Abstract
This study examines health outcomes in burn patients with sepsis. We hypothesized that burn patients with sepsis would have an increased odds risk for in-hospital death and longer intensive care unit (ICU) stays. This was a retrospective cohort of consecutive patients admitted to the burn ICU with total BSA (TBSA) ≥10% and/or inhalation injury between January 2008 and March 2015. Overall 407 burn patients were included; the case-rate for sepsis was 39.1% (n = 159); 20.1% (n = 82) patients were septic and 18.9% (n = 77) patients experienced septic shock. Patients with septic shock had the highest mortality rate (13.31% no sepsis vs 3.7% sepsis vs 49.4% septic shock, P < .01). Median 28-day ICU-free days was higher in patients without sepsis (23 days [Interquartile range (IQR) 14–27] no sepsis vs 0 days [IQR 0–10] sepsis vs 0 days [IQR 0–0] septic shock, P < .01). Sepsis (with or without shock) increased odds of in-hospital death (odds ratio 7.04, 95% confidence interval 1.93–25.7) in reference to the no sepsis group. With each incremental Sequential Organ Failure Assessment (SOFA) score or 10% TBSA increase, the odds risk for in-hospital death increased by 56 and 75%, respectively. Our study characterized outcomes in patients with sepsis after severe burn injury. The odds risk for in-hospital death was greater in patients with sepsis, increasing burn severity according to TBSA and SOFA score.
Severe burn-injured patients ubiquitously develop a systemic inflammatory response syndrome, limiting the utility of sepsis definitions used in critically ill patients.1–3 Multiple organ dysfunction syndrome, a direct response to sepsis, is the primary cause of mortality in burn-injured patients surviving initial burn injury.4 Burn-injured patients are at high risk for infection and sepsis.5 Burn care has greatly improved over the last decade and more patients encounter sepsis events; however, outcomes in this unique group of patients are not well described.
In 2007, the American Burn Association (ABA) developed guidelines to diagnose burn patients with sepsis, but little data exist on outcomes in this patient cohort.5 The ABA definition is based on expert opinion or pediatric data and is comprised of more stringent criteria than the Surviving Sepsis Campaign definition, including additional physiologic parameters, hyperglycemia, thrombocytopenia, and intolerance to enteral feeding.1,2,5–7 These criteria are distinct from both systemic inflammatory response syndrome and Sequential Organ Failure Assessment (SOFA), which are used to diagnosis sepsis in a nonburn population.1
Sepsis is a common complication after burn injury but little data are available that characterize risk factors and outcomes due to the complexity in application of the ABA Sepsis definition.8,9 We aim to compare patient outcomes and hypothesize that burn patients with sepsis have longer intensive care unit (ICU) and hospital stays and an increased odds of in-hospital death compared with patients without sepsis.
METHODS
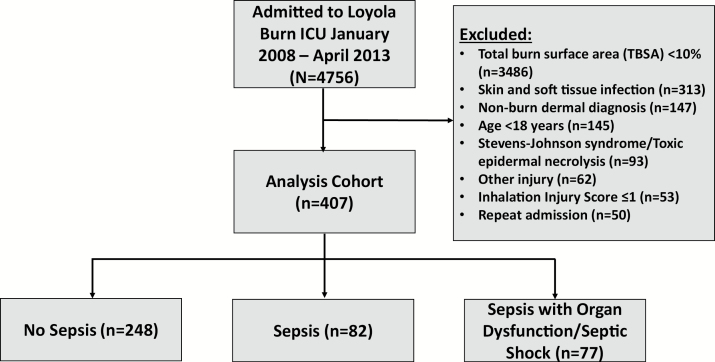
A cohort of 4761 consecutive patients admitted to the Burn Center between January 2008 and March 2015 at a large, academic, tertiary care burn center were reviewed. The Burn Center at Loyola University Medical Center is in the top tier for volume of cases in the region, treating over 600 patients annually on average. The following inclusion criteria were met for the analysis cohort: greater than or equal to 18 years of age; severe burn injury, defined as total BSA (TBSA) ≥10% and/or an abbreviated injury scale score corresponding to at least moderate inhalational injury based on fiberoptic bronchoscopic examination by the attending burn physician.10 Patients with admission to the burn ICU for a nonburn-related diagnosis were excluded. If admitted multiple times during the study period, only the first encounter was included. If sepsis occurred multiple times during an admission, only the first episode was examined (Figure 1). Infection control measures following best-practice guidelines were followed since the beginning of the study period for prevention of hospital-acquired infections.11 Admission to and discharge from the burn ICU were at the discretion of the treating physician.
