ABSTRACT
Background: VPM1002BC is a modified mycobacterium Bacillus Calmette Guérin (BCG) for the treatment of non-muscle invasive bladder cancer (NMIBC). The genetic modifications are expected to result in better immunogenicity and less side effects. We report on patient safety and immunology of the first intravesical application of VPM1002BC in human.
Methods: Six patients with BCG failure received a treatment of 6 weekly instillations with VPM1002BC. Patients were monitored for adverse events (AE), excretion of VPM1002BC and cytokines, respectively.
Results: No DLT (dose limiting toxicity) occurred during the DLT-period. No grade ≥3 AEs occurred. Excretion of VPM1002BC in the urine was limited to less than 24 hours. Plasma levels of TNFα significantly increased after treatment and blood-derived CD4+ T cells stimulated with PPD demonstrated significantly increased intracellular GM-CSF and IFN expression.
Conclusion: The intravesical application of VPM1002BC is safe and well tolerated by patients and results in a potential Th1 weighted immune response.
KEYWORDS: NMIBC (Non-muscle-invasive bladder cancer), BCG-failure, clinical trial, GMO (genetically modified organism), listeriolysin
Introduction
Clinical guidelines for high risk NMIBC recommend complete transurethral resection of the bladder tumor (TURB) followed by BCG induction and maintenance therapy for at least one year as the standard therapy.1 In addition to the prevention of recurrence and progression of NMIBC,2 the use of BCG as a mean of initiating anti-tumor immunity has also shown to prolong overall survival as compared to TURB alone3 and, in combination with maintenance therapy, superiority over intravesical chemotherapy.4
Bladder instillation of BCG induces a local infection involving attachment and internalization of mycobacteria into healthy and cancerous urothelial cells. Infected urothelial cells secrete a variety of pro-inflammatory chemokines and cytokines that attract neutrophils. Activation of the innate immune system is important for successful therapy and ideally induces a TH1 weighted immune response with induction of the adaptive immune system in terms of T cell responses.5,6
In case of failure of intravesical BCG therapy, current guidelines support radical cystectomy. If patients are unwilling or unfit to undergo cystectomy, a second round of BCG therapy, other immunotherapies, intravesical chemotherapies, device-assisted therapies, or combination therapies may be applied. Currently these therapies lack long-term results and solid prospective validation and are therefore considered oncologically inferior to cystectomy.1 Only scarce data is available from prospective studies regarding the outcome of a second BCG therapy round in patients with BCG failure. Di Lorenzo, et al. reported on 40 patients receiving BCG re-induction: 87.5% of patients failed to respond to BCG re-induction at one year; one patient died of systemic disease, 37.5% of the patients had disease progression and had to undergo cystectomy and 40% underwent radiation therapy plus systemic chemotherapy after one year.7
Several attempts of genetic modifications with the goal to enhance TH1 weighted immune responses in the bladder have been undertaken.8 One of these genetically modified vaccines, VPM1002, is currently tested in vaccine trials for tuberculosis9 and is now being developed as VPM1002BC for intravesical application.
VPM1002BC is a live, recombinant Mycobacterium bovis BCG (rBCGΔureC::hly). It was generated to direct phagosomal antigens to the major histocompatibility complex I pathway and enhance the induction of CD4+ and CD8+ T cell-mediated immune responses.10,11 To this end, the listeriolysin (hly) gene from Listeria monocytogenes was inserted into the urease C gene (ureC). Urease C activity is aborted by this insertion and the enzyme is no longer able to stabilize the pH in the phagosome of the host cell. Consequently, the pH drops and activates listeriolysin (LLO), which is most active at pH 5.5. LLO is a hemolysin able to perforate membranes. Its activity in the phagosome allows proteins to translocate to the cytosol of infected host or cancer cells.
Due to the unique mode of action, VPM1002BC is expected to be at least as potent as conventional BCG in evoking immune responses and should be rapidly cleared from the host, supporting the idea of lower side effects and lower systemic toxicity.11–13
Here, we report on the first intravesical application of VPM1002BC for the treatment of non-muscle invasive bladder cancer in patients with initial BCG therapy failure.
Patients and methods
Study design
The trial (NCT02371447) was designed as a multicenter, open-label, single arm, dose-de-escalation phase I/II study and conducted in compliance with the current version of the Declaration of Helsinki,14 the ICH-GCP15 and with national legal and regulatory requirements. The institutional review board at each participating center approved the trial. All patients provided written informed consent prior to enrollment.
