ABSTRACT
The tumor microenvironment (TME) provides necessary nutrition for tumor growth and recruits immunosuppressive factors including regulatory T cells and myeloid-derived suppressor cells (MDSCs) to inhibit the anti-tumor immune response induced by immunotherapy. As a main TME component, cancer associated fibroblasts (CAFs) can restrain T cell infiltration and activity through extracellular matrix remodeling. Vaccines targeting fibroblast-activating protein α (FAPα), which is mainly expressed on the CAF surface, can eliminate CAFs in tumors and regulate the TME, enhancing the potency of T cell-mediated anti-tumor effects. However, the anti-tumor effects were not fully realized as the tumor induces a large number of peripheral MDSCs during its growth, rendering the body of mice in an immunosuppressive state and preventing the vaccine from inducing effective anti-tumor immune responses. Here, we developed a dual-targeted DNA vaccine OsFS, targeting tumor matrix antigen FAPα and tumor cell antigen survivin simultaneously, exhibited enhanced antineoplastic effects in an established breast cancer model. Moreover, doxorubicin (Dox) pretreatment to remove the peripheral MDSCs induced to regulate the peripheral immune environment could further facilitate the anti-tumor activity of the vaccine. These results indicated that combination treatment of the tumor cells and the TME dual-targeting vaccine plus Dox could effectively realize the anti-tumor activity of the vaccine by decreasing immunosuppressive factors and inducing more tumor-infiltrating lymphocytes, which may offer important guidance for clinical research regarding the combination of the DNA vaccine with low-dose Dox.
KEYWORDS: Tumor microenvironment, fibroblast activating protein α, survivin, myeloid-derived suppressor cells, doxorubicin
Introduction
The recent clinical success of immunization checkpoint blockade therapy has initiated a new era of cancer immunotherapy.1,2 The premise for the effectiveness of programmed death-ligand 1 blockade is the presence of CD8+ T cells in tumors.3 Most cancer immunotherapies require T cells to perform anti-neoplastic effects. Moreover, numerous studies have shown that the quantity of T cells infiltrated in tumors positively correlates with the prognosis of patients with cancer across various cancer types.4–7 As a type of cancer immunotherapy, anti-cancer vaccines have attracted increasing attention because of their ability to induce a large number of tumor-specific CD8+ T cells, which are crucial for tumor regression.8,9 Nevertheless, a potential limitation of such vaccines is the inability to guarantee that the induced-CD8+ T cells can enter tumors, owing to the presence of the tumor microenvironment (TME). Therefore, destroying the TME to promote the infiltration of CD8+ T cells into the tumor is necessary to boost the effectiveness of immunotherapy such as cancer vaccines.
Cancer associated fibroblasts (CAFs), as a fundamental component of the TME, can promote the occurrence, development, metastasis, and drug resistance of cancer by synthesizing extracellular matrix components (such as collagen I and fibronectin) and interacting directly with cancer cells and other stromal cells.10–12 Moreover, CAFs can also recruit myeloid-derived suppressor cells (MDSCs) to infiltrate into tumors by secreting CCL2 and CXCL12 to inhibit the anti-tumor immune responses.13–15 In our previous work, we constructed a DNA vaccine targeting fibroblast activating protein α (FAPα), which expressed on the surface of CAFs. The DNA vaccine, OsF, could remodel the TME by inducing a large number of tumor-infiltrating lymphocytes to target FAPα-expressing CAFs.16 Most importantly, compared with other human matrix antigens, only FAPα has the ability to stimulate antigen-specific CTL responses in vitro from donor PBMC.17 So FAPα is an ideal target for cancer treatment. While the anti-tumor effect of the optimized vaccine was still not ideal.16 Notably, although FAPα-targeted vaccines can regulate the TME, they have no direct killing effects on cancer cells. Thus, in order to achieve better therapeutic results, FAPα-targeted vaccines may need to be combined with other vaccines and treatments.
Survivin, a novel member of the inhibitor of apoptosis protein family, represents another promising potential vaccine target for this approach, as it is overexpressed in various tumors and embryonic tissues but is not or rarely expressed in normal adult tissues.18 Moreover, convincing evidences for spontaneous cytotoxic T-cell responses19 and auto-antibody20 against survivin in cancer patients have been reported, which indicate that survivin is strongly immunogenic and may be suitable for the construction of cancer vaccine.
MDSCs comprise a heterogeneous population of cells consisting of immature myeloid cells and myeloid progenitor cells that are able to inhibit both innate and adaptive immunity.21,22 MDSCs function as effective inhibitory factors for T cell proliferation and activation in both humans and mice.21–24 Accumulation of MDSCs in the blood, spleen, lymph nodes, and tumors has been detected in a variety of mouse tumor models and human patients with cancer.25–27 Accordingly, elimination or inhibition of MDSCs may significantly improve the effects of cancer immunotherapies.23 Notably, many chemotherapeutic drugs have the effect of decreasing MDSCs, such as gemcitabine,28 5-Fluorouracil,29 and doxorubicin (Dox).30 In particular, Dox has been widely utilized as an anti-neoplastic drug in the treatment of various cancers including breast cancer.31 In addition, non-therapeutic doses of Dox can effectively eliminate the effect of MDSCs induced by tumors and promote CD8+ T cell proliferation.30,32 Nevertheless, it has not been reported that if low-dose Dox pretreatment could enhance the anti-tumor effect of DNA vaccine by depleting peripheral MDSCs.
A recent study has shown that a DNA vaccine targeting FAPα combined with another cancer vaccine exhibited synergistic anti-tumor effects.33 However, FAPα+ CAFs and tumor cells co-targeting DNA vaccines have not been reported, which may have excellent anti-tumor activities and facilitate preparation and administration. Thus, we proposed that a DNA vaccine co-targeting FAPα and survivin might modulate the TME in addition to directly killing tumor cells. Toward this end, we constructed a fusion DNA vaccine, OsFS, containing both FAPα and survivin to further enhance tumor death and inhibition through dual targeting effects against the TME and tumor cells. Notably, even though OsFS could reduce the infiltration of MDSCs in 4T1 tumors, a large number of MDSCs remained in the spleen and blood of mice, which might strongly inhibit the anti-tumor immune response induced by OsFS. Hence, we additionally hypothesized that Dox might more fully realize the anti-tumor activity of OsFS by decreasing peripheral MDSCs. The experimental results showed that OsFS and Dox act synergistically in subcutaneous and orthotopic 4T1 breast cancer models.
Materials and methods
Cell lines
Murine breast cancer cell lines (4T1 and EMT6) and colon carcinoma cell line (CT26) were used to construct the mouse cancer models, the 293 T cell line was used to detect protein expression of plasmids, and P815 murine mastocytoma cells used as target cells were incubated with or without peptides for cytotoxicity assays in vitro. All cell lines were preserved by the National Engineering Laboratory for AIDS Vaccine, Jilin University. All experiments were performed with mycoplasma-free cells.
Vaccine construction
The plasmid OsF was constructed previously in our laboratory (previously termed OshF(m)).16 For generating plasmid OS, the OS fragment (human survivin with deletion of 7 N-terminal amino acid residues following codon optimization) was cloned into the PstI/BamHI sites of the CpVR vector (VR1012 with a CpG motif). Plasmid OsFS was constructed using the Seamless Assembly Cloning Kit through homologous recombination following manufacturer protocol. All plasmids contained a Kozak sequence and a tPA signal sequence at the N-terminus of the antigen fragment.
