Abstract
Human adenovirus (HAdV) commonly causes infections of respiratory, gastrointestinal, genitourinary, and ocular surface mucosae. Although most adenovirus eye infections are mild and self-limited, specific viruses within human adenovirus species D (HAdV-D) are associated with epidemic keratoconjunctivitis (EKC), a severe and highly contagious ocular surface infection, which can lead to chronic and/or recurrent, visually disabling keratitis. In this review, we discuss the links between adenovirus ontogeny, genomics, immune responses, and corneal pathogenesis, for those viruses that cause EKC.
Keywords: Conjunctivitis, human adenovirus, epidemic keratoconjunctivitis, genomics, homologous recombination, inflammation, ocular infection, pink eye, tropism, virus evolution
Introduction
Adenoviruses are currently classified by their ancestral host, genomic organization, GC-content, DNA polymerase amino acid sequence distances, and oncogenic properties.[1] There are currently more than 100 recognized human adenovirus (HAdV) genotypes, differentiated by phylogenomics into seven species (A-G). HAdVs contribute to a variety of disease conditions, including respiratory, gastrointestinal, genitourinary, and ocular infections. Given the capacity of adenoviruses to infect and replicate in disparate cell types, adenovirus-derived vectors are commonly used in vaccination and gene therapy applications. Adenovirus research has also played an important role in the study of human biology, where the mechanisms of viral oncogenesis,[2,3] RNA splicing,[4,5] eukaryotic DNA replication,[6] antigen presentation, and immune evasion,[7] were first discovered and/or extensively studied using this model agent. Adenovirus is also a pathogen of increasing importance.
Infections likely due to adenovirus were originally described in the 1880’s but not formally associated with a specific viral etiology until the early 1950’s, following the first identification of the adenovirus. A group of viruses isolated from human adenoids and tonsils, and from pharyngeal and conjunctival secretions obtained from acute infections, was initially characterized as adenoidal-pharyngeal-conjunctival (A.P.C.) viruses.[8-10] In an attempt to isolate “the virus of the common cold”, Wallace Rowe and colleagues [11] made in 1953 the seminal isolation of adenovirus from latently infected human adenoids in organotypic culture. Subsequent studies revealed the fundamental physical features of HAdV, its distinct disease associations, and its capacity for persistent infection.
New viral lineages evolve to overcome host selection pressures and to adapt to new hosts, potentially leading to extended tissue tropisms and more virulent infections. HAdV evolves principally by homologous recombination, with a relatively higher recombination propensity within HAdV species D (HAdV-D) than for other species.[12] Not coincidentally, HAdV-D is the most prevalent of the seven species, and to date comprises 73 (71%) of the 103 validated types. The majority of viruses within HAdV-D do not cause clinically significant eye infections. However, a few specific types within HAdV-D are associated with epidemic keratoconjunctivitis (EKC), a severe and highly contagious ocular surface infection. The major EKC pathogens are HAdV-D8, 37, 53, 54, 56, and 64,[10,13-17] with three of these identified in the last decade. Despite the continued emergence of new viruses, the molecular mechanisms that drive recombination and adenovirus ontogeny are not precisely known. Clues from epidemiological investigations, including the emergence of numerous new members of HAdV-D in AIDS patients and those otherwise immunosuppressed,[18-21] suggests that an impaired host-defense either contributes to or is permissive of homologous recombination and the evolution of new adenoviruses.
A major drawback of adenovirus-mediated gene delivery is the activation of type-specific acquired immune responses that result in the loss of vector genomes soon after vector administration.[22] Since the majority of the human population has been exposed and is therefore immune to the more prevalent HAdV types, pre-existing immunity limits their use as adenoviral vectors for vaccination and gene therapy. Additionally, the absence of immunologic memory is important for successful use of adenoviral vectors. Therefore, HAdV types with relatively low seroprevalence, including A31,[23] B35,[24,25] C6,[26-28] D26,[29-31] E4,[32-34] other even less common HAdV types,[35] and simian adenoviruses,[36-38] have attracted attention as potential vector chassis for molecular therapies. In contrast, an innate immune response to adenovirus-based vaccine vectors appears to be critical to development of acquired immunity to the epitope expressed by the transgene.[39] In naturally infected individuals without preexisting exposure and humoral immunity, innate immune mechanisms also typically underlie clinical disease presentations. In EKC, simple binding of the virion to its host target cell is sufficient to initiate inflammatory responses,[40,41] suggesting that initial host immune responses to the physical virion contribute significantly to ocular pathogenesis. Because host immune responses appear important for adenoviral evolution, disease pathogenesis, and vector implementation, understanding the activation of virus induced inflammatory responses is a paramount undertaking.
In this review, we begin with the story of the discovery of HAdV and its association with ocular infection following a very large outbreak involving more than 10,000 cases during the Second World War. We then focus on the genomics and biology of HAdV evolution with special attention to EKC viral pathogenesis, and illustrate in vitro and in vivo models of corneal tropism and immune responses. Finally, we highlight the scope of HAdV-D evolution and its impact on ocular pathogenesis.
History of epidemic keratoconjunctivitis – the story of shipyard eye
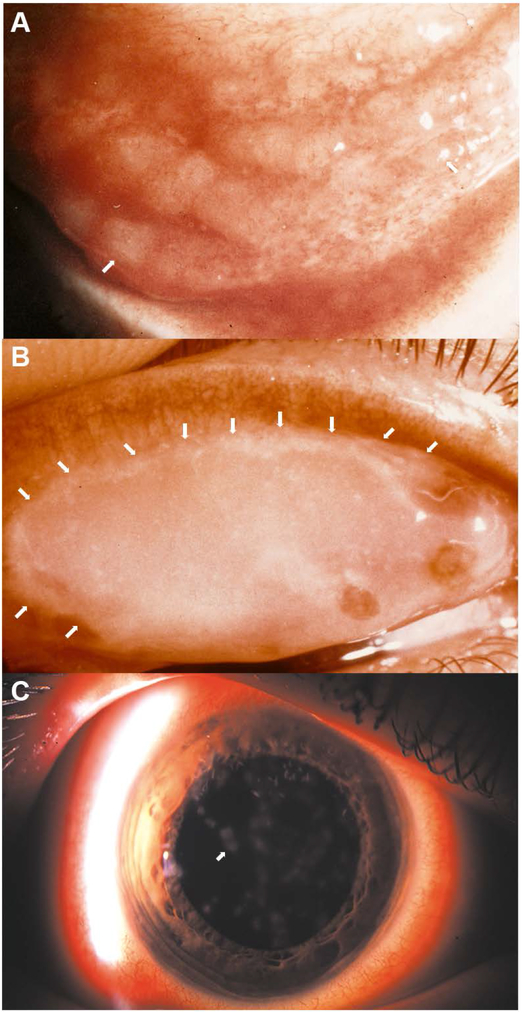
Adenovirus infection is one of the most common causes of conjunctivitis world-wide. EKC is a particularly severe form, that as can be discerned by its name, includes corneal inflammation. EKC was first described in Austria in 1889,[42] and although suspected over the years to have a viral etiology, was attributed to various other causes due to lack of techniques for virus characterization. The classic triad of clinical signs in EKC consists of membranous conjunctivitis, corneal epithelial erosions, and delayed-onset corneal subepithelial infiltrates (Fig. 1). Other common signs and symptoms of adenovirus conjunctivitis include conjunctival edema, redness, and discharge, eye pain, and blurred vision.[43] In 1930, Wright reported over 900 cases in an epidemic in Madras, India and then experimentally transmitted the disease to unwitting volunteers using filtered conjunctival washings with a filter size too small to pass bacteria or other nonviral pathogens.[44,45]
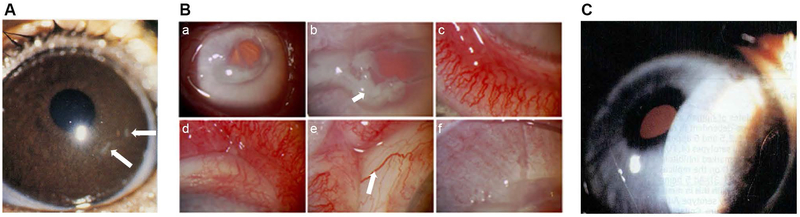
Fig. 1.
Photomicrographs of common clinical manifestations of ocular surface in epidemic keratoconjunctivitis (EKC). (A) Inferior conjunctival fornix of a patient with acute EKC shows conjunctival lymphoid hyperplasia presenting as jelly bean-shaped milky elevations of the mucosa (arrows point to two out of many follicles). (B) Superior eyelid tarsal surface with a conjunctival membrane (with visible margins of the membrane delineated by white arrows). (C) Corneal subepithelial infiltrates (arrow points to one of many such infiltrates). Image in C reproduced with permission.[264]
In 1941, a large outbreak with more than 10,000 cases occurred in the naval shipyards of Pearl Harbor, Hawaii. Thereafter, several thousand cases were seen in San Francisco, with most of the affected persons working in shipyards. Thus, the name “shipyard eye” was tied to the affliction, indicating a suspected occupational association. In 1942, Hogan and Crawford coined the name “epidemic keratoconjunctivitis” after an investigation of 125 cases.[46] However, the name “shipyard eye” remained popular even after other industrial workers were found to be affected.[47] In 1954 at the University of California, San Francisco, a new adenovirus was identified from the eye of a seaman named Trim. This virus was the eighth HAdV isolated with a unique serum neutralization profile, and was named serotype 8, strain Trim,[48] the latter reflecting a naming practice that today would violate patient privacy regulations. The index case presented with acute conjunctivitis, and developed subepithelial corneal opacities typical of EKC. Ten days after the initial contact, the patient’s nurse contracted a similar infection and also developed typical corneal opacities.[48] By 1957, 80% of EKC cases were documented as associated with adenovirus type 8.[49] Mitsui and coworkers [50] subsequently inoculated HAdV-D8 into the eyes of human volunteers who then showed typical clinical and serological responses. HAdV-D8 was re-isolated from the study subjects, thus adding evidence for an etiological role of HAdV-8 in EKC. In Jawetz’s classic description of shipyard eye,[43] he described EKC as an iatrogenic infection spread principally by contamination at ophthalmological clinics; this description was mirrored by the observations of others.[51-53] Today, despite standard infection control protocols mandated by clinic and hospital regulations, adenovirus outbreaks from ophthalmologic equipment continue to occur in health care settings.[54,55] Extracellular adenovirions are extremely stable and can persist at room temperature for weeks, suggesting that epidemics of adenovirus conjunctivitis and EKC will continue to evade routine prevention measures.
Human adenovirus-disease associations
HAdV infections tend to show species and type specificities. HAdV species B, C and E are associated principally with respiratory disease.[56-58] The illness appears flu-like with symptoms of pneumonia, croup, and bronchitis. HAdV-B, E and several EKC viruses within HAdV-D, also cause self-limited urinary tract infections, presenting as painful passage of blood-stained urine.[59-63] HAdV-F causes gastroenteritis, with severe diarrhea, vomiting, and fever. HAdV-G52, the sole virus within HAdV species G, was also isolated from a patient with gastroenteritis, but the latter association remains unconfirmed [64-66]. Most infections involving HAdV-F occur in young children.[67,68] Adenovirus infections are also a major concern in immunocompromised individuals.[20] In particular, HAdV-A, B, and C have all been associated with infections of allogenic transplant recipients, and with a high mortality rate.[69] HAdV-B, D and E can all cause ocular infections.[70-72]
HAdV infection of the ocular surface presents as one of three main clinical syndromes: simple follicular conjunctivitis, pharyngoconjunctival fever, or EKC. The first two conditions are self-limited and do not result in long term sequelae. However, the keratitis associated with EKC can be chronic and/or recurrent in up to one-third of cases.[73] Follicular conjunctivitis or “ pink eye” is an inflammation of the conjunctiva in which hyperplasia of conjunctival lymphoid follicles, a tertiary lymphoid tissue, is prominent (Fig. 1A). In addition to nonspecific signs of ocular redness and watery discharge, follicular conjunctivitis is characterized by palpable, tender, regional (preauricular) lymphadenopathy. These signs are also common to other acute viral infections of the ocular surface. Adenoviral conjunctivitis is particularly contagious, and involvement in one eye may spread within several days to other eye, to family members, and to coworkers. Acute hemorrhagic conjunctivitis is a variant of acute follicular conjunctivitis, in which subconjunctival hemorrhages are a prominent feature. This infection is caused by HAdV-B11, enterovirus 70, and coxsackie virus A24 variant.[74-76] Although adenovirus infection of the conjunctiva is typically self-limited, serious and potentially fatal consequences can occur in immunocompromised individuals. A recent outbreak of conjunctivitis due to HAdV-B3 infection in a neonatal intensive care unit affected 23 neonates and nine adults, and was found to have originated from use of contaminated ophthalmologic equipment.[54,55] Eleven neonates (48%) and all nine adults had conjunctivitis symptoms, with the same genotype of HAdV-B3 identified as the causal agent across all cases. Four neonates died with respiratory infection complicating their underlying conditions.
Pharyngoconjunctival fever is characterized by the triad of fever, pharyngitis, and acute follicular conjunctivitis, and causes epidemics in school-aged children.[77-79] This infection is caused by HAdV-B and E, and presents as a flu-like illness with conjunctivitis. Although many viral respiratory infections are associated with concomitant follicular conjunctivitis, in pharyngoconjunctival fever, the follicular conjunctivitis is particularly severe.[80,81] The lacrimal drainage system connects the ocular surface with the nasopharyngeal and respiratory mucosa,[82] possibly accounting for infection at both sites.