Patients included in the trial presented with recurrent NMIBC with high risk for progression (score 7–23 based on the European Organization for Research and Treatment of Cancer scoring system) after BCG therapy, for whom radical cystectomy was indicated but were unfit or unwilling to undergo cystectomy. We used the EAU 2008 Guideline definition of BCG failure to ensure comparability of outcome with our reference study.7 All patients had histologically confirmed diagnosis of recurrent NMIBC. A repeat TURB confirming a tumor-free state was mandatory, except for carcinoma in situ (CIS). In patients presenting with CIS, selective upper tract cytologies and biopsies of the prostatic urethra were recommended. At the start of the planned treatment, cytology by bladder wash had to be negative, except for patients with concomitant CIS.
Exclusion criteria for the trial were stage ≥ T2 urothelial carcinoma of the bladder, concomitant urothelial carcinoma of the upper urinary tract, or non-prostatic urethra. The detailed inclusion and exclusion criteria can be found here (Hyperlink: https://clinicaltrials.gov/ct2/show/NCT02371447). The primary endpoint was defined as the occurrence of DLT of intravesical VPM1002BC instillations. The DLT period was defined as the time for the first three instillations plus one week. DLTs were defined as Common Terminology Criteria for Adverse Events (CTCAE) grade 3 events related to the trial treatment and persisting more than 12 days despite adequate supportive measures or CTCAE ≥ grade 4 events related to trial treatment occurring during the DLT period. The secondary endpoint was the tolerability of the intravesical instillation of VPM1002BC. Tolerability during induction phase was defined as finishing at least five instillations of VPM1002BC within 12 weeks after treatment initiation.
A dose de-escalation and not a dose escalation design was chosen based on the regular starting dose of BCG (1–20 × 108 CFU BCG/50 ml NaCl).1 The 3 + 3 dose de-escalation strategy defined dose level 1 at 1–19.2 × 108 CFU/50 ml and dose level −1 at 1–19.2 × 107 CFU/46.4 ml.
Patients were scheduled for a standard treatment of six weekly instillations with VPM1002BC, followed by a maintenance regimen of one year with three weekly instillations at 3, 6 and 12 months after start of treatment.
Patients
Between September 2015 and May 2016, six patients were included into the trial at two Swiss centers. Patient characteristics are summarized in Table 1.
Table 1.
Patient baseline characteristics.
| Variable | Total (N = 6) n (%) |
|---|---|
| Age at registration (years) | 74.0 (min. 71.0, max. 82.0) |
| Sex | |
| Male | 6 (100.0%) |
| WHO performance score | |
| 0 | 6 (100.0%) |
| 1973 WHO grading | |
| Grade 2: moderately differentiated | 1 (16.7%) |
| Grade 3: poorly differentiated | 5 (83.3%) |
| 2004 WHO grading | |
| High-grade urothelial carcinoma | 6 (100.0%) |
| Current T classification after last TURB | |
| pT0 | 3 (50.0%) |
| pTis | 3 (50.0%) |
| Current N classification after last TURB | |
| cN0 | 6 (100.0%) |
| Current M classification after last TURB | |
| cM0 | 6 (100.0%) |
| T classification for recurrence in bladder | |
| pTa | 1 (16.7%) |
| pT1 | 2 (33.3%) |
| pTis | 3 (50.0%) |
| N classification for recurrence in bladder | |
| cN0 | 6 (100.0%) |
| M classification for recurrence in bladder | |
| cM0 | 6 (100.0%) |
| T classification for recurrence in prostatic urethra | |
| pT0 | 4 (66.7%) |
| pTX | 2 (33.3%) |
| Localization of the tumor at recurrence (more than one applicable) | |
| Anterior wall | 2 (33.3%) |
| Dome | 2 (33.3%) |
| Left wall | 2 (33.3%) |
| Posterior wall | 1 (16.7%) |
| Prostatic urethra | 0 (0.0%) |
| Right wall | 3 (50.0%) |
| Trigone and neck of the bladder | 1 (16.7%) |
Excretion profile of VPM1002BC
Blood and sputum samples were collected approximately one hour before instillations and at 24 hours after instillations 1 and 6. Urine samples were collected before instillation 1 and 6, and 2, 4, 8 and 24 hours after instillations 1 and 6.
