Abstract
Type 1 diabetes (T1D) results from an autoimmune-mediated destruction of pancreatic β-cells. The incidence of T1D is on the rise globally around 3–5% per year and rapidly increasing incidence in younger children is of the greatest concern. Currently there is no way to cure or prevent T1D, hence a deeper understanding of the underlying molecular mechanisms of this disease is essential to the development of new effective therapies. The endoplasmic reticulum (ER) is an organelle with multiple functions that are essential for cellular homeostasis. Excessive demand on the ER, chronic inflammation and environmental factors lead to ER stress and to re-establish cellular homeostasis, the adaptive unfolded protein response (UPR) is triggered. However, chronic ER stress leads to a switch from a pro-survival to a pro-apoptotic UPR, resulting in cell death. Accumulating data have implicated ER stress and defective UPR in the pathogenesis of inflammatory and autoimmune diseases, and ER stress has been implicated in β-cell failure in type 2 diabetes. However, the role of ER stress and the UPR in β-cell pathophysiology and in the initiation and propagation of the autoimmune responses in T1D remains undefined. This review will highlight the current understanding and recent in vivo data on the role of ER stress and adaptive responses in T1D pathogenesis and the potential therapeutic aspect of enhancing β-cell ER function and restoring UPR defects as novel clinical strategies against this disease.
Keywords: type 1 diabetes, ER stress, UPR, beta cell, NOD mice
Introduction
Type 1 diabetes (T1D) results from the immune-mediated destruction of pancreatic β-cells leading to loss of insulin production, unsuppressed glucose production, and hyperglycemia(1–3). The prevalence of T1D between the ages 0–19 years was reported to be 1.7/1,000, and has been rising globally and by as much as a 5.3% annually in the United States(4). Despite intensive research in the type 1 diabetes field, the initial signals that trigger autoimmunity, the intracellular mediators that lead to destruction of β-cells and the crosstalk between β-cells and immune cells in T1D still remain poorly understood, which hampers the development of effective preventive and/or therapeutic strategies against this disease.
Autoimmune T1D and metabolic disorder Type 2 diabetes (T2D) have long been thought to show clear differences in disease prevalence, onset, weight, presence of autoantibodies and insulitis. However, emerging data suggest that T1D and T2D may show many more similarities in disease pathogenesis than previously thought(5, 6). It is now becoming more evident that T2D patients also present with inflammation, earlier disease onset and islet autoimmunity(7–11). In contrast, increased childhood obesity is seen more often in T1D patients(12) and was once called juvenile diabetes. T1D can be detected in adult patients as well. This convergence or the overlap between autoimmune T1D and metabolic disorder T2D suggest the possibility of common cellular and/or immunometabolic mechanisms.
In T1D, autoimmune responses lead to production of cytokines that cause an inflammatory state in β-cells. In T2D, obesity induced chronic low-grade systemic inflammation is observed in metabolic organs such as liver, skeletal muscle, adipose tissue, hypothalamus and β-cells. As accumulating data suggest a significant role for inflammation in diabetes pathogenesis, conditions that induce inflammation and/or disrupt cellular homeostasis have been critically important for understanding the molecular mechanism of this disease. One such condition has been proposed to be the stress in the endoplasmic reticulum (ER). Although a growing body of work linked ER stress to T2D and obesity pathogenesis both in mouse models as well as in human patients(13–15), the role of ER stress and cellular adaptive responses in T1D pathogenesis still remains unclear.
