Abstract
Background and Aims
Vedolizumab is an anti-α4β7 biologic approved for ulcerative colitis [UC] and Crohn’s disease [CD]. We aimed to examine the association of maintenance vedolizumab concentrations with remission.
Methods
We performed a cross-sectional multi-centre study of inflammatory bowel disease [IBD] patients on maintenance vedolizumab. A homogeneous mobility shift assay [HMSA] was used to determine trough serum concentrations of vedolizumab and anti-drug antibodies [ATVs]. The primary outcome was corticosteroid-free clinical and biochemical remission defined as a composite of clinical remission, normalized C-reactive protein [CRP] and no corticosteroid use in 4 weeks. Secondary outcomes included corticosteroid-free endoscopic and deep remission. Vedolizumab concentrations were compared between patients in remission and with active disease. Logistic regression, adjusting for confounders, assessed the association between concentrations and remission.
Results
In total, 258 IBD patients were included [55% CD and 45% UC]. Patients in clinical and biochemical remission had significantly higher vedolizumab concentrations [12.7 µg/mL vs 10.1 µg/mL, p = 0.002]. Concentrations were also higher among patients in endoscopic and deep remission [14.2 µg/mL vs 8.5 µg/mL, p = 0.003 and 14.8 µg/mL vs 10.1 µg/mL, p = 0.01, respectively]. After controlling for potential confounders, IBD patients with vedolizumab concentrations >11.5 µg/mL were nearly 2.4 times more likely to be in corticosteroid-free clinical and biochemical remission. Only 1.6% of patients had ATVs.
Conclusions
In a large real-world cohort of vedolizumab maintenance concentrations, IBD patients with remission defined by objective measures [CRP and endoscopy] had significantly higher trough vedolizumab concentrations and immunogenicity was uncommon.
1. Introduction
Crohn’s disease [CD] and ulcerative colitis [UC] are the two major phenotypes of inflammatory bowel disease [IBD] that carry substantial health-related costs and diminished quality of life for patients.1,2 Vedolizumab is a humanized immunoglobulin G1 monoclonal antibody to the α4β7 integrin that modulates intestinal but not central nervous system lymphocyte trafficking. It is more effective than placebo in the induction and maintenance of remission in patients with UC and CD.3,4
However, a significant number of patients only partially respond or lose response to vedolizumab therapy with only 39% and 41.8% in clinical remission at week 52 in CD and UC, respectively.3,4 Experience with anti-tumour necrosis factor [TNF] biologics has shown that many patients with no response or loss of response have low drug concentrations and/or antibodies against the drug.5,6 In the pivotal GEMINI phase 3 clinical trials looking at the efficacy of vedolizumab in UC and CD, higher vedolizumab trough concentration quartiles at weeks 6 and 52 appeared to be associated with higher clinical response and remission rates.3,4 More data on how vedolizumab drug concentrations and the presence of anti-drug antibodies are correlated with disease activity are needed, as data with other biologics have shown that it can influence and help optimize the management of patients with IBD.7 One meta-analysis observed that dose intensification of vedolizumab can restore responsiveness in over half of patients with loss of response.8 A few studies have investigated the impact of vedolizumab concentrations during induction on clinical outcomes. An observational study from France found that patients with lower trough vedolizumab concentrations at weeks 2 and 6 were significantly more likely to need dose escalation by 6 months.9 Another real-world cohort from Israel suggested that week 6 trough vedolizumab concentrations were inversely correlated with week 14 C-reactive protein [CRP] concentrations.10 There are limited data on the impact of maintenance vedolizumab concentrations on clinical and objective outcomes. This scenario is particularly relevant as the recent American Gastroenterological Association [AGA] guidelines on therapeutic drug monitoring only advocated for reactive drug monitoring, at time of loss of response, as opposed to proactively checking concentrations during induction.11 Understanding the relationship between maintenance vedolizumab concentrations and disease activity will help to guide treatment decisions at time of loss of response. The aim of this study was to assess the association between trough serum vedolizumab concentrations and antibodies to vedolizumab [ATVs] during maintenance therapy with corticosteroid-free clinical, biochemical and endoscopic disease activity in a multi-centre cohort using a commercially available drug-tolerant assay.
