ABSTRACT
Immune checkpoint blockade has not yet been effective in patients with mismatch repair proficient metastatic colorectal cancer. Targeting immunosuppressive metabolic pathways is being explored as a new immunotherapeutic approach. We assessed whether CD73, the rate limiting enzyme that catalyzes the degradation of extracellular AMP into immunosuppressive adenosine, could be an immunological determinant of colorectal liver metastases (CRLMs). By immunofluorescence on tissue microarrays, intratumoral CD73 expression (tCD73) was analyzed in 391 CRLMs resected in 215 patients, and soluble CD73 (sCD73) was measured by ELISA in the pre-operative serum of 193 patients. High tCD73 was associated with worse pathological features, such as multiple and larger CRLMs, and poorer pathologic response to pre-operative chemotherapy. The median time to recurrence and disease-specific survival after CRLM resection was significantly shorter in patients with high tCD73 (11.0 and 46.4 months, respectively) compared with low tCD73 (19.0 and 61.5 months, respectively). tCD73 was strongly associated with patient outcomes independently of clinicopathological variables. sCD73 did not correlate with tCD73. Patients with high levels of sCD73 also had shorter disease-specific survival. Our results suggested that CD73 in CRLMs may be prognostically informative and may help select patients more likely to respond to adenosine pathway blocking agents.
KEYWORDS: CD73-Adenosine pathway, biomarker, colorectal cancer, immune checkpoint, liver metastasis
Introduction
Despite progress made over the past decades combining more effective cytotoxic agents, blockade of VEGF/R and EGFR with therapeutic antibodies, and more extensive resection of liver-confined metastases, metastatic colorectal cancer (CRC) remain in the top three leading cause of cancer-related deaths.1 Although PD-1 immune checkpoint blockade can lead to objective response in approximately 50% of patients with mismatch repair (MMR) deficient CRC,2 95% of metastatic CRC patients have MMR proficient tumors. Why metastatic CRC has been refractory to immunotherapy thus far is not well understood, as data accumulates supporting its interplay with the adaptive immune system,3-5 including the detection of tumor-infiltrating T cells reactive to autologous cancer cell lines6 and neoantigens.7 It is thus conceivable that T cell-based immunotherapy could work in selected metastatic CRC patients targeting biologically-relevant immune suppressive mechanisms.
In addition to the needs for new effective systemic therapies in this common malignancy, there currently lack robust biomarkers to guide adjuvant treatment and surveillance strategy in patients who undergo colorectal liver metastasis (CRLM) resection with curative intent. Improving our understanding of the immune determinants in CRC metastases may lead to the discovery of new biomarkers for prognostication and predictive of response to targeted immunotherapy.
Targeting the immunosuppressive adenosine pathway is being tested as a new immunotherapeutic approach.8 Stress-related adenosine triphosphate (ATP) release by cancer cells occurs through various mechanisms. While extracellular ATP is converted by the cell surface enzyme CD39 into adenosine monophosphate (AMP), CD73, a glycosyl-phosphatidylinositol (GPI) anchored cell surface protein encoded by NT5E, is the rate-limiting enzyme that catalyzes the degradation of AMP into adenosine. Although relatively ubiquitous, CD73 can be significantly overexpressed by cancerous and non-cancerous cells within the tumor microenvironment (TME). There, extracellular adenosine can mediate immunosuppression by binding to A2A and A2B receptors inhibiting CD8+ T-cell and NK-cell effector function. CD73 can also exert a non-enzymatic function as an adhesive and signaling molecule involved in cancer invasion and metastasis.9-13 CD73 overexpression in cancer cell lines has also been associated with resistance to cytotoxic agents.9 Soluble CD73, shed from cell membranes, conserves its enzymatic function, was found at higher level in cancer patient compared to heathy volunteer sera, and is associated with resistance to anti-PD-1 therapy in melanoma patients.14 High tumoral CD73 expression has been documented in various primary tumors, including primary CRC, and associated with worse clinical outcomes.15 CD73 in CRC metastases, however, remains largely unexplored.
Here, we investigated the expression of intratumoral CD73 (tCD73) in CRLMs resected with curative intent in 215 patients and the level of pre-operative soluble CD73 (sCD73) for their relationship with pre-operative chemotherapy, clinicopathological characteristics and outcomes.
Patients and methods
Patients
This study was approved by the Institutional Review Board. From our prospectively maintained database, we retrieved clinicopathological data from a cohort of 215 patients who underwent CRLM resection with curative intent at the Centre hospitalier de l’Université de Montréal between 2006 and 2014 (Table S1).
Pathological review
CRLM hematoxylin and eosin (H&E) whole slides were reviewed by hepatobiliary pathologists blinded to patient outcomes. Histopathologic response to chemotherapy was determined by tumor regression grade (TRG) scoring as described by Rubbia-Brandt et al.16
Tissue microarrays
Tissue microarrays (TMAs) were constructed with 391 morphologically representative formalin-fixed, paraffin-embedded tissue blocks with six 0.6 mm diameter cores per CRLM, for up to 3 metastases per patient prioritized by size. The surface of necrosis on each core was manually delineated on digitalized core images and calculated as percent of the total assessable core area (Visiomorph v.6 software, Visiopharm), then averaged with the other cores to represent the degree of necrosis in each CRLM.
Immunohistochemistry
To morphologically contextualize CD73 expression in CRLMs, a semi-automated IHC protocol with H&E counterstaining was used on selected whole and TMA slides. With a BenchMark XT automated stainer (Ventana Medical System), TMA slides were deparaffinized and rehydrated with xylene and alcohols. Endogenous peroxidases were blocked using diluted hydrogen peroxide and antigens retrieved in boiling target retrieval solution pH 9 for 40 min (Dako) followed by staining with anti-CD73 (Abcam ab91086, 1:400 dilution). UltraView Universal DAB Detection Kit (Ventana) was used for revelation prior to H&E counterstaining, dehydrated, and mounted as published.17 High resolution digital images were obtained with NanoZoomer-XR (Hamamatsu).