Mice and tumor model
Female BALB/c mice (6–8 weeks old) were purchased from Beijing Huafukang Biology Technology Co., Ltd. (Beijing, China) and raised in the animal experiment platform of the College of Life Sciences, Jilin University. A total of 2 × 104 murine 4T1 tumor cells were injected subcutaneously into the right lower flank or thotopically in the 4th mammary fat pad of mice on day 0. Mouse body weights and tumor sizes were measured using an electronic scale and a Vernier caliper every two days, respectively. Tumor size was calculated by the formula: V = (length·width2)/2 (mm3). Mice were euthanized for the following ethical reasons: tumor ulceration, tumor size reaching 2000 mm3, or decline in health (mice did not eat or exhibited severe weight loss). All animal experiments were performed in accordance with China law and with approval of the Ethics Committees of Jilin University (Changchun, China).
Strategies for treatment in mice
Following tumor establishment (7 days after tumor challenge), mice were randomly assigned to different groups. To determine therapeutic efficacy, mice were immunized three times by intramuscular injection with vaccine plasmids or CpVR vector (as a control) into the tibialis anterior muscles of both hind limbs (50 μg each limb) on days 7, 9, and 12. For detecting the proportion of MDSCs in splenocytes of immunogenic mice, the non-tumor-bearing mice (n = 3) were sacrificed following 7 days of immunization with a single vaccine and the splenocytes were separated for detection. For detecting the doxorubicin dosing schedule to remove MDSCs, mice in group 1 were administered with doxorubicin (5 mg/kg, intravenous (i.v.)) on day 7, whereas those in groups 2 and NS were administered with doxorubicin (5 mg/kg, i.v.) or normal saline (i.v.) on days 7 and 12, respectively. For investigating the anti-tumor effect of the vaccine combined with doxorubicin, mice were injected with doxorubicin (5 mg/kg, i.v.) on days 7 and 12, and then immunized with vaccine (100 μg, intramuscular) on days 13, 15, and 18. In order to explore the effect of different chemicals on vaccine effectiveness, mice were injected with 5-Fluorouracil (50 mg/kg, intraperitoneal (i.p.)) or oxaliplatin (5 mg/kg, i.p.) on day 12, and then immunized with vaccine. In all experiments, the first DNA immunization was administrated intramuscularly followed by electroporation.
(The remaining materials and methods are available in Supplementary material.)
Statistical analysis
All in vivo and in vitro experiments were performed at least two times. Data were analyzed using one-way analysis of variance, and presented as mean ± SD. Differences between groups were assessed for statistical significance using the unpaired t-test. The statistical analysis of survival data was performed using log-rank test. P < .05 was considered significant. All statistical analyzes were implemented in GraphPad Prism 8.0 (La Jolla, CA, USA).
Results
Construction of a survivin-targeted vaccine and verification of anti-tumor effects
To test whether a human survivin-based vaccine could inhibit the growth of 4T1 tumors in mice, we generated a DNA vaccine, OS (Figure 1(a)), containing codon-optimized human survivin deleted for the 7 N-terminal amino acid residues critical for its anti-apoptosis function.34 The expression of a 16-kDa protein encoded by the DNA vaccine was detected by western blotting (Supplementary Figure 1). Tumor-bearing female BALB/c mice were immunized according to the schedule (Supplementary Figure 2a). Compared to the Vector, OS resulted in slower tumor growth (Supplementary Figure 2b) and lighter tumor weight (Supplementary Figure 2c). Moreover, numbers of antigen-specific T cells in the OS group were nearly four-fold those in the Vector group (Supplementary Figure 2d). Together, these data suggested that the human survivin-based vaccine could induce antigen-specific T cells in mice and inhibit the growth of wild-type 4T1 tumors.
Figure 1.
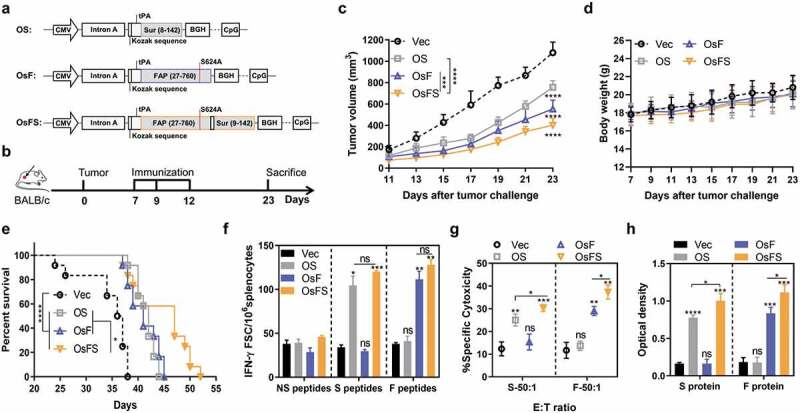
Inhibition of tumor growth and detection of anti-tumor immune response. (a) Schematic diagram of the three DNA vaccines: OS, OsF, and OsFS. (b) Therapeutic setting. BALB/c mice (n = 10) were challenged with 2 × 104 4T1 tumor cells on day 0 and then treated on days 7, 9, and 12. (c, d) The tumor volume (c) and body weight (d) were measured every two days following tumor challenge for 23 days. (e) Survival time was monitored for 52 days (n = 12). Mean survival times were as follows: Vector (Vec) group = 34.6 days; OS group = 41.6 days; OsF group = 41.2 days; OsFS group = 44.8 days. (f) Splenocytes separated from vaccinated mice were stimulated with FAPα peptides (F peptides), survivin peptidesm (S peptides), and unrelated human MUC1 peptides (NS peptides), and frequencies of antigen specific IFN-γ-secreting T cells were measured using ELISPot. (g) For the in vitro CTL assay, splenocytes of immunized tumor-bearing mice were incubated with P815 cells pulsed with FAP or survivin peptides as target cells at the E:T ratio (ratio of effecter cells to target cells) of 50:1. (h) Serum was collected upon sacrifice. Specific antibodies against survivin and FAPα were detected by ELISA. (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Antitumor activity of a co-targeting FAPα and survivin fusion DNA vaccine
Our early work demonstrated that codon-optimized human FAPα-based DNA vaccine, OsF (Figure 1(a)), could inhibit the growth of 4T1 tumor in mice by targeting FAPα+ CAFs,16 Therefore, we constructed a DNA vaccine that combines human FAPα and survivin, termed OsFS (Figure 1(a)), which could target both CAFs and tumor cells. The expression of the 105-kDa fusion protein encoded by OsFS was detected by western blotting (Supplementary Figure 3). To verify the anti-tumor effect of OsFS, mice were immunized as shown in Figure 1(b). OsFS could inhibit tumor growth substantially better than single antigen vaccine (Figure 1(c)). And none of the three vaccines affected the growth of mice (Figure 1(d)). Moreover, immunization with OsFS revealed an obviously enhanced survival benefit compared to that of immunization with other vaccines (Figure 1(e)). Compared with Vector, OsFS could induce greater FAPα and survivin-specific T cell immune responses (Figure 1(f,g)), the intensities of which did not notably differ from those of the single antigen vaccines, even stronger on CTL (Figure 1(g)) and antigen-specific antibody levels (Figure 1(h)). These findings indicated that the fusion of antigens did not affect the body’s immune responses against individual antigens; rather, the fusion vaccine OsFS induced stronger antigen-specific cellular and humeral immune responses and better antitumor activity compared with those of OsF and OS vaccines.
The fusion vaccine alters the TME and promotes greater CD3+CD8+ T cell infiltration
OsF could reduce CCL2 and CXCL12 expression in the TME by inducing more CD3+CD8+ T cells to remove FAPα+ CAFs, thereby reducing the proportion of MDSCs infiltrated in tumors.16 To investigate whether OsFS still has the effects on altering the TME, we detected the proportions of FAPα+ CAFs, CD3+CD8+ T cells, and MDSCs in tumors. Compared with OsF, OsFS further reduced the number of FAPα+ CAFs in the tumor (Figure 2(a)). All vaccines could induce more CD3+CD8+ T cells to infiltrate into tumors compared with Vector; however, OsFS could induce greater CD3+CD8+ T cell infiltration than that of individual antigen vaccines (Figure 2(b)). Furthermore, OsFS reduced the proportion of intratumoral MDSCs more effectively than OsF (Figure 2(c)).
