ABSTRACT
Staphylococcus aureus
and its toxins have been linked to disease progression and mortality in advanced stages of cutaneous T-cell lymphoma (CTCL). CD8+ T cells play a crucial role in anti-cancer responses and high CD8+ T cell numbers in tumor lesions are associated with a favorable prognosis in CTCL. Here, we show that CD8+ T cells from both healthy donors and Sézary syndrome patients are highly susceptible to cell death induced by Staphylococcal alpha-toxin, whereas malignant T cells are not. Importantly, alpha-toxin almost completely blocks cytotoxic killing of CTCL tumor cells by peptide-specific CD8+ T cells, leading to their escape from induced cell death and continued proliferation. These findings suggest that alpha-toxin may favor the persistence of malignant CTCL cells in vivo by inhibiting CD8+ T cell cytotoxicity. Thus, we propose a novel mechanism by which colonization with Staphylococcus aureus may contribute to cancer immune evasion and disease progression in CTCL.
KEYWORDS: Alpha-toxin, Staphylococcus aureus, CTCL, CD8+ T cells, cytotoxicity
Introduction
Cutaneous T-cell lymphoma (CTCL) is a family of non-Hodgkin lymphomas characterized by the accumulation of malignant CD4+ T cells in the skin.1 Mycosis fungoides (MF) is the most common form of CTCL, characterized by inflamed skin lesions with a complex microenvironment.1–3 Primary CD30+ lymphoproliferative disorders (LPD) are the second largest subtype of CTCL and Sézary syndrome (SS) is a highly aggressive leukemic variant of the disease.1
CTCL is associated with a compromised skin barrier, bacterial colonization, and subsequent infections, which are a major cause of morbidity and mortality in advanced disease stages.4,5 Bacteria have also been shown to promote disease progression in a mouse model of CTCL.6 The pathogen most frequently found to be associated with CTCL is Staphylococcus aureus (S. aureus).7 S. aureus releases a variety of toxins, including super-antigens, which have been proposed to play an important role in CTCL progression.8–10 Another important toxin produced by S. aureus is the pore forming alpha-toxin, also known as alpha-hemolysin and hla, which is known to induce cell death in T cells.11 Alpha-toxin production correlates both with Staphylococcal virulence in animal infection models and disease severity in humans.12,13 Alpha-toxin is expressed by the majority of S. aureus strains and has shown to be produced by clinical isolates from CTCL lesions.14 We have recently shown that, in contrast to healthy CD4+ T cells, malignant T cells are relatively resistant to cell death induced by alpha-toxin.15 However, the effect of alpha-toxin on CD8+ T cells from CTCL patients, the impact of this toxin on anti-tumor responses and its potential pathogenic role in the disease has not been investigated.
Antigen-specific CD8+ T cells play an important role in immune surveillance and the defense against cancer. Upon activation, CD8+ T cells differentiate into cytotoxic T lymphocytes (CTLs) that can specifically lyse transformed cells in an MHC class I restricted manner. Malignant cells in cancer are known to acquire various immune evasion mechanisms that subvert CTL function. The observed presence of CTLs with the potential to kill autologous tumor cells in CTCL and the finding that high numbers of CD8+ T cells have been linked to a favorable prognosis, have sparked the hypothesis that CD8+ T cells are important in controlling the early indolent stages of the disease and in preventing disease progression.16,17 It has become clear that malignant T cells display ectopic expression of numerous cancer associated antigens such as embryonic stem cell and meiosis-specific antigens, suggesting that these cancer-associated neo-epitopes may play an important role in immune surveillance by CD8+ T cells.18–20
In this study, we report that primary CD8+ T cells from SS patients and healthy donors are potently killed by alpha-toxin, whereas malignant cells are largely resistant. In addition, we show that the presence of alpha-toxin dramatically inhibits peptide-specific CD8+ T cell lysis of CTCL cells, allowing for continued malignant proliferation. To our knowledge, this is the first study to demonstrate that S. aureus alpha-toxin can block CD8 cytotoxicity and thus potentially facilitate cancer immune evasion.
Materials and methods
Cell culture and isolation of peripheral blood mononuclear cells (PBMCs)
Peptide specific CD8+ T cells targeting MART-1, PD-L1 and FOXP3 were all maintained in X-VIVO media (Lonza, #BE02-060F) supplemented with 5% Human serum (HS) (Copenhagen Hospital Blood Bank) and 2 × 103 U/ml IL-2 (Novartis, #004184). All CD8+ T cell lines were established from HLA-A2 positive melanoma or breast cancer patients.21–24 Mac1 and Mac2a cell lines were derived from a patient suffering from a primary CD30+ LPD, manifesting as anaplastic large-cell lymphoma and MF, respectively.25,26 Both cell lines were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich, #R204), supplemented with 10% Heat inactivated fetal bovine serum (FBS) (Biological Industries, #04-007-1A) and 1% penicillin-streptomycin (Sigma, #P7539). Cells cultures used were mycoplasma tested using the MycoAlertTM PLUS Mycoplasma Detection Kit (Lonza, #LZ-LT07-710) and MycoAlertTM Assay Control Set (Lonza, #LT07-518). Cells were tested after thawing and experiments were only performed if negative for mycoplasma. CD8+ T cells were grown for maximal two weeks, while Mac1 and Mac2a cell lines were kept for up to four weeks in culture until the last experiment was performed. PBMCs from healthy donors and SS patients were obtained in accordance with the Declaration of Helsinki. After approval by the Committee on Health Research Ethics (H-16025331), written informed consent was obtained from all SS patients (Supplementary Table 1 patient characteristics). PBMCs were isolated by Ficoll-based density-gradient centrifugation. Positive selection of CD8+ T cells was performed using CD8 MicroBeads (Miltenyi Biotec, #130-045-201) and LS columns (Miltenyi Biotec, #130-041-306) following the manufacturer’s instructions.