2. Methods
2.1. Study population and design
This was a cross-sectional study performed in paediatric and adult patients with CD or UC receiving maintenance therapy with vedolizumab at the Mount Sinai Feinstein IBD Clinical Center [New York, NY] or the Medical College of Wisconsin/Froedtert Hospital [Milwaukee, WI]. The study was approved by the Institutional Review Boards at each site and all patients [or their parent] signed informed consent. Inclusion criteria were age 6 years or older, a confirmed diagnosis of UC or CD, and indication for vedolizumab being active disease defined as clinical symptoms [per patient report and medical record review] and elevated CRP. Patients with an ostomy or ileal pouch anal anastomosis were excluded. All patients had received induction with vedolizumab [300 mg at weeks 0, 2 and 6] and were in the maintenance phase of treatment [week 14 or later]. Patients were all receiving 300 mg every 8 or 4 weeks depending on the decision of the treating physicians. Consecutive patients were enrolled with prospective collection of data. At the time of enrolment, a complete medical history and assessment of clinical disease activity was completed. Clinical characteristics, laboratories and endoscopic data were extracted from the electronic medical record. CRP is routinely drawn prior to infusions at the study sites. Medical and medication history reported by patients was verified through review of the electronic medical record. All serum samples were drawn immediately prior to administration of vedolizumab maintenance dose. Coded samples were then sent to Prometheus Laboratories Inc. [San Diego, CA] for analysis blinded to clinical status. Primary data were analysed by the authors independently from the study sponsor.
2.2. Measurement of vedolizumab concentrations and ATVs
Serum levels of vedolizumab and ATVs were measured using a validated, homogenous mobility shift drug tolerant assay [HMSA, Anser VDZ, Prometheus Laboratories]. In brief, vedolizumab levels were measured by calculating the shift in antigen-bound vedolizumab complex on a size exclusion column by high-performance liquid chromatography [HPLC]. AVA levels were quantified using a size exclusion chromatography-based mobility shift assay, run on an HPLC system with fluorescence detection. The lower limits of quantification for vedolizumab and ATV were 1.6 µg/mL and 1.6 U/mL, respectively. Vedolizumab and ATV concentrations below the detectable limit were reported as <1.6 U/mL and for the purposes of data analysis were considered as no detectable vedolizumab [0]. The laboratory conducting the measurements was blinded to all patient clinical data.
2.3. Outcomes and variables
The primary outcome was corticosteroid-free clinical and biochemical remission defined as a composite of clinical remission, a normalized CRP and no oral corticosteroid use in the previous 4 weeks. Clinical remission was defined as a Harvey Bradshaw Index [HBI] score of <5 for CD or a partial Mayo Score [pMS] of ≤1 for UC.12,13 Normal serum CRP levels were defined as per local assays [≤5.0 mg/L at Mount Sinai or ≤0.5 mg/L at Medical College of Wisconsin]. Secondary outcomes included corticosteroid-free clinical remission, corticosteroid-free endoscopic remission and corticosteroid-free deep remission. Endoscopic remission was defined as a Simple Endoscopic Score for Crohn’s Disease <4 or absence of ulcerations in CD patients or an endoscopic Mayo score <2 in UC patients on endoscopy performed within 8 weeks of infusion. Deep remission was defined as corticosteroid-free clinical remission with normal CRP and endoscopic remission. An overview of outcome definitions is given in Supplementary Table 1.
Independent variables included age, sex, race, smoking status, prior biologic exposure, prior IBD surgery, concomitant immunomodulator [methotrexate, 6-mercaptopurine, azathioprine] use, albumin level, vedolizumab infusion number and disease location. Disease location and behaviour was classified according to the Montreal classification with CD categorized as ileal [L1], colonic [L2] or ileo-colonic [L3] with inflammatory [B1], stricturing [B2] or penetrating [B3] behaviour, and UC categorized as proctitis [E1], left-sided disease [E2] or pancolitis [E3].14
2.4. Statistical analysis
Descriptive statistics were performed to describe baseline characteristics of the study population, which were reported as proportions or means for categorical and continuous variables, respectively. Average vedolizumab concentrations were reported as median with interquartile range [IQR] as they were non-parametric. Comparisons between vedolizumab concentrations in patients in remission and not in remission were performed using the Wilcoxon rank-sum test. Univariable analyses looking at the association between vedolizumab concentrations and independent variables with the predefined remission outcomes were performed using logistic regression. Receiver operating characteristic [ROC] analysis with determination of area under the curve [AUC] was also performed to assess the association of remission with vedolizumab concentrations. Optimal threshold values were chosen based on the Youden index method in the ‘OptimalCutpoints’ R package. Multivariable logistic regression was then performed to assess the association of vedolizumab concentrations with each definition of remission while adjusting for potential confounders. Independent variables that were associated with the predefined remission outcomes that were significant at the p ≤ 0.1 level were incorporated into the multivariable models. All analyses were performed using Stata 14.1 software [StataCorp] and RStudio version 1.1.419 [R Development Core Team]. Two-sided p values < 0.05 were considered statistically significant.