ER stress and the UPR
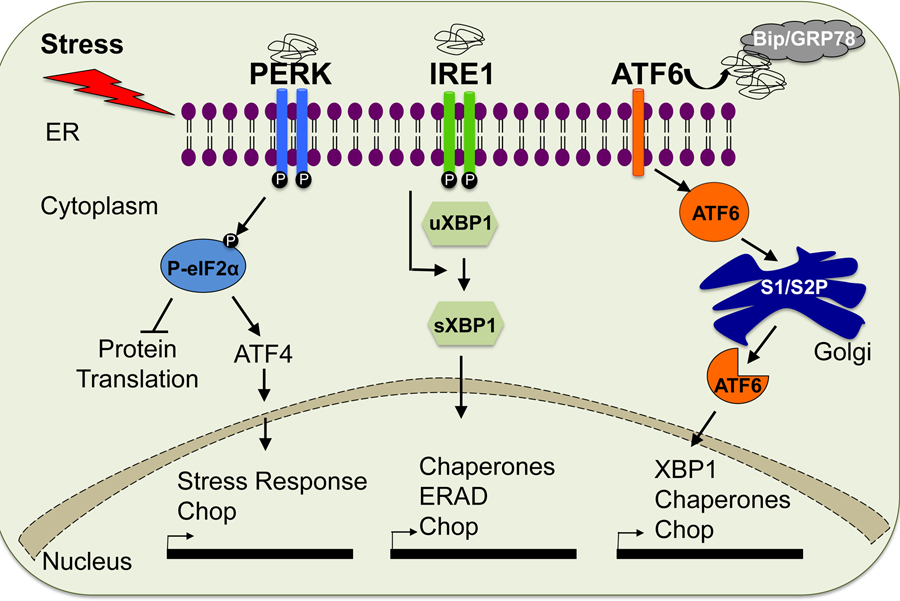
ER stress and the adaptive unfolded protein response (UPR) have been of great interest lately due to their crucial role in the pathogenesis of multiple diseases including neurodegenerative diseases, cancer and metabolic disorders. Surplus nutrition, viruses, environmental toxins, and chronic inflammation can alter calcium levels, protein glycosylation, and redox status in the ER leading to abnormal protein folding and secretion. Accumulation of unfolded and misfolded proteins results in ER stress and the cells react to this stress conditions by activating, an evolutionarily conserved adaptive response, the unfolded protein response or UPR(16). The UPR responds to ER stress primarily by arresting protein translation and activating signaling pathways that lead to expression of molecular chaperones that assist protein folding or facilitate degradation of misfolded proteins. To date, three major branches of the UPR have been identified. RNA-dependent protein kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring ER-to-nucleus signal kinase 1 (IRE1) are all located in the ER membrane and are activated in response to the accumulation of unfolded proteins within the ER. Activation of PERK leads to phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), which causes general inhibition of protein synthesis. In response to ER stress, ATF6 is translocated to the Golgi apparatus where it is cleaved by site-1 and site-2 proteases, yielding an active transcription factor. Similarly, activated IRE1 catalyzes removal of a small intron from the mRNA of the gene encoding X-box binding protein 1 (XBP1). This splicing event creates a translational frame shift in XBP1 mRNA to produce an active transcription factor (sXBP1). Active ATF6 and sXBP1 subsequently bind to the ER stress response element (ERSE) and the UPR element (UPRE) of target genes to regulate their expression (Figure 1). Upon acute stress, these branches of the UPR engage pro-survival and adaptive signaling, leading to re-establishment of cellular homeostasis; however if ER stress is prolonged and unresolved, the UPR switches from a pro-adaptive to pro-apoptotic outcome. Thus, the UPR determines the homeostatic vs. apoptotic fate of a cell(17, 18).
Figure 1. Unfolded Protein Response Pathway (UPR).
The UPR is triggered upon ER stress and mediated by three sensors (PERK, IRE1/SXBP1 AND ATF6) that are localized at the ER membrane.
Inflammation, autoimmunity and the UPR
It has been demonstrated that chronic ER stress can engage the canonical branches of the UPR to elevate inflammation through activation of the JNK and IKK pathways. Reciprocally, inflammatory cytokines such as IL1-β, TNF-α and IFN-γ were shown to induce ER stress through nitric oxide (NO)(19). Production of NO leads to the depletion of ER calcium and inhibition of ER chaperone function resulting in a severe disruption in ER homeostasis(20, 21). In addition, recent data support that UPR mediators can activate the NLRP3 inflammasome and promote programmed cell death under chronic ER stress conditions(22–24). Thus, these data indicate a tight interplay between inflammation and ER stress, and point towards a possible connection with the immune response. Consistent with this notion, accumulating experimental evidence indicates that a dysregulated UPR may promote autoimmunity in several different disease models including ankylosing spondylitis, rheumatoid arthritis and Sjögren’s syndrome(25–27). The link between ER stress and autoimmune responses has been proposed to be related to (a) misfolded proteins recognized as antigens by autoreactive immune cells, (b) dysregulation of immune-tolerance mechanisms in immune cells by ER stress, (c) ER stress-mediated death of cells releasing UPR-related autoantigens and/or neo-autoantigens, or (d) ER stress conferring a survival advantage to autoreactive cells(27). Whether β-cell ER stress and a defective UPR can initiate autoimmunity or are induced as a consequence of chronic inflammation in T1D remains largely unknown.