The third major adenovirus ocular syndrome, EKC, is the only infection that also involves the cornea.[9,46,48] It is caused mainly by viruses within HAdV-D but is also – less commonly – associated with other adenoviruses, including HAdV-B3, B7, C2, and E4, as well as other as yet unidentified agents.[83,84] EKC is characterized by acute membranous conjunctivitis (Fig. 1B) and delayed-onset subepithelial corneal infiltrates (Fig. 1C). The third component classically associated with the infection, acute corneal epithelial erosions, does not occur in most cases.[73]
Human adenovirus species and type specific features and genomic correlations
Given HAdV species and type specific disease associations, it is reasonable to assume the existence of genomic features that correlate specifically with tropism and virulence. The linear HAdV genome is ~36 kb in length and largely conserved with average nucleotide identities of 84.5-94.8% across species.[85] HAdVs commonly contain nearly 50 known and hypothetical genes.[86,87] The three major capsid proteins (penton base, hexon, and fiber), four minor cement proteins (IIIa, VI, VIII, and IX), six other proteins (V, VII, μ, IVa2, terminal protein, and adenovirus protease), and the viral dsDNA molecule, constitute the physical virion.[88] Adenovirus capsid protein structures share an ancient common ancestor with bacteriophage PRD1, suggesting remote evolutionary relationships.[89,90] Both capsids are organized on a T=25 (pseudo) icosahedral symmetry, the only known models of this arrangement.[91-95] The HAdV hexon protein is closely related to the bacteriophage PRD1 P3 protein; both contain a beta sandwich with four-stranded sheets arranged in a jelly-roll fold.[96] The hexon protein comprises the majority of the capsid with 240 hexon trimers. Twelve penton base pentamers in a ring configuration form the vertices of the icosahedron. A trimeric fiber protein protrudes from each penton base capsomer.
The hexon gene contains seven hypervariable regions (HVRs) comprising (specifying) two adjacent hypervariable loops (HVLs), L1 and L2 on the hexon protein. These HVLs together form the epsilon (ε) determinant, consisting of five serotype-specific B-cell epitopes, that is responsible for serum neutralization.[97-99] Although hexon is the most abundant adenoviral structural protein, it is the trimeric fiber protein that is critical to initial binding of the virion to one of several potential host cell receptors, including the coxsackie adenovirus receptor (CAR), membrane cofactor CD46, and GD1a glycan.[100-105] The fiber protein consists of an N-terminal tail, a central shaft of repeating sequences, and a C-terminal globular knob domain. The three conserved N-terminal tails of the fiber trimer bind to the penton base capsomer.[106] The central shaft domains consist of varying β-repeats that are highly rigid and stable,[107] and connect the N-terminal tail with the c-terminal fiber knob. The fiber knob is the first molecule to interact with the host cell during initial infection, and is also known as the gamma (γ) determinant,[108,109] because antibodies to the fiber knob inhibit hemagglutination of red blood cells in serological assays.[110] Historically, this was used as an accompaniment to typing by serum neutralization, as it permitted the identification of putatively recombinant viruses in which serum neutralization suggested one type while hemagglutination inhibition pointed to a different type. Such viruses were then referred to as “intertypic”,[19,111-113] a term now obsolete given recent revelations about the role of homologous recombination in HAdV evolution.[114] Structurally, the penton base protein has a basal jelly roll domain similar to that of the hexon,[106] and includes a crucial Arg-Gly-Asp (RGD) motif on one of its two hypervariable loops, HVL2, that interacts with αvβ1, αvβ3, and αvβ5 integrins.[115-117] This interaction in turn induces secondary intracellular signaling that is necessary for viral internalization.[115] Species F enteric HAdVs (F40 and F41), that lack the RGD motif, instead utilize laminin-binding α2, α3, and α6 integrins.[118,119] Both penton base and fiber knob proteins elicit antibody responses that have been shown to have a synergistic effect in neutralizing assays.[120]
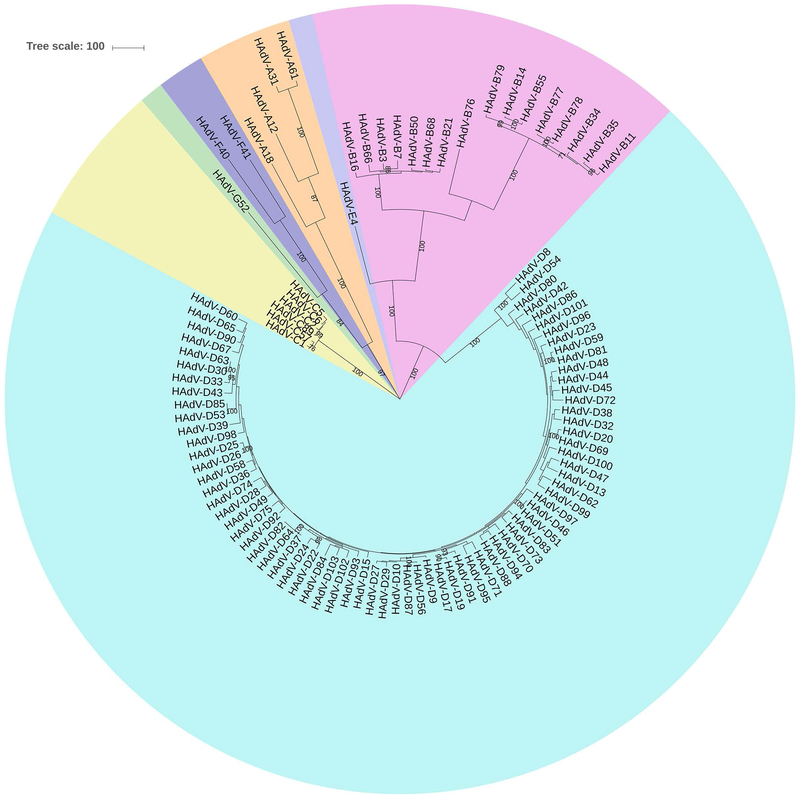
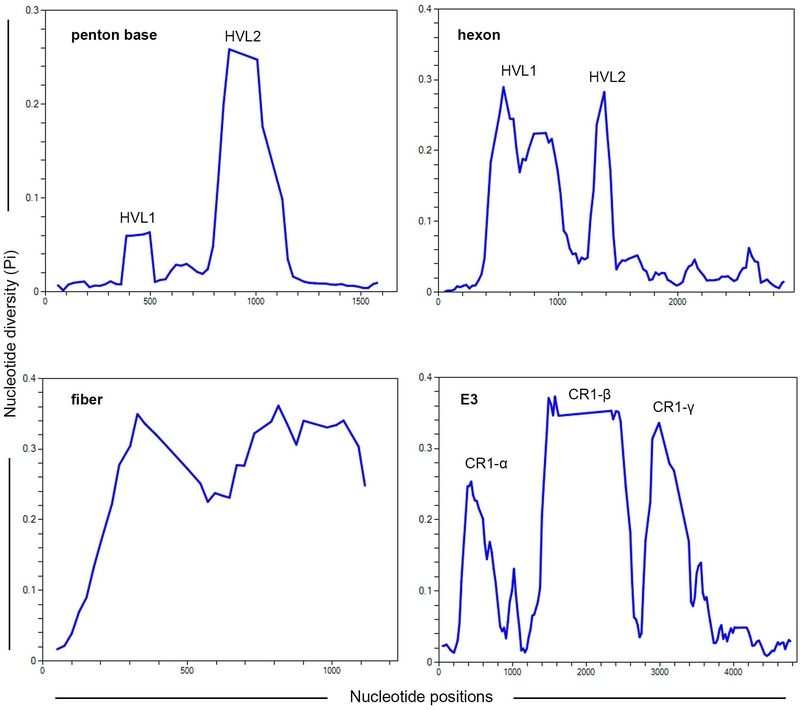
A phylogenetic analysis of the highly conserved DNA polymerase gene is shown (Fig. 2) for all 103 currently recognized HAdV genotypes. Of the seven HAdV species, HAdV-D contains the most types, but this may be due to ascertainment bias. However, HAdV-D also shows distinct genomic features that distinguish it from the other species.[12] For DNA polymerase, the types within the HAdV-D clade show a close evolutionary relationship with very short nucleotide distances between types. However, the relatively high conservation among HAdV-D genomes, as exemplified by the DNA polymerase gene, is interrupted by hypervariability at particular regions of the genome, specifically at the three major capsid genes and at three genes within the E3 transcription unit, E3 CR1α, β, and γ (Fig. 3).
Fig. 2.
Phylogenetic analysis of human adenovirus (HAdV) DNA polymerase gene, including 103 genotypes, for HAdV species A-G. The tree was constructed in MEGA7 using the Maximum parsimony analysis with a bootstrap test of 500 replicates. HAdV-D is the largest species. The tree was obtained using the Tree-Bisection-Regrafting (TBR) algorithm with search level 1 in which the initial trees were obtained by the random addition of sequences (10 replicates). The tree is drawn to scale with branch lengths calculated using the average pathway method and is in the units of the number of changes over the whole sequence. All positions with less than 95% site coverage were eliminated and there were a total of 3270 positions in the final dataset.
Fig. 3.
Nucleotide diversity Pi (π) plots showing the average number of nucleotide differences per site for the known hypervariable regions in HAdV-Ds, including penton base, hexon, fiber, and E3 CR1 genes, and performed with all 73 HAdV-D genotypes. The plot was constructed using DnaSP v6 with 100 nt window and 25 nt step size (gaps are excluded).
By comparison of whole genome sequences, marked differences are seen between species in the major capsid genes.[85] For example, HAdV-A and B penton base genes are largely hypervariable across their entire coding region, while the HAdV-C penton base gene is highly conserved. Uniquely, HAdV-D penton base genes are characterized by general conservation interrupted by two HVRs approximately 450 nucleotides apart. These code for penton base HVL1 and HVL2. HVL2 contains the RGD motif critical to viral internalization,[106] including for those viruses associated with EKC.[117,121] HAdV-D60, which was cultured from a 4 year-old child with bronchiolitis, has a unique deletion of the RGD motif.[122] The HAdV-D60 penton base gene is otherwise highly similar to that of HAdV-D37, a major EKC pathogen. HAdV-D60 productively infected human corneal epithelial cells in vitro, but more slowly, and paradoxically induced more severe disease in the mouse model of adenovirus keratitis (discussed below), with increased leukocyte infiltration into the cornea and elevated cytokine expression when compared to HAdV-D37. It may be that the RGD motif is important but dispensable for viral entry, and that delayed viral entry and trafficking [123] are conducive to enhanced host cell innate immune responses, as has been previously suggested.[124]
Homologous recombination of the hexon HVRs appears to be a major mechanism of adenovirus evolution. Hexon recombination between the renal pathogen HAdV-B11 and the respiratory pathogen HAdV-B14 generated the Trojan horse HAdV-B55, a severe respiratory pathogen on a B14 chassis but with the hexon (and associated humoral immune responses) of B11.[125-127] Thus HAdV-B55 avoids herd immunity to HAdV-B14, while retaining its respiratory pathogenicity.[128-131] Recently isolated and genome-characterized in China, HAdV-B55 causes severe acute respiratory disease in immunocompetent adults world-wide,[132-134] and of sufficient severity to warrant efforts to include it in a vaccine against other fatal respiratory adenoviruses, HAdV-B3 and B7.[135,136] In contrast to marked hypervariability within HVRs of the HAdV-D penton base gene and the entire fiber gene (see below), nucleotide diversity in the HAdV-D hexon gene HVRs is comparatively less in HAdV-Ds as compared to the other HAdV species.[137]
The HAdV fiber gene is entirely hypervariable across all species except HAdV-F, which has two fiber genes. Fiber length is largely due to the variable numbers of β-repeats in the central shaft domain. HADV-B and D fiber genes are shorter and therefore code for shorter fiber proteins than those of HAdV-A or C. Length of the fiber has been inversely correlated with fiber rigidity and hindrance of receptor binding by the fiber knob,[138,139] but shorter fibers are more permissive of docking between penton base RGD and host cell integrins and of subsequent host cell entry without requiring fiber knob binding.[140,141] In comparison to other species, HAdV-D utilizes CD46 and GD1a glycan as primary host cell receptors.[104,105] CD46 also is the major cellular receptor for HAdV-B.[142,143] It was demonstrated that although the motif for CAR binding is present on the HAdV-D37 fiber knob, HAdV-D37’s relatively short and more rigid fiber protein provides steric hindrance that restricts interaction with CAR.[139] Binding to CAR may be further impeded by extended, semi-flexible loop structures on the HAdV-D37 fiber knob, as also shown for HAdV-D26 and D48.[144]
Homologous recombination in human adenovirus evolution
Homologous recombination between adenovirus genomes was first discovered in the 1970’s by two different groups, with HAdV-C5 and HAdV-A12 as test viruses.[145,146] The first study employed complementation tests in which cells were doubly infected with wild type and temperature sensitive (ts) mutants to reveal recombination. The second study utilized co-infection of parental cytopathic virus (cyt+) and cyt mutants;[147] after recombination cytopathogenicity was conferred to the mutants. There is now general recognition of a link between DNA replication and recombination.[148-153] Recombination occurs soon after the initiation of DNA replication, increases with time after infection, extends into the late phase of infection, and takes place at multiple sites within a single genome.[154-156] It has been suggested that the process of adenovirus DNA replication directly promotes homologous recombination.[157] Two models for adenovirus recombination were proposed: the Meselson and Radding strand invasion model of recombination, and recombination mediated by complete strand annealing and mismatch repair.[153] However, to date the mechanisms that drive homologous recombination are not completely understood.
It appears from multiple studies that viruses within HAdV-D undergo homologous recombination more frequently than other species, often with important clinical consequences. This also can generate confusion about the infecting strain identity (type) when serology or limited molecular typing methods are used. For example, an outbreak of EKC was reported with a virus that serotyped as HAdV-D22, not otherwise known to be a causative agent for EKC. Limited molecular typing showed hexon identity with D22 but fiber knob identity with HAdV-D8, suggesting a novel recombinant.[158] Subsequent whole genome sequencing demonstrated a genome chassis of HAdV-D37 and D8, with only a small contribution from HAdV-D22, specifically the region of the hexon gene encoding HVL1 and 2 responsible for seroimmunity. Typed as HAdV-D53,[14] this virus was the second to be acknowledged by the adenovirus community as something beyond a mere “intertypic”, a term imputed by many as implying illegitimacy or something uncommon, i.e., not a distinct viral entity. Previous to HAdV-D53, HAdV-D29 was the sole “intertypic” allotted a serotype number by sero-taxonomists.[159] HAdV-D15 and D29 exhibit similar serum neutralization responses but different hemagglutination inhibition reactions, suggesting similar hexon proteins but different fibers. HAdV-D53 was also only the second virus acknowledged as unique based on genomics, after HAdV-G52,[65] which was sufficiently distinct to also justify its designation in a (new) seventh species (G).