Alpha-toxin treatment
Purified alpha-toxin was obtained from List Biological Laboratories (#120). Cells were treated with alpha-toxin at 37°C for the duration and with concentrations as stated in the respective figures and figure legends.
LDH activity assay
Cells were grown in RPMI 1640 (Gibco, #11835-063) or X-VIVO (Lonza, #BE02-061Q) media without phenol red prior to measuring Lactate Dehydrogenase (LDH) release with the LDH Cytotoxicity Detection Kit (TaKaRa, #MK401). LDH release was quantified from culture supernatants following the manufacturer’s instructions. Values were normalized to max release measured in Triton X-100 treated samples prior to subtracting the background absorbance.
Flow cytometry
Cell surface staining was performed in FACS-PBS (PBS + 1% FBS + 0.02% NaN3) or Brilliant Stain Buffer (BD Bioscience, #563794), using primary conjugated antibodies (listed in supplementary Table 2). Malignant cells were gated as CD3+CD4+TCRVβ#+ followed by subsequent exclusion of CD26+CD7+ cells. In patients where the dominant TCRVβ clone could not be targeted, malignant cells were gated as CD3+CD4+CD7− and/or CD26−, as previously described.27 Intracellular staining with FOXP3-APC (eBioscience, #17-4777-42) was performed using the eBioscience FOXP3/Transcription Factor Staining Buffer Set (ThermoFisher, #00-5523-00). Dead cells were excluded using propidium iodide (eBioscience, #MBS500PI) and/or Annexin V-FITC/PE (BioLegend, #640945/#640908) or Fixable Viability Dye eFluor780 (eBiosciences, #65-0865-14). Annexin V staining was conducted in Annexin V Binding Buffer (BD Bioscience, #556454).
Peptide specificity of CD8+ T cells was quantified with HLA-A2 tetramers. Cells were incubated with PE and APC conjugated tetramer complexes (MART-1, PD-L1, FOXP3) for 20 minutes at 37°C prior to cell surface and viability staining. HIV tetramers were included as negative controls. All flow cytometric analyses were performed using a 3 or 5 laser BD LSR-Fortessa. Flow cytometry data were visualized and analyzed using FlowJo (TreeStar) software.
Co-culture
Peptide specific CD8+ T cells and Mac1/Mac2a cells were labeled with CellTrace Blue (ThermoFisher, #C34574) and CellTrace Violet (ThermoFisher, #C34557), respectively. In short cells were washed in PBS, re-suspended in 0.5 ml PBS containing 0.2% HS and 0.5 ml PBS containing 10 µM of the respective dye were added. Cells were incubated for 5 minutes at room temperature before staining was halted by addition of 10 ml X-VIVO media containing 10% HS. After 5 minutes of incubation, cells were spun down and re-suspended in complete culture media. Effector cells were treated with alpha-toxin and target cells were pulsed with 1 nM of peptide for 2 hours at 37°C when indicated. Effector and target cells were co-cultured for 4 hours at 37°C analyzed by flow cytometry (gating strategy Supplementary Figure 1).
Proliferation was measured using 5-ethynyl-2`-deoxyiridine (EdU) incorporation. EdU was added to the co-culture 24 hours before analysis. Click-iT Plus EdU Alexa Fluor 488 assay (ThermoFisher, #C10632) was performed following the manufacturer’s instructions with the modification of substituting the provided fixative with the BD Cytofix/Cytoperm (BD Bioscience, #554722).
Results
First, we determined whether CD8+ T cells were sensitive to alpha-toxin-induced cell death. We exposed CD8+ T cells from healthy donors and SS patients to alpha-toxin and analyzed toxin-induced cell death by LDH release assay and/or flow cytometry. Regardless of the source, CD8+ T cells were highly susceptible to alpha-toxin, while the malignant CTCL cell line MyLa2059 was resistant (Figure 1(a–d)). Similarly, primary malignant T cells from SS patients were more resistant to alpha-toxin than the CD8+ T cells from the same patient (Figure 1(e)). Hence, exposure to alpha-toxin drastically increased the ratio of malignant to CD8+ T cells in eight of the ten tested SS patients (Figure 1(e)).
Figure 1.