Urine and sputum samples were decontaminated with N-acetyl-L-cystein-NaOH as described previously16 to avoid bacterial overgrowth. Heparin blood was analyzed without decontamination. The resuspended sample was then used for quantitative mycobacterial culture as well as M. tuberculosis complex (MTB)-specific in-house real-time PCR.16 Quantitative mycobacterial culture consisted of ten-fold dilutions of the sample up to 1.0 × 10−6. For resuspension and dilution, sterile PBS buffer plus 0.05% Tween-80 was used. Hundred microliters of each dilution were inoculated in triplicates on 7H10 agar plates. The plates were stored in an incubator at 37°C for six weeks and read weekly to enumerate the CFU. In addition, 1 ml of heparin blood was inoculated in BACTEC™Myco/F Lytic medium (Becton Dickinson) and incubated for six weeks in a BACTEC 9050 unit. Cultured isolates were identified and confirmed as VPM1002BC using a VPM1002BC hly-specific conventional PCR (35 cycles, PCR product length: 1415 bp) developed by Vakzine Projekt Management GmbH, Hannover, Germany.
Cytokine measurement in blood and urine
Kinetics of individual cytokine production by blood cells and in the urine taken before and after VPM1002BC treatments were analyzed. Urine samples were taken before instillation 1 (H0), and 4 hours (H4) after instillations 1, 3 and 6 of the induction phase and frozen immediately. Blood from patients was taken before instillations 1, 3 and 6 using TruCulture® (Myriad RBM, Austin, TX, USA) tubes containing, Mtb Purified Protein derivative (PPD; Staten Serum Institute, Copenhagen, Denmark). Staphylococcal Enterotoxin B (SEB; Bernhard-Nocht-Institut, Hamburg, Germany) and medium (Myriad RBM, Austin, TX, USA) only served as positive and negative controls, respectively. After 24 h at 37°C on a dry heat block, the supernatants were separated from the cells with a valve and stored at −20°C. Concentrations of IL-2, IFNγ, TNFα, IL-17, GM-CSF and IL-13 were quantified in supernatants and urine using a multiplexed particle-based flow cytometric assay (Bioplex, Bio-Rad Laboratories, Hercules, CA, USA) according to manufacturer’s instructions and acquisition performed using a Luminex 100 (Luminex Corporation, Austin, TX, USA). Creatinin was measured in urines for volume adjustment.17
Mtb-PPD-specific T cell response
Blood samples were taken before instillations and during induction at instillations 1, 3 and 6 and during the three maintenance cycles at instillations 1 and 3. Peripheral blood mononuclear cells (PBMC) were separated on a density gradient using Vacutainer CPT (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), washed and stored in liquid nitrogen until analysis. PBMCs were thawed and cultured (3 × 105 cells per well in duplicates) in 96 U-bottom well PP plate in presence of 2.5 μg/ml PPD, SEB or unstimulated (NS) as positive and negative controls, respectively. After six days, cells were re-stimulated 4 h with PMA/ionomycin (Sigma-Aldrich), 5 ng/ml and 500 ng/ml respectively, in presence of brefeldin A (BFA, 10 μg/mL). Cells were stained for viability (LiveDead-Aqua, Thermo Fisher Scientific, Waltham, MA, USA) then fixed (FACS Lysing Solution, Beckton, Dickenson and Company) and stored at −80°C. After permeabilization (BD Cytofix/CytopermTM), cells were labeled with fluorescently labeled antibodies to identify subsets and determine cytokine expression (Supplementary Methods).
Statistical analyses
Continuous variables were summarized using median and range, categorical variables using frequency and percentage. Adverse events were assessed according to NCI CTCAE v4.0.
For the exploratory immunology measurements the differences to pre-treatment were calculated and illustrated graphically using line plots.
For the VPM1002BC-specific T cell responses the area under the curve (AUC) of the differences to pre-treatment was calculated for each patient and cytokine. The median AUC and corresponding 95% confidence interval was calculated and compared to 0 using Wilcoxon signed-rank tests.
Two-tailed tests with a significance level of 0.05 were used for all analyses. Because no adjustment for multiple testing for these analyses was made, they were exploratory and hypothesis generating. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R 3.4.3 (http://www.r-project.org).
Results
Patients
Adverse events during the induction phase are listed in Table 2. No CTCAE grade ≥ 3 adverse events (AE) occurred during induction phase. CTCAE grade 2 AEs were observed in 4 out of 6 patients. The grade 2 AEs mainly consisted of asymptomatic urinary tract infections. All but one urinary tract infection (UTI) presented without symptoms and were detected and treated due to routine urine cultures taken in the context of the trial setting. The symptomatic UTI presented in one patient with prostatitis and did not lead to a stop of trial treatment.