3. Results
3.1. Study cohort characteristics
A total of 258 patients on vedolizumab maintenance therapy were enrolled in the study, 142 [55%] with CD and 116 [45%] with UC. Patient characteristics are described in Table 1. In total, 55% had CD, the vast majority were white, 66% had prior biologic exposure and median disease duration was 8 years; 22 patients [7.9%] were paediatric [age < 18 years]. The median number of prior vedolizumab infusions was five, corresponding to 38 weeks on the drug. In total, 49 patients [19.3%] were on escalated [every 4 weeks] vedolizumab. The median vedolizumab concentration for our study cohort was 10.7 µg/mL [IQR 6.7–17] with a range from 0 to 100.1 µg/mL. ATVs were detected in four patients [1.6%]. Three of these patients had UC and one had CD while none was on an immunomodulator. Despite the presence of ATVs, all had detectable vedolizumab concentrations. Median vedolizumab concentrations in patients on immunomodulators were not significantly higher than those on monotherapy [11.1 µg/mL, IQR 5.8–17.2 vs 10.6 µg/mL, IQR 6.9–16.8, p = 0.68]. Patients receiving vedolizumab every 4 weeks had significantly higher median concentrations than those on 8-week dosing [15.0 µg/mL, IQR 8.5–24.1 vs 10.2 µg/mL, IQR 6.3–15.2, p = 0.003]. There was no significant difference in trough median vedolizumab concentrations comparing patients who were biologic-naïve to those with prior biologic exposure [11 µg/mL, IQR 8.4–15.8 vs 10.4 µg/mL, IQR 5.9–17.2, p = 0.24].
Table 1.
Summary of patient characteristics [total n = 258]
| Variable | Crohn’s disease [n = 142], N [%] | Ulcerative colitis [n = 116], N [%] |
|---|---|---|
| Male | 73 [51.4] | 62 [53.5] |
| Race | ||
| White | 130 [92.9] | 104 [91.2] |
| Black | 6 [4.3] | 5 [4.4] |
| Asian | 3 [2.1] | 4 [4.4] |
| Other | 1 [0.7] | 0 [0] |
| Disease location by Montreal classification | ||
| L1 | 79 [59] | N/A |
| L2 | 38 [28] | |
| L3 | 18 [13] | |
| E1 | N/A | 10 [9] |
| E2 | 37 [32] | |
| E3 | 69 [59] | |
| Disease behaviour by Montreal classification | ||
| B1 | 88 [62.1] | N/A |
| B2 | 38 [26.7] | |
| B3 | 12 [8.7] | |
| B2/B3 | 4 [2.5] | |
| Prior biologic exposure | 101 [71.1] | 68 [58.6] |
| Prior IBD surgery | 60 [42.3] | 4 [3.5] |
| Current smoker | 10 [7.0] | 2 [1.7] |
| Combination therapy | 45 [31.7] | 33 [28.5] |
| Median [IQR] | Median [IQR] | |
| Age [years] | 33.1 [24–43.7] | 33 [22–52] |
| Disease duration [years] | 11 [6–17] | 5 [3–11] |
| Infusion number | 5 [4–9] | 6 [5–9] |
| Harvey Bradshaw Index | 2 [1–5] | N/A |
| Partial Mayo Score | N/A | 1 [0–2] |
| Albumin [g/dL] | 3.9 [3.5–4.2] | 3.9 [3.6–4.1] |
Abbreviations: IBD, inflammatory bowel disease; IQR, interquartile range; N/A, not applicable.