Aberrant UPR and type 1 diabetes
It has been suggested that disruption of ER homeostasis may contribute to β-cell dysfunction and diabetes. Consistent with this notion, misfolded insulin was shown to cause diabetes in both mouse models and humans(28–33). Moreover, in experimental animal models as well as in humans, mutations in genes critical for ER function result in β-cell failure and early onset, severe diabetes(34–42). The UPR has been implicated in β-cell biology and pathogenesis of diabetes. A loss of function mutation of the Perk gene has been shown to cause permanent neonatal insulin-dependent diabetes (Wolcott-Rallison Syndrome) in humans and mice(34, 36). Moreover, the IRE1 axis of the UPR has been shown to have a crucial function in insulin biosynthesis in vitro and in vivo(40). XBP-1 deficiency in β-cells causes modest hyperglycemia and glucose intolerance resulting from decreased insulin secretion from β-cells(42). Finally, Wolfram syndrome, caused by an autosomal-recessive mutation in the WSF1 gene, leads to non-autoimmune loss of β cells and insulin-dependent diabetes mellitus. In rodent and human cell lines, WFS1 was shown to mitigate ER stress by negatively regulating ATF6(43).
The indication that the UPR may play a role in the pathogenesis of autoimmune diabetes came from recent reports that demonstrated the presence of some of the ER stress markers in inflamed islets of both diabetes-prone NOD mice(44) and patients with autoimmune diabetes(45). In a recent study, we analyzed the protein levels of the three branches of the UPR in two different mouse models of T1D, and in human patients with T1D in situ. This work has demonstrated that expression of adaptive UPR mediators sXBP1 and ATF6 were significantly diminished in β-cells of NOD mice and human patients during the disease progression(46). Interestingly, in a follow-up study we showed that these markers were also down-regulated in the β-cells of both genetic and dietary mouse models of T2D, as well as in human T2D patients before the onset of hyperglycemia(47). Thus, these data suggested that highly secretory β-cells require an intact UPR and the loss of adaptive responses correlates with β-cell dysfunction and apoptosis.
Since, in experimental models of both type 2 diabetes and atherosclerosis, chemical chaperones such as tauroursodeoxycholic acid (TUDCA) and phenyl butyric acid (PBA) reduce ER stress and alleviate disease symptoms(48–50), we hypothesized that by increasing ER capacity, improving folding defects, or promoting the pro-survival and/or adaptive effects of the UPR, chemical chaperones might also protect pancreatic β-cells in T1D. To test this hypothesis, we administered TUDCA to two different mouse models of type 1 diabetes at the pre-diabetic stage, and detected a striking reduction in diabetes with TUDCA treatment in these models. Histological analyses indicated a markedly decreased insulitis and immune-mediated destruction in the islet area of TUDCA-treated mice. In line with reduced insulitis, β-cell survival and islet architecture were preserved upon TUDCA treatment. Immunophenotyping of the spleens, pancreata and lymph nodes of TUDCA treated animals revealed that systemic administration of TUDCA did not globally affect the relative representation of immune cell populations critically implicated in T1D while reducing the overall infiltration of islets. This could perhaps reflect decreased local antigen presentation or chemokine expression from islets, migratory and/or invasive defects of immune cells and/or potential alterations in T cell subtypes. Better understanding of the mechanism(s) by which alleviation of ER stress leads to decreased immune cell infiltration remains to be elucidated.
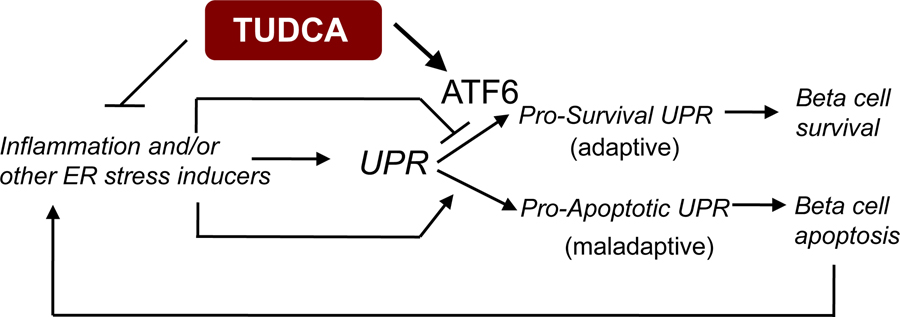
To determine whether TUDCA’s effects were altered in the genetic deficiency of the UPR, we induced diabetes in β-cell-specific ATF6α-deficient mice and wild-type controls that were intercrossed with RIP-LCMV-GP mice in the presence or absence of TUDCA treatment. There was no difference in the diabetes incidence between the ATF6α-deficient and control mice treated with vehicle. However, TUDCA’s protective effects were completely lost in mice with β-cell-specific ATF6α-deficiency, demonstrating that the effect of TUDCA in T1D was dependent on the intact function of ATF6 in β-cells. These data suggest that ATF6 acts as a pro-survival mediator of the UPR to maintain the function and survival of β-cells in type 1 diabetes and that TUDCA uses the ATF6 branch to induce the adaptive UPR and restore β-cell homeostasis (Figure 2).