To complete the study of HAdV-D53, the D22 genome was also fully sequenced, and analysis of the D22 penton base disclosed high identity between HAdV-D22 penton base HVR1 with that of HAdV-D37, and between HAdV-D22 penton base HVR2 (RGD loop) with that of HAdV-D19.[160] Penton base gene recombination also was then identified in many other viruses within HAdV-D, suggesting a recombination hotspot between the two penton base HVRs.
Further evidence for the importance of homologous recombination as a mechanism of adenovirus evolution arose from whole genome sequencing and analysis of another emergent ocular pathogen, HAdV-D56.[16] HAdV-D56 was associated with a lethal pneumonia in a neonate and keratoconjunctivitis in three caregivers.[161] The viral isolate was initially identified as similar to HAdV-D15/29 by sequencing of hexon HVR1-6 and as HAdV-D9 by hemagglutination inhibition. Subsequent whole genome sequencing and computational analysis demonstrated 100% identity between the virus isolated from the neonate and virus isolated from one of the caregivers with EKC.[16] HAdV-D56 was shown to have a novel penton base HVR1, the penton base HVR2 of HAdV-D9, the hexon gene of HAdV-D15, and the fiber gene of HAdV-D9. A later study identified a fourth virus with the HAdV-D15 hexon gene, HAdV-D69; as with HAdV-D29 and D56, D69 was otherwise disparate for the remainder of its genome.[162]
The prototype HAdV-D19 (HAdV-D19p), originally isolated in 1955,[163] was not an EKC virus. However, a virus isolated from patients with EKC in the 1970’s also serotyped as type 19 (HAdV-D19a),[164-166] and was only later whole genome sequenced.[167] Recombination analysis showed that HAdV-D19a contained the penton base gene of HAdV-D22, the hexon gene of HAdV-D19p, and the fiber gene of HAdV-D37, with the majority of genome identical to HAdV-D37 (98.6% overall identity).[17] This led to its reclassification as HAdV-D64. In experimental infections, HAdV-D64 was shown to enter corneal epithelial cells and cause keratitis in the mouse model, while HAdV-D19 did not. HAdV-D64 remains the most common cause of EKC in many parts of the world. These discoveries underscore the importance of whole genome sequencing to establish unique identities of new viruses, and the role of homologous recombination in HAdV-D evolution and EKC pathogenesis.
The HAdV E3 transcription unit codes principally for proteins that mediate immune evasion by the virus,[168-171] including down-regulation of interferon signaling, interference with tumor necrosis factor-mediated cytotoxicity, and prevention of antigen presentation to cytotoxic T lymphocytes. Homologous recombination within the HAdV-D E3 transcription unit was identified as common and specific to the E3 CR1α, CR1β, and CR1γ genes.[172] A notable instance of HAdV-D E3 recombination was identified in a mixed infection with two different viruses containing same hexon, HAdV-D29 and HAdV-D30, in which a single recombination event just prior to the E3 transcription unit resulted in a novel virus, HAdV-D63, with the fiber gene of D29.[173] Remarkably, every unique HAdV-D analyzed to date shows evidence for at least two recombination events.[12] It is noteworthy that the major recombination sites within HAdV-D all code for proteins that interact with the immune system, either by direct interaction between the major capsid proteins and antibodies and/or T cells,[174] or indirectly through modulation of immune functions, as with the E3 encoded proteins. These observations suggest that recombination is an important means of adenoviral adaptation to host immune pressures. Further, although the current typing methodology accepted by GenBank defines a new HAdV type by molecular typing of the three major capsid genes, the addition of E3 identities would undoubtedly lead to an even more granular differentiation between HAdV genomes.
Historically, homologous recombination was thought to occur within HAdV species but not between them. However, interspecies recombination has been demonstrated for HAdV-E4,[175] the only member of species E. The HAdV-E4 genome was shown to have laterally transferred its hexon gene to the genome of HAdV-B16. The HAdV-E4 genome is highly similar to a simian (chimpanzee) adenovirus (SAdV)-E26. Subsequently, two highly similar isolates of another virus, SAdV-B35, one isolated from a chimpanzee and the other from a bonobo, were shown to share genomic regions with SAdV-B21 and SAdV-B27, but also had regions of high similarity to HAdV-B21 and HAdV-B16, suggesting prior zoonotic or anthroponotic transmission with subsequent recombination events.[176] Since both HAdV-B21 and -B16 are human respiratory pathogens, it was suggested that SAdV-B35 could be a nascent human respiratory pathogen. This idea proved prescient when a recent analysis of an adenovirus originally isolated from a fatal respiratory infection in a 6 year-old boy in 1965 revealed a virus genome nearly identical to SAdV-B35.[177] This inter-species recombinant was typed as HAdV-B76. In contrast, no HAdV-D genome has shown evidence for recombination with other HAdV species or with adenoviruses from simian hosts.
Molecular mechanisms underlying HAdV-D recombination in the penton base gene
As discussed above, binding of the RGD motif on penton base HVL2 to host cell integrins mediates intracellular signaling and viral internalization. Using a mouse model (described below), injection of a 15-mer synthetic RGD-containing peptide into the cornea simultaneous to viral infection competitively inhibited HAdV-D37 keratitis, while injection of a RGD-replaced control peptide had no effect.[178] This suggested a vital role for penton base RGD interaction with corneal cells as a determinant of corneal pathogenesis in EKC. In contrast, HAdV-D60 with a natural deletion of the RGD loop induced significantly more inflammation in the mouse keratitis model.[122] However, the delayed entry and slower trafficking of HAdV-D60 as compared to that of HAdV-D37 may have initiated a more sustained intracellular signaling cascade, as has been suggested to be important to chemokine gene expression induced by adenovirus infection.[179] Regardless, the relatively high frequency of penton base recombination in HAdV-D suggests substantial host pressure and an important role for the HAdV-D penton base protein in viral pathogenesis.
The penton base gene HVRs 1 and 2, which code respectively for penton base protein HVLs 1 and 2, are separated by ~ 450 nucleotides. These have been shown to recombine independently of one another,[85,160] suggesting the possibility that a nucleotide recombination signal or motif might exist between HVR1 and HVR2 that facilitates homologous recombination. In several bacterial species and in bacteriophage lambda, the crossover hot spot instigator or “Chi” nucleotide sequence mediates homologous recombination using bacterial Rec enzymes.[180-183] In brief, the RecBCD protein complex with both DNA-unwinding (helicase) and DNA hydrolysis (nuclease) activities initiates recombination by binding and unwinding DNA. Upon Chi-site recognition, the RecBCD nicks the Chi-strand and then RecA enzyme is loaded to create an ssDNA-protein filament, which invades homologous dsDNA, leading to homologous recombination.[184-186] This Chi site-mediated recombination fits the aforementioned Meselson and Radding strand invasion model for adenovirus recombination.[153] Remarkably, Chi-like nucleotide sequences were identified in HAdV-Ds immediately adjacent to the 5’ side of penton base HVR2.[187] When co-infected in the presence of an E. Coli lysate containing Rec proteins, HAdV-Ds showed increased homologous recombination; with RecA depletion or with a RecA mutant, recombination was reduced. These data suggest that HAdV-D evolution might be facilitated by bacterial flora present at mucosal surfaces, particularly in the gastrointestinal and respiratory tracts, where persistence and co-infection by adenoviruses are common.[188,189]
HAdV-D genomic signatures – determinants of corneal tropism
HAdV-D15, D29, D56, and D69 all have highly similar hexon genes with otherwise disparate genomes.[162] When injected into the mouse cornea, all four types induced keratitis, but only HAdV-D56, the sole member of this group associated with EKC, was shown to infect human corneal epithelial cells and human corneal fibroblasts in vitro. Therefore, although the presence of virions in the corneal stroma, even from disparate HAdV-D types, will variably induce inflammation, tropism of specific HAdV-Ds for the cornea is clearly not determined by the hexon protein. The fiber knob is the specific ligand on the adenovirus that first engages the host target cell, and its binding is currently a prime target for experimental therapies against EKC.[190-193] Previous data showed a single amino acid substitution in HAdV-D19 fiber knob, from glutamine to lysine at amino acid 240 (numbered according to HAdV-D37), conferred binding to the immortalized Chang C human conjunctival cell line (now known to be HeLa cell contaminated).[194] A reverse substitution in the HAdV-D37 fiber knob abrogated binding. Given its importance to host cell receptor binding, a comprehensive phylogenetic analysis of HAdV-D fiber knobs revealed a pattern wherein the six EKC-associated viruses form a distinct “EKC clade” that was not seen for other genomic regions.[195] Detailed amino acid alignments showed that a lysine or alanine residue at position 240 differentiates EKC from non-EKC viruses. The ratio of nonsynonymous substitutions (dN) to synonymous substitutions (dS) identified amino acid 240 to be under positive selection (dN/dS>1). HAdV-Ds with this amino acid residue also readily entered human corneal epithelial cells in vitro. Productive infection of ocular surface cells including the corneal epithelium is presumably requisite to the development of EKC. Therefore, selection pressure in the HAdV fiber knob is a key determinant of corneal epithelial cell tropism.
Prediction of the next EKC pathogen
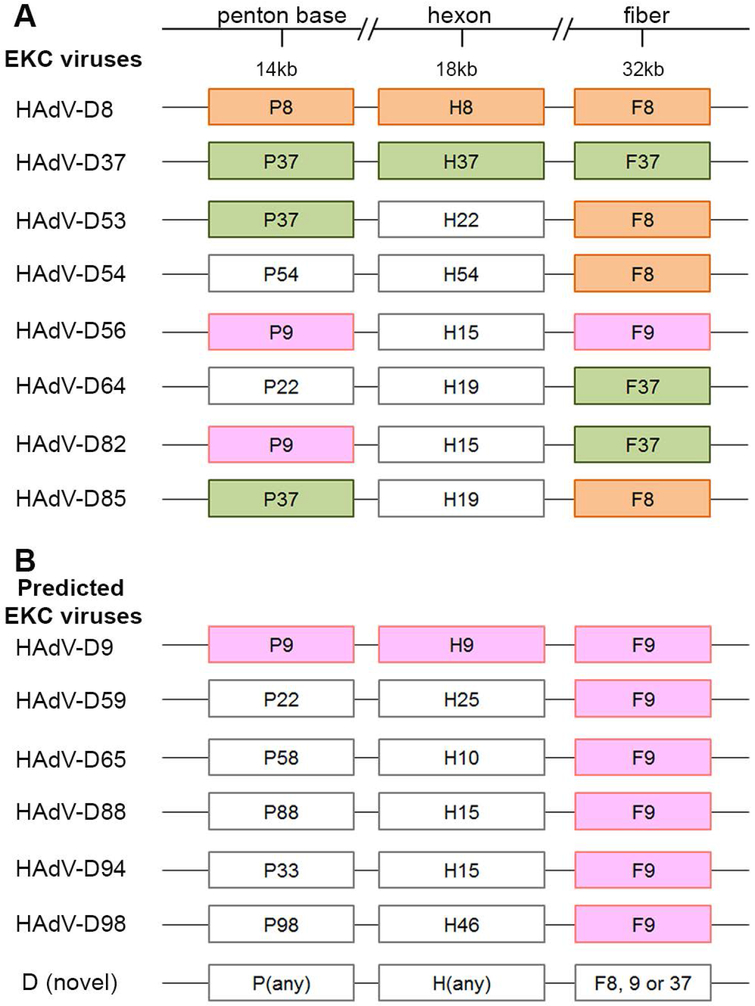
Given that the first step in corneal infection by adenovirus is fiber knob binding to its receptor on corneal epithelial cells, analysis of fiber knob binding motifs might be useful to predict which HAdVs can infect the cornea and cause EKC, even in the absence of a previously known clinical association. With only one exception, the known EKC types within HAdV-D contain the fiber gene of either HAdV-D8 or D37 (Fig. 4A). The sole exception is HAdV-D56, which through recombination acquired the fiber gene of HAdV-D9,[16] the latter not known to be a cause of EKC. Using HAdV-D8 and D37 as paradigm EKC pathogens, it was suggested that a lysine or alanine at amino acid 240 was necessary for corneal epithelial tropism;[195] HAdV-D56 (and D9) has an alanine at this site. Real-world confirmation of the association between the HAdV-D8 and D37 fiber knobs and corneal tropism, derives from analysis of HAdV-D85,[196] a recently characterized virus that caused EKC, and HAdV-D82, which was suggested to be an emerging EKC pathogen in one report.[197] Remarkably, HAdV-D85 has the fiber gene of D8, while HAdV-D82 has the fiber gene of D37. However, there must be other factors associated with the potential to cause EKC, as although HAdV-D9 readily enters human corneal epithelial cells in vitro, it has not been shown to cause EKC. Interestingly, the HAdV-D8 Trim strain housed at the American Type Culture Collection (ATCC, Manassas, VA) was found to be contaminated with HAdV-D9 and D10,[198] and both D8 and D9 were isolated together from a patient with ocular infection (not specifically characterized as EKC).[198,199] These data raise the possibility that HAdV-D9 might, in certain circumstances, cause EKC. Newer HAdV-D isolates with the HAdV-D9 fiber gene, not known to be associated with EKC, but predicted as potential EKC pathogens, are listed in Fig. 4B.
Fig. 4.
Molecular HAdV-D types known to cause epidemic keratoconjunctivitis (EKC) and those predicted to be possible causes. (A) The known EKC viruses all have a fiber gene from either type HAdV-D8, D9, or D37, with otherwise disparate hexon genes. (B) Putative EKC-causing viruses based on the aforementioned fiber gene association. It is proposed that any virus with the fiber knob of HAdV-D8, D9, or D37 is a potential cause of EKC.
Modeling host immune responses in EKC
Although the current emphasis in drug discovery for ocular surface infections by adenoviruses has been focused on the identification of antiviral drugs, managing host immune responses to the infection is likely critical to control of clinical symptoms. In this section, we briefly report findings from basic studies on adenovirus-induced cell signaling using human corneal cells. We then describe two novel models of ocular adenovirus infection: an in vitro model of the human cornea in four dimensions, and an in vivo model of adenovirus keratitis in the mouse. Experiments performed in these two models generally confirm prior observations from those performed on monolayer cell culture of human corneal cells.