3.2. Corticosteroid-free clinical and biochemical remission
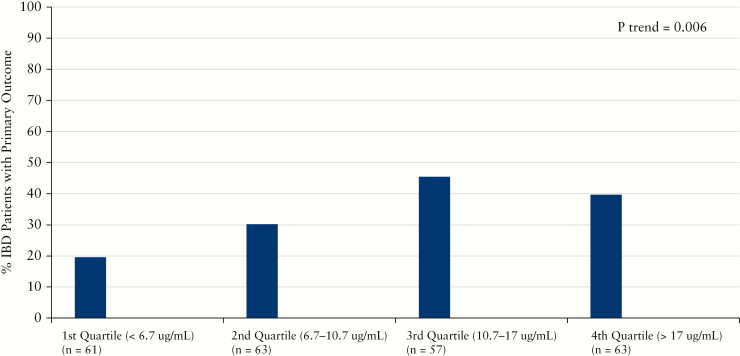
A total of 83 [34%] patients met the primary outcome of corticosteroid-free clinical and biochemical remission while 161 [66%] did not. Ten patients who did not have CRP at the time of trough vedolizumab concentration were excluded. Median vedolizumab concentrations were significantly higher in IBD patients meeting the primary outcome compared to those not in remission [12.7 µg/mL vs 10.1 µg/mL, p = 0.002, Table 2]. This difference in vedolizumab concentrations was more pronounced and significant in UC compared to CD patients [Table 2]. When examining the individual components of the primary outcome, the results appear to be primarily driven by corticosteroid-free biochemical remission, as vedolizumab concentrations did not significantly differ in IBD patients in corticosteroid-free clinical remission [Supplementary Table 2]. Vedolizumab concentrations were nearly identical when stratified by clinical remission in CD; however, trough vedolizumab concentrations were marginally significantly higher among UC patients in clinical remission [13.1 µg/mL vs 10.2 µg/mL, p = 0.05]. We then analysed the primary outcome remission rates in IBD patients, stratifying trough vedolizumab concentrations by quartile. Rates of corticosteroid-free remission were significantly higher with increasing vedolizumab quartile, with the 3rd and 4th quartiles having similar proportions of IBD patients with the composite outcome of corticosteroid-free clinical and biochemical remission [Figure 1]. Similar findings were seen with corticosteroid-free biochemical remission but not with corticosteroid-free clinical remission [data not shown].
Table 2.
Median vedolizumab concentrations and primary outcome [clinical and CRP corticosteroid-free remission]
| Median vedolizumab concentration, µg/mL [IQR] | |
|---|---|
| IBD [p = 0.002] | |
| Remission [n = 82] | 12.7 [8.4–19.4] |
| No remission [n = 162] | 10.1 [5.9–15.5] |
| UC [p = 0.007] | |
| Remission [n = 36] | 15.0 [11–21.8] |
| No remission [n = 75] | 10.7 [7.4–17.4] |
| CD [p = 0.056] | |
| Remission [n = 46] | 10.9 [7.5–17.0] |
| No remission [n = 87] | 8.6 [5.3–14.9] |
Abbreviations: IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; IQR, interquartile range.
Figure 1.
Increasing vedolizumab concentration quartiles and rates of corticosteroid-free clinical and biochemical remission [primary outcome] during maintenance therapy.