Table 2.
Adverse events during induction phase with VPM1002BC graded according to common terminology criteria for adverse events (CTCAE) Version 4.0.
| CTCAE SOC | Term | Grade 1 n (%) | Grade 2 n (%) |
|---|---|---|---|
| Blood and lymphatic system disorders | Anemia | 2 (33.3%) | |
| Gastrointestinal disorders | Abdominal pain | 1 (16.7%) | |
| Infections and infestations | Asymptomatic bladder infection | 1 (16.7%) | |
| Prostate infection | 1 (16.7%) | ||
| Asymptomatic urinary enterococcus faecalis | 1 (16.7%) | ||
| Asymptomatic urinary tract infection | 1 (16.7%) | ||
| Investigations | Creatinine increased | 1 (16.7%) | |
| Nervous system disorders | Headache | 1 (16.7%) | |
| Renal and urinary disorders | Alguria | 2 (33.3%) | |
| Decreased GFR/Chronic kidney disease* | 1 (16.7%) | ||
| Hematuria | 1 (16.7%) | ||
| Nycturia | 1 (16.7%) | ||
| Skin and subcutaneous tissue disorders | Temporary redness of the skin | 1 (16.7%) | |
| Vascular disorders | Hematoma | 1 (16.7%) |
* grade 1 at baseline.
One case of grade 2 decreased GFR was observed. The patient who experienced this AE presented with chronic kidney disease grade 1 at baseline and the kidney function recovered to baseline after treatment.
No DLT-defining AE occurred in the first three patients during the DLT period as assessed by an independent safety committee. Consequently, patients 4–6 were treated at the same dose level 1. Again, no DLT occurred in these patients.
All patients concluded all six intravesical instillations within a median duration of 5.8 weeks (min. 5.0 weeks, max 7.1 weeks). Based on these results, the independent safety committee considered intravesical instillations with VPM1002BC to be safe and well tolerated.
Excretion profile of VPM1002BC
Blood, urine and sputum samples were collected to analyze putative routes of transmission and of intracorporal persistence of VPM1002BC. All blood and sputum samples, as well as all urine samples before the first and the sixth instillation showed no growth and no DNA of VPM1002BC (Figures 1 and 2). VPM1002BC DNA was detectable 24 hours after instillation 1 and 6 (Figure 2). However, no cultivable bacteria were detectable 24 h after the respective instillations (Figure 1). All isolated bacteria were characterized by VPM1002BC hly-specific PCR and all cultured isolates were indicative of VPM1002BC except for one patient. In this patient, the urine culture after 4 and 8 hours of the first instillation identified conventional BCG mycobacteria.
Figure 1.

Colony forming units (cfu) of VPM1002BC in urine after the first intravesical VPM1002BC instillation (blue dots) and the 6th intravesical VPM1002BC instillation (red squares).
Figure 2.

Cycle threshold (ct) values of VPM1002BC PCR in urine measured 2, 4, 8 and 24 hours after the first intravesical VPM1002BC instillation (blue dots) and the 6th intravesical VPM1002BC instillation (red squares). Ct-values > 45 were set as 45 in the figure.
Exploratory immunology
Cytokines (IL-2, IFNγ, TNFα, IL-17, GM-CSF and IL-13) were measured in urines pre and post instillations and in plasma after stimulation of whole blood with PPD. After the first instillation, levels of all cytokines in urines increased by a median factor of 4.9 [range 1.93; 9.63] (Supplementary Figure 1) and, prior to the third instillation, the levels of Th1 cytokines (IL-2, IFNγ and TNFα) in plasma increased by a factor of 1.64 [range 1.63; 1.65] (Supplementary Figure 2). Of note, TNFα increase in plasma was significant (p = .03).
VPM1002BC-specific T cell responses from PBMCs were assessed by flow cytometry by measuring frequencies of T cell subsets expressing cytokine (IFNγ, TNFα, IL-17, GM-CSF, and IL-2) and proliferating (Ki67+) after stimulation with PPD. Analysis of the kinetics of proliferating cells showed a significant increase of GM-CSF+ Ki67+ CD4+ T cells after the first instillation (p = .0313) with a trend for IFNγ (p = .06) (Supplementary Figure 4). Furthermore, analysis of total CD4+ T cell responses after PPD showed a significant increase of IFNγ+ and GM-CSF+, and a trend for IL-17+ CD4+ T cells, compared to the pre-treatment status over the course of the induction treatment period (Figure 3). The same analysis of the CD3+ and CD8+ T cells and the pos. control stimulation with SEB did not reveal any significant changes over time (Supplementary figures 4–9).