Cell culture models of HAdV-D37 and D64 infection
HAdVs can be readily cultured from the corneal epithelium of infected patients.[200] However, studies of mRNA and protein expression by infected cells suggested that human keratocytes, which make up about 85% of all cells within the underlying corneal stroma, produce significantly more chemokines than human corneal epithelial cells.[41] In infected keratocytes, gene expression of CXCL8 and CCL2, key chemoattractants for neutrophils and monocytes respectively, was evident within 1-2 hours after HAdV-D64 infection,[201] prior to expression of E1A.[202] Induction of proinflammatory gene expression in HAdV-D64 infected human keratocytes is driven by a signaling cascade involving protein kinase C, Src, and focal adhesion kinase, with downstream activation of several mitogen-activated protein kinases (MAPKs) to differentially drive NFκB activation and nuclear translocation.[179,202-207] Both the duration and timing of upstream signaling determine the specifics of NFκB dimerization and transcriptional activity. Furthermore, upstream activation of the phosphoinositide 3-kinase/Akt pathway stabilizes the cell against apoptotic cell death and enables viral replication.[208] It appears that these signaling pathways are part of the same signaling network that mediates viral internalization in this cell type.[202,206,208-210]
Human cornea facsimile model of adenovirus keratitis
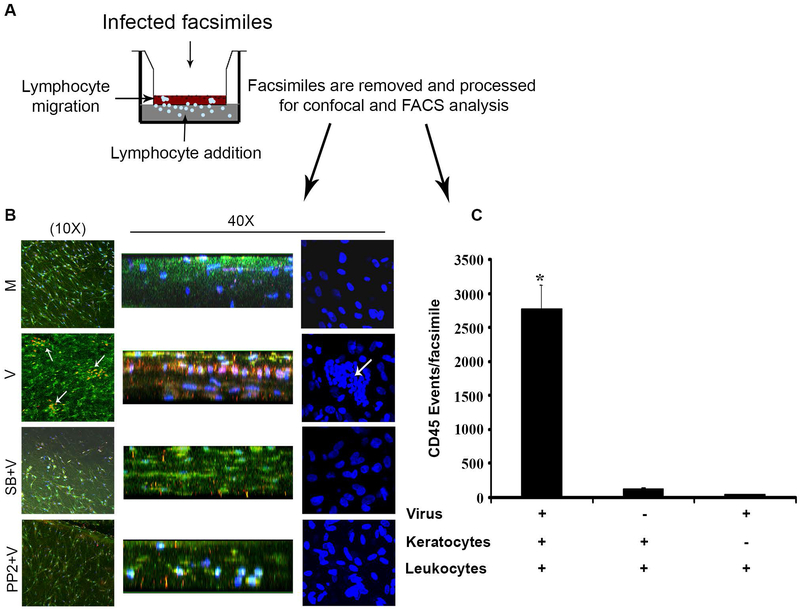
The human cornea, from anterior to posterior, consists of 1) a 5-cell layered, 50-micron thick, corneal epithelium, 2) a collagenous stroma of 500 to 1000 microns thickness, intermixed with keratocytes and a lesser population of bone marrow derived cells, mostly macrophages, 3) Descemet’s membrane and 4) a monolayered endothelium. Keratocytes assume a fibroblast phenotype in the presence of serum and are then referred as human corneal fibroblasts (HCFs).[41,211] An in vitro three-dimensional culture model of the human cornea was originally developed to study collagen degradation by keratocytes.[212] A similar system using primary, cultured, human keratocytes embedded in type I collagen and overlaid onto a tissue culture transwell insert was exploited to show the induction of CXCL8 upon HAdV-D64 infection.[41] The initial iteration of this model showed diffuse CXCL8 reactivity in the collagenous stroma that did not explain the subepithelial localization of infiltrates seen in human adenovirus keratitis. Because CXCL8 stably binds basement membranes,[213] the model was further adapted by overlaying Matrigel® on the upper surface of the collagen (Fig. 5A). Matrigel is a gelatinous protein complex secreted by cultured mouse sarcoma cells, containing many components of epithelial basement membrane, and previously shown to bind interferon gamma.[214] Immunohistochemistry performed on facsimiles overlaid with Matrigel and after overnight infection with HAdV-D37 showed foci of CXCL8 bound to the Matrigel, and colocalized to heparan sulfate.[215] Heparan sulfate is a component of corneal epithelial basement membrane [216] also present in Matrigel, and expected to bind to CXCL8 because of its negative charge.[213] This model was further extended by the addition of human peripheral blood leukocytes (PBLs) beneath the insert. After overnight infection, and just one hour after placing PBLs beneath the insert, myeloperoxidase positive neutrophils had migrated upwards against gravity to establish sub-Matrigel foci of inflammatory cells (Fig. 5B), similar to subepithelial corneal infiltrates observed in infected human patients. Pretreatment with chemical inhibitors of Src (PP2) and p38 MAPK (SB203580) completely blocked the formation of the neutrophil infiltrates. It was also shown that the virus alone in the absence of keratocytes was insufficient to induce leukocyte migration (Fig. 5C). Therefore, the human cornea facsimile model of adenovirus keratitis recapitulates the formation of subepithelial infiltrates in HAdV-D infected human cornea and confirms a role for corneal cell signaling in corneal inflammation.
Fig. 5.
Cornea facsimile model of adenovirus keratitis. (A). Facsimiles shown in the sketch were generated in 12-ml transwell plates with a 3um pore size using primary human keratocytes mixed with collagen type 1, and layered with reduced growth factor containing Matrigel®. Infection with HAdV-D37 was performed overnight, and leukocytes then added to the lower chamber for one hour prior to harvest for confocal microscopy and flow cytometry for myeloperoxidase and CD45, respectively. (B) By myeloperoxidase staining (red/orange), as shown both en face (left column) and in cross section (stacked 3-D view, middle column), infected (V) corneal facsimiles developed neutrophil infiltration, whereas mock (M) infected facsimiles did not. DAPI staining (blue) to show cell nuclei (right column) showed similar pockets of cellular infiltration only in virus infected wells. Pretreatment with inhibitors of p38 (SB203580) and Src (PP2) blocked infiltration. (C) Flow cytometry for CD45 positive cells (leukocytes) in HAdV-D37 infected facsimiles showed CD45 events only in the presence of both virus and keratocytes. Therefore, virus alone was insufficient to induce chemotaxis. Figure modified with permission from Rajaiya et al.[215]
Mouse model of adenovirus keratitis
Animal models of adenovirus keratoconjunctivitis are limited by species-specific tropisms of the virus. The cotton rat (Sigmodon hispidus) (Fig. 6A),[217,218] New Zealand White rabbit (Oryctolagus cuniculus) (Fig. 6B),[219-221] and Hollander rabbit (Oryctolagus cuniculus) (Fig. 6C),[222] have all been used in experimental studies of ocular surface infection by adenoviruses. All require both topical and intrastromal (corneal) viral inoculation. Cotton rats in particular are difficult to breed and handle, and although they develop keratitis upon HAdV-D8 inoculation, these animals have not proven tractable for immune studies. HAdV-Ds do not replicate in rabbit models. Complicating this, antibodies to rabbit immune markers are only sparsely available. With rare exception,[217] most studies in rabbits have focused on trials of antiviral agents,[219,221,223-227] rather than studies of immune responses. These limit the use of these models in studies of adenoviral immunopathogenesis. Importantly though, it was shown in the rabbit model that intrastromal inoculation of HAdV-B14 could induce keratitis despite an absence of viral replication.[228]
Fig. 6.
Cotton rat and rabbit models of adenovirus ocular infection. (A) The cotton rat model, figure adapted with permission from Tsai et al.[217] The image shown is of an adult cotton rat cornea 18 days after inoculation with HAdV-D8 (1×105 plaque-forming units). Arrows indicate subepithelial opacities observed two weeks after inoculation. (B). New Zealand White (NZW) rabbit ocular model, figure adapted with permission from Clement et al.[221] Image of Ad5-infected NZW rabbit eye at 7 days post-infection. a. Intact cornea. b. Discharge and exudates on the cornea (arrow). c. Lower eyelid inflammation/vascular dilation. d. Upper eyelid inflammation/vascular dilation. e. Prominent blood vessel (arrow) close to the caruncle of the eye. f. Corneal neovascularization. (C) Hollander rabbit corneal model, figure adapted with permission from Hauwere et al.[222] Rabbits were infected with HAdV-C5 by intracorneal and subconjunctival injections and topical instillation. The infected eye developed subepithelial opacities by day 56 post infection.
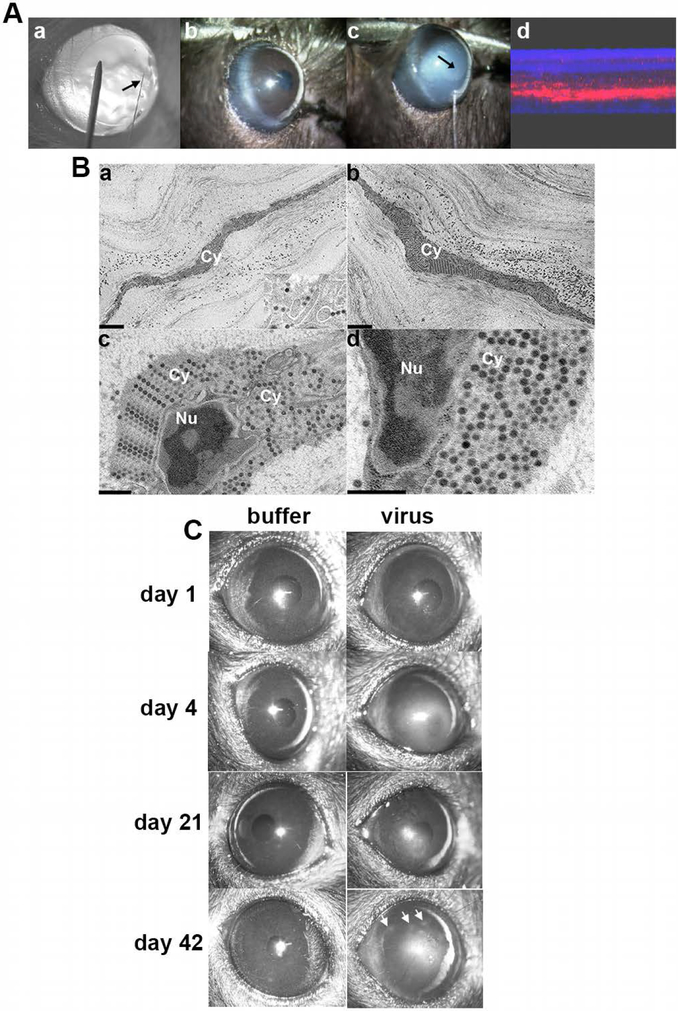
HAdV does not replicate in mice, but Eggerding and coworkers showed that early viral genes are transcribed in permissive mouse cells,[229] consistent with viral attachment and entry. Ginsberg and colleagues infected the lungs of C57Bl/6N mice with HAdV-C5 to study adenovirus pneumonia – they showed that HAdV-C5 does not replicate in the murine lung but that infection with very high titers of virus (1010 plaque forming units) can induce substantial inflammation.[230] The latter observation was applied to develop a mouse model of HAdV-D37 keratitis in which virus enters corneal stromal cells, early viral gene expression is induced, and clinical keratitis develops similar to human patients; this most optimally occurred in female C57Bl/6J mice.[231,232] Corneal injections are performed with heat-pulled glass micro-pipette needles, similar to needles used for single cell injections, and at least 105 TCID50 of purified, endotoxin free HAdV-D37 is required to reproducibly induce clinically evident keratitis (Fig. 7A). Successful corneal stromal injection is evident by transient whitening of the corneal stroma. Stromal inflammation is evident beginning at 1 day post-infection, and peaks at 4 days. Ultrastructural studies subsequently established robust viral entry into stromal keratocytes (Fig. 7B). However, capsids appeared to “stack up” in the cells, suggesting failure of capsid disassembly and a scarcity of nuclear entry.[233] It was postulated that failure of HAdV-D37 to replicate in the mouse cornea might be due to disordered intracellular trafficking or difficulty interacting with key nuclear pore proteins.[210] Inflammation is initially diffuse and then clears, but at approximately 6 weeks post-injection, a subset of mice develop corneal subepithelial infiltrates similar to patterns for recurrent adenovirus keratitis in human patients (Fig. 7C).
Fig. 7.