Immunofluorescence
We optimized a multiplex immunofluorescence (IF) panel on a test CRLM TMA to concurrently detect CD73, cytokeratins to compartmentalize stromal vs. epithelial expression patterns, and DAPI for nuclear staining of cells. As for other tumor types, 18-21 the non-patchy expression of CD73 made it suitable for larger scale TMA quantification. We used standard deparaffinization and rehydratation protocols, antigen retrieval (Dako S1699) in sub-boiling conditions for 40 min, and protein-block (Dako X0909) followed by specific staining with primary antibodies against CD73 (Abcam ab91086, 1:300 dilution), and cytokeratins 8/18 (Dako IR094, 1:2 dilution). We used anti-mouse IgG1 Alexa-Fluor 647 (Life technology, A21240; 1/800) and anti-rabbit Alexa-Fluor 488 (Life technology, A21206; 1/400) as secondary antibodies, DAPI, and mounted the slides with ProLong Gold (ThermoFisher). We then stained the full CRLM TMA. Slides were digitalized and core images were imported with TMA maps and identifiers into Visiomorph v.6 software (Visiopharm) for automated quantification. CD73 positive areas and mean fluorescence intensity (MFI), in stromal and cancer cell areas using cytokeratin masks, were computed. tCD73 was defined as Intratumoral total expression of CD73 considering both stromal and cancer cell compartments. Mean values were calculated per CRLM and concatenated into the clinicopathological database.
ELISA
Soluble CD73 was detected in the sera of 193 patients. First, Nunc Maxisorp plates were coated overnight with 1 µg/ml of anti-human CD73 (clone AD2, BioLegend, 344002). Blocking solution (PBS, 0.01% Tween-20, 0.1% BSA) was subsequently added for 1 h at room temperature (RT). Samples were then incubated 2 h at 100 μl per well, undiluted. Detection antibody (anti-CD73 clone 1E9, Santa Cruz, sc-32299, 5 µg/ml) was incubated for 1 h. HRP-conjugated anti-mouse IgG3 detection antibody (1:4000 dilution; Southern Biotech 1101–05) was added for 1 h at RT, followed by tetramethylbenzidine substrate. Finally, the reaction was stopped with 2 N HCl. Absorbance was read at 450 and corrected at 570 nm using a Versamax microplate reader.
Statistics
Proportions were compared with Chi-squared test, and continuous variables with Wilcoxon paired-t or Mann–Whitney tests. Correlation between continuous variables was assessed with Spearman Rank Order. Patient disease status was updated until October 2017. Disease-specific survival (DSS) and time to recurrence (TTR) were calculated by the Kaplan-Meier method, from the time of hepatectomy until colorectal cancer-specific death and recurrence, respectively. Groups were compared by the log-rank test. Hazards ratios with a 95% confidence interval were calculated using univariate Cox proportional hazards regression modeling. Variables with a univariate Cox P value <.05 were included in multivariate analysis with forward-stepwise conditional multivariate modeling (SPSS v.24.0, IBM; Prism v.8, GraphPad Software).
Results
Clinicopathological characteristics of CRLM patients
A total of 215 patients underwent hepatic CRLM resections with curative intent (Table S1). Mean age was 63.0 years (range: 32–84), and 64% were males. Pre-operative chemotherapy was received by 78.6% of patients, consisting of 5-FU, leucovorin and oxaliplatin (FOLFOX) with a mean of 6 cycles, most often combined with the anti-VEGF blocker bevacizumab. Pathologic response to chemotherapy was assessed by TRG ;16 complete or major histopathological response (TRG 1–2) was found in 54 CRLMs (14.5%), while partial (TRG 3) or lack of response (TRG 4–5) were found in 238 CRLMs (85.5%). TRG per patient was defined by the metastatic lesion with the worst score, resulting in 135 patients (81.8%) with TRG 3-4-5. At a median follow-up of 44.8 months (range 0.2 to 130.4 months), 71.6% of patients had recurred; median TTR and DSS were 15.4 and 56.7 months, respectively.
CD73 intratumoral expression patterns and serum detection
Using immunofluorescence, we evaluated tCD73 in 391 CRLMs by calculating the percentage of surface area stained by CD73 antibody relative to the total core biopsy area, as well as measuring CD73 MFI in the total core. A broad range of CD73 expression was detected, ranging from 0.2 to 63.2 positive surface area (mean 6.4%), and 124.6 to 2336.1 MFI (mean 386.0) (Supp. Fig. S1A). There was a strong correlation between CD73 positive surface area and MFI (Supp. Figure 1(b)), thus CD73 MFI in CRLMs was selected for further analysis. Cytokeratin expression enabled us to differentiate stromal vs. cancer cell CD73 expression. There was a strong correlation between stromal and epithelial CD73 expression (Spearman r = 0.715, p < .001). As shown in Figure 1(a), CD73 expression was detected in the stromal compartment of metastases and in some cases in the lumens of tumor pseudoglands, which contained eosinophilic material of necrotic cell debris and neutrophils consistent with “dirty” necrosis.22 Immunohistochemical analysis showed a strong association between CD73 apical glandular cancer cell surface expression and its detection in the pseudoglandular lumens (Figure 1(a), right), supporting detection of shed CD73 or membrane-bound to immune cells. CD73 antibody did not bind nonspecifically to necrotic area; CD73 was not detected in some highly necrotic metastases, while high CD73 expression was also found in non-necrotic metastases (Figure 1(b)). In a subset analysis of 14 patients who underwent surgery for recurrence, tCD73 levels were not significantly different in recurrent compared to initial CRLM (Supp. Figure 1(c)). By ELISA, a broad range of sCD73 was measured in pre-hepatectomy serum of 193 patients (mean 2.9 ng/ml, range, 0 to 13.2 ng/ml) (Supp. Figure 1(d)). Notably, sCD73 levels were not correlated with tCD73 expression levels (Figure 1(c)).