Figure 3.
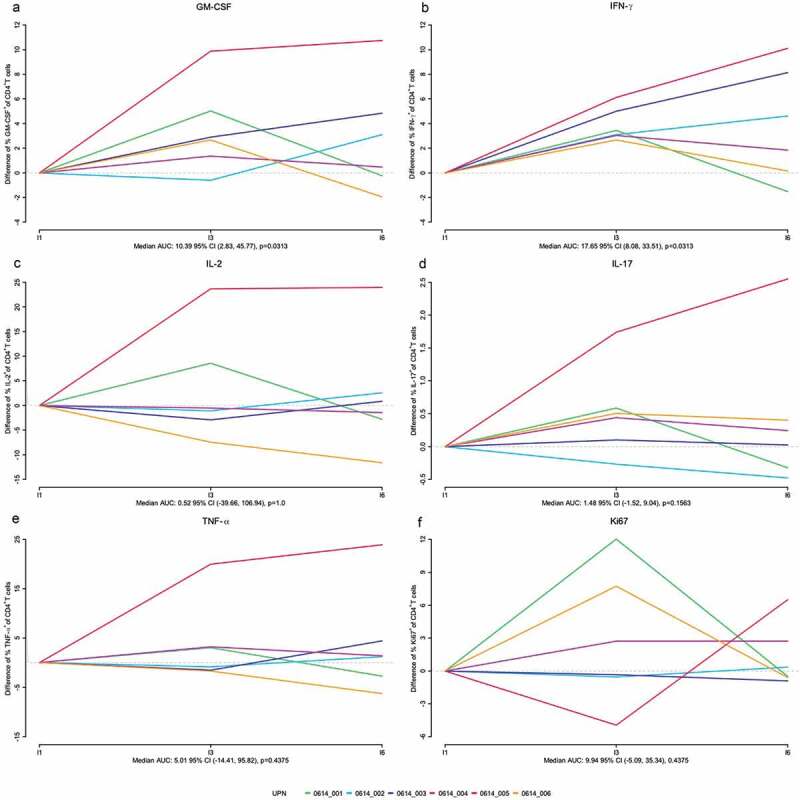
Intracellular cytokine expression in peripheral blood mononuclear cell (PBMC) derived CD4+ T cells after re-stimulation with purified protein derivative (PPD). Patient-derived CD4+ T cells from PBMCs before (I1 = instillation 1), and during the course of the induction phase (I3, I6) were assessed for the indicated intracellular cytokine concentrations. The colored lines depict the individual development per patient. Fold-changes compared to the pre-treatment status (I1) of the percentage of CD4+ T cells producing the cytokine of interest are shown.
Discussion
We report the phase I results of the first intravesical application of VPM1002BC for the treatment of cancer. VPM1002BC was well tolerated and no DLT occurred during the treatment. All patients were able to finish the 6 weekly instillations of VPM1002BC and within the predefined timeframe. The grade 2 adverse finding of asymptomatic urinary tract infections in 3 out of 6 patients as well as prostatitis in 1 out of 6 patients needs further attention during the phase II of the study. However, asymptomatic bacteriuria is a common finding during BCG therapy and has been reported in up to 30% of the treated patients.3 Regarding the single case of grade 2 impaired renal function the affected patient presented with chronic kidney disease grade 1 at baseline and like all patients undergoing BCG therapy was asked not to drink in the morning prior to the instillation. This may have resulted in lowered pre-treatment estimated GFR values that resolved after treatment.
To assess putative routes of transmission of VPM1002BC into the environment as well as persistence in the body, urine, blood and sputum were collected and analyzed by the CFU counts method and by MTB PCR. In all these samples of the six patients, no traces of VPM1002BC DNA and no CFU of VPM1002BC before the initial and before the 6th instillation were detected. This analysis showed no persistence of VPM1002BC 24 hours after the sixth instillation in the urine. In one patient, culture of conventional BCG in the urine was obtained at the first instillation. This is indicative of a persistence of conventional BCG of 6.6 months after the preceding BCG therapy cycle. Such long lasting persistence has been reported before in bladder granulomas after conventional BCG treatment.18 A previous study demonstrated CFUs in up to 40% of patients treated with BCG Connaught strain after 24 h and after 6 days in 27.1% of patients. A positive polymerase chain reaction was evident up to 24 months post instillation in between 4.2% and 37.5% of the investigated bladder biopsies.18 It’s likely that the implemented genetic changes in VPM10002BC with increased exposure of mycobacterial antigen by perforation of the phagosome membrane11 leads to quicker clearance of the bacteria from the bladder.