Figure 2.

Regulation of the tumor microenvironment by vaccines. (a, b, c) Representative flow cytometry plots (left) and quantification (right) of frequencies of intratumoral FAPα+ CAFs (a), CD3+CD8+ T cells (b), and CD11b+Gr-1+ MDSCs (c). (d, e) Relative mRNA expression levels of survivin (BIRC5), FAPα, collagen I (COL1A1), CXCL12, CCL2 (d), and IL-2, TNF-α, IFN-γ, IL-6, IL-10, GM-CSF (CSF2), and G-CSF (CSF3) (e) in tumors isolated from vaccinated tumor-bearing mice were detected by qRT-PCR. Gapdh was used as an endogenous control. (*P < .05, **P < .01, ***P < .001, ****P < .0001).
We next analyzed the mRNA expression levels of antigens and collagen I, CXCL12, and CCL2 in tumors (Figure 2(d)). OsFS significantly reduced the mRNA expression levels of survivin (BIRC5), FAPα, and collagen I (COL1A1) compared to those of individual antigen vaccines. The expression of Cxcl12 in mice immunized with OsFS or OsF was approximately 2-fold lower than that in mice immunized with Vector, with the expression of Ccl2 reduced by 4-fold. Moreover, OsFS was more effective than OsF to down-regulate the expression of Ccl2 in the tumor. Although compared with Vector, OS could reduce the level of Cxcl12 expression, it did not reduce the level of Ccl2. Moreover, OsFS or OsF notably reduced the mRNA expression levels of GM-CSF (CSF2) and G-CSF (CSF3) (associated with induction and activation of MDSCs) compared to those of Vector, whereas OS only moderately reduced the expression of G-CSF (Figure 2(e)). These findings might underlie why OS was less able to reduce the proportion of MDSCs in tumors compared to OsF and OsFS (Figure 2(c)). Additionally, OsFS appreciably increased the mRNA expression of IL-2, TNF-α, and IFN-γ and decreased that of IL-6 and IL-10 compared with the other vaccines (Figure 2(e)). These results revealed that OsFS had a stronger ability to regulate the TME and promoted the infiltration of large numbers of CD3+CD8+ T cells compared to single antigen vaccines.
Tumors induce a large number of peripheral MDSCs
Although the anti-tumor activity of OsFS was significantly stronger than that of the individual antigen vaccines, the antitumor effect was still not ideal. To discover the underlying mechanisms, we detected immunosuppression-related cells in the splenocytes of tumor-bearing mice. In tumor-bearing mice, the proportion of CD11b+Gr-1+ MDSCs in splenocytes exceeded 50%; this was > 40% even in splenocytes of vaccine-immunized tumor-bearing mice (Figure 3(a)). However, the proportion of MDSCs in splenocytes of vaccinated healthy mice (no tumor bearing mice) was about only 3% (Figure 3(b)). These results suggested that the large number of MDSCs in tumor-bearing mouse splenocytes was induced by tumors rather than vaccines. And, consistent with other studies, the majority of MDSCs in the spleen of tumor-bearing mice were PMN-MDSCs (CD11b+Ly-6 G+Ly-6Clow), not M-MDSCs (CD11b+Ly-6 G−Ly-6Chi) (Figure 3(c)). 25,35 Moreover, both types of MDSCs could inhibit the secretion of IL-2 by splenocytes of immunized mice (Figure 3(d)).
Figure 3.
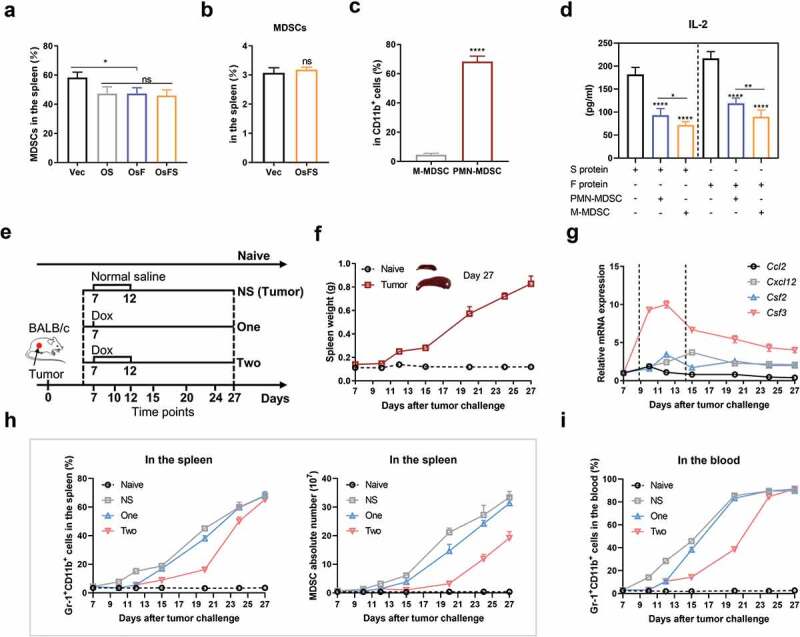
Decrease of tumor-induced MDSCs by doxorubicin. (a) The proportions of CD11b+Gr-1+ MDSCs in the spleens of vaccinated tumor-bearing mice on day 23. (b) CD11b+Gr-1+ MDSC frequencies in splenocytes of non-tumor-bearing mice 7 days after immunization. (c) 4T1 tumors mainly induced PMN-MDSC production in the spleen. Representative flow cytometry plots (left) and quantification (right) of frequencies of different types of MDSCs in the spleens of tumor-bearing mice on day 23. PMN-MDSCs, CD11b+Ly-6 G+Ly-6Clow; M-MDSCs, CD11b+Ly-6 G−Ly-6Chi. (d) 1 × 107 splenocytes isolated from immunized non-tumor-bearing mice were co-cultured with and without MDSCs (splenocytes:MDSC = 1:1) in 12-well plates and stimulated with FAPα or survivin protein (0.2 μg/well) for 5 days. The IL-2 concentration in culture supernatants was detected by ELISA. (e-i) Effects of Dox on MDSCs in tumor-bearing mice. (e) Experimental design, treatment schedule, and detection time points. (f) The spleen weight of 4T1 tumor-bearing mice following the tumor progression. (g) Changes of MDSC-related factors with tumor progression. Relative mRNA expression levels of CCL2, CXCL12, GM-CSF (CSF2), and G-CSF (CSF3) in tumors were detected by qRT-PCR. Gapdh was used as an endogenous control. (h) Frequency (left) and absolute number (right) of MDSCs in the spleen. (i) Proportion of MDSCs in the blood. (*P < .05, **P < .01, ****P < .0001).
Peripheral MDSCs increase with tumor growth and can be decreased by Dox
As MDSCs might inhibit the anti-tumor effect of OsFS, we examined whether removing peripheral MDSCs would enhance the OsFS anti-tumor effect. In mouse models, two doses of Dox (5 mg/kg) could selectively kill MDSCs by triggering apoptosis,30 but one dose did have little effect on MDSCs.29 To explore the effects of different cancer stages and different administration times of Dox on peripheral MDSCs, 4T1 tumor-bearing mice were treated with Dox (5 mg/kg) according to the strategy shown in Figure 3(e). The proportion of MDSCs in the spleen and blood of mice was detected by flow cytometry on days 7, 10, 12, 15, 20, 24, and 27. With tumor growth, the spleen weight of mice also increased (Figure 3(f)). We also found that the mRNA expression levels of CCl2, CXCL12, GM-CSF, and G-CSF in tumors increased sharply around 9–15 days, and then declined but remained at a relatively high level (Figure 3(g)). Compared with naive mice, the proportion of MDSCs in the spleen and blood of 4T1 tumor-bearing mice increased significantly with tumor progression (Figure 3(h,i)). Moreover, the ratio of MDSCs in the spleen (Figure 3(h)) and blood (Figure 3(i)) of mice treated with Dox significantly decreased compared to that of the NS group on days 15 and 20. Mice treated twice with Dox had fewer MDSCs than mice treated with a single Dox administration. On days 24 and 27, the proportion of MDSCs in the spleen and blood of mice treated with Dox increased gradually; however, the absolute number of MDSCs in the spleen was still lower than that in the NS group (Figure 3(h)). In addition, there were differences in the ability of different tumor types (CT26, EMT6 and 4T1) to induce MDSCs, but Dox administration could effectively reduce the content of MDSCs in the spleen of tumor-bearing mice (Supplementary Figure 4).