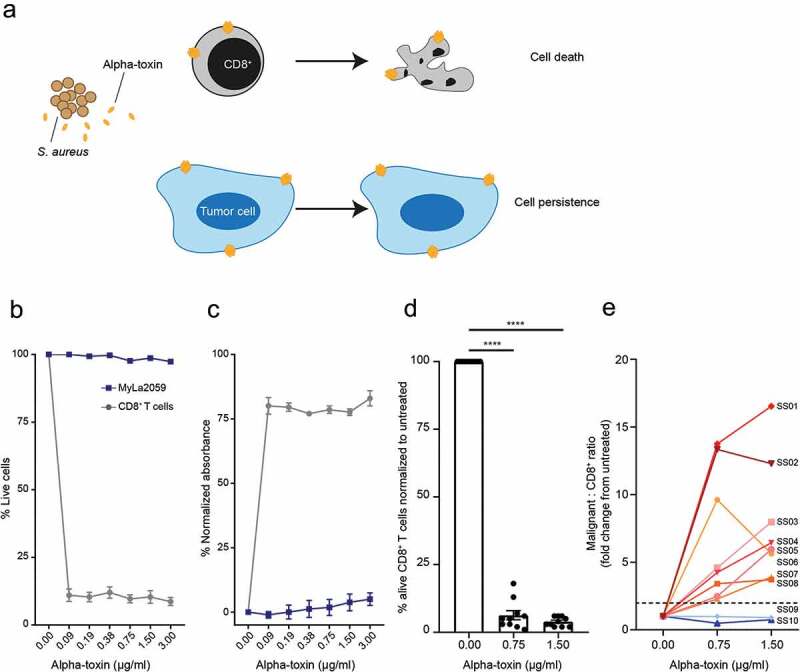
CD8+ T cells are sensitive to alpha-toxin-induced cell death at concentrations where malignant CTCL cells are not. Malignant CTCL cells and CD8+ T cells from healthy donors or SS patients were exposed to alpha-toxin for 6 hours at 37°C. Viability was assessed by flow cytometry and LDH release was measured in the culture supernatant. (a) Schematic hypothesis for alpha-toxin favoring malignant cells over CD8+ T cells. (b,c) Purified primary CD8+ T cells from healthy donors and the malignant CTCL cell line, MyLa2059 (n = 3). (d) CD8+ T cells from SS patients (n = 10). (e) Change in Malignant SS cells to CD3+CD8+ T cell ratio (n = 10) by alpha-toxin exposure relative to untreated control. Line and bar plots display mean ± standard error of mean. Paired students t-tests using GraphPad Prism version 7.00. * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001.
The difference in sensitivity between malignant CTCL cells and CD8+ T cells suggested that alpha-toxin producing S. aureus within the tumor microenvironment could abrogate tumor-specific CD8+ T cell responses toward malignant CTCL cells. To test this, we first used a well-established MART-1 peptide-based model to study MART-1 specific CD8+ T cell-mediated killing of MART-1 pulsed cancer cells (described in23). The strength of this anti-tumor CD8+ T cell cytotoxicity assay is that it is highly specific and robust. Presentation of the MART-1 peptide to CD8+ T cells is restricted to HLA-A2 molecules and we only observed specific killing of MART-1 pulsed HLA-A2+ Mac-1 cell lines, but not of MART-1 pulsed HLA-A2- CTCL lines such as MyLa2059.23 Accordingly, we pulsed Mac1 cells with MART-1 peptide and co-cultured them with HLA-A2 restricted MART-1 specific CD8+ T cells that were pre-treated with alpha-toxin or vehicle as a control. Specific cytotoxicity was determined by LDH release and flow cytometric analysis of remaining Mac1 cells. We found that pre-treatment with alpha-toxin strongly inhibited CD8+ T cell-mediated killing of MART1-pulsed malignant T cells (Figure 2(a–d), Supplementary Figure 2(a,b)).
Figure 2.
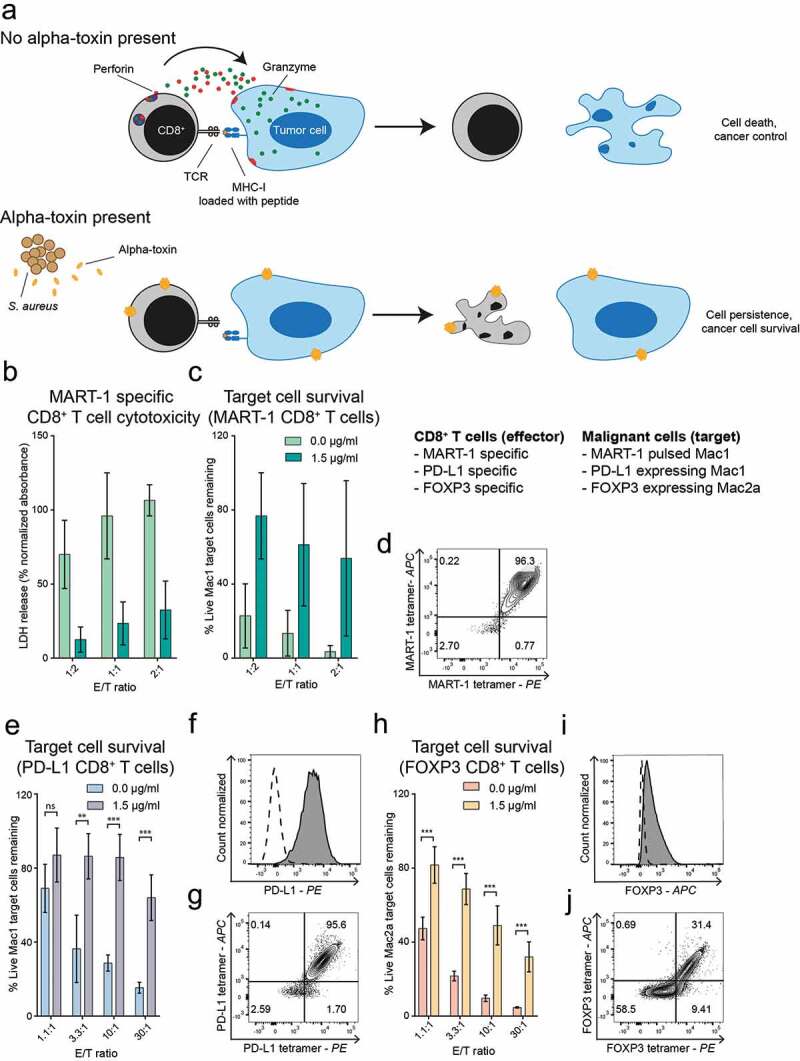
Alpha-toxin inhibits anti-cancer responses of peptide specific CD8+ T cells. Peptide specific CD8+ T cells were cultured for 2 hours at 37°C in the presence or absence of alpha-toxin before being co-cultured for 4 hours with their respective target cells. (a) Schematic hypothesis of effect of alpha-toxin in co-cultures of CD8+ T cell effector cells and target CTCL cells. B-D: MART-1 specific CD8+ T cells (n = 2). (b) LDH release after co-culture of MART-1 specific CD8+ T cells with MART-1 peptide pulsed Mac1 cells. Specific cell lysis was calculated after subtracting the background release from both effector and target cells. (c) Normalized percentage of remaining target cells after exposure to MART-1 specific CD8+ T cells. (d) Tetramer staining of MART-1-specific