Mouse model of adenovirus keratitis. (A) Induction of the mouse model of adenovirus keratitis. a. BALB/c mouse cornea retroilluminated to show size of heat-pulled, glass micropipette needle (arrow) as compared to a 33 gauge metal needle. b. Diffuse illumination view of mouse cornea prior to, and c. during injection with HAdV-D37 or virus-free dialysis buffer using the glass needle (arrow) and a gas-powered microinjection system. The injection causes transient whitening of the corneal stroma (arrow points to tip of glass needle within the corneal stroma. d. Composite confocal image of a mouse cornea taken immediately after injection of Cy3 dye demonstrates successful intrastromal injection. (B) Thin-section electron microscopy of C57Bl/6j mouse corneal stroma at intermediate time points after intrastromal injection of HAdV-D37. Micrographs show the corneal stroma at a, 4 hours, and b-d, 8 hours after injection. Intracellular structures are labeled as follows: Cy, cytoplasm; Nu, nucleus. The inset in a shows a higher magnification of intracellular virus. All micrographs show densely packed intracellular viral arrays. Scale bars: 2 μm in a and b, and 0.5 μm in c and d, and the inset in a. Adapted with permission from Mukherjee et al.[233] (C) Representative clinical photographs of C57BL/6j mouse corneas after mock infection with dialysis buffer or 105 TCID HAdV-D37 at days shown post infection. Buffer-injected corneas remained clear at all times. Opacities in HAdV-D37 injected corneas were seen as early as 1 day after infection, and appeared to peak at 4 days. The opacities then regressed slowly but in approximately one-third of mice recurred as characteristic subepithelial infiltrates at about 6 weeks after infection (day 42, right panel, arrows). Figure adapted with permission from Chintakuntlawar et al.[232]
Using this model, it was shown that neutrophils constitute the first wave of infiltrating immune cells upon corneal stromal infection, and that this is due to CXCL1 expression by infected corneal stromal cells.[234] The cells productive of CXCL1 were presumed to be keratocytes although myeloid lineage cells also are present in small numbers in the corneal stroma.[235] It was then demonstrated that the HAdV-D37 capsid, specifically the penton base RGD, was a major molecular pattern responsible for the initial innate immune response to infection.[178] Activation of MyD88 and Src were identified as key to the activation of downstream proinflammatory mediators.[236]
Viral persistence, host immune suppression, and the evolution of new human adenovirus D types
Homologous recombination between HAdVs requires co-infection of the same cell by two viruses with significant homology. After infection, even at disparate physical sites, HAdV infection persists in nasopharyngeal and gastrointestinal lymphoid tissues, with continued viral shedding for months to years after the initial infection.[11,237-245] Adenovirus persistence allows continuous production of progeny virus over an extended period, albeit at lower levels than during acute infection.[246] Viral persistence increases the odds of co-infection, and indirectly promotes homologous recombination and viral evolution. A study of HAdV persistence in human T-lymphocyte cell lines provided evidence for persistence being type-dependent, suggesting there are viral genetic determinants of HAdV persistence.[247]
In 1995, Khoo and coworkers estimated the 1-year actuarial risk of adenovirus infection in persons infected with HIV to be 28%.[199] Multiple, previously unrecognized HAdV-Ds were discovered during the global AIDS epidemic, including HAdV-D43-51, D58, D59, D62, and D75.[18,248-258] In addition, numerous reports detail previously identified viruses that were found to infect AIDs patients.[21,199,248,251,258] Other novel, so-called “intertypic” HAdV-D recombinants were identified from patients immunosuppressed due to other causes.[18,19] These observations suggest that immune compromise may offer a platform for the emergence of new HAdV-D types by recombination. Using a persistent adenovirus infection model in vitro, Zheng and colleagues demonstrated that interferon gamma inhibits the association between the transactivator GABP with the HAdV EIA enhancer region, suppressing E1A expression,[259] and promoting persistent infection. In parallel experiments, blockade of interferon signaling and removal of E1A repression led to a transition from a persistent to a lytic infection. Relevant to these findings, HIV infected individuals may show a dramatic decline in interferon gamma levels at late-stages of infection.[260-262] One might predict that immunosuppressive drugs that function by depleting interferon gamma producing T and NK lymphocytes, or directly repressing cytokine signaling pathways, in addition to their association with disseminated adenovirus infections,[245] could promote the emergence of new HAdVs through enhancement of viral DNA replication. These ideas are consistent with the notion that loss of host interferon responses leads to HAdV reactivation and dissemination in immunocompromised persons,[263] and indirectly promotes the emergence of new HAdVs through homologous recombination.
Conclusions
In this review, we described the original association of HAdV with severe eye infections, identified special aspects of HAdV genomics, provided observations indicating recombination as a driving force for evolution specific to those viruses that cause severe keratoconjunctivitis, presented two unique experimental models of HAdV keratitis, and showed how the intersection of HAdV genomics and biology can illuminate concepts in HAdV ocular pathogenesis and evolution.
Acknowledgments
Funding
This work was funded by National Institutes of Health grants EY013124, EY021558, and EY014104, a Senior Scientific Investigator Award grant (to JC) from Research to Prevent Blindness, Inc., New York, NY, and the Massachusetts Lions Eye Research Fund.
Abbreviations
- CXCL8
interleukin 8
- CCL2
monocyte chemoattractant protein-1
- CsCl
ceasium chloride
- EKC
epidemic keratoconjunctivitis
- HAdV
human adenovirus
- AIDS
acquired immunodeficiency syndrome
- HIV
human immunodeficiency virus
- A.P.C viruses
adenoidal-pharyngeal-conjunctival viruses
- HVL
hypervariable loop
- HVR
hypervariable region
References
- [1].Harrach B, Benkö M, Both GW, Brown M, Davison AJ, Echavarría M, Hess M, Jones MS, Kajon A, Lehmkuhl HD, Mautner V, Mittal SK and Wadell G (2012) Family - Adenoviridae In Virus taxonomy : classification and nomenclature of viruses : ninth report of the International Committee on Taxonomy of Viruses (Andrew MQ King MJA, Carstens Eric B., and Lefkowitz Elliot J., ed.êds), pp. 125–141. Elsevier, Oxford United Kingdom, Amsterdam. [Google Scholar]
- [2].Trentin JJ, Yabe Y and Taylor G (1962). The quest for human cancer viruses. Science 137, 835–41. [DOI] [PubMed] [Google Scholar]
- [3].Huebner RJ, Rowe WP and Lane WT (1962). Oncogenic effects in hamsters of human adenovirus types 12 and 18. Proc Natl Acad Sci U S A 48, 2051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Berget SM, Moore C and Sharp PA (1977). Spliced segments at the 5’ terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A 74, 3171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chow LT, Gelinas RE, Broker TR and Roberts RJ (1977). An amazing sequence arrangement at the 5’ ends of adenovirus 2 messenger RNA. Cell 12, 1–8. [DOI] [PubMed] [Google Scholar]
- [6].Challberg MD and Kelly TJ Jr. (1979). Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A 76, 655–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burgert HG and Kvist S (1985). An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell 41, 987–97. [DOI] [PubMed] [Google Scholar]
- [8].Zaiman E (1956). The A.P.C. viruses. Postgrad Med J 32, 426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jawetz E, Kimura S, Nicholas AN, Thygeson P and Hanna L (1955). new type of APC virus from epidemic keratoconjunctivitis. Science 122, 1190–1. [DOI] [PubMed] [Google Scholar]
- [10].Jawetz E, Thygeson P, Hanna L, Nicholas A and Kimura S (1956). Antibodies to APC virus type 8 in epidemic keratoconjunctivitis. Proc Soc Exp Biol Med 92, 91–5. [DOI] [PubMed] [Google Scholar]
- [11].Rowe WP, Huebner RJ, Gilmore LK, Parrott RH and Ward TG (1953). Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 84, 570–3. [DOI] [PubMed] [Google Scholar]
- [12].Robinson CM et al. (2013). Molecular evolution of human adenoviruses. Sci Rep 3, 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Jong JC, Wigand R, Wadell G, Keller D, Muzerie CJ, Wermenbol AG and Schaap GJ (1981). Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J Med Virol 7, 105–18. [DOI] [PubMed] [Google Scholar]
- [14].Walsh MP et al. (2009). Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS One 4, e5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Akiyoshi K, Suga T, Fukui K, Taniguchi K, Okabe N and Fujimoto T (2011). Outbreak of epidemic keratoconjunctivitis caused by adenovirus type 54 in a nursery school in Kobe City, Japan in 2008. Jpn J Infect Dis 64, 353–5. [PubMed] [Google Scholar]
- [16].Robinson CM et al. (2011). Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology 409, 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou X, Robinson CM, Rajaiya J, Dehghan S, Seto D, Jones MS, Dyer DW and Chodosh J (2012). Analysis of human adenovirus type 19 associated with epidemic keratoconjunctivitis and its reclassification as adenovirus type 64. Invest Ophthalmol Vis Sci 53, 2804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hage E, Dhingra A, Liebert UG, Bergs S, Ganzenmueller T and Heim A (2017). Three novel, multiple recombinant types of species of human mastadenovirus D (HAdV-D 73, 74 & 75) isolated from diarrhoeal faeces of immunocompromised patients. J Gen Virol, Nov 2. doi: 10.1099/jgv.0.000968 [DOI] [PubMed] [Google Scholar]
- [19].Kajon AE, Lamson D, Shudt M, Oikonomopoulou Z, Fisher B, Klieger S, St George K and Hodinka RL (2014). Identification of a novel intertypic recombinant species D human adenovirus in a pediatric stem cell transplant recipient. J Clin Virol 61, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lion T (2014). Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 27, 441–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hierholzer JC (1992). Adenoviruses in the immunocompromised host. Clin Microbiol Rev 5, 262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu Q and Muruve DA (2003). Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther 10, 935–40. [DOI] [PubMed] [Google Scholar]
- [23].Hofmayer S, Madisch I, Darr S, Rehren F and Heim A (2009). Unique sequence features of the Human adenovirus 31 complete genomic sequence are conserved in clinical isolates. BMC Genomics 10, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Omosa-Manyonyi G et al. (2015). A Phase I Double Blind, Placebo-Controlled, Randomized Study of the Safety and Immunogenicity of an Adjuvanted HIV-1 Gag-Pol-Nef Fusion Protein and Adenovirus 35 Gag-RT-Int-Nef Vaccine in Healthy HIV-Uninfected African Adults. PLoS One 10, e0125954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ouedraogo A et al. (2013). A phase 1b randomized, controlled, double-blinded dosage-escalation trial to evaluate the safety, reactogenicity and immunogenicity of an adenovirus type 35 based circumsporozoite malaria vaccine in Burkinabe healthy adults 18 to 45 years of age. PLoS One 8, e78679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Capone S et al. (2006). A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J Virol 80, 1688–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Crosby CM and Barry MA (2014). IIIa deleted adenovirus as a single-cycle genome replicating vector. Virology 462-463, 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Crosby CM, Matchett WE, Anguiano-Zarate SS, Parks CA, Weaver EA, Pease LR, Webby RJ and Barry MA (2017). Replicating Single-Cycle Adenovirus Vectors Generate Amplified Influenza Vaccine Responses. J Virol, January 3;91(2). pii: e00720–16. doi: 10.1128/JVI.00720-16.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Milligan ID et al. (2016). Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. JAMA 315, 1610–23. [DOI] [PubMed] [Google Scholar]
- [30].Baden LR et al. (2013). First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 207, 240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barouch DH et al. (2013). Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001). J Infect Dis 207, 248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lubeck MD et al. (1989). Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci U S A 86, 6763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gurwith M et al. (2013). Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis 13, 238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hsu KH et al. (1992). Immunogenicity of recombinant adenovirus-respiratory syncytial virus vaccines with adenovirus types 4, 5, and 7 vectors in dogs and a chimpanzee. J Infect Dis 166, 769–75. [DOI] [PubMed] [Google Scholar]
- [35].Mennechet FJD et al. (2019). A review of 65 years of human adenovirus seroprevalence. Expert Rev Vaccines 18, 597–613. [DOI] [PubMed] [Google Scholar]
- [36].Roy-Chowdhury J and Horwitz MS (2002). Evolution of adenoviruses as gene therapy vectors. Mol Ther 5, 340–4. [DOI] [PubMed] [Google Scholar]
- [37].Colloca S et al. (2012). Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med 4, 115ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ewer K et al. (2016). A Monovalent Chimpanzee Adenovirus Ebola Vaccine Boosted with MVA. N Engl J Med 374, 1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rhee EG et al. (2011). Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J Virol 85, 315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bruder JT and Kovesdi I (1997). Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol 71, 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chodosh J, Astley RA, Butler MG and Kennedy RC (2000). Adenovirus keratitis: a role for interleukin-8. Invest Ophthalmol Vis Sci 41, 783–9. [PubMed] [Google Scholar]
- [42].Fuchs E (1889). Keratitis punctata superficialis. Wien Klin Wochenschr, 837–842. [Google Scholar]
- [43].Jawetz E (1959). The story of shipyard eye. Br Med J 1, 873–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wright RE and Co W (1930). Superficial Punctate Keratitis. Br J Ophthalmol 14, 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wright RE (1930). Superficial punctate keratitis: A record of an epidemic of superficial punctate keratitis which appeared in Madras about May, 1928, and continued during the year that followed, embodying observations relating to the clinical appearances, biomicroscopy, epidemiology, aetiology and histopathology, of the disease, based on the investigation of over 900 cases. Br J Ophthalmol 14, 257–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hogan MJ and Crawford JW (1942). Epidemic Keratoconjunctivitis: (Superficial Punctate Keratitis, Keratitis Subepithelialis, Keratitis Maculosa, Keratitis Nummularis) With a Review of the Literature and a Report of 125 Cases. Am J Ophthalmol 25, 1059–1078. [DOI] [PubMed] [Google Scholar]