Combination of OsFS with Dox leads to marked improvement of anti-tumor activities
As MDSCs up-regulation could be detected in the spleen and blood of tumor-bearing mice 7 days following tumor inoculation (Supplementary Figure 5a, b), and expression of factors associated with MDSCs induction increased markedly in 7–15 days (Figure 3(g)), we decided to treat the mice in the combination group with Dox first followed by vaccination (Figure 4(a)). During the treatment, the weight of all experimental mice was within the normal range, and Dox administration only had a slight effect on the weight of the mice (Figure 4(b)). OsFS combined with Dox led to a greater tumor inhibition rate compared to that of other groups in the 4T1 tumor model (Figure 4(c,d)). The tumor inhibition rate of the Dox+OsFS group reached 78.3% compared with that of the Vec group, which was improved over those of the Dox (51.4%) and OsFS (51.3%) groups. And the combination therapy also showed stronger antitumor effect than the other two monotherapies in the EMT6 tumor model (Supplementary Figure 6). The numbers of FAPα and survivin-specific IFN-γ+ T cells in the combination group increased by 0.5- and 1.3-fold, respectively, compared with that in individual vaccine group (Figure 4(e)). In addition, when splenocytes were stimulated with survivin and FAPα proteins, the level of secreted IL-2 of the combination group was nearly 2.3- and 2.5-fold higher, respectively, than that of the vaccine group (Figure 4(f)). And combination therapy further reduced the proportion of FAPα+ CAFs in the tumor compared to OsFS alone (Figure 4(g)). Dox also prolonged the survival time of mice in the combination group by 46.2% compared with the Vector group. Furthermore, the Dox and OsFS groups exhibited prolonged survival time by 15.6% and 19.6%, respectively, compared with that of the Vector group (Figure 4(h)).
Figure 4.
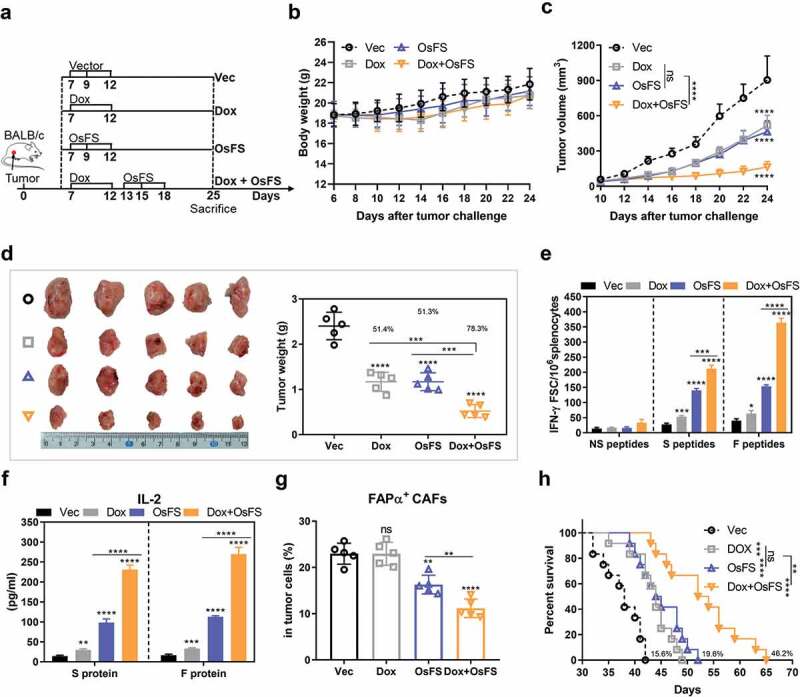
Anti-tumor effects of fusion vaccine combined with doxorubicin treatment. (a) Schematic of the therapeutic regimen. All treatments began 7 days after mice (n = 10) were inoculated with 4T1 tumor cells. Mice (n = 5) were euthanized on the 25th day. (b, c) Body weights (b) and tumor volumes (c) were recorded for 24 days. (d) Schematic diagrams of tumor nodes (left) and tumor weight analysis (right) (n = 5). Tumor weights denoted as the means ± SD were as follows: Vector (Vec) group = 2.402 ± 0.3043; Dox group = 1.168 ± 0.2112; OsFS group = 1.17 ± 0.1966; Dox+OsFS group = .522 ± 0.142. (e) ELISpot assays were conducted on day 25 using splenocytes from vaccinated mice stimulated with FAPα peptides, survivin peptides, and unrelated human MUC1 peptides. (f) Secretion of IL-2 from splenocytes stimulated with FAPα or survivin protein was detected by ELISA. (g) Frequencies of FAPα+ CAFs in tumor cells of vaccinated tumor-bearing mice was detected by flow cytometry. (h) Survival curve showing results of mice treated with OsFS+Dox compared with mice treated with each agent alone (n = 12). Mean survival times were as follows: Vec group = 37.7 days; Dox group = 43.6 days; OsFS group = 45.1 days; Dox+OsFS group = 55.1 days. (*P < .05, **P < .01, ***P < .001, ****P < .0001).
The effects of combination therapy on different immune cells vary
In addition to MDSCs, Tregs and tumor-associated macrophages (TAMs) are also involved in cancer-induced immunosuppression. Moreover, Dox not only selectively scavenges MDSCs but also promotes tumor-specific CD8+ T cell proliferation.30 To investigate the effects of combination therapy on CD8+ T cells and immunosuppressive cells in peripheral regions and the TME of mice, we performed flow cytometry to identify these cells in spleens and primary tumors. The results showed that the proportion of CD3+CD8+ T cells in splenocytes of the combination group was more than twice that of the OsFS group (Figure 5(a)), whereas the proportion of MDSCs in splenocytes of the combination group was significantly lower than that in the separate treatment groups (Figure 5(b)). The proportion of CD3+CD69+ T cells in splenocytes of the combination group was markedly higher than that of the separate treatment groups (Supplementary Figure 7). Additionally, compared with the Dox group, the combination group and OsFS group had fewer Tregs in the spleen (Figure 5(c)). Similar to the flow cytometry results for the spleen, vaccine combined with Dox immensely increased the relative percentage of CD3+CD8+ T cells in the TME (Figure 5(d)). In addition, combination therapy significantly decreased Tregs in tumors (Figure 5(f)). However, the combination therapy did not further reduce MDSCs in tumors (Figure 5(e)), which might be due to factors other than CAFs in this model that affect the infiltration of MDSCs. Notably, OsFS or combination therapy increased the proportion of M1 macrophages (F4/80+CD206−) in the TME, which was beneficial for anti-tumor immunity. However, the proportion of M2 macrophages (F4/80+CD206+) in the TME of the combination group was 2-fold higher than that of the OsFS group (Figure 5(g)). Consistent with this, the qRT-PCR result of Il6, which constitutes a main factor promoting the M2-like differentiation of macrophages, in the combination group was significantly higher than that in the single vaccine group (Supplementary Figure 8).
Figure 5.