Figure 1.

CD73 expression in CRLM and soluble CD73 serum level. (a) Representative examples of CD73 detection by multiplex immunofluorescence, near absent (left) and high in the stroma and within lumens of cancer pseudoglands. By immunohistochemistry (IHC), detection of membrane-bound CD73 on the apical border of cancer pseudoglands (arrow) in conjunction with shed or immune-cell bound detection within pseudogland lumens (right). Hematoxylin and eosin staining are shown for morphological reference in upper left corners. Bars represent 50 μm. (b) Correlation between percent necrotic CRLM surface area and intratumoral CD73 detection (tCD73). (c) Correlation between soluble CD73 (sCD73) serum level and tCD73. (d) tCD73 levels according to pre-operative chemotherapy status (left) and histologic pathological response to chemotherapy, assessed by the Tumor Regression Grade (TRG) system, where 1 represent complete response and 5 absence of response. Correlations assessed with Spearman method. Means compared with Mann-Whitney test and One-Way ANOVA test. MFI, Mean Fluorescence Intensity; CK, Cytokeratins; DAPI, 4ʹ.6ʹ-Diamidino-2-Phenylindol.
CD73 association with clinicopathological features
Higher tCD73 was associated with more aggressive CRLM clinicopathological features, such as multiple and larger metastases (Table 1). Although tCD73 expression level was similar whether patients had received pre-operative chemotherapy or not, resistance to pre-operative chemotherapy (TRG score 3-4-5), characterized by more necrosis than fibrosis and more abundant tumor cells, was associated with higher tCD73 expression (Figure 1(d)). No correlation was found with tumor budding. In 71 patients with available KRAS status, higher tCD73 expression was found in mutated KRAS tumors compared to wild type (MFI 434.1 vs 368.1 respectively, p = .031, Mann-Whitney test). Of note, the study cohort represented patients with non-familial, sporadic, MMR proficient CRC, with no bona fide microsatellite unstable MSI. Finally, circulating sCD73 levels were not significantly associated with clinicopathological features (Table 1).
Table 1.
Association between CD73 expression in CRLM and soluble CD73 serum level with clinicopathological factors.
| Variable | Patients N (%) | tCD73 (MFI) | SEM | P value | Patients N (%) | sCD73 (ng/mL) | SEM | P value |
|---|---|---|---|---|---|---|---|---|
| Age at hepatectomy | ||||||||
| ≤65 years | 111 (53.6) | 450.2 | 31.7 | 0.469 | 105 (54.4) | 2.97 | 0.23 | 0.651 |
| >65 years | 96 (46.4) | 405.6 | 24.6 | 88 (45.6) | 2.85 | 0.24 | ||
| Gender | ||||||||
| Male | 135 (65.2) | 401.4 | 21.7 | 0.093 | 126 (65.3) | 2.82 | 0.19 | 0.521 |
| Female | 72 (34.8) | 482.2 | 42.1 | 67 (34.7) | 3.09 | 0.30 | ||
| Timing of metastasis | ||||||||
| Synchronous | 79 (38.2) | 415.8 | 35.0 | 0.469 | 77 (39.9) | 2.68 | 0.23 | 0.150 |
| Metachronous | 128 (61.8) | 437.9 | 25.2 | 116 (60.1) | 3.07 | 0.22 | ||
| Number of metastases | ||||||||
| Single | 86 (41.5) | 424.0 | 37.7 | 0.050 | 78 (40.4) | 2.64 | 0.21 | 0.448 |
| Multiple | 121 (58.5) | 433.4 | 22.7 | 115 (59.6) | 3.09 | 0.24 | ||
| Diameter of largest metastasis | ||||||||
| ≤5 cm | 168 (81.2) | 402.1 | 20.9 | 0.027 | 157 (81.3) | 2.72 | 0.16 | 0.180 |
| >5 cm | 39 (18.8) | 547.7 | 58.2 | 36 (18.7) | 3.74 | 0.54 | ||
| Disease-free interval | ||||||||
| ≥12months | 63 (30.4) | 448.4 | 39.2 | 0.920 | 58 (30.1) | 3.26 | 0.28 | 0.020 |
| <12months | 144 (69.6) | 421.2 | 24.0 | 135 (69.9) | 2.76 | 0.20 | ||
| CEA level | ||||||||
| ≤200 ng/mL | 198 (96.6) | 433.0 | 21.3 | 0.933 | 187 (97.4) | 2.84 | 0.16 | 0.014 |
| >200 ng/mL | 7 (3.4) | 385.3 | 57.9 | 5 (2.6) | 5.90 | 1.35 | ||
| Clinical Risk Score* | ||||||||
| Low risk | 122 (60.7) | 431.4 | 30.1 | 0.276 | 115 (61.2) | 2.62 | 0.17 | 0.203 |
| High risk | 79 (39.3) | 417.3 | 24.6 | 73 (38.8) | 3.38 | 0.33 | ||
| Margin liver resection | ||||||||
| Negative | 190 (91.8) | 418.7 | 19.1 | 0.645 | 177 (91.7) | 2.81 | 0.16 | 0.226 |