- [47].Sprague JB, Hierholzer JC, Currier RW 2nd, Hattwick MA and Smith MD (1973). Epidemic keratoconjunctivitis. A severe industrial outbreak due to adenovirus type 8. N Engl J Med 289, 1341–6. [DOI] [PubMed] [Google Scholar]
- [48].Jawetz E, Kimura SJ, Hanna L, Coleman VR, Thygeson P and Nicholas A (1955). Studies on the etiology of epidemic keratoconjunctivitis. Am J Ophthalmol 40, 200–9; discussion, 209-11. [DOI] [PubMed] [Google Scholar]
- [49].Hanna L, Jawetz E, Mitsui Y, Thygeson P, Kimura SJ and Nicholas C (1957). Continuing studies on the association of adenovirus type 8 with epidemic keratoconjunctivitis. Am J Ophthalmol 44, 66–74. [DOI] [PubMed] [Google Scholar]
- [50].Mitsui Y, Hanabusa J, Minoda R and Ogata S (1957). Effect of inoculating adenovirus (APC virus) type 8 into human volunteers. Am J Ophthalmol 43, 84–90. [DOI] [PubMed] [Google Scholar]
- [51].Kjer P and Mordhorst CH (1961). Studies on an epidemic of keratoconjunctivitis caused by adenovirus type 8. II. Clinical and epidemiological aspects. Acta Ophthalmol (Copenh) 39, 984–92. [DOI] [PubMed] [Google Scholar]
- [52].Dawson C and Darrell R (1963). Infections due to adenovirus type 8 in the United States. I. An outbreak of epidemic keratoconjunctivitis originating in a physician’s office. N Engl J Med 268, 1031–4. [DOI] [PubMed] [Google Scholar]
- [53].Wegman DH, Guinee VF and Milian SJ (1970). Epidemic keratoconjunctivitis. Am J Public Health Nations Health 60, 1230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sammons JS et al. (2019). Outbreak of Adenovirus in a Neonatal Intensive Care Unit: Critical Importance of Equipment Cleaning During Inpatient Ophthalmologic Examinations. Ophthalmology 126, 137–143. [DOI] [PubMed] [Google Scholar]
- [55].Chodosh J (2019). Neonatal Intensive Care Eye. Ophthalmology 126, 144–145. [DOI] [PubMed] [Google Scholar]
- [56].Wurzel DF et al. (2014). Adenovirus species C is associated with chronic suppurative lung diseases in children. Clin Infect Dis 59, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Scott MK et al. (2016). Human Adenovirus Associated with Severe Respiratory Infection, Oregon, USA, 2013-2014. Emerg Infect Dis 22, 1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kajon AE, Lamson DM, Bair CR, Lu X, Landry ML, Menegus M, Erdman DD and St George K (2018). Adenovirus Type 4 Respiratory Infections among Civilian Adults, Northeastern United States, 2011-2015(1). Emerg Infect Dis 24, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Utz JP (1964). Viruria in Man. Prog Med Virol 6, 71–81. [PubMed] [Google Scholar]
- [60].Numazaki Y, Shigeta S, Kumasaka T, Miyazawa T, Yamanaka M, Yano N, Takai S and Ishida N (1968). Acute hemorrhagic cystitis in children. Isolation of adenovirus type II. N Engl J Med 278, 700–4. [DOI] [PubMed] [Google Scholar]
- [61].[No authors listed] (1968). Adenovirus infection of the urinary tract. Lancet 1, 1242. [PubMed] [Google Scholar]
- [62].Numazaki Y, Kumasaka T, Yano N, Yamanaka M, Miyazawa T, Takai S and Ishida N (1973). Further study on acute hemorrhagic cystitis due to adenovirus type 11. N Engl J Med 289, 344–7. [DOI] [PubMed] [Google Scholar]
- [63].Hanaoka N, Ito S, Konagaya M, Nojiri N, Yasuda M, Fujimoto T and Deguchi T (2019). Infectious human adenoviruses are shed in urine even after disappearance of urethral symptoms. PLoS One 14, e0212434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Reis TA et al. (2016). The role of human adenoviruses type 41 in acute diarrheal disease in Minas Gerais after rotavirus vaccination. Braz J Microbiol 47, 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jones MS 2nd et al. (2007). New adenovirus species found in a patient presenting with gastroenteritis. J Virol 81, 5978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wood DJ (1988). Adenovirus gastroenteritis. Br Med J (Clin Res Ed) 296, 229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Richmond SJ, Caul EO, Dunn SM, Ashley CR, Clarke SK and Seymour NR (1979). An outbreak of gastroenteritis in young children caused by adenoviruses. Lancet 1, 1178–81. [DOI] [PubMed] [Google Scholar]
- [68].Uhnoo I, Wadell G, Svensson L and Johansson ME (1984). Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol 20, 365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kojaoghlanian T, Flomenberg P and Horwitz MS (2003). The impact of adenovirus infection on the immunocompromised host. Rev Med Virol 13, 155–71. [DOI] [PubMed] [Google Scholar]
- [70].Van Der Veen J and Van Der Ploeg G (1958). An outbreak of pharyngoconjunctival fever caused by types 3 and 4 adenovirus at Waalwijk, The Netherlands. Am J Hyg 68, 95–105. [DOI] [PubMed] [Google Scholar]
- [71].Ariga T, Shimada Y, Ohgami K, Tagawa Y, Ishiko H, Aoki K and Ohno S (2004). New genome type of adenovirus serotype 4 caused nosocomial infections associated with epidemic conjunctivitis in Japan. J Clin Microbiol 42, 3644–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Centers for Disease, C. and Prevention. (2013). Adenovirus-associated epidemic keratoconjunctivitis outbreaks--four states, 2008-2010. MMWR Morb Mortal Wkly Rep 62, 637–41. [PMC free article] [PubMed] [Google Scholar]
- [73].Butt AL and Chodosh J (2006). Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea 25, 199–202. [DOI] [PubMed] [Google Scholar]
- [74].Maitreyi RS, Dar L, Muthukumar A, Vajpayee M, Xess I, Vajpayee RB, Seth P and Broor S (1999). Acute hemorrhagic conjunctivitis due to enterovirus 70 in India. Emerg Infect Dis 5, 267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Oh MD et al. (2003). Acute hemorrhagic conjunctivitis caused by coxsackievirus A24 variant, South Korea, 2002. Emerg Infect Dis 9, 1010–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Swenson PD, Wadell G, Allard A and JC H. (2003) Adenoviruses. In Manual of clinical microbiology (Murray PR, Baron EJ, Jorgensen JH, Pfaller MA and RH Y, eds), pp. 1407–17. ASM Press, Washington, DC. [Google Scholar]
- [77].Payne SB, Grilli EA, Smith AJ and Hoskins TW (1984). Investigation of an outbreak of adenovirus type 3 infection in a boys’ boarding school. J Hyg (Lond) 93, 277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Harley D, Harrower B, Lyon M and Dick A (2001). A primary school outbreak of pharyngoconjunctival fever caused by adenovirus type 3. Commun Dis Intell 25, 9–12. [DOI] [PubMed] [Google Scholar]
- [79].Giladi N and Herman J (1984). Pharyngoconjunctival fever. Arch Dis Child 59, 1182–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Artieda J, Pineiro L, Gonzalez M, Munoz M, Basterrechea M, Iturzaeta A and Cilla G (2009). A swimming pool-related outbreak of pharyngoconjunctival fever in children due to adenovirus type 4, Gipuzkoa, Spain, 2008. Euro Surveill 14. [PubMed] [Google Scholar]
- [81].Xie L et al. (2012). Two adenovirus serotype 3 outbreaks associated with febrile respiratory disease and pharyngoconjunctival fever in children under 15 years of age in Hangzhou, China, during 2011. J Clin Microbiol 50, 1879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Paulsen F, Thale A, Kohla G, Schauer R, Rochels R, Parwaresch R and Tillmann B (1998). Functional anatomy of human lacrimal duct epithelium. Anat Embryol (Berl) 198, 1–12. [DOI] [PubMed] [Google Scholar]
- [83].Zhang L, Zhao N, Sha J, Wang C, Jin X, Amer S and Liu S (2016). Virology and epidemiology analyses of global adenovirus-associated conjunctivitis outbreaks, 1953-2013. Epidemiol Infect 144, 1661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lee CS et al. (2018). Determinants of Outcomes of Adenoviral Keratoconjunctivitis. Ophthalmology 125, 1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ismail AM et al. (2018). Genomic analysis of a large set of currently-and historically-important human adenovirus pathogens. Emerg Microbes Infect 7, 10. doi: 10.1038/s41426-017-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lauer KP et al. (2004). Natural variation among human adenoviruses: genome sequence and annotation of human adenovirus serotype 1. J Gen Virol 85, 2615–25. [DOI] [PubMed] [Google Scholar]
- [87].Robinson CM, Shariati F, Gillaspy AF, Dyer DW and Chodosh J (2008). Genomic and bioinformatics analysis of human adenovirus type 37: new insights into corneal tropism. BMC Genomics 9, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Reddy VS and Nemerow GR (2014). Structures and organization of adenovirus cement proteins provide insights into the role of capsid maturation in virus entry and infection. Proc Natl Acad Sci U S A 111, 11715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bahar MW, Graham SC, Stuart DI and Grimes JM (2011). Insights into the evolution of a complex virus from the crystal structure of vaccinia virus D13. Structure 19, 1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Abrescia NG et al. (2008). Insights into virus evolution and membrane biogenesis from the structure of the marine lipid-containing bacteriophage PM2. Mol Cell 31, 749–61. [DOI] [PubMed] [Google Scholar]
- [91].Butcher SJ, Bamford DH and Fuller SD (1995). DNA packaging orders the membrane of bacteriophage PRD1. EMBO J 14, 6078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Roberts MM, White JL, Grutter MG and Burnett RM (1986). Three-dimensional structure of the adenovirus major coat protein hexon. Science 232, 1148–51. [DOI] [PubMed] [Google Scholar]
- [93].Reddy VS, Natchiar SK, Stewart PL and Nemerow GR (2010). Crystal structure of human adenovirus at 3.5 A resolution. Science 329, 1071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Prasad BV and Schmid MF (2012). Principles of virus structural organization. Adv Exp Med Biol 726, 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Benson SD, Bamford JK, Bamford DH and Burnett RM (1999). Viral evolution revealed by bacteriophage PRD1 and human adenovirus coat protein structures. Cell 98, 825–33. [DOI] [PubMed] [Google Scholar]
- [96].Khayat R and Johnson JE (2011). Pass the jelly rolls. Structure 19, 904–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Crawford-Miksza L and Schnurr DP (1996). Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol 70, 1836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Roberts DM et al. (2006). Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441, 239–43. [DOI] [PubMed] [Google Scholar]
- [99].Yuan X et al. (2009). Molecular modeling and epitopes mapping of human adenovirus type 3 hexon protein. Vaccine 27, 5103–10. [DOI] [PubMed] [Google Scholar]
- [100].Louis N, Fender P, Barge A, Kitts P and Chroboczek J (1994). Cell-binding domain of adenovirus serotype 2 fiber. J Virol 68, 4104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bergelson JM et al. (1997). Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–3. [DOI] [PubMed] [Google Scholar]
- [102].Bewley MC, Springer K, Zhang YB, Freimuth P and Flanagan JM (1999). Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286, 1579–83. [DOI] [PubMed] [Google Scholar]
- [103].Gaggar A, Shayakhmetov DM and Lieber A (2003). CD46 is a cellular receptor for group B adenoviruses. Nat Med 9, 1408–12. [DOI] [PubMed] [Google Scholar]
- [104].Wu E, Trauger SA, Pache L, Mullen TM, von Seggern DJ, Siuzdak G and Nemerow GR (2004). Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J Virol 78, 3897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nilsson EC et al. (2011). The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med 17, 105–9. [DOI] [PubMed] [Google Scholar]
- [106].Zubieta C, Schoehn G, Chroboczek J and Cusack S (2005). The structure of the human adenovirus 2 penton. Mol Cell 17, 121–35. [DOI] [PubMed] [Google Scholar]
- [107].van Raaij MJ, Mitraki A, Lavigne G and Cusack S (1999). A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 401, 935–8. [DOI] [PubMed] [Google Scholar]
- [108].Rosen L (1958). Hemagglutination by adenoviruses. Virology 5, 574–7. [DOI] [PubMed] [Google Scholar]
- [109].Madisch I, Harste G, Pommer H and Heim A (2005). Phylogenetic analysis of the main neutralization and hemagglutination determinants of all human adenovirus prototypes as a basis for molecular classification and taxonomy. J Virol 79, 15265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rosen L (1960). A hemagglutination-inhibition technique for typing adenoviruses. Am J Hyg 71, 120–8. [DOI] [PubMed] [Google Scholar]
- [111].Kaneko H et al. (2011). Complete genome analysis of a novel intertypic recombinant human adenovirus causing epidemic keratoconjunctivitis in Japan. J Clin Microbiol 49, 484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kajon AE, Dickson LM, Murtagh P, Viale D, Carballal G and Echavarria M (2010). Molecular characterization of an adenovirus 3-16 intertypic recombinant isolated in Argentina from an infant hospitalized with acute respiratory infection. J Clin Microbiol 48, 1494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Enomoto M et al. (2012). Isolation of an intertypic recombinant human adenovirus (candidate type 56) from the pharyngeal swab of a patient with pharyngoconjunctival fever. Jpn J Infect Dis 65, 457–9. [DOI] [PubMed] [Google Scholar]
- [114].Ismail AM, Lee JS, Lee JY, Singh G, Dyer DW, Seto D, Chodosh J and Rajaiya J (2018). Adenoviromics: Mining the Human Adenovirus Species D Genome. Front Microbiol 9, 2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wickham TJ, Mathias P, Cheresh DA and Nemerow GR (1993). Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73, 309–19. [DOI] [PubMed] [Google Scholar]
- [116].Li E, Brown SL, Stupack DG, Puente XS, Cheresh DA and Nemerow GR (2001). Integrin alpha(v)beta1 is an adenovirus coreceptor. J Virol 75, 5405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Storm RJ et al. (2017). Human Adenovirus Type 37 Uses alphaVbeta1 and alpha3beta1 Integrins for Infection of Human Corneal Cells. J Virol 91 (5). pii: e02019–16. doi: 10.1128/JVI.02019-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Albinsson B and Kidd AH (1999). Adenovirus type 41 lacks an RGD alpha(v)-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res 64, 125–36. [DOI] [PubMed] [Google Scholar]
- [119].Rajan A et al. (2018). Enteric Species F Human Adenoviruses use Laminin-Binding Integrins as Co-Receptors for Infection of Ht-29 Cells. Sci Rep 8, 10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gahery-Segard H, Farace F, Godfrin D, Gaston J, Lengagne R, Tursz T, Boulanger P and Guillet JG (1998). Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol 72, 2388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Xiao J, Natarajan K, Rajala MS, Astley RA, Ramadan RT and Chodosh J (2005). Vitronectin: a possible determinant of adenovirus type 19 tropism for human corneal epithelium. Am J Ophthalmol 140, 363–9. [DOI] [PubMed] [Google Scholar]