The analysis of blood and sputum before and after intravesical instillations of VPM1002BC showed no CFUs and no traces of VPM1002BC DNA. As such, a systemic uptake of VPM1002BC could not be demonstrated in the study setting.
In order to gain preliminary insight into the immune responses induced by VPM1002BC in the treated patients, cytokines in urine and in whole blood were determined. Despite the limited sample size (6 patients) and the sample variability, a clear trend in TH1 cytokines induction was observed both in urine and whole blood in a global analysis of cytokine secretion with a significant induction of TNFα in whole blood (Supplementary Figure 1 and 2). Furthermore, responses to PPD either from whole CD4+ or Ki67+CD4+ T cells were significant for GM-CSF and IFNγ. Our data is in agreement with previous findings. It has been indeed demonstrated that TNFα may play an ambiguous role since it was associated with tumor growth and poor prognosis,19 but also increased in urine of patients undergoing BCG treatment. In this regard, urinary neutrophils, strongly induced during BCG treatment, express large amounts of TNFα.20 Moreover, under the stimulation of INFγ, TNF-related apoptosis-inducing ligand (TRAIL), a member of the TNFα family, expressed by a number of cells such as macrophages, dendritic cells, natural killer cells, B cells and T cells is able to confer to these cells enhanced anti-tumor abilities.21 Finally, TNFα and GM-CSF were characterized in recent studies as playing a role in the prevention of bladder cancer.22 Taken together, our data, including a trend in IL-17 secretion by PBMC, a cytokine also associated by some authors to the recruitment of neutrophils after BCG treatment, brings arguments for VPM002BC in reproducing some aspects of the immunological cascade induced by BCG. Although the role of TNFα, IFNγ, IL-17 and GM-CSF cannot be definitively established at that stage, it could be of interest to postulate that these TH1 cytokines may play a role in the anti-tumor effect of VPM1002BC. Furthermore, in subsequent efficacy trials, it would be interesting to examine the variations of these cytokines as markers of protection. Elsäßer, et al. reported that IFNγ-producing CD4+ T cells of patients with a preexisting immunity against BCG increased to a much lesser extent as compared to BCG-naive patients.23 Since all patients included in the trial already experienced a previous BCG treatment course, the clear increase in the frequency of these cells in our trial is interesting and potentially indicative for the induction of an immune response by VPM1002BC. A more detailed analysis on a larger number of patients will be assessed in the phase II of the trial in order to corroborate these results.
Conclusion
The intravesical application of VPM1002BC is safe, well tolerated by the patients and shows promising immunological results. Phase II of this trial with the aim to test the preliminary efficacy in BCG-non-responding patients has completed recruitment and final results are expected for 2019.
Funding Statement
Serum Institute of India Pvt. Ltd., Pune, India. State Secretary for Education, Research and Innovation (SERI). Swiss Cancer Research Foundation (SCS). Swiss Cancer League (SCL).
Acknowledgments
We are in debt to all patients who participated in the study. We thank Clarisse Straub, Magdalena Schneider, Sabrina Stammler, and Andrea Egli Berini for excellent technical assistance during microbiological analyses.
Author contributions
C. A. R. Conception of the trial, data collection, data analysis and interpretation, critical revision of the article, drafting the manuscript, final approval of the manuscript for publication.
P. B. Data collection, data analysis and interpretation, drafting the manuscript, critical revision of the article, final approval of the manuscript for publication.
G. M. Recruitment of patients, data collection, critical revision of the article, final approval of the manuscript for publication.
M. R. Recruitment of patients, data collection, critical revision of the article, final approval of the manuscript for publication.
H. P. Recruitment of patients, data collection, critical revision of the article, final approval of the manuscript for publication.
G. W. Recruitment of patients, data collection, critical revision of the article, final approval of the manuscript for publication.
R. C. Recruitment of patients, data collection, critical revision of the article, final approval of the manuscript for publication.
G. P. P. Data analysis and interpretation, drafting the manuscript, critical revision of the article, final approval of the manuscript for publication.