CD8+ T cells. E-G: PD-L1 specific CD8+ T cells (n = 3). (e) Mac1 cells remaining after co-culture with PD-L1 specific CD8+ T cells. (f) PD-L1 expression of Mac1 cells. The dashed line representing the expression levels of the FMO control. (g) Tetramer staining of PD-L1-specific CD8+ T cells. H-J: FOXP3 specific CD8+ T cells (n = 3). (h) Displaying normalized percentage of Mac2a cells remaining after the exposure to FOXP3-specific CD8+ T cells. (i) Foxp3 expression of Mac2a cells. The dashed line represents the expression levels of the isotype control. (j) Tetramer staining of FOXP3-specific CD8+ T cells. Bar plots display mean ± standard error of mean. Paired students t-tests using GraphPad Prism version 7.00. * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001.
Next, we addressed whether alpha-toxin inhibits CD8+ T cell-mediated cytotoxicity against endogenous antigens expressed by malignant T cells in CTCL. To this end, we utilized HLA-A2 restricted CD8+ T cells recognizing peptides derived from Programmed cell death 1 ligand (PD-L1) or Forkhead box P3 (FOXP3), both representing peptides frequently found to be dysregulated in CTCL.28–30 Similar to the inhibition of cytotoxicity in the MART-1 model, peptide-specific CD8+ T cells targeting endogenous CTCL peptides showed similar decrease in their specific killing when cultured in the presence of alpha-toxin (Figure 2(e–j) and Supplementary Figure 2(c–f)).
Finally, we wanted to investigate if the presence of alpha-toxin could facilitate immune evasion by eliminating cancer-specific CD8+ T cells, while allowing continued CTCL cell proliferation. We co-cultured MART-1 peptide-pulsed Mac1 cells with MART-1 specific CD8+ T cells in the presence or absence of alpha-toxin and measured the proliferation of malignant target cells using flow cytometry (Figure 3(a)). In the presence of alpha-toxin, peptide-pulsed malignant CTCL cells continued to proliferate when co-cultured with CTLs, whereas proliferation was almost completely abrogated when co-cultured with reactive CTLs in the absence of alpha-toxin (Figure 3(b–d)).
Figure 3.
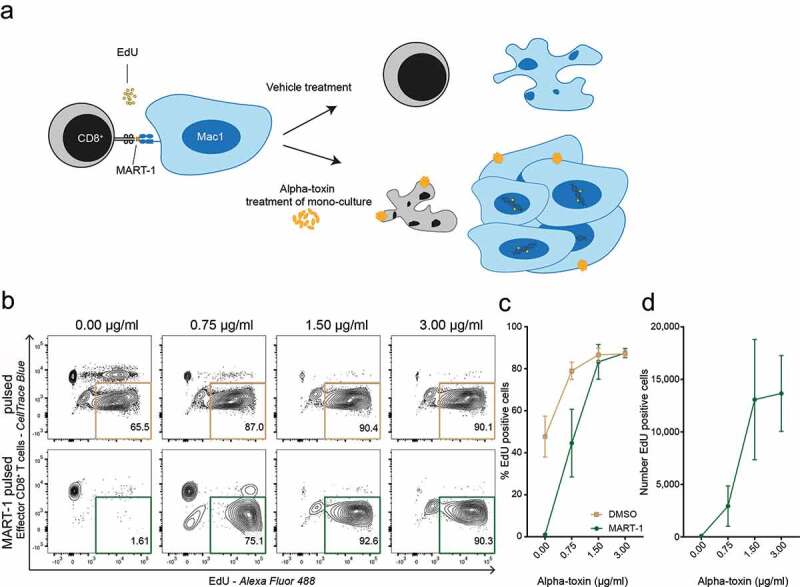
Alpha-toxin facilitates immune evasion and allows continued malignant proliferation. (a) Schematic hypothesis of alpha-toxin mediated immune evasion. Target CTCL (Mac1) cells and effector CD8+ T cells were pre-treated with alpha-toxin or vehicle control before being co-cultured for 48 hours. During the last 24 hours EdU was added to the culture. (b–d) Percentage and number of proliferating EdU+ Mac1 cells after 48 hours of co-culture with MART-1 specific CD8+ T cells (n = 3). Both target and effector cells were pre-treated with 2-fold increasing concentrations of alpha-toxin. (b) Representative flow cytometry contour plot. (c) Percentage of EdU+ target cells. (d) Number of EdU+ target cells. Bar and line plots depict mean ± standard error of mean for three independent replicates.