| Positive | 17 (8.2) | 550.4 | 129.4 | 16 (8.3) | 4.03 | 0.90 | ||
| Tertiary lymphoid structure | ||||||||
| No | 184 (88.9) | 426.8 | 21.8 | 0.481 | 174 (90.6) | 2.91 | 0.17 | 0.972 |
| Yes | 23 (11.1) | 451.2 | 60.2 | 18 (9.4) | 3.03 | 0.53 | ||
| Primary tumor | ||||||||
| Left colon | 153 (74.3) | 418.1 | 21.2 | 0.865 | 137 (71.4) | 3.06 | 0.20 | 0.074 |
| Right colon | 53 (25.7) | 446.0 | 49.1 | 55 (28.6) | 2.54 | 0.30 | ||
| pT category | ||||||||
| pT1-pT3 | 161 (83.9) | 422.8 | 24.2 | 0.069 | 150 (83.8) | 2.83 | 0.18 | 0.573 |
| pT4 | 31 (16.1) | 452.9 | 36.8 | 29 (16.2) | 3.19 | 0.50 | ||
| pN category | ||||||||
| pN0 | 73 (36) | 436.1 | 37.2 | 0.887 | 69 (36.5) | 2.82 | 0.28 | 0.639 |
| pN+ | 130 (64) | 420.3 | 24.6 | 120 (63.5) | 2.89 | 0.20 | ||
| Preop chemotherapy | ||||||||
| No | 45 (21.7) | 426.1 | 49.0 | 0.289 | 41 (21.2) | 2.62 | 0.27 | 0.684 |
| Yes | 162 (78.3) | 430.4 | 22.5 | 152 (78.8) | 2.99 | 0.19 | ||
| 5FU, leucovorin, oxaliplatin-based | ||||||||
| No | 20 (12.3) | 441.5 | 67.9 | 0.943 | 19 (12.5) | 3.17 | 0.47 | 0.491 |
| Yes | 142 (87.7) | 428.9 | 23.9 | 133 (87.5) | 2.96 | 0.21 | ||
| Bevacizumab | ||||||||
| No | 35 (21.6) | 394.3 | 37.7 | 0.441 | 34 (22.4) | 2.87 | 0.41 | 0.652 |
| Yes | 127 (78.4) | 440.4 | 26.7 | 118 (77.6) | 3.02 | 0.22 | ||
| Tumor regression grade | ||||||||
| 1-2 | 27 (17) | 351.1 | 36.1 | 0.032 | 29 (19.6) | 2.34 | 0.32 | 0.077 |
| 3-4-5 | 132 (83) | 449.1 | 26.3 | 119 (80.4) | 3.21 | 0.23 | ||
| Extrahepatic recurrence | ||||||||
| No | 63 (42.9) | 420.9 | 39.0 | 0.344 | 59 (43.7) | 2.58 | 0.23 | 0.462 |
| Yes | 84 (57.1) | 473.0 | 35.2 | 76 (56.3) | 3.05 | 0.28 |
Abbreviations: tCD73, tumoral CD73 expression in CRLM; sCD73, soluble CD73 measured in serum; CEA, Carcinoembryoinic Antigen; T, Tumor; N, Node; SEM, Standard error of the mean. *Clinical Risk Score calculated giving one point for each of the following clinicopathologic characteristics, and where low risk is defined as 0 to 2 points, high risk as 3 to 5 points: node-positive (N+) primary cancer, disease-free interval (time between resection of primary and liver recurrence) <12 months, more than 1 liver metastasis, largest liver metastasis >5 cm, and prehepatectomy serum carcinoembryonic antigen (CEA) level >200 ng/ml.
CD73 association with patient outcome
Both high CD73 expression in the stroma or on cancer cells were associated with poorer outcomes. The best prognostic value was however obtained when both compartments were considered for CD73 quantification, herein defined as tCD73. High tCD73 average expression from all metastatic lesions in given patients was significantly associated with poor prognosis (Supp. Fig. 2A). Interestingly, as shown in Figure 2(a), the metastasis with the highest mean tCD73 expression appeared to drive patient prognosis, as it better discriminated survival curves compared to using the average CD73 expression of all metastatic lesions in patients with multiple metastases. Using the upper tertile (MFI > 398.3) as a cutoff for high versus low tCD73 expression, high tCD73 expression was associated with both shorter TTR (11.0 vs. 19.0 months, P = .002) and DSS (46.4 vs. 61.5 months, P < .001) (Figure 2(a)).
Figure 2.
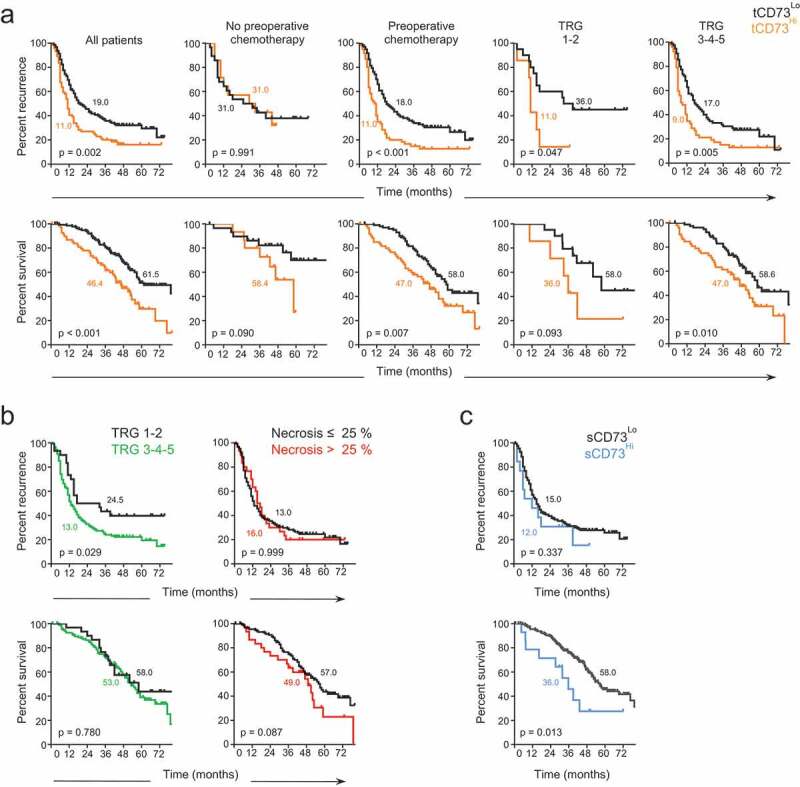
Prognostic value of CD73 in colorectal cancer liver metastasis. Time to recurrence (upper panels) and disease-specific survival (lower panels) of patients according to (a) high (yellow) versus low (black) intra-tumoral CD73 expression (tCD73), using the upper tertile as cutoff (MFI > 398.3), (b) histopathologic response (Tumor Regression Grade, TRG) (left) and the degree of necrosis (right) in patients who received pre-operative chemotherapy, and (c) high (blue) versus low (black) soluble CD73 serum level (sCD73), using a cutoff value of 7.2 ng/mL (minimal p-value approach). Median time to recurrence and disease-specific survival are annotated on graphs. Log-rank test.