Effects of combination therapy on some types of immune cells infiltrated into the spleen and tumor. (a-d) The proportions of CD3+CD8+ T cells (a), MDSCs (b), and Tregs (in CD4+ cells) (c) in spleens were detected by flow cytometry. Flow cytometry plots from a representative sample (left) and quantitative data (right) of four different groups are shown. (d-f) The proportions of CD3+CD8+ T cells (in CD45+ cells) (d), MDSCs (e), and Tregs (in CD4+ cells) (f) infiltrated into tumors. (g) Representative flow cytometry plots (left) and quantification (right) of frequencies of different types of tumor-infiltrating TAMs (F4/80+CD206− M1 and F4/80+CD206+ M2). (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Combination therapy significantly inhibits spontaneous lung metastasis
To assess whether the vaccine combined with Dox inhibits metastases in mice, tumor-bearing mice (n = 5) were sacrificed on day 32 following subcutaneous tumor challenge, and the lungs of all mice were separated to examine the metastatic foci. Metastatic foci in the Dox group, the OsFS group, and the Dox+OsFS group were reduced by 70.5%, 77.3%, and 96.6%, respectively, compared with that in the Vector group (Supplementary Figure 9a). Moreover, only one mouse in the combination group exhibited lung metastasis. Tumor weight analysis showed that the combination treatment still had a stronger role in inhibiting tumor growth compared with the separate treatments on day 32 (Supplementary Figure 9b).
The synergistic anti-4T1 effect of DOX and OsFS depends on MDSCs depletion
Studies have shown that some chemical agents, including Dox, can promote anti-tumor immune responses by triggering immunogenic cell death (ICD).36–38 In this study, Dox pretreatment induced weak FAPα and survivin-specific immune responses in 4T1 tumor-bearing mice (Figure 4(e)), which may also enhance the antitumor effect of OsFS. 5-fluorouracil (5FU) has been shown to selectively kill MDSCs without inducing ICD, while oxaliplatin (Ox) has been proved to induce ICD without affecting MDSCs.29,38 To clarify the antitumor mechanism of OsFS combined with Dox, we compared the effects of Dox, 5FU, and Ox on the antitumor effect of OsFS (Figure 6). Meanwhile, we set up a Dox+OsFS+MDSCs group to further explore the effect of MDSCs on the synergy of Dox and OsFS, which received an adoptive transfer of MDSC after 1 day of Dox injection. 4T1 tumor-bearing mice were grouped and treated according to Figure 6(a). Although Ox can induce the production of a small number of antigen-specific T cells, it did not significantly enhance the antitumor effect of OsFS, and 5FU had a synergistic effect with OsFS without the induction of an antigen-specific immune response (Figure 6(b,c, and e)). What’s more, MDSCs transfer eliminated the synergy between Dox and OsFS (Figure 6(d,e)). These data indicated that Dox enhanced the antitumor effect of OsFS mainly though depleting MDSCs.
Figure 6.
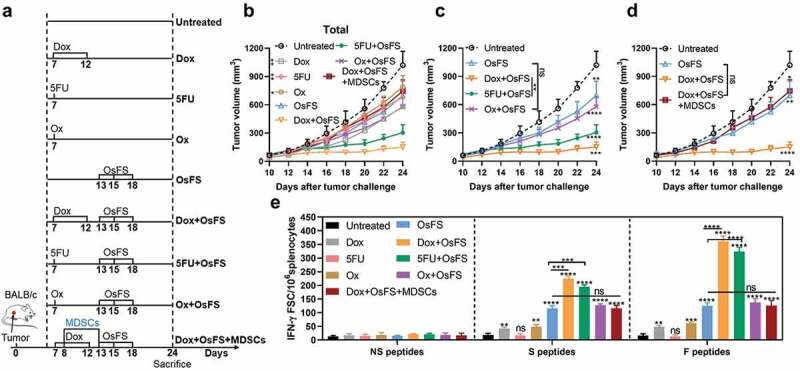
Dox pretreatment mainly enhanced the effect of OsFS by depleting peripheral MDSCs rather than inducing ICD. (a) Mice were subcutaneously inoculated with 20,000 4T1 tumor cells on day 0. After 7 days, the mice were evenly divided into 9 groups, including: Untreated group, Dox group, 5-fluorouracil (5FU) group, oxaliplatin (Ox) group, OsFS group, Dox+OsFS group, 5FU+OsFS group, Ox+OsFS group, and Dox+OsFS+MDSCs group (n = 5). Groups Dox, Dox+OsFS, and Dox+OsFS+MDSCs received Dox (5 mg/kg, i.v.) on days 7 and 12. Groups 5FU and 5FU+OsFS received 5FU (50 mg/kg, i.p.) on day 12. Groups Ox and Ox+OsFS received Ox (5 mg/kg, i.p.) on day 12. Groups OsFS, Dox+OsFS, 5FU+OsFS, Ox+OsFS, and Dox+OsFS+MDSCs received vaccine on days 13, 15, and 18. Mice in the Dox+OsFS+MDSCs group received an adoptive transfer of 1 × 107 MDSCs isolated from 4T1 tumor-bearing mice on days 8 and 13. (b,c and d) Tumor volumes were measured every 2 days from day 10 to day 24. In order to facilitate data analysis, we reorganize part of the data in (b) into (c) and (d). (e) ELISpot assays were conducted on day 24. Splenocytes from tumor-bearing mice were stimulated with FAPα peptides (F peptides), survivin peptides (S peptides), and unrelated human MUC1 peptides (NS peptides). (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Two rounds of Dox therapy can further enhance the anti-tumor effect of the vaccine
The number of peripheral MDSCs tended to recover with the progression of tumor growth and disappearance of Dox efficacy (Figure 3(h,i)).30 Therefore, we examined whether a second round of Dox therapy after vaccination might further enhance the anti-tumor effect of the vaccine. To test this hypothesis, mice were divided into four groups (n = 10): Untreated, Dox+Dox, Dox+OsFS, and Dox+OsFS+Dox. The mice in groups Dox+Dox and Dox+OsFS+Dox received the second round of Dox treatment on days 21 and 26 (Figure 7(a)). Compared to the other groups, the treatment regimen in group Dox+OsFS+Dox significantly inhibited tumor growth (Figure 7(b)), and no deaths occurred in this group until day 52. And two rounds of Dox therapy did not cause significant weight loss of mice in the Dox+OsFS+Dox group (Figure 7(c)).
Figure 7.
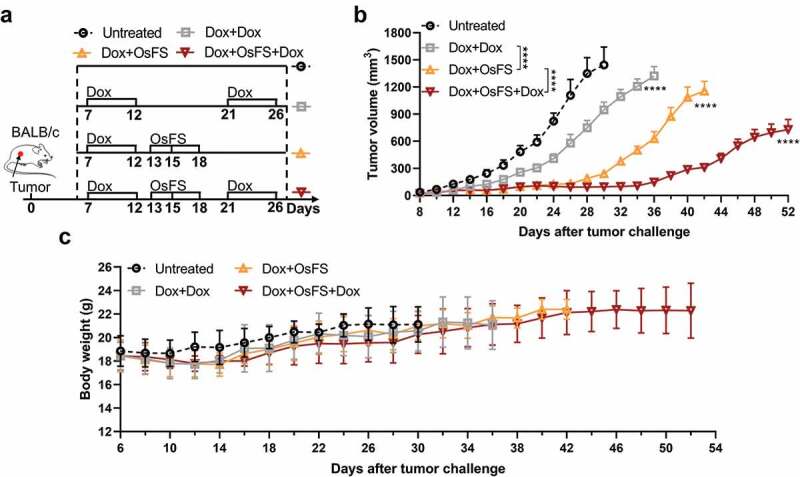
Vaccine combined with two cycles of Dox exhibited better tumor inhibition effect. BALB/c mice (n = 10) were grouped and treated according to the schematic of the therapeutic regimen (a). Tumor volumes (b) and body weights (c) were recorded for 52 days. (***P < .0001).