Discussion
Bacterial infections are a major cause of morbidity and mortality in advanced stage CTCL.4 Notably, several lines of evidence suggest that S. aureus fuels disease progression in this malignant disease, although the underlying mechanism is only partially understood.8 We have previously shown that malignant CD4+ T cells are relatively resistant to alpha-toxin-induced cell death compared to their nonmalignant CD4+ T cell counterparts.15 This suggests that alpha-toxin released during staphylococcal infections may tilt the balance between malignant and nonmalignant CD4+ T cells, favoring the persistence of malignant cells in CTCL.
In this study, we demonstrate that CD8+ T cells are highly sensitive toward alpha-toxin-induced toxicity, and that alpha-toxin selects for malignant cells in the majority of the investigated SS patients. Furthermore, we show that alpha-toxin effectively inhibits CD8+ T cell-mediated anti-cancer responses, while allowing malignant CTCL cells to persist and proliferate. This was the case irrespective of whether peptide specific CD8+ T cells were directed against MART-1 or PD-L1 and FOXP3 peptides, which are endogenously expressed in CTCL. The current study focused on the direct effect of alpha-toxin on cytotoxic CD8+ T cells using cell lines and primary cells isolated from the blood of ten SS patients. Thus, our results do not directly address whether similar effects occur in the skin of S. aureus infected patients. While we have previously shown that cell lines from various subtypes of CTCL are all resistant to alpha-toxin induced cell death,15 this study was limited to primary cells from SS patients and CD30+ LPD (Mac1 and Mac2a) cell lines. These cell lines were chosen as they have been previously used in CD8+ T cell mediated cytotoxicity assays targeting exogenous or CTCL-relevant endogenous peptides.21–24
High numbers of CD8+ T cells are significantly correlated with increased survival in CTCL, suggesting that CD8+ T cells are actively involved in restraining malignant cells.16,31 Indeed, CTCL patients harbor cytotoxic T cells with the potential to kill autologous cancer cells in an HLA-Class I restricted manner.17 The present findings outline a novel mechanism whereby S. aureus infections can contribute to immune evasion in CTCL through the release of alpha-toxin. As shown here and elsewhere,15 we observed inhibitory effects of alpha-toxin on primary CD4+ and CD8+ T cells at concentrations above 0.1 μg/ml and maximal effects at around 1.5 μg/ml. However, little is known about the physiological concentrations of alpha-toxin within lesional skin of CTCL patients. Of notice, alpha-toxin concentrations in supernatants from community-associated alpha-toxin producing S. aureus isolated from nasal swabs of colonized individuals can vary between 1–60 μg/ml (mean of 40 μg/ml). Although these figures cannot be extrapolated to concentrations in situ, they suggest that the profound effects reported here at alpha-toxin concentrations of 0.75–1.50 μg/ml are likely well within the physiologically relevant range that we can expect in the tumor micro-environment of CTCL skin lesions.32 Thus, our findings suggest that environmental factors such as staphylococcal toxins may directly interfere with anti-cancer responses and facilitate disease progression. As such, alpha-toxin could be a potential target for future therapies by vaccination or neutralizing antibodies. Encouragingly, a neutralizing antibody targeting alpha-toxin (MEDI4893) has been shown to be effective against S. aureus infection in a murine dermonecrosis model and is currently being evaluated in clinical trials of ventilator-associated pneumonia patients.12 However, the effect of neutralizing alpha-toxin in CTCL patients has not yet been tested.