3.3. Corticosteroid-free endoscopic and deep remission
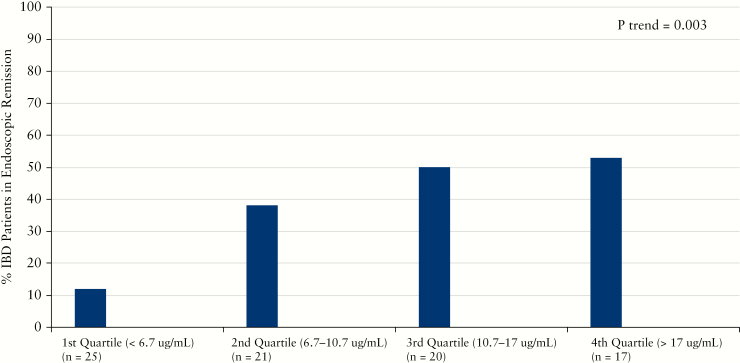
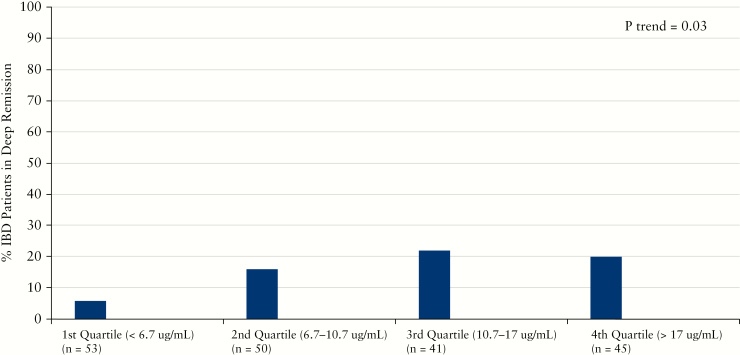
A subset of patients had sufficient data to evaluate corticosteroid-free endoscopic and deep remission. A total of 83 patients had endoscopic data [30 in remission and 53 with active disease] while deep remission was attained in 29 patients. Median vedolizumab concentrations were significantly higher in IBD patients in corticosteroid-free endoscopic remission [13.1 µg/mL vs 8.5 µg/mL, p = 0.01, Table 3]. While vedolizumab concentrations were numerically higher in UC patients in corticosteroid-free endoscopic remission, the difference was not statistically significant. In contrast, CD patients in corticosteroid-free endoscopic remission had significantly higher concentrations than those not in remission [15.0 µg/mL vs 7.5 µg/mL, p = 0.008]. In quartile analysis, higher vedolizumab concentration quartiles were associated with significantly higher rates of corticosteroid-free endoscopic remission [Figure 2]. A similar association with increased median vedolizumab concentrations was seen for those patients in corticosteroid-free deep remission [14.8 µg/mL vs 9.4 µg/mL, p = 0.005, Table 4]. UC patients in corticosteroid-free deep remission had a non-significant but numerically higher vedolizumab trough concentration while vedolizumab was significantly higher in CD patients in corticosteroid-free deep remission compared to those with active disease [11.1 µg/mL vs 10.7 µg/ml, p = 0.4 and 15.0 µg/mL vs 8.8 µg/mL, p = 0.01]. There was no statistically significant difference in vedolizumab concentrations when alternatively defining corticosteroid-free endoscopic remission as a Mayo score of 0 [data not shown]. In quartile analysis, higher vedolizumab concentration quartiles were also associated with significantly higher rates of corticosteroid-free deep remission [Figure 3].
Table 3.
Median vedolizumab concentrations and corticosteroid-free endoscopic remission
| Median vedolizumab concentration, µg/mL [IQR] | |
|---|---|
| IBD [p = 0.003] | |
| Remission [n = 30] | 14.2 [8.4–21.2] |
| No remission [n = 53] | 8.5 [5.5–13.8] |
| UC [p = 0.26] | |
| Remission [n = 10] | 11.3 [8.8–21.2] |
| No remission [n = 18] | 9.75 [5.5–13.8] |
| CD [p = 0.008] | |
| Remission [n = 20] | 15.0 [8.3–20.7] |
| No remission [n = 35] | 7.5 [5.0–13.8] |
Abbreviations: IBD, inflammatory bowel disease; UC, ulcerative colitis; CD: Crohn’s disease; IQR, interquartile range.
Figure 2.
Increasing vedolizumab concentration quartiles and rates of corticosteroid-free endoscopic remission during maintenance therapy.
Table 4.
Median vedolizumab concentrations and corticosteroid-free deep remission
| Median vedolizumab concentration, µg/mL [IQR] | |
|---|---|
| IBD [p = 0.01] | |
| Remission [n = 29] | 14.8 [8.4–21.2] |
| No remission [n = 160] | 10.1 [5.9–15.1] |
| UC [p = 0.40] | |
| Remission [n = 9] | 11.1 [8.8–21.2] |
| No remission [n = 70] | 10.7 [7.2–15.2] |
| CD [p = 0.01] | |
| Remission [n = 20] | 15.0 [8.3–20.5] |
| No remission [n = 90] | 8.8 [5.3–15] |
Abbreviations: IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; IQR, interquartile range.
Figure 3.
Increasing vedolizumab concentration quartiles and rates of corticosteroid-free deep remission during maintenance therapy.