Therapeutic effect of combination therapy in an orthotopic model of breast cancer
To better mimic the human breast cancer condition, 4T1 tumor cells were injected thotopically in mammary fat pad of female BALB/c mice (Figure 8). In the combination group, 4T1 tumor-bearing mice were treated with Dox first followed by OsFS (Figure 8(a)). After 26 days of 4T1 injection, the mice were euthanized and the tumors were stripped and weighed (Figure 8(c)). The tumor inhibition rate of the combination group reached 76.8% compared with that of the Vec group, which was improved over those of the Dox (39.8%) and OsFS (49.5%) groups (Figure 8(c)). Furthermore, combination therapy prolonged the survival time of mice by 53.4% compared with the Vector group (Dox: 15.3%, OsFS: 24.1%) (Figure 8(d)). ELISpot results showed that Dox combination significantly increased the number of antigen-specific IFN-γ+ T cells induced by OsFS (Figure 8(e)). To investigate the effects of Dox combination on CD8+ cells, Tregs, MDSCs, and TAMs in the TME of 4T1 orthotopic injection model, we performed flow cytometry to identify these cells in primary tumors (Figure 8(f-i)). Similar to the experimental results in the 4T1 subcutaneous injection model, Dox combination further promoted the infiltration of CD3+CD8+ T cells (Figure 8(f)), decreased Tregs (Figure 8(g)), and did not further reduce the number of MDSCs in tumors (Figure 8(h)). Moreover, the proportion of M2 macrophages in the TME of the combination group was still higher than that of the other groups (Figure 8(i)). These results thus indicated that the combination therapy still had a strong anti-tumor effect in the orthotopic breast cancer model, but may also need to be combined with other immunomodulators to boost the effect. Anti PD-L1 has FDA approval with taxane in triple negative metatatic breast cancer. However, it may be due to the low expression of PD-L1 in 4T1 tumor line,39 anti-PD-1 did not further boost the effects of Dox+OsFS therapy and Paclitaxel+OsFS therapy (Supplementary Figures 10 and 11).
Figure 8.
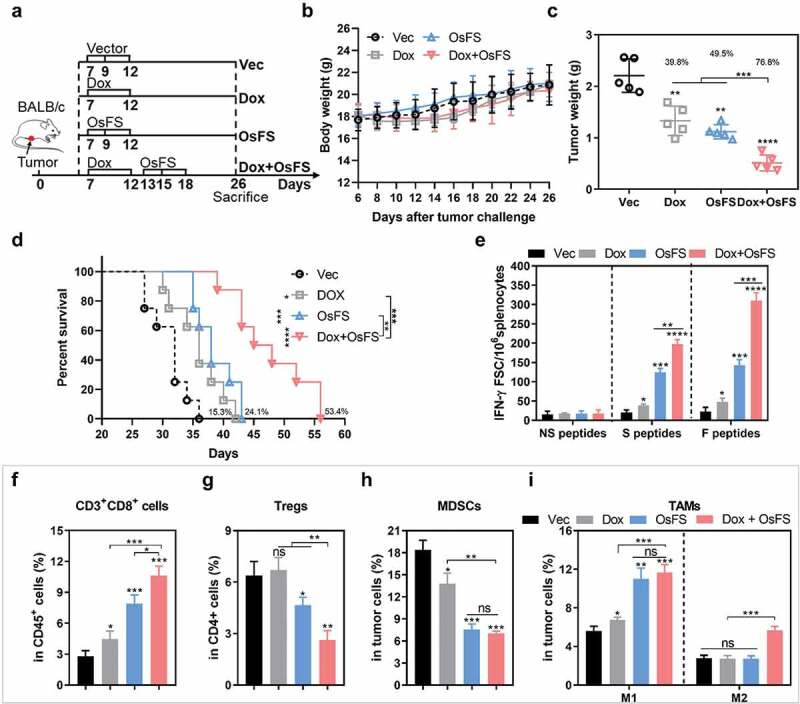
Anti-tumor effects of combination therapy in a 4T1 orthotopic injection model. (a) Schematic of the therapeutic regimen. 2 × 104 4T1 tumor cells were injected orthotopically in the mammary fat pad of BALB/c mice on day 0, and all treatments began 7 days after tumor injection. (b) There was no obvious decrease in body weight weight of mice in response to the treatments. (c) Mice (n = 5) were euthanized, and tumor weights were measured on the 26th day. (Vec group = 2.208 ± 0.3231; Dox group = 1.330 ± 0.2852; OsFS group = 1.116 ± 0.1372; Dox+OsFS group = .5120 ± 0.1547). (d) Survival time was monitored for 56 days (n = 8). Mean survival times were as follows: Vector (Vec) group = 31.1 days; OS group = 35.9 days; OsF group = 38.6 days; OsFS group = 47.8 days. (e) Splenocytes from vaccinated tumor-bearing mice were stimulated with FAPα peptides, survivin peptides, and unrelated human MUC1 peptides. The frequencies of IFN-γ-producing T cells were measured by ELISpot assays. (f-i) The proportions of CD3+CD8+ T cells (in CD45+ cells) (f), Tregs (in CD4+ cells) (g), MDSCs (h), and TAMs (F4/80+CD206− M1 and F4/80+CD206+ M2) (i) infiltrated into tumors. (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Discussion
FAPα+ CAFs alter the extracellular matrix by secreting CXCL12, CCl2, collagen I, and other factors, which renders the TME suitable for tumor growth but not for tumor-infiltrating lymphocytes to destroy tumors, and prevents additional effector T cells from entering tumors.14,15,40,41 Deleting FAPα+ cells from tumors causes rapid hypoxic necrosis of tumors.42 Additionally, studies by ourselves and others have shown that DNA vaccines targeting FAPα can alter the TME by inducing FAPα-specific CD8+ T cells to infiltrate into tumors and target FAPα+ CAFs.16,43–45 Moreover, vaccines targeting tumor-associated antigens exhibit better tumor suppression ability upon combination with an FAPα-targeting vaccine.33
We hypothesized that a DNA vaccine targeting both FAPα and tumor cell antigens would exhibit enhanced tumor killing activity, along with being more convenient to apply. Survivin was selected as an ideal tumor-associated antigen as it is overexpressed in various tumors and shows high homology between human and mouse protein sequences. Here, we first demonstrated that a human survivin-based vaccine could stimulate survivin-specific immune responses in mice to inhibit 4T1 tumor growth. What’s more, no difference was observed in the ability of human or mouse survivin46 (or FAPα16) DNA vaccines to induce a specific immune response. This allowed construction of a DNA vaccine containing both human FAPα and human survivin to realize the dual functions of TME regulation and tumor killing ability of the vaccine in mice.
As expected, the fusion vaccine (OsFS) induced more tumor-infiltrating lymphocytes and significantly reduced FAPα+ CAFs and related cytokines and chemokines in tumors. Moreover, it significantly reduced the proportions of FAPα+ CAFs and MDSCs in tumors. However, the large number of peripheral MDSCs induced by tumors limited the anti-tumor effect of the vaccine. Consistent with this phenomenon, most cancer vaccines exhibit good therapeutic effects on prevention models and adjuvant treatment models after surgery, albeit minimal effects on established cancer models. Numerous studies have indicated that a potential important reason for vaccine failure is that tumors induce a large number of immunosuppressive cells, such as Tregs, TAMs, or/and MDSCs.8
This hypothesis was also confirmed in our experiments. We found that the spleen of the tumor-bearing mice gradually expanded as the tumor progressed, and flow cytometry analysis revealed a large number of MDSCs in the spleen and blood of 4T1 tumor-bearing mice, especially in those with advanced tumors. Notably, MDSCs can block T-cell activation by nitrating lymphocyte-specific protein tyrosine kinase, leading to reduced IL-2 production and proliferation47 and inhibit antigen-specific T cell responses in peripheral lymphoid organs.21,22 Moreover, a large number of MDSCs exist in the peripheral immune environment of mice at the beginning of the vaccine treatment. And when the splenocytes of immunogenic mice were incubated with MDSCs in vitro, their ability to secrete IL-2 was significantly inhibited.