The prognostic value of tCD73 appeared mainly accounted for by patients who received pre-operative chemotherapy (78.3% of patients, n = 162). When substratifying these patients according to major (TRG 1–2) or poor (TRG 3, 4, or 5) histopathological response to chemotherapy, high tCD73 was still prognostically discriminant (Figure 2(a), right). tCD73high patients with major response to chemotherapy had a median time to recurrence similar to tCD73high patients with poor response to chemotherapy, respectively of 11.0 and 9.0 months. By itself, TRG scoring in patients who received pre-operative chemotherapy was predictive of TTR but not of DSS, and necrosis had no prognostic value (Figure 2(b)). Pre-operative sCD73 was not as closely associated with prognosis as intratumoral tCD73. Nonetheless, using a cutoff value of 7.2 ng/mL determined with the minimal p-value approach, 23 the 14 patients (7.2%) with the highest sCD73 levels had significantly shorter DSS compared to the rest of the cohort (36.0 vs. 58.0 months, P = .013) (Figure 2(c)).
Of 207 patients analyzable for tCD73, 121 (58.5%) had multiple metastases (>1). In this subgroup, 27 patients (22.3%) presented with heterogeneous tCD73 expression levels in different metastases form the same patient. Mean tCD73 expression in CRLMs of patients who had heterogeneous metastases was higher than in patients who presented with homogenous metastases (MFI 598.2 vs 385.7 respectively, P < .001). Interestingly, patients with heterogenous metastases had significantly shorter DSS than patients with homogenous metastases (Supp. Fig. 2B).
By multivariate analysis, high tCD73 expression was associated with shorter TTR (HR = 1.55; 95%-CI 1.09–2.21; P = .014) and DSS (HR = 1.94; 95%-CI 1.26–2.98; P = .003), independent of clinicopathological variables (Table 2). Interestingly, in the subgroup of patients who received pre-operative chemotherapy, high tCD73 expression singled as the strongest independent prognostic factor for TTR (HR = 2.00; 95%-CI, 1.37–2.94, P < .001), while TRG scoring and all other clinicopathological variables did not reach significance. Consistent with previous observation, sCD73 was not significantly associated with patient outcomes by multivariate analysis.
Table 2.
Univariate and multivariate analysis of CD73 and clinicopathological variables with outcomes.
| Time to recurrence | Disease specific survival | |||
| |
HR (95% CI) |
P value |
HR (95% CI) |
P value |
| Total cohort (N = 215) | ||||
| Univariate Analysis | ||||
| Age at hepatectomy (≤65 vs >65 years) | 0.94 (0.68 - 1.31) | 0.718 | 1.38 (0.92 - 2.07) | 0.115 |
| Gender (male vs female) | 1.04 (0.74 - 1.47) | 0.818 | 1.25 (0.83 - 1.88) | 0.292 |
| Timing of metastasis (synchronous vs metachronous) | 0.82 (0.59 - 1.14) | 0.239 | 0.81 (0.54 - 1.21) | 0.300 |
| Number of metastases (single vs multiple) | 1.72 (1.23 - 2.41) | 0.002 | 1.34 (0.88 - 2.03) | 0.176 |
| Diameter of largest metastasis (≤5 vs >5 cm | 1.41 (0.90 - 2.20) | 0.130 | 1.25 (0.69 - 2.24) | 0.464 |
| Disease-free interval <12 months (no vs yes) | 1.79 (1.23 - 2.61) | 0.002 | 1.87 (1.14 - 3.06) | 0.013 |
| CEA level (≤200 vs >200 ng/mL) | 5.32 (2.13 - 13.29) | <0.001 | 3.98 (1.73 - 9.14) | 0.001 |
| Liver resection margin (negative vs positive for cancer cells) | 1.43 (0.81 - 2.53) | 0.221 | 2.36 (1.31 - 4.25) | 0.004 |
| Tertiary lymphoid structure (no vs yes) | 0.86 (0.50 - 1.50) | 0.603 | 0.35 (0.15 - 0.81) | 0.014 |
| Necrosis (≤ 25 vs > 25 %) | 0.97 (0.64 - 1.46) | 0.876 | 1.55 (0.95 - 2.52) | 0.077 |
| Primary tumor (left vs right) | 0.91 (0.63 - 1.33) | 0.637 | 1.16 (0.73 - 1.83) | 0.530 |
| pT category (T1-T2-T3 vs T4) | 2.12 (1.39 - 3.23) | 0.001 | 2.17 (1.36 - 3.48) | 0.001 |
| pN category (N0 vs N+) | 1.43 (1.01 - 2.02) | 0.044 | 1.60 (1.02 - 2.52) | 0.041 |
| Preop chemotherapy (no vs yes) | 1.51 (0.98 - 2.30) | 0.059 | 1.78 (1.01 - 3.13) | 0.047 |
| tCD73 (low vs high) | 1.70 (1.22 - 2.36) | 0.002 | 1.92 (1.28 - 2.86) | 0.002 |
| sCD73 (low vs high) | 1.00 (0.89 - 1.13) | 0.985 | 1.08 (0.94 - 1.23) | 0.270 |