3.4. ROC analysis
Last, we performed ROC analysis to further characterize the performance of vedolizumab concentration with different definitions of remission. For the primary outcome, vedolizumab concentrations had an AUC of 0.62 (95% confidence interval [CI] 0.55, 0.69). The optimal cutoff for remission as defined by the primary outcome, according to the Youden index, was 11.5 µg/mL with a sensitivity of 57.3%, specificity of 63.0%, positive predictive value of 44% and negative predictive value of 74.5%. Among UC patients the optimal cutoff for the primary outcome based on Youden index analysis was 10.1 µg/mL [sensitivity 88.9% and specificity 45.3%] and for CD it was 6.8 µg/mL [sensitivity 82.6% and specificity 37.9%]. For secondary outcomes, vedolizumab concentrations had an AUC of 0.67 [95% CI 0.53, 0.81] for corticosteroid-free endoscopic remission and an AUC of 0.64 [95% CI 0.54, 0.75] for corticosteroid-free deep remission. The optimal cutoff for corticosteroid-free endoscopic remission according to the Youden index was 10.7 µg/mL with a sensitivity of 68.0%, specificity of 62.9%, positive predictive value of 56.7% and negative predictive value of 73.3%. The optimal cutoff for corticosteroid-free deep remission for the Youden index was 14.8 µg/mL with a sensitivity of 51.7%, specificity of 73.1%, positive predictive value of 25.9% and negative predictive value of 89.3%.
3.5. Multivariable analysis of remission
To understand if trough vedolizumab concentrations were independently associated with measures of corticosteroid-free remission, we next looked at other clinical variables that may impact corticosteroid-free remission rates in univariable logistic regression. Results for these variables for the primary outcome, corticosteroid-free endoscopic remission and corticosteroid-free deep remission are shown in Supplementary Tables 3–5. Variables significant at the preset p value were then analysed with trough vedolizumab concentration in multivariable models for each outcome of interest. Trough vedolizumab concentration was dichotomized as above or below the optimal cutoff concentration selected by the Youden index [11.5 µg/mL for primary outcome, 10.7 µg/mL for corticosteroid-free endoscopic remission and 14.8 µg/mL for corticosteroid-free deep remission]. After controlling for possible confounding variables, the trough vedolizumab concentration above the identified optimal cutoffs was still significantly associated with an increased chance of achieving remission in each of the examined outcomes [Table 5].
Table 5.
Multivariable association of vedolizumab concentration above optimal cutoff based on Youden index with different definitions of remission
| Adjusted OR [95% CI] | p | |
|---|---|---|
| Primary outcome [>11.5 µg/mL] | 2.45 [1.37–4.38] | 0.002 |
| Endoscopic remission [>10.7 µg/mL] | 3.99 [1.42–11.20] | 0.009 |
| Deep remission [>14.8 µg/mL] | 3.72 [1.54–8.98] | 0.004 |
Adjusting for all covariables significant at p ≤ 0.10 in univariable analysis. Included all IBD patients. All end points are corticosteroid free. Covariables adjusted for in analyses [based on univariable association] with adjusted OR [aOR] from multivariable analysis: Primary outcome: age [aOR 0.98, 95% CI 0.97–1.00], escalated vedolizumab dosing [aOR 0.39, 95% CI 0.17–0.92], prior biologic [aOR 0.52, 95% CI 0.29–0.95]. Endoscopic remission: combination therapy [aOR 1.77, 95% CI 0.64–4.88], prior biologic [aOR 0.29, 95% CI 0.10–0.83]. Deep remission: prior biologic [aOR 0.61, 95% CI 0.25–1.48], number of prior infusions [aOR 0.85, 95% CI 0.71–1.02], escalated vedolizumab dosing [aOR 0.37, 95% CI 0.09–1.48].
4. Discussion
In a large multi-centre cohort of IBD patients, we observed that trough vedolizumab concentrations measured using a drug-tolerant assay were significantly associated with corticosteroid-free remission during maintenance vedolizumab treatment. Immunogenicity was very low, with only 1.6% of patients having ATVs. Patients with vedolizumab concentrations above 11.5 µg/mL were 2.4 times more likely to be in corticosteroid-free clinical and biochemical remssion after adjusting for potential confounders. Those with vedolizumab concentrations >14.8 µg/mL were nearly four times more likely to be in corticosteroid-free deep remission. To our knowledge, this is the largest reported real-world cohort on vedolizumab concentrations to date and the first to use a drug-tolerant assay. Our results offer data to help guide clinical decision-making during maintenance therapy, particularly at the time of loss of response to vedolizumab when checking drug concentrations is advocated by national gastroenterology societies.11 If a patient is not responding to vedolizumab during maintenance, our observations suggest that dose escalation should be considered in order to achieve a concentration of at least 11.5 µg/mL. Higher concentrations [>14.8 µg/mL] may be needed for deep remission.