IL-17 family cytokines are expressed in skin lesions in a substantial fraction of CTCL patients and are associated with progressive disease and poor prognosis.33,34 However, it is unknown whether or not IL-17 family members play a pathogenic role in disease progression.35 IL-17 cytokines are an important part of host T helper 17 responses against S. aureus in healthy individuals (reviewed in 36). Likewise, S. aureus toxins trigger STAT3 mediated IL-17 expression in cultures of primary malignant and nonmalignant T cells.8 Therefore, it is possible that IL-17 expression in skin lesions is a consequence of anti-S. aureus responses by malignant and/or nonmalignant T cells in CTCL. Since S. aureus infections are associated with a poor prognosis, it is possible that IL-17 is a marker of infection rather than a primary driver of disease progression. An alternative, but not mutually exclusive, explanation might be that IL-17 family cytokines promote a pro-tumorigenic microenvironment in CTCL. For example, IL-17F released by malignant T cells promotes angiogenesis in vitro37 and IL-17A/F stimulates expression of IL-6, IL-8, COX2/PGE2, MCP-1 and G-CSF in keratinocytes, fibroblasts, and endothelial cells.38,39 As PGE2 is a growth factor for malignant T cells,40 IL-17 cytokines may drive malignant proliferation via induction of PGE2 and other growth factors in the microenvironment.41
S. aureus and its toxins have previously been associated with CTCL disease progression by inducing STAT3 activation, an oncogene implicated in the survival and proliferation of malignant T cells.6,8,42 In addition, our studies suggest that the Staphylococcal alpha-toxin further contributes to CTCL pathogenesis by tilting the balance between malignant and nonmalignant CD4+ T cells15 and by interfering with the anti-cancer response of CD8+ T cells. The current findings support the use of antibiotic treatment in CTCL patients and suggest a benefit of using a combinatorial treatment approach including antibiotics and neutralizing antibodies targeting alpha-toxin, therapies which have previously been shown to have synergistic effects.13 In addition, the selective effect of a bacterial toxin on malignant versus anti-cancer immune cells may also have implications for immune therapies aimed at augmenting CD8-mediated killing of cancer cells (reviewed in43,44) where bacterial infections are involved. S. aureus infections are not limited to CTCL patients and are also a matter of concern in other cancers as well as benign skin diseases, where an effect of alpha-toxin on protective immune cells could also play an important role.45,46
In conclusion, we provide first evidence that S. aureus-derived alpha-toxin can effectively eliminate cytotoxic T cell-mediated killing of malignant cells, allowing immune evasion and continued growth of malignant CTCL cells. Thus, our findings suggest a novel mechanism by which alpha-toxin-producing S. aureus may promote disease persistence and progression in CTCL and suggests that neutralizing alpha-toxin may represent a promising therapeutic approach.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the patients that donated blood for this study as well as the photophoresis team at Copenhagen University Hospital Bispebjerg for providing the patient samples.
Funding Statement
This work was supported by the Novo Nordisk Research Foundation [NNF14OC0012345]; LEO Foundation [NA]; Danish Research Council (Danmarks Frie Forskning Fond) [NA]; Danish Cancer Society (Kraeftens Bekaempelse) [NA]; Lundbeck Foundation [NA]; LINAK A/S Nordborg [NA]; Aage Bangs Foundation [NA]; Kraeftfonden [NA]; Fight Cancer Program (Knaek Cancer) [NA].
Authors’ contributions
E.B. performed experiments; E.B., T.B.B. analysed the data and made the figures; E.B., S.M.A, C.N., A.W-O., M.G., S.F., T.H., B.G.J.S., L.M.L., H.F., S.B.K., L.M.R.G., R.C., L.I., T.K., C.M.B., C.G., J.C.B., A.W., M.H.A, T.B.B., and N.Ø. designed the research. E.B., T.B.B. and N.Ø. wrote the original draft of the paper. E.B., S.M.A, C.N., A.W-O., M.G., S.F., T.H., B.G.J.S., L.M.L., H.F., S.B.K., L.M.R.G., R.C., L.I., T.K., C.M.B., C.G., J.C.B., A.W., M.H.A, T.B.B., and N.Ø. reviewed and edited the manuscript. T.B.B., M.H.A. and N.Ø. supervised the project. N.Ø. acquired the funding.
Disclosure of Potential Conflicts of Interest
Jürgen C. Becker declares the association with the pharma firm Takeda as an external advisor. Niels Ødum has an advisory consultant honorarium from Micreos human Health B.V. All other authors declare no potential conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, Jaffe ES.. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703–8. doi: 10.1182/blood-2018-11-881268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vieyra-Garcia P, Crouch JD, O’Malley JT, Seger EW, Yang CH, Teague JE, Lowry EL. Benign T cells drive clinical skin inflammation in cutaneous T cell lymphoma. JCI Insight. 2019;4(1). doi: 10.1172/jci.insight.124233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litvinov IV, Tetzlaff MT, Thibault P, Gangar P, Moreau L, Watters AK, Provost N. Gene expression analysis in cutaneous T-cell lymphomas (CTCL) highlights disease heterogeneity and potential diagnostic and prognostic indicators. Oncoimmunology. 2017;6(5):e1306618. doi: 10.1080/2162402X.2017.1306618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and Sezary syndrome. JAMA. 1992;267(10):1354–1358. doi: 10.1001/jama.1992.03480100060031. [DOI] [PubMed] [Google Scholar]