Figure 1.
Study diagram
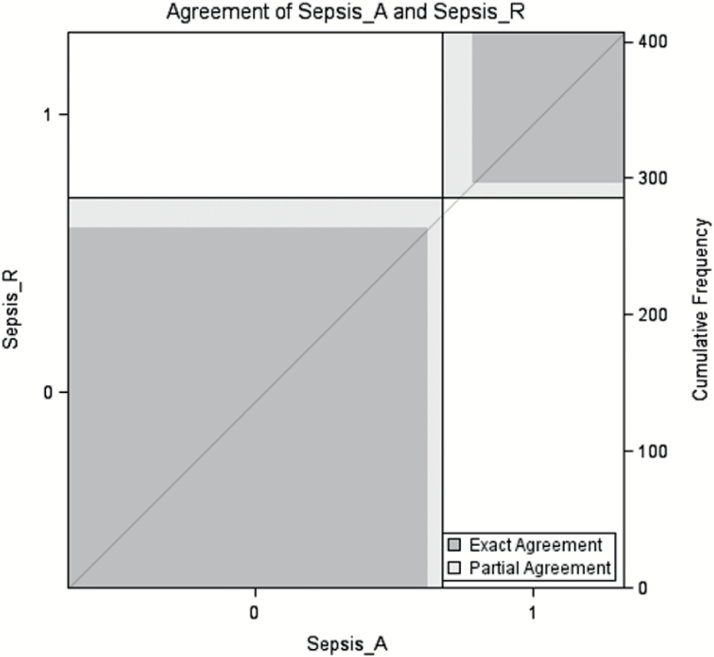
Sepsis identification was performed by two independent reviewers who treat sepsis and incorporating the ABA definition (M.A.R., M.A.). The reviewers independently reviewed the same electronic medical records to identify cases and noncases. Interobserver reliability of ABA sepsis case identification was measured using Cohen’s kappa coefficient. In cases of discordant patient assessments, a third, independent burn critical care surgeon (M.J.M.) was used as an arbitrator. Excellent interobserver agreement was observed with κ coefficient 0.82 (95% confidence interval [CI] 0.76–0.88, P = .031). Case agreement is displayed in Figure 2. Adjudication of group classification was needed in 32 cases (7.9%). ABA Sepsis criteria were met if at least three criteria were identified in addition to a documented infection, defined as a positive culture, a pathologic tissue source, or a clinical response to antimicrobial therapy.5 The SOFA criteria were used to identify septic shock patients with the following criteria in accordance with the sepsis-3 definition: PaO2: FiO2 ratio <400 mm Hg, platelets <150,000/mcl, bilirubin ≥1.2 mg/dl, mean arterial pressure (MAP) <70 mm Hg, and serum creatinine ≥1.2 mg/dl.1 Organ dysfunction by Glasgow Coma Score was omitted due to too many missing data. A score of 0 was imputed for all patients to provide a conservative estimate.
Figure 2.
Case agreement.
The primary objective of this study was to characterize in-hospital death between patients with no sepsis and sepsis. Secondary endpoints included hospital and ICU length of stay (LOS), 28-day ICU-free days, hyperglycemia and acute respiratory distress syndrome (ARDS) between groups. Twenty-eight-day ICU-free days are defined as the number of days alive and outside the ICU during the 28-day period from ICU admission. Patients discharged from the hospital within 28 days were assumed to be alive.
Statistical Analysis
Baseline demographics were analyzed using descriptive statistics. Continuous variables were expressed as medians and interquartile ranges (IQR) and analyzed using the Kruskal–Wallis test by ranks. Proportions were analyzed using a chi-square test or Fischer’s exact test. A multivariable logistic regression was conducted to determine predictors of in-hospital mortality. Candidate variables were evaluated in univariable analysis and those with P values ≤.2 were included in the multivariable analysis. The following candidate variables were identified a priori for evaluation based on prior literature: sepsis (yes/no), SOFA score on day 1, age, TBSA, and inhalation injury.12–15
Analysis was preformed utilizing SAS, version 9.4 (SAS Institute, Cary, NC) and STATA version 12 (College Station, TX). Approval of this study with a waiver of consent and Health Insurance Portability and Accountability Act authorization was provided by the institutional review board at Loyola University Medical Center.