L. G. Critical revision of the article, final approval of the manuscript for publication.
B. E. Critical revision of the article, final approval of the manuscript for publication.
H. S. Final approval of the manuscript for publication.
M. G. Final approval of the manuscript for publication.
S. G. Final approval of the manuscript for publication.
U. S. Final approval of the manuscript for publication.
D. G. Data analysis and interpretation, critical revision of the article, final approval of the manuscript for publication.
F. S. Data analysis and interpretation, critical revision of the article, final approval of the version to be published.
R. A. Data analysis and interpretation, drafting the manuscript, critical revision of the article, final approval of the version to be published.
M. E. Data analysis and interpretation, critical revision of the article, final approval of the version to be published.
S. B. Data analysis and interpretation, Critical revision of the article, Final approval of the version to be published.
S. H. Data analysis and interpretation, Critical revision of the article, Final approval of the version to be published.
A. W. Critical revision of the article, Final approval of the version to be published.
Conflicts of interest
Cyrill A. Rentsch: Coordinating investigator of this trial; Company Consultant to medac GmbH.
Piet Bosshard: No conflict of interest to declare.
Grégoire Mayor: No conflict of interest to declare.
Malte Rieken: No conflict of interest to declare.
Heike Püschel: No conflict of interest to declare.
Grégory Wirth: No conflict of interest to declare.
Richard Cathomas: Advisory role to MSD, BMS, Janssen, Astellas, Sanofi, Bayer, Roche, Pfizer, Eisai, Ipsen, Astra Zeneca. Speaker honoraria by Astellas, Debiopharm, BMS.
Gerald P. Parzmair: Employed by Vakzine Projekt Management GmbH, which is involved with the development of VPM1002BC.
Leander Grode: Co-inventor/patent holder of BCGΔureC::hly (VPM1002BC); Employed by Vakzine Projekt Management GmbH, which is involved with the development of VPM1002BC.
Bernd Eisele: Employed by Vakzine Projekt Management GmbH, which is involved with the development of VPM1002BC.
Hitt Sharma: Employed by Serum Institute of India Pvt. Ltd., which is the license holder and manufacturer of VPM1002BC.
Manish Gupta: Employed by Serum Institute of India Pvt. Ltd., which is the license holder and manufacturer of VPM1002BC.
Sunil Gairola: Employed by Serum Institute of India Pvt. Ltd., which is the license holder and manufacturer of VPM1002BC.
Umesh Shaligram: Employed by Serum Institute of India Pvt. Ltd., which is the license holder and manufacturer of VPM1002BC.
Daniel Goldenberger: No conflict of interest to declare.
François Spertini: No conflict of interest to declare.
Régine Audran: No conflict of interest to declare.
Milica Enoiu: No conflict of interest to declare.
Simona Berardi: No conflict of interest to declare.
Stefanie Hayoz: No conflict of interest to declare.
Andreas Wicki: No conflict of interest to declare.
Trial registration
ClinicalTrials.gov NCT02371447.
Supplementary material
Supplemental data for this article can be accessed on the publisher’swebsite.
References
- 1.Babjuk M, Burger M, Compérat E, Gontero P, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Sylvester R, et al. European association of urology guidelines 2018 Edition. Arnhem (The Netherlands): European Association of Urology Guidelines Office; 2018 [Google Scholar]
- 2.Sylvester RJ, van der MA, Lamm DL.. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168(5):1964–8. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 3.Herr HW, Schwalb DM, Zhang ZF, Sogani PC, Fair WR, Whitmore WF Jr, Oettgen HF. Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;13(6):1404–1408. doi: 10.1200/JCO.1995.13.6.1404. [DOI] [PubMed] [Google Scholar]
- 4.Malmstrom PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, Solsona E, Di Stasi SM, Witjes JA. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Biot C, Rentsch CA, Gsponer JR, Birkhauser FD, Jusforgues-Saklani H, Lemaitre F, Auriau C, Bachmann A, Bousso P, Demangel C, et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med. 2012;4(137):137ra72. doi: 10.1126/scitranslmed.3003586. [DOI] [PubMed] [Google Scholar]
- 6.Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol. 2018;15(10):615–625. doi: 10.1038/s41585-018-0055-4. [DOI] [PubMed] [Google Scholar]