- [122].Robinson CM et al. (2013). Predicting the next eye pathogen: analysis of a novel adenovirus. MBio 4, e00595–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Shayakhmetov DM, Eberly AM, Li ZY and Lieber A (2005). Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J Virol 79, 1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Teigler JE, Kagan JC and Barouch DH (2014). Late endosomal trafficking of alternative serotype adenovirus vaccine vectors augments antiviral innate immunity. J Virol 88, 10354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Walsh MP, Seto J, Jones MS, Chodosh J, Xu W and Seto D (2010). Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J Clin Microbiol 48, 991–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Seto D, Jones MS, Dyer DW and Chodosh J (2013). Characterizing, typing, and naming human adenovirus type 55 in the era of whole genome data. J Clin Virol 58, 741–2. [DOI] [PubMed] [Google Scholar]
- [127].Hierholzer JC, Pumarola A, Rodriguez-Torres A and Beltran M (1974). Occurrence of respiratory illness due to an atypical strain of adenovirus type 11 during a large outbreak in Spanish military recruits. Am J Epidemiol 99, 434–42. [DOI] [PubMed] [Google Scholar]
- [128].Yang Z et al. (2009). Genomic analyses of recombinant adenovirus type 11a in China. J Clin Microbiol 47, 3082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kajon AE, Dickson LM, Metzgar D, Houng HS, Lee V and Tan BH (2010). Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training cAMP. J Clin Microbiol 48, 1438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zhang Q, Seto D, Cao B, Zhao S and Wan C (2012). Genome sequence of human adenovirus type 55, a re-emergent acute respiratory disease pathogen in China. J Virol 86, 12441–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Zhao S et al. (2014). Re-emergent human adenovirus genome type 7d caused an acute respiratory disease outbreak in Southern China after a twenty-one year absence. Sci Rep 4, 7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lafolie J et al. (2016). Severe Pneumonia Associated with Adenovirus Type 55 Infection, France, 2014. Emerg Infect Dis 22, 2012–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Yoo H et al. (2017). Febrile Respiratory Illness Associated with Human Adenovirus Type 55 in South Korea Military, 2014-2016. Emerg Infect Dis 23, 1016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Salama M et al. (2016). Outbreak of adenovirus type 55 infection in Israel. J Clin Virol 78, 31–5. [DOI] [PubMed] [Google Scholar]
- [135].Liu T et al. (2018). A recombinant trivalent vaccine candidate against human adenovirus types 3, 7, and 55. Vaccine 36, 2199–206. [DOI] [PubMed] [Google Scholar]
- [136].Tian X et al. (2018). A tetravalent vaccine comprising hexon-chimeric adenoviruses elicits balanced protective immunity against human adenovirus types 3, 7, 14 and 55. Antiviral Res 154, 17–25. [DOI] [PubMed] [Google Scholar]
- [137].Robinson CM, Seto D, Jones MS, Dyer DW and Chodosh J (2011). Molecular evolution of human species D adenoviruses. Infect Genet Evol 11, 1208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Chiu CY, Wu E, Brown SL, Von Seggern DJ, Nemerow GR and Stewart PL (2001). Structural analysis of a fiber-pseudotyped adenovirus with ocular tropism suggests differential modes of cell receptor interactions. J Virol 75, 5375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wu E, Pache L, Von Seggern DJ, Mullen TM, Mikyas Y, Stewart PL and Nemerow GR (2003). Flexibility of the adenovirus fiber is required for efficient receptor interaction. J Virol 77, 7225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Roelvink PW, Kovesdi I and Wickham TJ (1996). Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol 70, 7614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Seki T, Dmitriev I, Kashentseva E, Takayama K, Rots M, Suzuki K and Curiel DT (2002). Artificial extension of the adenovirus fiber shaft inhibits infectivity in coxsackievirus and adenovirus receptor-positive cell lines. J Virol 76, 1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Segerman A, Arnberg N, Erikson A, Lindman K and Wadell G (2003). There are two different species B adenovirus receptors: sBAR, common to species B1 and B2 adenoviruses, and sB2AR, exclusively used by species B2 adenoviruses. J Virol 77, 1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Fleischli C, Sirena D, Lesage G, Havenga MJ, Cattaneo R, Greber UF and Hemmi S (2007). Species B adenovirus serotypes 3, 7, 11 and 35 share similar binding sites on the membrane cofactor protein CD46 receptor. J Gen Virol 88, 2925–34. [DOI] [PubMed] [Google Scholar]
- [144].Baker AT, Greenshields-Watson A, Coughlan L, Davies JA, Uusi-Kerttula H, Cole DK, Rizkallah PJ and Parker AL (2019). Diversity within the adenovirus fiber knob hypervariable loops influences primary receptor interactions. Nat Commun 10, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Williams JF and Ustacelebi S (1971). Complementation and recombination with temperature-sensitive mutants of adenovirus type 5. J Gen Virol 13, 345–8. [DOI] [PubMed] [Google Scholar]
- [146].Takemori N (1972). Genetic studies with tumorigenic adenoviruses. 3. Recombination in adenovirus type 12. Virology 47, 157–67. [DOI] [PubMed] [Google Scholar]
- [147].Takemori N, Riggs JL and Aldrich C (1968). Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology 36, 575–86. [DOI] [PubMed] [Google Scholar]
- [148].Haber JE (1999). DNA recombination: the replication connection. Trends Biochem Sci 24, 271–5. [DOI] [PubMed] [Google Scholar]
- [149].Kogoma T (1996). Recombination by replication. Cell 85, 625–7. [DOI] [PubMed] [Google Scholar]
- [150].Radding C (2001). Colloquium introduction. Links between recombination and replication: vital roles of recombination. Proc Natl Acad Sci U S A 98, 8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Alberts B (2003). DNA replication and recombination. Nature 421, 431–5. [DOI] [PubMed] [Google Scholar]
- [152].Syeda AH, Hawkins M and McGlynn P (2014). Recombination and replication. Cold Spring Harb Perspect Biol 6, a016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Young CSH (1995) Homologous Recombination in the Replicative Cycle of Adenoviruses and Its Relationship to DNA Replication In The Molecular Repertoire of Adenoviruses II (Walter Doerfler and Böhm P, eds), pp. 89–108. Springer, Berlin Heidelberg. [Google Scholar]
- [154].Young CSH and Silverstein SJ (1980). The kinetics of adenovirus recombination in homotypic and heterotypic genetic crosses. Virology 101, 503–15. [DOI] [PubMed] [Google Scholar]
- [155].Munz PL, Young C and Young CSH (1983). The genetic analysis of adenovirus recombination in triparental and superinfection crosses. Virology 126, 576–86. [DOI] [PubMed] [Google Scholar]
- [156].Young CSH, Cachianes G, Munz P and Silverstein S (1984). Replication and recombination in adenovirus-infected cells are temporally and functionally related. J Virol 51, 571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Young CSH and Fisher PB (1980). Adenovirus recombination in normal and repair-deficient human fibroblasts. Virology 100, 179–84. [DOI] [PubMed] [Google Scholar]
- [158].Engelmann I, Madisch I, Pommer H and Heim A (2006). An outbreak of epidemic keratoconjunctivitis caused by a new intermediate adenovirus 22/H8 identified by molecular typing. Clin Infect Dis 43, e64–6. [DOI] [PubMed] [Google Scholar]
- [159].Rosen L, Baron S and Bell JA (1961). Four newly recognized adenoviruses. Proc Soc Exp Biol Med 107, 434–7. [DOI] [PubMed] [Google Scholar]
- [160].Robinson CM, Rajaiya J, Walsh MP, Seto D, Dyer DW, Jones MS and Chodosh J (2009). Computational analysis of human adenovirus type 22 provides evidence for recombination among species D human adenoviruses in the penton base gene. J Virol 83, 8980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Henquell C et al. (2009). Fatal adenovirus infection in a neonate and transmission to health-care workers. J Clin Virol 45, 345–8. [DOI] [PubMed] [Google Scholar]
- [162].Singh G et al. (2015). Recombination of the epsilon determinant and corneal tropism: Human adenovirus species D types 15, 29, 56, and 69. Virology 485, 452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Bell SD Jr., Mc CD, Murray ES, Chang RS and Snyder JC (1959). Adenoviruses isolated from Saudi Arabia. I. Epidemiologic features. Am J Trop Med Hyg 8, 492–500. [DOI] [PubMed] [Google Scholar]
- [164].Irving L, Kennett M, Lewis F, Birch C and Donaldson A (1981). Adenovirus eye infections in an Australian city, 1972-9. J Hyg (Lond) 86, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Hierholzer JC, Guyer B, O’Day D and Schaffner W (1974). Letter: Adenovirus type 19 keratoconjunctivitis. N Engl J Med 290, 1436. [DOI] [PubMed] [Google Scholar]
- [166].Guyer B, O’Day DM, Hierholzer JC and Schaffner W (1975). Epidemic keratoconjunctivitis: a community outbreak of mixed adenovirus type 8 and type 19 infection. J Infect Dis 132, 142–50. [DOI] [PubMed] [Google Scholar]
- [167].Robinson CM, Shariati F, Zaitshik J, Gillaspy AF, Dyer DW and Chodosh J (2009). Human adenovirus type 19: genomic and bioinformatics analysis of a keratoconjunctivitis isolate. Virus Res 139, 122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Fessler SP, Delgado-Lopez F and Horwitz MS (2004). Mechanisms of E3 modulation of immune and inflammatory responses. Curr Top Microbiol Immunol 273, 113–35. [DOI] [PubMed] [Google Scholar]
- [169].Windheim M, Hilgendorf A and Burgert HG (2004). Immune evasion by adenovirus E3 proteins: exploitation of intracellular trafficking pathways. Curr Top Microbiol Immunol 273, 29–85. [DOI] [PubMed] [Google Scholar]
- [170].Burgert HG, Ruzsics Z, Obermeier S, Hilgendorf A, Windheim M and Elsing A (2002). Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol 269, 273–318. [DOI] [PubMed] [Google Scholar]
- [171].Mahr JA and Gooding LR (1999). Immune evasion by adenoviruses. Immunol Rev 168, 121–30. [DOI] [PubMed] [Google Scholar]
- [172].Singh G, Robinson CM, Dehghan S, Jones MS, Dyer DW, Seto D and Chodosh J (2013). Homologous recombination in E3 genes of human adenovirus species D. J Virol 87, 12481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Singh G, Robinson CM, Dehghan S, Schmidt T, Seto D, Jones MS, Dyer DW and Chodosh J (2012). Overreliance on the hexon gene, leading to misclassification of human adenoviruses. J Virol 86, 4693–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zaiss AK, Machado HB and Herschman HR (2009). The influence of innate and pre-existing immunity on adenovirus therapy. J Cell Biochem 108, 778–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Dehghan S, Seto J, Liu EB, Walsh MP, Dyer DW, Chodosh J and Seto D (2013). Computational analysis of four human adenovirus type 4 genomes reveals molecular evolution through two interspecies recombination events. Virology 443, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Dehghan S, Seto J, Jones MS, Dyer DW, Chodosh J and Seto D (2013). Simian adenovirus type 35 has a recombinant genome comprising human and simian adenovirus sequences, which predicts its potential emergence as a human respiratory pathogen. Virology 447, 265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Dehghan S et al. (2019). A Zoonotic Adenoviral Human Pathogen Emerged through Genomic Recombination among Human and Nonhuman Simian Hosts. J Virol 93(18). pii: e00564–19. doi: 10.1128/JVI.00564-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Chintakuntlawar AV, Zhou X, Rajaiya J and Chodosh J (2010). Viral capsid is a pathogen-associated molecular pattern in adenovirus keratitis. PLoS Pathog 6, e1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Rajaiya J, Sadeghi N and Chodosh J (2009). Specific NFkappaB subunit activation and kinetics of cytokine induction in adenoviral keratitis. Mol Vis 15, 2879–89. [PMC free article] [PubMed] [Google Scholar]
- [180].Lam ST, Stahl MM, McMilin KD and Stahl FW (1974). Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics 77, 425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Clark AJ and Margulies AD (1965). Isolation and Characterization of Recombination-Deficient Mutants of Escherichia Coli K12. Proc Natl Acad Sci U S A 53, 451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].McKittrick NH and Smith GR (1989). Activation of Chi recombinational hotspots by RecBCD-like enzymes from enteric bacteria. J Mol Biol 210, 485–95. [DOI] [PubMed] [Google Scholar]
- [183].Biswas I, Maguin E, Ehrlich SD and Gruss A (1995). A 7-base-pair sequence protects DNA from exonucleolytic degradation in Lactococcus lactis. Proc Natl Acad Sci U S A 92, 2244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Taylor AF, Schultz DW, Ponticelli AS and Smith GR (1985). RecBC enzyme nicking at Chi sites during DNA unwinding: location and orientation-dependence of the cutting. Cell 41, 153–63. [DOI] [PubMed] [Google Scholar]
- [185].Anderson DG and Kowalczykowski SC (1997). The translocating RecBCD enzyme stimulates recombination by directing RecA protein onto ssDNA in a chi-regulated manner. Cell 90, 77–86. [DOI] [PubMed] [Google Scholar]
- [186].Smith GR (2012). How RecBCD enzyme and Chi promote DNA break repair and recombination: a molecular biologist’s view. Microbiol Mol Biol Rev 76, 217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Lee JY et al. (2018). Bacterial RecA Protein Promotes Adenoviral Recombination during In Vitro Infection. mSphere 3 pii: e00105–18. doi: 10.1128/mSphere.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Berciaud S et al. (2012). Adenovirus infections in Bordeaux University Hospital 2008-2010: clinical and virological features. J Clin Virol 54, 302–7. [DOI] [PubMed] [Google Scholar]
- [189].Vora GJ et al. (2006). Co-infections of adenovirus species in previously vaccinated patients. Emerg Infect Dis 12, 921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Chandra N, Frangsmyr L, Imhof S, Caraballo R, Elofsson M and Arnberg N (2019). Sialic Acid-Containing Glycans as Cellular Receptors for Ocular Human Adenoviruses: Implications for Tropism and Treatment. Viruses 11 pii: E395. doi: 10.3390/v11050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Chandra N et al. (2019). Sulfated Glycosaminoglycans as Viral Decoy Receptors for Human Adenovirus Type 37. Viruses 11 pii: E247. doi: 10.3390/v11030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Chandra N, Frangsmyr L and Arnberg N (2019). Decoy Receptor Interactions as Novel Drug Targets against EKC-Causing Human Adenovirus. Viruses 11 pii: E242. doi: 10.3390/v11030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Caraballo R et al. (2015). Triazole linker-based trivalent sialic acid inhibitors of adenovirus type 37 infection of human corneal epithelial cells. Org Biomol Chem 13, 9194–205. [DOI] [PubMed] [Google Scholar]