Together, these observations indicate that the mouse immune system was already in an immunosuppressive state prior to vaccine administration, thus limiting the vaccine’s ability to provoke an immune response and dampening its potential anti-tumor activity. Additionally, during the time required from initial vaccination to emerging specific immune response, tumors would continue to induce more MDSCs. Therefore, in order to achieve the desired anti-tumor effect of the vaccine, it is necessary to first remove the peripheral MDSCs to disrupt the immunosuppressive state of the peripheral immune environment.
Non-therapeutic doses of Dox have been shown to selectively kill MDSCs and enhance the efficacy of Th1 or Th17 cell therapy.30 Therefore, we ascertained whether Dox might act synergistically with OsFS. When OsFS was combined with Dox, the ratio of antigen-specific IFN-γ-secreting CD8+ T cells in the spleen was significantly increased. And the ability of splenocytes to secrete IL-2 was restored. In addition, the tumor growth inhibition rate and survival time of the combination group were significantly higher than those of the separate treatment groups in both 4T1 subcutaneous and orthotopic breast cancer models. Moreover, combination therapy significantly inhibited lung metastasis as it afforded a stronger ability for killing tumor cells; furthermore, the number of MDSCs in spleens of the combination group was less than that of other groups at the detection time point. However, the number of MDSCs in the Dox group subsequently increased owing to the disappearance of drug efficacy. We also revealed that the therapeutic effect of Dox+OsFS+Dox was much better than that of Dox+Dox, which indicated that antigen-specific T cells induced by the vaccine play an important role in inhibiting tumor growth, although its efficacy could be inhibited by MDSCs. In addition, we found that Dox in this study mainly enhanced the antitumor effect of OsFS by decreasing MDSCs rather than inducing ICD. This may be due to the short half-life (t1/2) of free Dox (5 mg/kg) in vivo, which limits the uptake of Dox by tumors.48
Notably, both the vaccine and combination treatments increased the proportion of M1 TAMs. However, the proportion of M2 TAMs in the combination group increased compared with that in the vaccine group, which might explain the inability of the combination of two rounds of Dox to completely eliminate the tumors. Specifically, when the tumor is attacked by T cells, it might inhibit the anti-tumor immune response by transforming more macrophages into M2 TAMs or inducing large numbers of peripheral MDSCs and other immunosuppressive factors. Therefore, in order to achieve better anti-tumor effect, it is likely necessary for the combination therapy to act in conjunction with other immunomodulators, such as metformin, which could skew TAM polarization from an M2- to M1-like phenotype.49 Further experimental verification is required to identify the optimal combination schemes and anti-tumor effects.
Numerous cancer vaccines can produce antigen-specific immune responses in the healthy human body, but are ineffective or weak in the treatment of patients with cancer.8 This may be due to the large number of peripheral MDSCs or other immunosuppressive factors induced in the course of cancer progression, which render the vaccine unable to trigger effective anti-tumor immune responses in vivo. Several studies including our work have demonstrated that chemotherapeutic agents (such as carboplatin/paclitaxel,50,51 gemcitabine,52,53 and cyclophosphamide54-56) have the effect of further enhancing the anti-tumor effects of DNA vaccine and other immunotherapies. Therefore, in order to achieve better therapeutic effect, vaccines need to be used in combination with appropriate immunomodulators, such as Dox in clinical application in breast cancer.
Taken together, our results showed that a DNA vaccine co-targeting FAPα and survivin could promote the infiltration of T cells into tumors to simultaneously kill tumor cells and regulate the TME. However, the presence of a large number of peripheral MDSCs limited the anti-tumor effect of OsFS, with the number of MDSCs increasing with tumor progression. Pre-treatment with Dox to decrease MDSCs significantly improved the anti-tumor effect of OsFS, effectively inhibited lung metastasis, and prolonged the survival of mice. Thus, the experimental results of this study may provide a basis for translation of the combination therapy, OsFS+Dox, into clinical research.
Funding Statement
Supported by the Key R & D Projects of Science and Technology Department of Jilin Province [no. 20180201001YY], Major Projects of Science and Technology Innovation in Changchun City [no. 17YJ002], the Specialized Research Fund for the National Natural Science Foundation of China [no. 31300765], the Jilin Province Science and Technology Development Program [no. 20160519018JH], and the National Science and Technology Major Project of the Ministry of Science and Technology of China [no. 2014ZX09304314-001].
Abbreviations
- CAFs
cancer associated fibroblasts
- collagen I (COL1A1)
collagen, type I, alpha 1
- CCL2
C-C motif chemokine ligand 2
- CTL
cytotoxic lymphocyte
- CXCL12
C-X-C motif chemokine ligand 12
- Dox
doxorubicin
- ELISA
enzyme-linked immunosorbent assay
- FAPα
fibroblast activating protein α
- G-CSF (CSF3)
colony stimulating factor 3
- GM-CSF (CSF2)
colony stimulating factor 2
- IFN-γ
interferon γ
- IL
interleukin
- i.p.
intraperitoneal
- i.v.
intravenous
- MDSCs
myeloid-derived suppressor cells
- Ox
oxaliplatin
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative real-time polymerase chain reaction
- survivin (BIRC5)
baculoviral IAP repeat-containing 5
- TAMs
tumor-associated macrophages
- TME
tumor microenvironment
- TNF-α
tumor necrosis factor α
- Tregs
regulatory T cells
- 5FU
5-Fluorouracil
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–14. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, NY). 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J clin oncol. 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Wang J, Chen R, Bai Y, Lu X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8(9):15621–15631. doi: 10.18632/oncotarget.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Tan Y, Qian Y, Xue W, Wang Y, Du J, Jin L, Ding W. Clinicopathologic and prognostic significance of tumor-infiltrating CD8+ T cells in patients with hepatocellular carcinoma: a meta-analysis. Medicine. 2019;98(2):e13923. doi: 10.1097/MD.0000000000013923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16(4):219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 9.Finn OJ. The dawn of vaccines for cancer prevention. Nat Rev Immunol. 2018;18(3):183–194. doi: 10.1038/nri.2017.140. [DOI] [PubMed] [Google Scholar]
- 10.Brouty-Boye D. Developmental biology of fibroblasts and neoplastic disease. Prog Mol Subcell Biol. 2005;40:55–77. [DOI] [PubMed] [Google Scholar]
- 11.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev. 2009;19(1):67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel). 2015;7(4):2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams SA, Harata-Lee Y, Comerford I, Anderson RL, Smyth MJ, McColl SR. Multiple functions of CXCL12 in a syngeneic model of breast cancer. Mol Cancer. 2010;9(1):250. doi: 10.1186/1476-4598-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouyang L, Chang W, Fang B, Qin J, Qu X, Cheng F. Estrogen-induced SDF-1alpha production promotes the progression of ER-negative breast cancer via the accumulation of MDSCs in the tumor microenvironment. Sci Rep. 2016;6(1):39541. doi: 10.1038/srep39541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, Dang Y, Chu Y, Fan J, He R. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 2016;76(14):4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 16.Geng F, Guo J, Guo QQ, Xie Y, Dong L, Zhou Y, Liu CL, Yu B, Wu H, Wu JX, et al. A DNA vaccine expressing an optimized secreted FAPalpha induces enhanced anti-tumor activity by altering the tumor microenvironment in a murine model of breast cancer. Vaccine. 2019;37(31):4382–4391. doi: 10.1016/j.vaccine.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Fassnacht M, Lee J, Milazzo C, Boczkowski D, Su Z, Nair S, Gilboa E. Induction of CD4(+) and CD8(+) T-cell responses to the human stromal antigen, fibroblast activation protein: implication for cancer immunotherapy. Clin Cancer Rese. 2005;11(15):5566–5571. doi: 10.1158/1078-0432.CCR-05-0699. [DOI] [PubMed] [Google Scholar]