| Multivariate Analysis | ||||
| CEA level (≤200 vs >200 ng/mL) | 4.83 (1.90 - 12.27) | 0.001 | 3.83 (1.48 - 9.89) | 0.006 |
| Disease-free interval <12 months (no vs yes) | 1.73 (1.17 - 2.56) | 0.006 | 2.05 (1.22 - 3.44) | 0.007 |
| Liver resection margin (negative vs positive for cancer cells) | - | - | 2.39 (1.28 - 4.49) | 0.007 |
| Tertiary lymphoid structure (no vs yes) | - | - | 0.34 (0.15 - 0.79) | 0.013 |
| pT category (T1-T2-T3 vs T4) | 1.99 (1.30 - 3.06) | 0.002 | 2.35 (1.45 - 3.80) | 0.001 |
| tCD73 (low vs high) |
1.55 (1.09 - 2.21) |
0.014 |
1.94 (1.26 - 2.98) |
0.003 |
| Preoperative chemotherapy (n = 169) | ||||
| Univariate Analysis | ||||
| Age at hepatectomy (≤65 vs >65 years) | 0.97 (0.67 - 1.39) | 0.852 | 1.46 (0.94 - 2.25) | 0.094 |
| Gender (male vs female) | 1.03 (0.70 - 1.50) | 0.882 | 1.30 (0.83 - 2.03) | 0.251 |
| Timing of metastasis (synchronous vs metachronous) | 0.96 (0.67 - 1.37) | 0.817 | 0.93 (0.61 - 1.43) | 0.748 |
| Number of metastases (single vs multiple) | 1.52 (1.03 - 2.23) | 0.035 | 1.30 (0.80 - 2.09) | 0.287 |
| Diameter of largest metastasis (≤5 vs >5 cm | 1.53 (0.93 - 2.53) | 0.097 | 1.73 (0.91 - 3.27) | 0.095 |
| Disease-free interval <12 months (no vs yes) | 1.42 (0.90 - 2.24) | 0.134 | 1.21 (0.68 - 2.14) | 0.525 |
| CEA level (≤200 vs >200 ng/mL) | 3.81 (1.19 - 12.19) | 0.024 | 5.42 (1.94 - 15.15) | 0.001 |
| Liver resection margin (negative vs positive for cancer cells) | 1.78 (0.97 - 3.24) | 0.061 | 2.54 (1.37 - 4.71) | 0.003 |
| Tertiary lymphoid structure (no vs yes) | 0.82 (0.43 - 1.57) | 0.548 | 0.47 (0.20 - 1.08) | 0.075 |
| Necrosis (≤ 25 vs > 25 %) | 0.99 (0.64 - 1.56) | 0.980 | 1.56 (0.93 - 2.61) | 0.091 |
| Primary tumor (left vs right) | 0.80 (0.54 - 1.21) | 0.291 | 0.90 (0.55 - 1.47) | 0.662 |
| pT category (T1-T2-T3 vs T4) | 1.74 (1.10 - 2.75) | 0.018 | 2.01 (1.22 - 3.32) | 0.006 |
| pN category (N0 vs N+) | 1.19 (0.81 - 1.75) | 0.368 | 1.48 (0.90 - 2.41) | 0.120 |
| Oxaliplatin-based chemotherapy pre-op (no vs yes) | 1.04 (0.60 - 1.79) | 0.894 | 1.53 (0.74 - 3.18) | 0.253 |
| Bevacuzimab pre-op (no vs yes) | 1.21 (0.77 - 1.91) | 0.404 | 1.43 (0.81 - 2.52) | 0.217 |
| Tumor Regression Grade (1-2 vs 3-4-5) | 1.64 (0.98 - 2.74) | 0.061 | 1.09 (0.60 - 1.97) | 0.780 |
| tCD73 (low vs high) | 1.96 (1.36 - 2.82) | <0.001 | 1.78 (1.15 - 2.75) | 0.009 |
| sCD73 (low vs high) | 0.99 (0.86 - 1.13) | 0.844 | 1.14 (1.00 - 1.30) | 0.053 |
| Multivariate Analysis | ||||
| CEA level (≤200 vs >200 ng/mL) | - | - | 5.48 (1.65 - 18.19) | 0.005 |
| pT category (T1-T2-T3 vs T4) | - | - | 2.15 (1.29 - 3.58) | 0.003 |
| Liver resection margin (negative vs positive for cancer cells) | - | - | 2.62 (1.36 - 5.03) | 0.004 |
| tCD73 (low vs high) | 2.00 (1.37 - 2.94) | <0.001 | 1.72 (1.08 - 2.74) | 0.022 |
Abbreviations: HR, Hazard ratio; CI, Confidence interval; tCD73, tumoral CD73 expression in CRLM ; sCD73, soluble CD73 measured in serum; CEA, Carcinoembryoinic Antigen; T, Tumor; N, Node.
Discussion
The final step in the conversion of pro-inflammatory extracellular ATP to immunosuppressive adenosine is catalyzed by the rate-limiting membrane-bound and soluble ectonucleotidase CD73, and constitutes an important negative-feedback mechanism that prevents excessive immune responses.24 Recent evidence support that many solid tumors usurp this pathway as an immune escape mechanism. In this study, we found a broad range of intratumoral CD73 expression in CRLMs by cancer and stromal cells. High CD73 in the TME of CRLMs was associated with shorter TTR and DSS, multiple and larger metastases, and resistance to preoperative chemotherapy. Intriguingly, sCD73 was not correlated with tCD73. While sCD73 was not significantly associated with clinicopathological features, patients with higher sCD73 levels (top 7.2%) displayed shorter DSS. To our knowledge, this is the first study investigating tCD73 and sCD73 in the metastatic setting of a significantly large cohort of patients with a common epithelial malignancy.