RESULTS
During the study period, 4761 patients were admitted to the burn ICU, of which 8.6% (n = 407) met inclusion criteria (Figure 1). The large majority of patients (73.2%) were excluded for TBSA <10% (n = 3486; 73.2%). Of those included, the case-rate for sepsis was 39.1% (n = 159); 20.1% (n = 82) of the patients were septic and 18.9% (n = 77) of the patients experienced septic shock in accordance with the sepsis-3 criteria.1 Patient characteristics (Table 1) were similar between patients with sepsis, septic shock, and without sepsis with the exception of an incrementally higher median TBSA in both sepsis groups (14.7% no sepsis vs 22.2% sepsis vs 35.5% septic shock, P < .01), higher median SOFA scores in both sepsis groups (0 no sepsis vs 2 sepsis vs 4 septic shock, P < .01) and more concomitant burn and inhalation injury in both sepsis groups (6.1% no sepsis vs 23.2% sepsis vs 31.2% septic shock, P < .01). Additionally, sources of infection differed between groups, with significantly more bacteremia, genitourinary infections and pneumonia in both sepsis groups, and more abdominal infections in septic patients (Table 1).
Table 1.
Characteristics
| Characteristic | No Sepsis (n = 248) | Sepsis (n = 82) | Septic Shock (n = 77) | P |
|---|---|---|---|---|
| Age, median (IQR) | 47 (33–60) | 43 (30–55) | 53 (40–62) | .05 |
| Sex, male, n (%) | 174 (70.2) | 65 (79.3) | 47 (61.0) | .04 |
| Race, white n (%) | 159 (64.1) | 49 (59.8) | 50 (64.9) | .74 |
| SOFA score (median, IQR) | 0 (0–2) | 2 (1–3) | 4 (3–7) | <.01 |
| Comorbid Conditions, n (%) | ||||
| Coronary artery disease | 16 (6.5) | 3 (3.7) | 8 (10.4) | .23 |
| Diabetes | 31 (12.5) | 9 (11.0) | 10 (13.0) | .92 |
| Cancer | 7 (3) | 1 (1.2) | 4 (5.2) | .33 |
| Hypertension | 88 (35.5) | 31 (37.8) | 32 (41.6) | .62 |
| Tobacco use | 63 (25.4) | 28 (34.2) | 24 (31.9) | .26 |
| Burn mechanism, n (%) | .08 | |||
| Flame | 166 (66.9) | 65 (79.3) | 62 (80.5) | |
| Scald | 37 (14.9) | 5 (6.1) | 7 (9.1) | |
| Chemical | 9 (3.6) | 1 (1.2) | 1 (1.3) | |
| Grease | 8 (3.2) | 0 (0) | 0 (0) | |
| Electrical | 2 (0.8) | 2 (2.4) | 0(0) | |
| Other | 26 (10.5) | 9 (11) | 7 (9.1) | |
| Diagnosis, n (%) | <.01 | |||
| Burn | 209 (84.3) | 55 (67.1) | 47 (61) | |
| Burn + inhalation injury | 15 (6.1) | 19 (23.2) | 24 (31.2) | |
| Inhalation injury | 16 (6.5) | 8 (9.8) | 5 (6.5) | |
| TBSA, median (IQR) | 14.7 (11.8–19.5) | 22.2 (15.5–32.3) | 35.5 (20.1–56.2) | <.01 |
| Inhalation score (n = 89), median (IQR) | 2 (1–3) | 3 (2–3) | 2.5 (1–3) | .62 |
| Source of Infection, n (%) | ||||
| Abdominal | 0 (0) | 2 (2.4) | 0 (0) | .02 |
| Bacteremia | 0(0) | 20 (24.4) | 21 (27.3) | <.01 |
| Genitourinary | 3 (1.2) | 8 (9.8) | 7 (9.1) | <.01 |
| Pneumonia | 9 (3.6) | 45(54.8) | 38 (49.4) | <.01 |
| Skin/soft tissue | 17 (6.9) | 9 (11) | 7 (9.1) | .47 |
| Admission vitals, median (IQR) | ||||
| SBP, mmHg | 144 (125–160) | 144 (123–155) | 128 (106–160) | .03 |
| DBP, mmHg | 80 (67–89) | 80 (68–89) | 72 (60–87) | .08 |
| HR, bpm | 98 (90–100) | 99 (94–100) | 100 (95–114) | <.01 |
SOFA, Sequential Organ Failure Assessment; IQR, interquartile range; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
For the primary outcome of in-hospital death (Table 2), the proportion was 18.2% for the cohort and patients with septic shock had the greatest mortality (13.31% no sepsis vs 3.7% sepsis vs 49.4% septic shock, P < .01). A positive blood culture was identified in 95.6% (n = 155) of the septic cases. Median 28-day ICU-free days was higher in patients without sepsis (23 days [IQR 14–27] no sepsis vs 0 days [IQR 0–10] sepsis vs 0 days [IQR 0–0] septic shock, P < .01). Hospital and ICU LOS were longer in both sepsis groups compared to the no sepsis group. Both sepsis groups also had higher incidence of hyperglycemia and ARDS.