- 5.Mirvish ED, Pomerantz RG, Geskin LJ. Infectious agents in cutaneous T-cell lymphoma. J Am Acad of Dermatol. 2011;64(2):423–431. doi: 10.1016/j.jaad.2009.11.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanok MH, Sun A, Fogli LK, Narendran V, Eckstein M, Kannan K, Dolgalev I. Role of dysregulated cytokine signaling and bacterial triggers in the pathogenesis of cutaneous T-cell lymphoma. J Invest Dermatol. 2018. May;138(5):1116–1125. doi: 10.1016/j.jid.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen V, Huggins RH, Lertsburapa T, Bauer K, Rademaker A, Gerami P, Guitart J. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J Am Acad Dermatol. 2008;59(6):949–952. doi: 10.1016/j.jaad.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Willerslev-Olsen A, Krejsgaard T, Lindahl LM, Litvinov IV, Fredholm S, Petersen DL, Wasik MA. Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood. 2016;127(10):1287–1296. doi: 10.1182/blood-2015-08-662353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindahl LM, Fredholm S, Joseph C, Nielsen BS, Jønson L, Willerslev-Olsen A, Hu T. STAT5 induces miR-21 expression in cutaneous T cell lymphoma. Oncotarget. 2016;7(29):45730–45744. doi: 10.18632/oncotarget.10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krejsgaard T, Willerslev-Olsen A, Lindahl LM, Bonefeld CM, Koralov SB, Geisler C, Woetmann A. Staphylococcal enterotoxins stimulate lymphoma-associated immune dysregulation. Blood. 2014;124(5):761–770. doi: 10.1182/blood-2014-01-551184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nygaard TK, Pallister KB, DuMont AL, DeWald M, Watkins RL, Pallister EQ, Voyich JM. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One. 2012;7(5):e36532. doi: 10.1371/journal.pone.0036532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tkaczyk C, Semenova E, Shi YY, Rosenthal K, Oganesyan V, Warrener P, Sellman BR. Alanine scanning mutagenesis of the MEDI4893 (suvratoxumab) epitope reduces alpha toxin lytic activity in vitro and Staphylococcus aureus fitness in infection models. Antimicrob Agents Chemother. 2018;62(11):e01033–18. doi: 10.1128/AAC.01033-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Keller A. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother. 2014;58(2):1108–1117. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindahl LM, Willerslev-Olsen A, Gjerdrum LM, Nielsen PR, Blümel E, Rittig AH, Wasik MA. Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma. Blood. 2019;134(13):1072–1083. doi: 10.1182/blood.2018888107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blümel E, Willerslev-Olsen A, Gluud M, Lindahl LM, Fredholm S, Nastasi C, Persson JL. Staphylococcal alpha-toxin tilts the balance between malignant and non-malignant CD4+ T cells in Cutaneous T-cell lymphoma. Oncoimmunology. 2019;8(11):e1641387. doi: 10.1080/2162402X.2019.1641387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoppe RT, Medeiros LJ, Warnke RA, Wood GS. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J Am Acad Dermatol. 1995;32(3):448–453. doi: 10.1016/0190-9622(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 17.Berger CL, Wang N, Christensen I, Longley J, Heald P, Edelson RL. The immune response to class I-associated tumor-specific cutaneous T-cell lymphoma antigens. J Invest Dermatol. 1996;107(3):392–397. doi: 10.1111/1523-1747.ep12363378. [DOI] [PubMed] [Google Scholar]
- 18.Gantchev J, Martínez Villarreal A, Xie P, Lefrançois P, Gunn S, Netchiporouk E, Sasseville D. The Ectopic Expression of Meiosis Regulatory Genes in Cutaneous T-Cell Lymphomas (CTCL). Front Oncol. 2019. May 31;9:429. doi: 10.3389/fonc.2019.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefrançois P, Xie P, Wang L, Tetzlaff MT, Moreau L, Watters AK, Netchiporouk E. Gene expression profiling and immune cell-type deconvolution highlight robust disease progression and survival markers in multiple cohorts of CTCL patients. Oncoimmunology. 2018;7(8):e1467856. doi: 10.1080/2162402X.2018.1467856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litvinov IV, Netchiporouk E, Cordeiro B, Zargham H, Pehr K, Gilbert M, Zhou Y. Ectopic expression of embryonic stem cell and other developmental genes in cutaneous T-cell lymphoma. Oncoimmunology. 2014;3(11):e970025. doi: 10.4161/21624011.2014.970025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munir S, Andersen GH, Met Ö, Donia M, Frøsig TM, Larsen SK, Andersen MH. HLA-restricted CTL that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res. 2013;73(6):1764–1776. doi: 10.1158/0008-5472.CAN-12-3507. [DOI] [PubMed] [Google Scholar]
- 22.Larsen SK, Munir S, Woetmann A, Frøsig TM, Odum N, Svane IM, Andersen MH. Functional characterization of Foxp3-specific spontaneous immune responses. Leukemia. 2013;27(12):2332–2340. doi: 10.1038/leu.2013.196. [DOI] [PubMed] [Google Scholar]
- 23.Oelke M, Moehrle U, Chen JL, Behringer D, Cerundolo V, Lindemann A, Mackensen A. Generation and purification of CD8+ melan-A-specific cytotoxic T lymphocytes for adoptive transfer in tumor immunotherapy. Clin Cancer Res. 2000;6:1997–2005. [PubMed] [Google Scholar]
- 24.Munir Ahmad S, Martinenaite E, Hansen M, Junker N, Borch TH, Met Ö, Andersen MH. PD-L1 peptide co-stimulation increases immunogenicity of a dendritic cell-based cancer vaccine. Oncoimmunology. 2016;5(8):e1202391. doi: 10.1080/2162402X.2016.1202391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis TH, Morton CC, Miller-Cassman R, Balk SP, Kadin ME. Hodgkin’s disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N Engl J Med. 1992;326(17):1115–1122. doi: 10.1056/NEJM199204233261704. [DOI] [PubMed] [Google Scholar]
