. Cell. Immunol. 290:72. [DOI] [PubMed] [Google Scholar]
- 45. Pinchuk, I. V., Beswick, E. J., Saada, J. I.et al. 2011. Human colonic myofibroblasts promote expansion of CD4+ CD25high Foxp3+ regulatory T cells. Gastroenterology 140:2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gao, Q., Meijer, M. J., Kubben, F. J.et al. 2005. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig. Liver Dis. 37:584. [DOI] [PubMed] [Google Scholar]
- 47. Arihiro, S., Ohtani, H., Hiwatashi, N., Torii, A., Sorsa, T. and Nagura, H. 2001. Vascular smooth muscle cells and pericytes express MMP-1, MMP-9, TIMP-1 and type I procollagen in inflammatory bowel disease. Histopathology 39:50. [DOI] [PubMed] [Google Scholar]
- 48. McMahan, R. S., Birkland, T. P., Smigiel, K. S.et al. 2016. Stromelysin-2 (MMP10) moderates inflammation by controlling macrophage activation. J. Immunol. 197:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smigiel, K. S. and Parks, W. C. 2017. Matrix metalloproteinases and leukocyte activation. Prog. Mol. Biol. Transl. Sci. 147:167. [DOI] [PubMed] [Google Scholar]
- 50. Vafadari, B., Salamian, A. and Kaczmarek, L. 2016. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J. Neurochem. 139 (Suppl. 2):91. [DOI] [PubMed] [Google Scholar]
- 51. Biancheri, P., Brezski, R. J., Di Sabatino, A.et al. 2015. Proteolytic cleavage and loss of function of biologic agents that neutralize tumor necrosis factor in the mucosa of patients with inflammatory bowel disease. Gastroenterology 149:1564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.