Table 2.
Outcomes
| Outcome | No Sepsis (n = 248) | Sepsis (n = 82) | Septic Shock (n = 77) | P |
|---|---|---|---|---|
| In-hospital death, n (%) | 33 (13.3) | 3 (3.7) | 38 (49.4) | <.01 |
| LOS, median (IQR)* | ||||
| ICU LOS | 5 (1–14) | 31 (18–45) | 64 (35–102) | <.01 |
| Hospital LOS | 13 (7–18) | 33 (24–49) | 64.5 (36–106) | <.01 |
| Hyperglycemia, n (%) | 15 (10.1) | 11 (20.8) | 14 (22.3) | <.01 |
| Acute respiratory distress syndrome, n (%) | 21 (8.5) | 37 (45.2) | 58 (75.3) | <.01 |
| Discharge Disposition, n (%) | <.01 | |||
| Home | 178 (71.7) | 32 (39.0) | 7 (9.1) | |
| Skilled nursing facility | 14 (5.7) | 7 (8.5) | 7 (9.1) | |
| Rehabilitation facility | 11 (4.4) | 27 (32.9) | 15 (19.5) | |
| Other | 12 (4.8) | 14 (17.1) | 8 (10.4) |
ICU, intensive care unit; IQR, interquartile range; LOS, length of stay.
*n = 321 after excluding patients experiencing in-hospital mortality.
In a multivariable logistic regression analysis adjusted for age, sex, race, burn mechanism, TBSA, inhalation injury score, and comorbidities (heart failure, tobacco use), sepsis (with or without shock) increased odds of in-hospital death (odds ratio [OR] 7.04, 95% [CI] 1.93–25.7) in reference to the no sepsis group. The OR was also greater in patients with higher SOFA score on day one and TBSA. With each incremental SOFA score or 10% TBSA increase, the odds risk for in-hospital death increased by 56% (OR 1.56, 95% CI 1.32–1.84) and 75% (OR 1.75, 95% CI 1.44–2.21; P < .01), respectively.
DISCUSSION
This study evaluated outcomes in severe burn-injured patients with sepsis and found greater odds for in-hospital death in patients who developed sepsis (with or without shock) compared with patients who did not develop sepsis. In this study, patients with sepsis were more likely to have inhalation injury, higher TBSA and baseline SOFA scores. All types of infection occurred more frequently in septic patients, of which pneumonia, bacteremia, and genitourinary infections were most common.
Studies describing case-rates or outcomes in burn patients with sepsis were mainly performed at time of autopsy.16,17 One study included 144 pediatric patients over a 20-year period and found sepsis to be the cause of death in 47% of the patients.16 In the same study, the increasing rate of sepsis-related deaths over the study period was mainly attributed in part to increasing antimicrobial resistance (42 vs 86% between beginning and end of the study period). Another study comprised of autopsies performed in 334 burn-injured patients over a 5-year period found that septicemia was included in the cause of death in 65% of the patients.18 Our study is unique in that our sepsis assessment was performed using the ABA definition to identify sepsis cases, then divided into sepsis versus septic shock in accordance with the Sepsis-3 definition and we were able to characterize outcomes in hospitalized patients with more detailed information about LOS, organ dysfunctions, and sources of infection.1,5 Furthermore, we showed that sepsis remains a leading cause of in-hospital death in burn-injured patients, with mortality occurring in nearly half of the patients with septic shock.
In addition to sepsis, TBSA and SOFA score were noted to be important risk factors. Our finding that each 10% incremental increase in TBSA of burn injury was most strongly associated with death is consistent with prior evidence.14,19 TBSA is often used in tools to predict risk of death (eg, the Baux Score).17,19 SOFA score is another ICU severity score that is now incorporated into the new Sepsis-3 definition.1 While it has been used widely in critically ill patients, only one prior study has found it to be predictive of mortality in patients with burn injury.20 Our findings support further exploration of the predictive ability of SOFA score on mortality in burn-injured patients.
In our study cohort, we found that patients with sepsis had a lower mortality rate relative to patients without sepsis, counter to what would be expected. This may be due to patients without sepsis having a much shorter ICU and hospital LOS (by 26 and 20 days, respectively). Thus, patients in the sepsis group were in a hospital setting longer, with potential exposure to nosocomial pathogens leading to the development of sepsis during that time. Additionally, patients without sepsis were older and had a higher proportion of some comorbid conditions (eg, coronary artery disease), although none were statistically significant.