- 26.Netchiporouk E, Gantchev J, Tsang M, Thibault P, Watters AK, Hughes J-DM, Ghazawi FM. Analysis of CTCL cell lines reveals important differences between mycosis fungoides/Sézary syndrome vs. HTLV-1+ leukemic cell lines. Oncotarget. 2017;8(56):95981–95998. doi: 10.18632/oncotarget.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buus TB, Willerslev-Olsen A, Fredholm S, Blümel E, Nastasi C, Gluud M, Gniadecki R. Single-cell heterogeneity in Sézary syndrome. Blood Adv. 2018;2(16):2115–2126. doi: 10.1182/bloodadvances.2018022608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantekure K, Yang Y, Raghunath P, Schaffer A, Woetmann A, Zhang Q, Wasik M. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma (CTCL)/mycosis fungoides (MF). American J Dermatopath. 2012;34(1):126–128. doi: 10.1097/DAD.0b013e31821c35cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capriotti E, Vonderheid EC, Thoburn CJ, Wasik MA, Bahler DW, Hess AD. Expression of T-plastin, FoxP3 and other tumor-associated markers by leukemic T-cells of cutaneous T-cell lymphoma. Leuk Lymphoma. 2008;49(6):1190–1201. doi: 10.1080/10428190802064917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasprzycka M, Zhang Q, Witkiewicz A, Marzec M, Potoczek M, Liu X, Wang HY. γc-signaling cytokines induce a regulatory T cell phenotype in malignant CD4+ T lymphocytes. J Immunol. 2008;181:2506–2512. doi: 10.4049/jimmunol.181.4.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermeer MH, van Doorn R, Dukers D, Bekkenk MW, Meijer CJ, Willemze R. CD8+ T cells in cutaneous T-cell lymphoma: expression of cytotoxic proteins, Fas ligand, and killing inhibitory receptors and their relationship with clinical behavior. J Clin Oncol. 2001;19(23):4322–4329. doi: 10.1200/JCO.2001.19.23.4322. [DOI] [PubMed] [Google Scholar]
- 32.Mairpady Shambat S, Haggar A, Vandenesch F, Lina G, van Wamel WJ, Arakere G, Svensson M. Levels of alpha-toxin correlate with distinct phenotypic response profiles of blood mononuclear cells and with agr background of community-associated staphylococcus aureus isolates. PLoS One. 2014;9(8):e106107. doi: 10.1371/journal.pone.0106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krejsgaard T, Ralfkiaer U, Clasen-Linde E, Eriksen KW, Kopp KL, Bonefeld CM, Geisler C. Malignant cutaneous T-cell lymphoma cells express IL-17 utilizing the Jak3/Stat3 signaling pathway. J Invest Dermatol. 2011;131(6):1331–1338. doi: 10.1038/jid.2011.27. [DOI] [PubMed] [Google Scholar]
- 34.Krejsgaard T, Litvinov IV, Wang Y, Xia L, Willerslev-Olsen A, Koralov SB, Kopp KL. Elucidating the role of interleukin-17F in cutaneous T-cell lymphoma. Blood. 2013;122(6):934–950. doi: 10.1182/blood-2013-01-480889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Litvinov IV, Shtreis A, Kobayashi K, Glassman S, Tsang M, Woetmann A, Sasseville D. Investigating potential exogenous tumor initiating and promoting factors for Cutaneous T-Cell Lymphomas (CTCL), a rare skin malignancy. Oncoimmunology. 2016;5(7):e1175799. doi: 10.1080/2162402X.2016.1175799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11(8):505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauenborg B, Litvinov IV, Zhou Y, Willerslev-Olsen A, Bonefeld CM, Nastasi C, Fredholm S. Malignant T cells activate endothelial cells via IL-17 F. Blood Cancer J. 2017;7(7):e586. doi: 10.1038/bcj.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanda N, Koike S, Watanabe S. IL-17 suppresses TNF-alpha-induced CCL27 production through induction of COX-2 in human keratinocytes. J Allergy Clin Immunol. 2005;116(5):1144–1150. doi: 10.1016/j.jaci.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Interleukin-17. Int Rev Immunol. 1998;16(5–6):541–551. doi: 10.3109/08830189809043008. [DOI] [PubMed] [Google Scholar]
- 40.Kopp KL, Kauczok CS, Lauenborg B, Krejsgaard T, Eriksen KW, Zhang Q, Wasik MA. COX-2-dependent PGE(2) acts as a growth factor in mycosis fungoides (MF). Leukemia. 2010;24(6):1179–1185. doi: 10.1038/leu.2010.66. [DOI] [PubMed] [Google Scholar]
- 41.Krejsgaard T, Lindahl LM, Mongan NP, Wasik MA, Litvinov IV, Iversen L, Langhoff E. Malignant inflammation in cutaneous T-cell lymphoma-a hostile takeover. Semin Immunopathol. 2017;39(3):269–282. doi: 10.1007/s00281-016-0594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seffens A, Herrera A, Tegla C, Buus TB, Hymes KB, Ødum N. STAT3 dysregulation in mature T and NK cell lymphomas. Cancers (Basel). 2019;11(11). doi: 10.3390/cancers11111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arina A, Corrales L, Bronte V. Enhancing T cell therapy by overcoming the immunosuppressive tumor microenvironment. Semin Immunol. 2016;28(1):54–63. doi: 10.1016/j.smim.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 44.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 45.Kullander J, Forslund O, Dillner J. Staphylococcus aureus and squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2009;18(2):472–478. doi: 10.1158/1055-9965.EPI-08-0905. [DOI] [PubMed] [Google Scholar]
- 46.Blicharz L, Rudnicka L, Samochocki Z. Staphylococcus aureus: an underestimated factor in the pathogenesis of atopic dermatitis? Postepy Dermatol Alergol. 2019;36(1):11–17. doi: 10.5114/ada.2019.82821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