However, as with any study on associations of drug concentrations with clinical outcomes, prospective interventional trials will ultimately be needed to fully validate this approach. Our results are largely consistent with the few prior published studies that have observed a significant association between vedolizumab concentrations and clinical response to vedolizumab in IBD patients. However, the types of assays used previously varied [often not clinically available platforms] and end points differed. Much of the data currently available relate to early induction concentrations. In a post-hoc analysis of the GEMINI clinical trials, week 6 vedolizumab concentrations were significantly higher among both CD and UC patients in clinical remission after adjusting for potential confounders, although objective markers of inflammation were not explored.15 Additionally, real-world observational studies have suggested that early induction vedolizumab concentrations are associated with later remission defined by clinical activity indices, a need to escalate therapy within 6 months and endoscopic healing within the first year of treatment.9,10,16
Fewer data are available regarding maintenance vedolizumab concentrations. In a study from Israel that included 60 patients on maintenance therapy, those with normal CRP during maintenance had higher vedolizumab concentrations using an in-house assay [21.8 µg/mL vs 11.9 µg/mL, p = 0.0006].10 Similar to our findings, this group found no association with clinical remission and maintenance vedolizumab concentrations. A study from the Leuven group looked at induction and maintenance concentrations in 179 patients.17 Their study utilized a vedolizumab assay available in Europe but ATV assessment was only possible in samples in which vedolizumab was below the limit of detection. Week 14, 22 and 30 vedolizumab concentrations were significantly higher among IBD patients with biological and endoscopic remission, with a higher probability of remission when the vedolizumab concentration was >14 µg/mL. Our study adds to these data by investigating a drug-tolerant commercially available assay and through evaluation of objective measures of remission including deep remission.
Using a drug-tolerant assay, we observed very low rates of immunogenicity to vedolizumab. Clinical trial data suggested that 10–12% of patients developed ATVs, although this was measured 14 weeks after discontinuation of vedolizumab.18 Other observational studies have suggested low immunogenicity rates; however, this was principally done using drug-sensitive assays.10,17 Our results with a drug-tolerant assay in the largest maintenance cohort to date are convincing in showing that the rates of immunogenicity are very low with vedolizumab. The potential implication is that combination with immunomodulators may not be necessary to prevent immunogenicity with vedolizumab.
We did not observe any impact of concomitant immunomodulator therapy on vedolizumab concentrations. This is similar to prior observations from analysis of vedolizumab pharmacokinetic and pharmacodynamics data from clinical trials.19 It is unclear why this is the case but one possible explanation is that recent data suggest that the primary impact of immunomodulator therapy in combination with biologics is to limit immunogenicity to the biologic as opposed to synergistic effect of a different mechanism of action.20
A previous study suggested near complete saturation of α4β7 on peripheral blood T cells with serum vedolizumab concentrations of 3 µg/mL.10 Blocking homing of α4β7-positive T cells to gut is one of the purported likely mechanisms of vedolizumab.21 However, our data and those of others suggest that increasing vedolizumab concentrations, well above 3 µg/mL, are associated with improved response and remission rates. Therefore, the mechanism of vedolizumab may not solely depend on complete blockade of peripheral α4β7-expressing T cells. This is supported by a recent study that found that vedolizumab treatment resulted in significant changes in innate immune cells, including macrophages, as well as mucosal gene expression of many chemokines, innate immune receptors and T helper [Th] 17-associated cytokines.21 Further studies are needed to elucidate the mechanism of action of vedolizumab and the impact of higher vedolizumab concentrations on immune cell populations and gene expression.