Our study is limited in its retrospective, single-center design; thus future studies should externally validate our findings at other burn centers. In addition, SOFA scores are biased with a lower median value because we could not reliably calculate GCS for all patients.
CONCLUSION
Our study characterized outcomes in patients with sepsis with severe burn injury. The odds for in-hospital death were greater in patients with sepsis, increasing burn severity according to TBSA and SOFA score.
Conflict of interest statement
M.A. is currently receiving a grant (K23AA024503) from the NIH. E.J.K. is currently receiving a grant (R01GM115257) from the NIH. The remaining authors have no other conflicts of interest to disclose.
REFERENCES
- 1. Singer M, Deutschman CS, Seymour CW et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhodes A, Evans LE, Alhazzani W et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486–552. [DOI] [PubMed] [Google Scholar]
- 3. Mann-Salinas EA, Baun MM, Meininger JC et al. Novel predictors of sepsis outperform the American Burn Association sepsis criteria in the burn intensive care unit patient. J Burn Care Res 2013;34:31–43. [DOI] [PubMed] [Google Scholar]
- 4. Greenhalgh DG. Sepsis in the burn patient: a different problem than sepsis in the general population. Burns Trauma. 2017;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenhalgh DG, Saffle JR, Holmes JHt et al. American burn association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776–90. [DOI] [PubMed] [Google Scholar]
- 6. Housinger TA, Brinkerhoff C, Warden GD. The relationship between platelet count, sepsis, and survival in pediatric burn patients. Arch Surg 1993;128:65–6; discussion 66. [DOI] [PubMed] [Google Scholar]
- 7. Wolf SE, Jeschke MG, Rose JK, Desai MH, Herndon DN. Enteral feeding intolerance: an indicator of sepsis-associated mortality in burned children. Arch Surg 1997;132:1310–3; discussion 1313. [DOI] [PubMed] [Google Scholar]
- 8. Rehou S, Mason S, Burnett M, Jeschke MG. Burned adults develop profound glucose intolerance. Crit Care Med 2016;44:1059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rech MA, Mosier MJ, Zelisko S, Netzer G, Kovacs EJ, Afshar M. Comparison of automated methods versus the American Burn Association sepsis definition to identify sepsis and sepsis with organ dysfunction/septic shock in burn-injured adults. J Burn Care Res 2017;38:312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Endorf FW, Gamelli RL. Inhalation injury, pulmonary perturbations, and fluid resuscitation. J Burn Care Res 2007;28:80–3. [DOI] [PubMed] [Google Scholar]
- 11. Rafla K, Tredget EE. Infection control in the burn unit. Burns. 2011;37:5–15. [DOI] [PubMed] [Google Scholar]
- 12. Cassidy JT, Phillips M, Fatovich D, Duke J, Edgar D, Wood F. Developing a burn injury severity score (BISS): adding age and total body surface area burned to the injury severity score (ISS) improves mortality concordance. Burns. 2014;40:805–13. [DOI] [PubMed] [Google Scholar]
- 13. Ceniceros A, Pértega S, Galeiras R et al. Predicting mortality in burn patients with bacteraemia. Infection. 2016;44:215–22. [DOI] [PubMed] [Google Scholar]
- 14. Li BG, Hsu WS, Shih TS. Causes of death in aged burn patients: analysis of 36 cases. Burns. 1990;16:207–10. [DOI] [PubMed] [Google Scholar]
- 15. Shirani KZ, Pruitt BA Jr, Mason AD Jr. The influence of inhalation injury and pneumonia on burn mortality. Ann Surg 1987;205:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams FN, Herndon DN, Hawkins HK et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care 2009;13:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heng JS, Clancy O, Atkins J et al. Revised Baux Score and updated Charlson comorbidity index are independently associated with mortality in burns intensive care patients. Burns. 2015;41:1420–7. [DOI] [PubMed] [Google Scholar]
- 18. Sharma BR, Harish D, Singh VP, Bangar S. Septicemia as a cause of death in burns: an autopsy study. Burns. 2006;32:545–9. [DOI] [PubMed] [Google Scholar]
- 19. Nitzschke S, Offodile AC 2nd, Cauley RP et al. Long term mortality in critically ill burn survivors. Burns. 2017;43:1155–62. [DOI] [PubMed] [Google Scholar]
- 20. Lorente JA, Vallejo A, Galeiras R et al. Organ dysfunction as estimated by the sequential organ failure assessment score is related to outcome in critically ill burn patients. Shock. 2009;31:125–31. [DOI] [PubMed] [Google Scholar]