In line with our results, high expression of CD73 in colorectal primary cancers compared to normal peritumoral tissues has been reported.25 In an IHC study of 90 rectal cancer, high CD73 expression on cancerous cells but not on stromal cells was associated with shorter overall survival.26 Reports on other solid tumors have not specifically addressed the relationship between CD73 and the effect of chemotherapy.
Poor response to chemotherapy, rapid tumor growth, and larger tumors are characterized by the presence of necrotic areas which is the endpoint of chronic ischemia due to insufficient vascularization and inadequate oxygenation. In hypoxic conditions, stressed cancer cells release ATP that can then be degraded into adenosine by CD73 into the TME.11 Tumor growth requires concurrent suppression of immune response as well as the development of neoangiogenesis. In this context, accumulating data underscore that adenosine plays a key role in endothelial cell proliferation, survival, migration and vessel formation though promotion of pro-angiogenic factors such as vascular endothelial growth factor A (VEGFA), Interleukin 8 (IL-8), basic fibroblast growth factor (bFGF) and angiopoietin 1.27-29 Nonetheless, in our study, CD73 expression was independent of pathological response to chemotherapy and the degree of necrosis, and was found to be more strongly associated with patient outcomes (Figiure 2 and Table 2).
Some cytotoxic agents, such as platinum compounds, can induce stress-related ATP release by cancer cells, which is a key mediator of immunogenic cell death.30 In a TME rich in CD73 however, the resulting immunosuppressive adenosine may prevent effective immune-mediated destruction of residual cancer cells. In our data, the majority of patients received oxaliplatin-based chemotherapy prior to CRLM resection, and patients who obtained a complete or near complete eradication of tumor cells (TRG 1 or 2) had tumor bearing significantly lower levels of CD73 in resected CRLMs. CD73 expression in CRLM patients who did not receive preoperative chemotherapy was similar to those who did. Limited by their associative nature, these data could either signify that low intratumoral CD73 at baseline enhanced immune-mediated destruction of cancer cells in tumors susceptible to chemotherapy, but could also mean that “after the fact”, a metastatic deposit essentially replaced by fibrous tissue bears lower CD73 levels. Further study with pre- and post-chemotherapy biopsies could help understand the underlying biology.
In the present study, we have not found a correlation between tCD73 and its soluble form measured as sCD73 in the serum of patients. Lack of concordance may be explained by the not necessarily linear relationship between membrane-bound and soluble forms14 and beside tumors, multiple other cellular sources of CD73 in various normal organ tissues.31-33
Whether high tCD73 could serve as a predictive biomarker of response to immunotherapy with CD73 or A2A receptor inhibitors will have to be evaluated. Along the paradigm observed with PD-1/PDL1 blockade, CD73 alone may not be sufficient as a biomarker and could be combined with features of immune hot tumors (T-cell infiltration, Interferon gamma signaling), and in this case, expression of A2A receptors on infiltrating immune cells. Nevertheless, as a prognostic biomarker in CRLMs resected with curative intent, CD73 performed better than standard clinicopathological variables and TRG scoring, specifically in the subgroup of patients who received pre-operative chemotherapy. These findings need to be validated on independent cohort. It also remains to be investigated whether high expression of CD73 is an acquired phenomenon of some liver metastases by comparing to expression in matched primary tumors.
In conclusion, our findings provide new insights into the potential relevance of targeting CD73 in conjunction with cytotoxic chemotherapy in CRLM patients, and in using CD73 as a predictive and prognostic biomarker.
Funding Statement
This work was supported by a research grant from Surface Oncology, who had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, by the Chaire Roger des Groseillers en oncologie chirurgicale hépatobiliaire et pancréatique de l’Université de Montréal, and start-up funding from Institut du cancer de Montréal, the CRCHUM, and the Université de Montréal Surgery Department. S.T. holds a Clinical Research Scholarship from the Fonds de recherche du Québec - Santé. N.M. was supported by the International Hepato-Pancreato-Biliary Association (IHPBA) Kenneth Warren Research Fellowship and Ethicon, Inc., a subsidiary of Johnson & Johnson. D.T. holds a Clinical Research Scholarship from the Fonds de recherche du Québec – Santé.
Acknowledgments
We thank Louise Rousseau, Sophie Langevin and Julie Bilodeau from the CHUM Hepatopancreatobiliary and Colorectal Cancer Prospective Database & Biobank for patient recruitment, biospecimen handling, and maintenance of clinicopathological data; Lilianne Meunier and Véronique Barès from the CRCHUM histology platform for building the tissue microarray and technical assistance with slide handling and high-resolution scanning; and Aurélie Cleret-Bohot from the CRCHUM microscopy platform for technical assistance with automated immunofluorescence quantification.