Our study had several limitations and strengths. Limitations include the cross-sectional nature of the study with the possibility of reverse causation [patients in remission tend to have higher vedolizumab concentrations as opposed to patients with higher vedolizumab concentrations have higher rates of remission] for which prospective interventional studies are needed to provide further clarification. Endoscopic end points were not centrally read, although it is routine at the participating centres that endoscopic scores or detailed descriptions of ulcerated mucosa are recorded in endoscopy reports. The strengths of our study include the use of a large, multi-centre, real-world cohort of IBD patients with multiple definitions of remission. Further, we used a validated HMSA platform that can detect ATVs in the presence of detectable vedolizumab, which increases the validity of our immunogenicity findings. In addition, our clinical data were prospectively collected with standard data collection forms verified through chart review. Lastly, previous studies principally used internal assays or platforms that are not commercially available in North America, so our findings using a widely used commercial assay are more widely applicable.
In conclusion, we observed that maintenance vedolizumab concentrations are significantly associated with corticosteroid-free remission in IBD patients in a large real-world cohort. Vedolizumab appears to have low immunogenicity and, when controlling for potential confounders, trough vedolizumab concentrations of ≥11.5 µg/mL as determined with a drug-tolerant HMSA were significantly associated with corticosteroid-free clinical and biochemical remission. Further prospective interventional studies are needed to better understand the impact of concentration-based dosing and changes to vedolizumab dosing in response to low trough concentrations.
Funding
This study was funded by Prometheus Laboratories Inc., San Diego, CA. RCU is supported by a Career Development Award from the Crohn’s and Colitis Foundation.
Conflict of Interest
RCU: consultant for Takeda, Pfizer, and Janssen. AY: consultant and speaker bureau for Prometheus and Takeda. MCD: consultant for Prometheus and Takeda.
Author Contributions
RCU and AY: study concept and design, data acquisition, data analysis and interpretation, and writing of manuscript. JJ, BP, EC, PS, KK, AB, DS, PB-P, CF, AP, BB: data acquisition and revision of manuscript for important intellectual content. AJ: data acquisition, interpretation of data and revision of manuscript for important intellectual content. SN and MCD: study concept and design, interpretation of data, and revision of manuscript for important intellectual content. Guarantor of article: MCD.
Supplementary Material
References
- 1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feagan BG, Rutgeerts P, Sands BE, et al.; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Feagan BG, Rutgeerts P, et al.; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 5. Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2006;4:1248–54. [DOI] [PubMed] [Google Scholar]
- 6. Yarur AJ, Jain A, Hauenstein SI, et al.. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2016;22:409–15. [DOI] [PubMed] [Google Scholar]
- 7. Afif W, Loftus EV Jr, Faubion WA, et al.. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peyrin-Biroulet L, Danese S, Argollo M, et al. . Loss of response to vedolizumab and ability of dose intensification to restore response in patients with Crohn’s disease or ulcerative colitis: a systematic review and meta -analysis. Clin Gastroenterol Hepatol 2018. doi: 10.1016/j.cgh.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 9. Williet N, Boschetti G, Fovet M, et al.. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017;15:1750–1757.e3. [DOI] [PubMed] [Google Scholar]
- 10. Ungar B, Kopylov U, Yavzori M, et al.. Association of vedolizumab level, anti-drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:697–705.e7. [DOI] [PubMed] [Google Scholar]
- 11. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee American Gastroenterological Association Institute Guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34. [DOI] [PubMed] [Google Scholar]
- 12. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 13. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silverberg MS, Satsangi J, Ahmad T, et al.. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19 Suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- 15. Rosario M, French JL, Dirks NL, et al.. Exposure–efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis 2017;11:921–9. [DOI] [PubMed] [Google Scholar]
- 16. Yacoub W, Williet N, Pouillon L, et al.. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018;47:906–12. [DOI] [PubMed] [Google Scholar]
- 17. Dreesen E, Verstockt B, Bian S, et al.. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:1937–46.e8. [DOI] [PubMed] [Google Scholar]
- 18. Restellini S, Khanna R, Afif W. Therapeutic drug monitoring with ustekinumab and vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis 2018;24:2165–72. [DOI] [PubMed] [Google Scholar]
- 19. Rosario M, Dirks NL, Gastonguay MR, et al.. Population pharmacokinetics–pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther 2015;42:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colombel JF, Adedokun OJ, Gasink C, et al. . Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy-a post-hoc analysis. Clin Gastroenterol Hepatol 2018 Sep 26. doi: 10.1016/j.cgh.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 21. Zeissig S, Rosati E, Dowds CM, et al.. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019;68:25–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.