- 18.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8(1):61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 19.Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, Thor Straten P. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–5968. [PubMed] [Google Scholar]
- 20.Rohayem J, Diestelkoetter P, Weigle B, Oehmichen A, Schmitz M, Mehlhorn J, Conrad K, Rieber EP. Antibody response to the tumor-associated inhibitor of apoptosis protein survivin in cancer patients. Cancer Res. 2000;60(7):1815–1817. [PubMed] [Google Scholar]
- 21.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5(1):3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, Umansky V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120(1):16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176(1):284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 25.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 27.Jin G, Zhang Y, Chang X, Zhang Y, Xu J, Wei M, Zeng X. Increased percentage of mo-MDSCs in human peripheral blood may be a potential indicator in the diagnosis of breast cancer. Oncol Res Treat. 2017;40(10):603–608. doi: 10.1159/000478933. [DOI] [PubMed] [Google Scholar]
- 28.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9(7–8):900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 30.Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74(1):104–118. doi: 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 32.Alizadeh D, Katsanis E, Larmonier N. Chemotherapeutic targeting of myeloid-derived suppressor cells. Oncoimmunology. 2014;3(1):e27359. doi: 10.4161/onci.27359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duperret EK, Trautz A, Ammons D, Perales-Puchalt A, Wise MC, Yan J, Reed C, Weiner DB. Alteration of the tumor stroma using a consensus dna vaccine targeting fibroblast activation protein (FAP) synergizes with antitumor vaccine therapy in Mice. Clin Cancer Rese. 2018;24(5):1190–1201. doi: 10.1158/1078-0432.CCR-17-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Wang Y, Liu C, Zhang L, Xia Q, Zhang Y, Wu J, Jiang C, Chen Y, Wu Y, et al. DNA and adenovirus tumor vaccine expressing truncated survivin generates specific immune responses and anti-tumor effects in a murine melanoma model. Cancer Immunol Immunother. 2012;61(10):1857–1867. doi: 10.1007/s00262-012-1296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rong Y, Yuan C-H, Qu Z, Zhou H, Guan Q, Yang N, Leng X-H, Bu L, Wu K, Wang F-B, et al. Doxorubicin resistant cancer cells activate myeloid-derived suppressor cells by releasing PGE2. Sci Rep. 2016;6(1):23824. doi: 10.1038/srep23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawano M, Tanaka K, Itonaga I, Iwasaki T, Miyazaki M, Ikeda S, TSUMURA H. Dendritic cells combined with doxorubicin induces immunogenic cell death and exhibits antitumor effects for osteosarcoma. Oncol Lett. 2016;11(3):2169–2175. doi: 10.3892/ol.2016.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam G-H, Lee EJ, Kim YK, Hong Y, Choi Y, Ryu M-J, Woo J, Cho Y, Ahn DJ, Yang Y, et al. Combined Rho-kinase inhibition and immunogenic cell death triggers and propagates immunity against cancer. Nat Commun. 2018;9(1):2165. doi: 10.1038/s41467-018-04607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vacchelli E, Aranda F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2014;3(3):e27878. doi: 10.4161/onci.27878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu YX. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;30(3):500. doi: 10.1016/j.ccell.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Houthuijzen JM, Jonkers J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. 2018;37(4):577–597. doi: 10.1007/s10555-018-9768-3. [DOI] [PubMed] [Google Scholar]
- 41.Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15(11):669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 42.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science (New York, NY). 2010;330(6005):827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 43.Xia Q, Zhang FF, Geng F, Liu CL, Xu P, Lu ZZ, Yu B, Wu H, Wu JX, Zhang HH, et al. Anti-tumor effects of DNA vaccine targeting human fibroblast activation protein alpha by producing specific immune responses and altering tumor microenvironment in the 4T1 murine breast cancer model. Cancer Immunol Immunother. 2016;65(5):613–624. doi: 10.1007/s00262-016-1827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4(11):e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116(7):1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu K, Qin H, Cha SC, Neelapu SS, Overwijk W, Lizee GA, Abbruzzese JL, Hwu P, Radvanyi L, Kwak LW, et al. Survivin DNA vaccine generated specific antitumor effects in pancreatic carcinoma and lymphoma mouse models. Vaccine. 2007;25(46):7955–7961. doi: 10.1016/j.vaccine.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 47.Feng S, Cheng X, Zhang L, Lu X, Chaudhary S, Teng R, Frederickson C, Champion MM, Zhao R, Cheng L, et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc Natl Acad Sci U S A. 2018;115(40):10094–10099. doi: 10.1073/pnas.1800695115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu J, Liu X, Liao YP, Wang X, Ahmed A, Jiang W, Ji Y, Meng H, Nel AE. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano. 2018;12(11):11041–11061. doi: 10.1021/acsnano.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Wang JC, Sun X, Ma Q, Fu GF, Cong LL, Zhang H, Fan DF, Feng J, Lu SY, Liu JL, et al. Metformin’s antitumour and anti-angiogenic activities are mediated by skewing macrophage polarization. J Cell Mol Med. 2018;22(8):3825–3836. doi: 10.1111/jcmm.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welters MJ, van der Sluis TC, van Meir H, Loof NM, van Ham VJ, van Duikeren S, Santegoets SJ, Arens R, de Kam ML, Cohen AF, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med. 2016;8(334):334ra52. doi: 10.1126/scitranslmed.aad8307. [DOI] [PubMed] [Google Scholar]
- 51.Domingos-Pereira S, Galliverti G, Hanahan D, Nardelli-Haefliger D. Carboplatin/paclitaxel, E7-vaccination and intravaginal CpG as tri-therapy towards efficient regression of genital HPV16 tumors. J Immunother Cancer. 2019;7(1):122. doi: 10.1186/s40425-019-0593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang HY, Han BS, Kwon B, Sin JI. Optimized gemcitabine therapy in combination with E7 peptide immunization elicits tumor cure by preventing ag-specific ctl inhibition in animals with large established tumors. DNA Cell Biol. 2018;37(10):850–860. doi: 10.1089/dna.2018.4319. [DOI] [PubMed] [Google Scholar]
- 53.Tallon de Lara P, Cecconi V, Hiltbrunner S, Yagita H, Friess M, Bode B, Opitz I, Vrugt B, Weder W, Stolzmann P, et al. Gemcitabine synergizes with immune checkpoint inhibitors and overcomes resistance in a preclinical model and mesothelioma patients. Clin Cancer Rese. 2018;24(24):6345–6354. doi: 10.1158/1078-0432.CCR-18-1231. [DOI] [PubMed] [Google Scholar]
- 54.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich P-Y, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 55.Son CH, Bae JH, Lee HR, Shin DY, Yang K, Park YS. Enhanced dendritic cell-based immunotherapy using low-dose cyclophosphamide and CD25-targeted antibody for transplanted Lewis lung carcinoma cells. J Immunother (Hagerstown, Md: 1997). 2015;38(3):107–115. doi: 10.1097/CJI.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 56.Xia Q, Geng F, Zhang FF, Liu CL, Xu P, Lu ZZ, Xie Y, Sun B, Wu H, Yu B, et al. Cyclophosphamide enhances anti-tumor effects of a fibroblast activation protein alpha-based DNA vaccine in tumor-bearing mice with murine breast carcinoma. Immunopharmacol Immunotoxicol. 2017;39(1):37–44. doi: 10.1080/08923973.2016.1269337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