Disclosure of Potential Conflicts of Interest
J. S. is a permanent member of the scientific advisory board of Surface Oncology and holds stocks of Surface Oncology. S.T. is receiving non-clinical research funding from Bristol-Myers Squibb. All remaining authors have declared no conflicts of interest.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–8. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, Buttard B, Morgand E, Bruni D, Jouret-Mourin A, et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175(3):751–765 e716. doi: 10.1016/j.cell.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Mlecnik B, Van den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, et al. Comprehensive intrametastatic immune quantification and major impact of immunoscore on survival. J Natl Cancer Inst. 2018;110:97–108. [DOI] [PubMed] [Google Scholar]
- 5.Turcotte S, Katz SC, Shia J, Jarnagin WR, Kingham TP, Allen PJ, Fong Y, D’Angelica MI, DeMatteo RP. Tumor MHC class I expression improves the prognostic value of T-cell density in resected colorectal liver metastases. Cancer Immunol Res. 2014;2(6):530–537. doi: 10.1158/2326-6066.CIR-13-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turcotte S, Gros A, Tran E, Lee CCR, Wunderlich JR, Robbins PF, Rosenberg SA. Tumor-reactive CD8+ T cells in metastatic gastrointestinal cancer refractory to chemotherapy. Clin Cancer Res. 2014;20(2):331–343. doi: 10.1158/1078-0432.CCR-13-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran E, Ahmadzadeh M, Lu Y-C, Gros A, Turcotte S, Robbins PF, Gartner JJ, Zheng Z, Li YF, Ray S, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350(6266):1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709–724. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 9.Allard D, Chrobak P, Allard B, Messaoudi N, Stagg J. Targeting the CD73-adenosine axis in immuno-oncology. Immunol Lett. 2019;205:31–39. doi: 10.1016/j.imlet.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Wang R, Zhang Y, Lin X, Gao Y, Zhu Y. Prognositic value of CD73-adenosinergic pathway in solid tumor: A meta-analysis and systematic review. Oncotarget. 2017;8:57327–57336. doi: 10.18632/oncotarget.16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagg J, Beavis PA, Divisekera U, Liu MCP, Moller A, Darcy PK, Smyth MJ. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72(9):2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 13.Stagg J. The double-edge sword effect of anti-CD73 cancer therapy. Oncoimmunology. 2012;1:217–218. doi: 10.4161/onci.1.2.18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morello S, Capone M, Sorrentino C, Giannarelli D, Madonna G, Mallardo D, Grimaldi AM, Pinto A, Ascierto PA. Soluble CD73 as biomarker in patients with metastatic melanoma patients treated with nivolumab. J Transl Med. 2017;15:244. doi: 10.1186/s12967-017-1348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X-R, He X-S, Chen Y-F, Yuan R-X, Zeng Y, Lian L, Zou Y-F, Lan N, Wu X-J, Lan P, et al. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol. 2012;106(2):130–137. doi: 10.1002/jso.v106.2. [DOI] [PubMed] [Google Scholar]
- 16.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18(2):299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 17.Grosset AA, Loayza-Vega K, Adam-Granger E, Birlea M, Gilks B, Nguyen B, Soucy G, Tran-Thanh D, Albadine R, Trudel D. Hematoxylin and Eosin counterstaining protocol for immunohistochemistry interpretation and diagnosis. Appl Immunohistochem Mol Morphol. 2017;27(7):558–563. doi: 10.1097/PAI.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 18.Buisseret L, Pommey S, Allard B, Garaud S, Bergeron M, Cousineau I, Ameye L, Bareche Y, Paesmans M, Crown JPA, et al. Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial. Ann Oncol. 2018;29(4):1056–1062. doi: 10.1093/annonc/mdx730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudreau PO, Allard B, Turcotte M, Stagg J. CD73-adenosine reduces immune responses and survival in ovarian cancer patients. Oncoimmunology. 2016;5:e1127496. doi: 10.1080/2162402X.2015.1127496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclerc BG, Charlebois R, Chouinard G, Allard B, Pommey S, Saad F, Stagg J. CD73 expression is an independent prognostic factor in prostate cancer. Clin Cancer Res. 2016;22(1):158–166. doi: 10.1158/1078-0432.CCR-15-1181. [DOI] [PubMed] [Google Scholar]
- 21.Turcotte M, Allard D, Mittal D, Bareche Y, Buisseret L, José V, Pommey S, Delisle V, Loi S, Joensuu H, et al. CD73 promotes resistance to HER2/ErbB2 antibody therapy. Cancer Res. 2017;77(20):5652–5663. doi: 10.1158/0008-5472.CAN-17-0707. [DOI] [PubMed] [Google Scholar]
- 22.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–130. doi: 10.1111/his.2007.50.issue-1. [DOI] [PubMed] [Google Scholar]
- 23.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 24.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 25.Eroglu A, Canbolat O, Demirci S, Kocaoĝlu H, Eryavuz Y, Akgül H. Activities of adenosine deaminase and 5ʹ-nucleotidase in cancerous and noncancerous human colorectal tissues. Med Oncol. 2000;17:319–324. doi: 10.1007/BF02782198. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Song B, Wang X, Chang X-S, Pang T, Zhang X, Yin K, Fang G-E. The expression and clinical significance of CD73 molecule in human rectal adenocarcinoma. Tumour Biol. 2015;36(7):5459–5466. doi: 10.1007/s13277-015-3212-x. [DOI] [PubMed] [Google Scholar]
- 27.Ohta A, Ohta A, Madasu M, Kini R, Subramanian M, Goel N, Sitkovsky M. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J Immunol. 2009;183(9):5487–5493. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 28.Zarek PE, Huang C-T, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111(1):251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 31.Heuts DP, Weissenborn MJ, Olkhov RV, Shaw AM, Gummadova J, Levy C, Scrutton NS. Crystal structure of a soluble form of human CD73 with ecto-5ʹ-nucleotidase activity. Chembiochem. 2012;13:2384–2391. doi: 10.1002/cbic.201200426. [DOI] [PubMed] [Google Scholar]
- 32.Vaara ST, Hollmen M, Korhonen A-M, Maksimow M, Ala-Kokko T, Salmi M, Jalkanen S, Pettilä V. Soluble CD73 in Critically Ill septic patients - data from the prospective FINNAKI study. PLoS One. 2016;11(10):e0164420. doi: 10.1371/journal.pone.0164420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