Figure 2. TUDCA’s effects in type 1 diabetes.
Inflammation or ER stress inducers can initiate the UPR. If ER stress is chronic and unresolvable, maladaptive UPR leads to β-cell apoptosis and death. This in turn can induce inflammation and ER stress. TUDCA, by reducing inflammation and activating pro-survival UPR through ATF6 can give rise to β-cell survival and protection from diabetes.
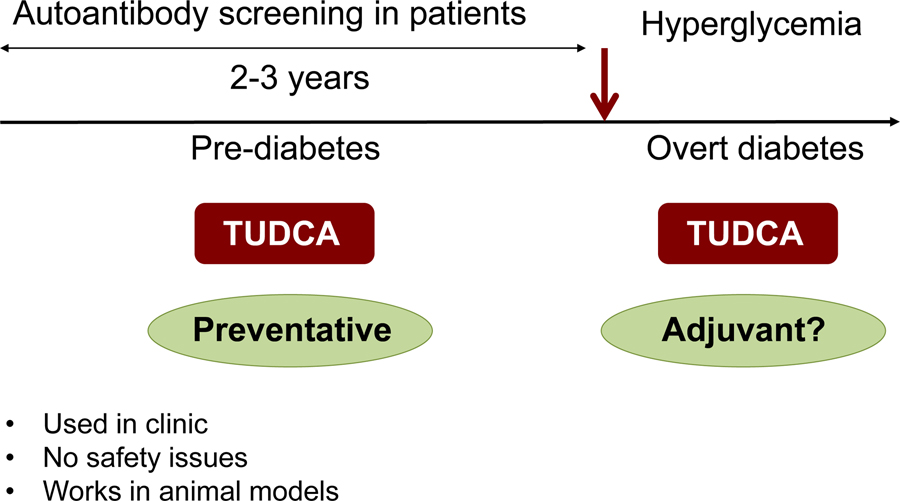
In addition to providing a direct link for the first time that the UPR plays an important role in β-cell survival, function and type 1 diabetes pathogenesis, this study provided proof of principle that the ER can be chemically targeted in an autoimmune disease to enhance its functional capacity. Although several interventions studies have been very successful in the NOD mouse model, these interventions failed to achieve similar effects in clinical studies. However, obtaining the same preventive effects in two different mouse models of T1D and demonstrating the similar UPR defects in human T1D patients, our study suggests that TUDCA, an agent that is already used safely in the clinic for liver diseases, might be useful as a preventative therapeutic agent for T1D as autoantibody screening allows detection of people at risk for T1D 2–3 years before the onset of the disease. Another interesting therapeutic possibility could involve using TUDCA as an adjuvant therapy. While our studies indicate that administration of TUDCA after the onset of diabetes does not show any effect, whether it can be effective in combination with other immune modulating agents remain to be tested (Figure 3).
Figure 3. The translational possibilities of TUDCA.
TUDCA or other agents that could enhance ER function and restore the UPR might be used as preventative therapeutic agents for T1D. By autoantibody screening, people at high risk for T1D can be detected several years before the onset of the disease and this can be an ideal therapeutic window for the application of ER modulating agents. In addition, TUDCA might be used as an adjuvant therapy and may provide better efficacy in combination with other immunomodulating agents.
Future directions
The role of ER stress and the UPR in type 1 diabetes pathogenesis is a newly emerging aspect of this field that is of great interest. Our recent work together with emerging data from other groups has established the importance of organelle stress and dysregulated adaptive UPR in the pathogenesis of autoimmune disease and raised the possibility that improving ER capacity and adaptive responses of the UPR in β-cells might be a preventive strategy applicable to those who are at high risk for type 1 diabetes. To date, several different strategies that focused mainly on immune modulation did not have a sustainable success in T1D clinical trials(51). Thus, enhancing β-cell ER function and the adaptive UPR alone or as a combination therapy might be a novel clinical approach. In addition to providing evidence that preserving in β-cell’s ER function can play a significant role in T1D pathogenesis, this study raised several interesting questions such as: (a) what are the functions of different branches of the UPR in T1D disease progression? (b) how do stressed β-cells crosstalk with immune cells? (c) are there biomarkers that can be indicative of β-cell ER stress or dysregulated UPR? (d) do immune cells undergo ER stress and have altered function during T1D progression? We believe addressing these questions will greatly improve our current understanding of β-cell ER stress and the UPR and may be effectively incorporated into development of better therapeutic and preventive approaches for type 1 diabetes.