- [194].Huang S, Reddy V, Dasgupta N and Nemerow GR (1999). A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J Virol 73, 2798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Ismail AM, Lee JS, Dyer DW, Seto D, Rajaiya J and Chodosh J (2016). Selection Pressure in the Human Adenovirus Fiber Knob Drives Cell Specificity in Epidemic Keratoconjunctivitis. J Virol 90, 9598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Hashimoto S, Gonzalez G, Harada S, Oosako H, Hanaoka N, Hinokuma R and Fujimoto T (2018). Recombinant type Human mastadenovirus D85 associated with epidemic keratoconjunctivitis since 2015 in Japan. J Med Virol 90, 881–9. [DOI] [PubMed] [Google Scholar]
- [197].Gonzalez G, Aoki K, Yawata N and Kitaichi N (2017). Epidemic Kerato-Conjunctivitis; New Era of Required Clinical Practices due to the Emergence of Novel Recombinant Types in Human mastadenovirus D. J Clin Ophthalmol Eye Disorders 1, 1–6. [Google Scholar]
- [198].Fahnestock PKS, Y.D., Benson J, Cooper J, Ikonomi P. (2008) Molecular authentication as a quality control tool in a biological repository In Am Soc Virol 27th Annu Meet ed.êds), pp. abstr P4-14, p 225, Cornell University, Ithaca, NY. [Google Scholar]
- [199].Khoo SH, Bailey AS, de Jong JC and Mandal BK (1995). Adenovirus infections in human immunodeficiency virus-positive patients: clinical features and molecular epidemiology. J Infect Dis 172, 629–37. [DOI] [PubMed] [Google Scholar]
- [200].Chodosh J, Miller D, Stroop WG and Pflugfelder SC (1995). Adenovirus epithelial keratitis. Cornea 14, 167–74. [PubMed] [Google Scholar]
- [201].Natarajan K, Shepard LA and Chodosh J (2002). The use of DNA array technology in studies of ocular viral pathogenesis. DNA Cell Biol 21, 483–90. [DOI] [PubMed] [Google Scholar]
- [202].Natarajan K, Rajala MS and Chodosh J (2003). Corneal IL-8 expression following adenovirus infection is mediated by c-Src activation in human corneal fibroblasts. J Immunol 170, 6234–43. [DOI] [PubMed] [Google Scholar]
- [203].Natarajan K, Ghalayini AJ, Sterling RS, Holbrook RM, Kennedy RC and Chodosh J (2002). Activation of focal adhesion kinase in adenovirus-infected human corneal fibroblasts. Invest Ophthalmol Vis Sci 43, 2685–90. [PubMed] [Google Scholar]
- [204].Rajaiya J, Xiao J, Rajala RV and Chodosh J (2008). Human adenovirus type 19 infection of corneal cells induces p38 MAPK-dependent interleukin-8 expression. Virol J 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Xiao J and Chodosh J (2005). JNK regulates MCP-1 expression in adenovirus type 19-infected human corneal fibroblasts. Invest Ophthalmol Vis Sci 46, 3777–82. [DOI] [PubMed] [Google Scholar]
- [206].Yousuf MA, Lee JS, Zhou X, Ramke M, Lee JY, Chodosh J and Rajaiya J (2016). Protein kinase C Signaling in Adenoviral Infection. Biochemistry 55:5938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Rajaiya J, Yousuf MA, Singh G, Stanish H and Chodosh J (2012). Heat shock protein 27 mediated signaling in viral infection. Biochemistry 51, 5695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Rajala MS, Rajala RV, Astley RA, Butt AL and Chodosh J (2005). Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J Virol 79, 12332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Yousuf MA, Zhou X, Mukherjee S, Chintakuntlawar AV, Lee JY, Ramke M, Chodosh J and Rajaiya J (2013). Caveolin-1 associated adenovirus entry into human corneal cells. PLoS One 8, e77462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Lee JS, Ismail AM, Lee JY, Zhou X, Materne EC, Chodosh J and Rajaiya J (2019). Impact of dynamin 2 on adenovirus nuclear entry. Virology 529, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Fini ME (1999). Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res 18, 529–51. [DOI] [PubMed] [Google Scholar]
- [212].Mishima H, Okamoto J, Nakamura M, Wada Y and Otori T (1998). Collagenolytic activity of keratocytes cultured in a collagen matrix. Jpn J Ophthalmol 42, 79–84. [DOI] [PubMed] [Google Scholar]
- [213].Witt DP and Lander AD (1994). Differential binding of chemokines to glycosaminoglycan subpopulations. Curr Biol 4, 394–400. [DOI] [PubMed] [Google Scholar]
- [214].Lortat-Jacob H, Kleinman HK and Grimaud JA (1991). High-affinity binding of interferon-gamma to a basement membrane complex (matrigel). J Clin Invest 87, 878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Rajaiya J, Zhou X, Barequet I, Gilmore MS and Chodosh J (2015). Novel model of innate immunity in corneal infection. In Vitro Cell Dev Biol Anim 51, 827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Hassell JR, Robey PG, Barrach HJ, Wilczek J, Rennard SI and Martin GR (1980). Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A 77, 4494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Tsai JC, Garlinghouse G, McDonnell PJ and Trousdale MD (1992). An experimental animal model of adenovirus-induced ocular disease. The cotton rat. Arch Ophthalmol 110, 1167–70. [DOI] [PubMed] [Google Scholar]
- [218].Trousdale MD, Goldschmidt PL and Nobrega R (1994). Activity of ganciclovir against human adenovirus type-5 infection in cell culture and cotton rat eyes. Cornea 13, 435–9. [DOI] [PubMed] [Google Scholar]
- [219].Gordon YJ, Romanowski E and Araullo-Cruz T (1992). An ocular model of adenovirus type 5 infection in the NZ rabbit. Invest Ophthalmol Vis Sci 33, 574–80. [PubMed] [Google Scholar]
- [220].Trousdale MD, Nobrega R, Wood RL, Stevenson D, dos Santos PM, Klein D and McDonnell PJ (1995). Studies of adenovirus-induced eye disease in the rabbit model. Invest Ophthalmol Vis Sci 36, 2740–8. [PubMed] [Google Scholar]
- [221].Clement C et al. (2011). Clinical and antiviral efficacy of an ophthalmic formulation of dexamethasone povidone-iodine in a rabbit model of adenoviral keratoconjunctivitis. Invest Ophthalmol Vis Sci 52, 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].De Hauwere B, Lutz A, Maudgal PC, Neyts J and de Clercq E (1995). An ocular model of Adenovirus type 5 infection in the rabbit. Bull Soc Belge Ophtalmol 259, 33–42. [PubMed] [Google Scholar]
- [223].Romanowski EG, Yates KA and Gordon YJ (2002). Topical corticosteroids of limited potency promote adenovirus replication in the Ad5/NZW rabbit ocular model. Cornea 21, 289–91. [DOI] [PubMed] [Google Scholar]
- [224].Kaneko H et al. (2004). The cotton rat model for adenovirus ocular infection: antiviral activity of cidofovir. Antiviral Res 61, 63–6. [DOI] [PubMed] [Google Scholar]
- [225].Romanowski EG, Yates KA and Gordon YJ (2001). Short-term treatment With a potent topical corticosteroid of an acute ocular adenoviral infection in the New Zealand white rabbit. Cornea 20, 657–60. [DOI] [PubMed] [Google Scholar]
- [226].Romanowski EG, Yates KA, Teuchner B, Nagl M, Irschick EU and Gordon YJ (2006). N-chlorotaurine is an effective antiviral agent against adenovirus in vitro and in the Ad5/NZW rabbit ocular model. Invest Ophthalmol Vis Sci 47, 2021–6. [DOI] [PubMed] [Google Scholar]
- [227].de Oliveira CB, Stevenson D, LaBree L, McDonnell PJ and Trousdale MD (1996). Evaluation of Cidofovir (HPMPC, GS-504) against adenovirus type 5 infection in vitro and in a New Zealand rabbit ocular model. Antiviral Res 31, 165–72. [DOI] [PubMed] [Google Scholar]
- [228].Trousdale MD, Nobrega R, Stevenson D, Nakamura T, dos Santos PM, Labree L and McDonnell PJ (1995). Role of adenovirus type 5 early region 3 in the pathogenesis of ocular disease and cell culture infection. Cornea 14, 280–9. [DOI] [PubMed] [Google Scholar]
- [229].Eggerding FA and Pierce WC (1986). Molecular biology of adenovirus type 2 semipermissive infections. I. Viral growth and expression of viral replicative functions during restricted adenovirus infection. Virology 148, 97–113. [DOI] [PubMed] [Google Scholar]
- [230].Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM and Prince GA (1991). A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A 88, 1651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Chodosh J (2006). Human adenovirus type 37 and the BALB/c mouse: progress toward a restricted adenovirus keratitis model (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 104, 346–65. [PMC free article] [PubMed] [Google Scholar]
- [232].Chintakuntlawar AV, Astley R and Chodosh J (2007). Adenovirus type 37 keratitis in the C57BL/6J mouse. Invest Ophthalmol Vis Sci 48, 781–8. [DOI] [PubMed] [Google Scholar]
- [233].Mukherjee S, Zhou X, Rajaiya J and Chodosh J (2015). Ultrastructure of adenovirus keratitis. Invest Ophthalmol Vis Sci 56, 472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Chintakuntlawar AV and Chodosh J (2009). Chemokine CXCL1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J Interferon Cytokine Res 29, 657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Ramke M, Zhou X, Materne EC, Rajaiya J and Chodosh J (2016). Resident corneal c-fms(+) macrophages and dendritic cells mediate early cellular infiltration in adenovirus keratitis. Exp Eye Res 147, 144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Zhou X, Ramke M, Chintakuntlawar AV, Lee JY, Rajaiya J and Chodosh J (2017). Role of MyD88 in adenovirus keratitis. Immunol Cell Biol 95, 108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Kosulin K et al. (2016). Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin Microbiol Infect 22, 381 e1–381 e8. [DOI] [PubMed] [Google Scholar]
- [238].Garnett CT, Talekar G, Mahr JA, Huang W, Zhang Y, Ornelles DA and Gooding LR (2009). Latent species C adenoviruses in human tonsil tissues. J Virol 83, 2417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Zhang Y, Huang W, Ornelles DA and Gooding LR (2010). Modeling adenovirus latency in human lymphocyte cell lines. J Virol 84, 8799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Roy S, Calcedo R, Medina-Jaszek A, Keough M, Peng H and Wilson JM (2011). Adenoviruses in lymphocytes of the human gastro-intestinal tract. PLoS One 6, e24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Fox JP, Brandt CD, Wassermann FE, Hall CE, Spigland I, Kogon A and Elveback LR (1969). The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol 89, 25–50. [DOI] [PubMed] [Google Scholar]
- [242].Hogg JC (2001). Role of latent viral infections in chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med 164, S71–5. [DOI] [PubMed] [Google Scholar]
- [243].Proenca-Modena JL et al. (2019). Human adenovirus replication and persistence in hypertrophic adenoids and palatine tonsils in children. J Med Virol 91, 1250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].de Mezerville MH, Tellier R, Richardson S, Hebert D, Doyle J and Allen U (2006). Adenoviral infections in pediatric transplant recipients: a hospital-based study. Pediatr Infect Dis J 25, 815–8. [DOI] [PubMed] [Google Scholar]
- [245].Lion T (2019). Adenovirus persistence, reactivation, and clinical management. FEBS Lett [DOI] [PubMed] [Google Scholar]
- [246].King CR, Zhang A and Mymryk JS (2016). The Persistent Mystery of Adenovirus Persistence. Trends Microbiol 24, 323–4. [DOI] [PubMed] [Google Scholar]
- [247].Markel D, Lam E, Harste G, Darr S, Ramke M and Heim A (2014). Type dependent patterns of human adenovirus persistence in human T-lymphocyte cell lines. J Med Virol 86, 785–94. [DOI] [PubMed] [Google Scholar]
- [248].Hierholzer JC, Wigand R, Anderson LJ, Adrian T and Gold JW (1988). Adenoviruses from patients with AIDS: a plethora of serotypes and a description of five new serotypes of subgenus D (types 43-47). J Infect Dis 158, 804–13. [DOI] [PubMed] [Google Scholar]
- [249].Reddy PS, Ganesh S, Knowles NJ, Kaleko M, Connelly S and Bristol A (2006). Complete sequence and organization of the human adenovirus serotype 46 genome. Virus Res 116, 119–28. [DOI] [PubMed] [Google Scholar]
- [250].Schnurr D and Dondero ME (1993). Two new candidate adenovirus serotypes. Intervirology 36, 79–83. [DOI] [PubMed] [Google Scholar]
- [251].De Jong JC, Wermenbol AG, Verweij-Uijterwaal MW, Slaterus KW, Wertheim-Van Dillen P, Van Doornum GJ, Khoo SH and Hierholzer JC (1999). Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J Clin Microbiol 37, 3940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Giordano MO et al. (1999). Diarrhea and enteric emerging viruses in HIV-infected patients. AIDS Res Hum Retroviruses 15, 1427–32. [DOI] [PubMed] [Google Scholar]
- [253].Ferreyra L et al. (2010). A novel human adenovirus hexon protein of species D found in an AIDS patient. Arch Virol 155, 27–35. [DOI] [PubMed] [Google Scholar]
- [254].Liu EB et al. (2011). Genetic analysis of a novel human adenovirus with a serologically unique hexon and a recombinant fiber gene. PLoS One 6, e24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Liu EB et al. (2012). Computational and serologic analysis of novel and known viruses in species human adenovirus D in which serology and genomics do not correlate. PLoS One 7, e33212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Al Qurashi YM, Alkhalaf MA, Lim L, Guiver M and Cooper RJ (2012). Sequencing and phylogenetic analysis of the hexon, fiber, and penton regions of adenoviruses isolated from AIDS patients. J Med Virol 84, 1157–65. [DOI] [PubMed] [Google Scholar]
- [257].Alissa Alkhalaf M, Al Qurashi YM, Guiver M and Cooper RJ (2014). Genome sequences of three species d adenoviruses isolated from AIDS patients. Genome Announc 2 pii: e01267–13. doi: 10.1128/genomeA.01267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Hierholzer JC, Adrian T, Anderson LJ, Wigand R and Gold JW (1988). Analysis of antigenically intermediate strains of subgenus B and D adenoviruses from AIDS patients. Arch Virol 103, 99–115. [DOI] [PubMed] [Google Scholar]
- [259].Zheng Y, Stamminger T and Hearing P (2016). E2F/Rb Family Proteins Mediate Interferon Induced Repression of Adenovirus Immediate Early Transcription to Promote Persistent Viral Infection. PLoS Pathog 12, e1005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Bailer RT, Holloway A, Sun J, Margolick JB, Martin M, Kostman J and Montaner LJ (1999). IL-13 and IFN-gamma secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression. J Immunol 162, 7534–42. [PubMed] [Google Scholar]
- [261].Huang XL, Fan Z, Kalinyak C, Mellors JW and Rinaldo CR Jr. (2000). CD8(+) T-cell gamma interferon production specific for human immunodeficiency virus type 1 (HIV-1) in HIV-1-infected subjects. Clin Diagn Lab Immunol 7, 279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Stacey AR et al. (2009). Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 83, 3719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Radke JR and Cook JL (2018). Human adenovirus infections: update and consideration of mechanisms of viral persistence. Curr Opin Infect Dis 31, 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Pennington MR, Saha A, Painter DF, Gavazzi C, Ismail AM, Zhou X, Chodosh J and Rajaiya J (2019). Disparate Entry of Adenoviruses Dictates Differential Innate Immune Responses on the Ocular Surface. Microorganisms 7 pii: E351. doi: 10.3390/microorganisms7090351. [DOI] [PMC free article] [PubMed] [Google Scholar]