- 7.Di Lorenzo G, Perdona S, Damiano R, Faiella A, Cantiello F, Pignata S, Ascierto P, Simeone E, De Sio M, Autorino R, et al. Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer. Cancer. 2010;116(8):1893–1900. doi: 10.1002/cncr.24914. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich G, Viret J-F, Hess J. Novel vaccination strategies based on recombinant Mycobacterium bovis BCG. Int J Med Microbiol. 2003;292(7–8):441–451. doi: 10.1078/1438-4221-00227. [DOI] [PubMed] [Google Scholar]
- 9.Nieuwenhuizen NE, Kulkarni PS, Shaligram U, Cotton MF, Rentsch CA, Eisele B, Grode L, Kaufmann SHE. The recombinant Bacille Calmette-Guerin vaccine VPM1002: ready for clinical efficacy testing. Front Immunol. 2017;8:1147. doi: 10.3389/fimmu.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen CS, Dietrich J, Agger EM, Lycke NY, Lovgren K, Andersen P. The combined CTA1-DD/ISCOMs vector is an effective intranasal adjuvant for boosting prior Mycobacterium bovis BCG immunity to Mycobacterium tuberculosis. Infect Immun. 2007;75(1):408–416. doi: 10.1128/IAI.01290-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115(9):2472–2479. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann SH, Cole ST, Mizrahi V, Rubin E, Nathan C. Mycobacterium tuberculosis and the host response. J Exp Med. 2005;201(11):1693–1697. doi: 10.1084/jem.20050842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grode L, Ganoza CA, Brohm C, Weiner J 3rd, Eisele B, Kaufmann SH. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013;31(9):1340–1348. doi: 10.1016/j.vaccine.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 14.World Medical A . World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 15.International Conference on Harmonization (ICH) (1996) E 6 guideline for good clinical practice. [accessed 10June 1996]. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf.
- 16.Hinic V, Feuz K, Turan S, Berini A, Frei R, Pfeifer K, Goldenberger D. Clinical evaluation of the Abbott RealTime MTB assay for direct detection of Mycobacterium tuberculosis-complex from respiratory and non-respiratory samples. Tuberculosis (Edinb). 2017;104:65–69. doi: 10.1016/j.tube.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Bisiaux A, Thiounn N, Timsit M-O, Eladaoui A, Chang -H-H, Mapes J, Mogenet A, Bresson J-L, Prié D, Béchet S, et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J Urol. 2009;181(4):1571–1580. doi: 10.1016/j.juro.2008.11.124. [DOI] [PubMed] [Google Scholar]
- 18.Durek C, Richter E, Basteck A, Rusch-Gerdes S, Gerdes J, Jocham D, Bohle A. The fate of bacillus Calmette-Guerin after intravesical instillation. J Urol. 2001;165(5):1765–1768. doi: 10.1016/S0022-5347(05)66410-5. [DOI] [PubMed] [Google Scholar]
- 19.Marsh HP, Haldar NA, Bunce M, Marshall SE, le Monier K, Winsey SL, Christodoulos K, Cranston D, Welsh KI, Harris AL, et al. Polymorphisms in tumour necrosis factor (TNF) are associated with risk of bladder cancer and grade of tumour at presentation. Br J Cancer. 2003;89(6):1096–1101. doi: 10.1038/sj.bjc.6601165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jinesh GG, Chunduru S, Kamat AM. Smac mimetic enables the anticancer action of BCG-stimulated neutrophils through TNF-alpha but not through TRAIL and FasL. J Leukoc Biol. 2012;92(1):233–244. doi: 10.1189/jlb.1211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simons MP, Nauseef WM, Griffith TS. Neutrophils and TRAIL: insights into BCG immunotherapy for bladder cancer. Immunol Res. 2007;39(1–3):79–93. doi: 10.1007/s12026-007-0084-1. [DOI] [PubMed] [Google Scholar]
- 22.Shintani Y, Sawada Y, Inagaki T, Kohjimoto Y, Uekado Y, Shinka T. Intravesical instillation therapy with bacillus Calmette-Guerin for superficial bladder cancer: study of the mechanism of bacillus Calmette-Guerin immunotherapy. Int J Urol. 2007;14(2):140–146. doi: 10.1111/j.1442-2042.2007.01696.x. [DOI] [PubMed] [Google Scholar]
- 23.Elsasser J, Janssen MW, Becker F, Suttmann H, Schmitt K, Sester U, Stöckle M, Sester M. Antigen-specific CD4 T cells are induced after intravesical BCG-instillation therapy in patients with bladder cancer and show similar cytokine profiles as in active tuberculosis. PLoS One. 2013;8(9):e69892. doi: 10.1371/journal.pone.0069892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- International Conference on Harmonization (ICH) (1996) E 6 guideline for good clinical practice. [accessed 10June 1996]. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf.