Acknowledgement
This symposium was supported in part by a grant from the National Center for Research Resources (R13 RR023236).
References
- 1.Lehuen A, Diana J, Zaccone P, and Cooke A. Immune cell crosstalk in type 1 diabetes. Nature reviews Immunology.10(7):501–13. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D, Vence L, and Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414(6865):792–8. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson MA, Eisenbarth GS, and Michels AW. Type 1 diabetes. Lancet. 2013. [DOI] [PMC free article] [PubMed]
- 4.van Belle TL, Coppieters KT, and von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91(1):79–118. [DOI] [PubMed] [Google Scholar]
- 5.Donath MY, Storling J, Maedler K, and Mandrup-Poulsen T. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J Mol Med (Berl). 2003;81(8):455–70. [DOI] [PubMed] [Google Scholar]
- 6.Odegaard JI, and Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harb Perspect Med 2012;2(3):a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 2014;13(6):465–76. [DOI] [PubMed] [Google Scholar]
- 8.Shoelson SE, Lee J, and Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116(7):1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks-Worrell B, Narla R, and Palmer JP. Islet autoimmunity in phenotypic type 2 diabetes patients. Diabetes Obes Metab 2013;15 Suppl 3(137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013;4(6):270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itariu BK, and Stulnig TM. Autoimmune aspects of type 2 diabetes mellitus - a mini-review. Gerontology. 2014;60(3):189–96. [DOI] [PubMed] [Google Scholar]
- 12.Minges KE, Whittemore R, and Grey M. Overweight and obesity in youth with type 1 diabetes. Annu Rev Nurs Res 2013;31(47–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, et al. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59(8):1899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, and Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–61. [DOI] [PubMed] [Google Scholar]
- 16.Bernales S, Papa FR, and Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol 2006;22(487–508. [DOI] [PubMed] [Google Scholar]
- 17.Walter P, and Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. [DOI] [PubMed] [Google Scholar]
- 18.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell.140(6):900–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storling J, Binzer J, Andersson AK, Zullig RA, Tonnesen M, Lehmann R, Spinas GA, Sandler S, Billestrup N, and Mandrup-Poulsen T. Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia. 2005;48(10):2039–50. [DOI] [PubMed] [Google Scholar]
- 20.Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, and Corbett JA. The role of nitric oxide and the unfolded protein response in cytokine-induced beta-cell death. Diabetes. 2008;57(1):124–32. [DOI] [PubMed] [Google Scholar]
- 21.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, and Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A 2001;98(19):10845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell metabolism. 2012;16(2):265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488(7413):670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, et al. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell metabolism. 2012;16(2):250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panayi GS, and Corrigall VM. BiP regulates autoimmune inflammation and tissue damage. Autoimmun Rev 2006;5(2):140–2. [DOI] [PubMed] [Google Scholar]
- 26.Blass S, Union A, Raymackers J, Schumann F, Ungethum U, Muller-Steinbach S, De Keyser F, Engel JM, and Burmester GR. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis and rheumatism. 2001;44(4):761–71. [DOI] [PubMed] [Google Scholar]
- 27.Todd DJ, Lee AH, and Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 2008;8(9):663–74. [DOI] [PubMed] [Google Scholar]
- 28.Stoy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A 2007;104(38):15040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, and Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 1999;103(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, and Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52(2):409–16. [DOI] [PubMed] [Google Scholar]
- 31.Colombo C, Porzio O, Liu M, Massa O, Vasta M, Salardi S, Beccaria L, Monciotti C, Toni S, Pedersen O, et al. Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. The Journal of clinical investigation. 2008;118(6):2148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molven A, Ringdal M, Nordbo AM, Raeder H, Stoy J, Lipkind GM, Steiner DF, Philipson LH, Bergmann I, Aarskog D, et al. Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes. 2008;57(4):1131–5. [DOI] [PubMed] [Google Scholar]
- 33.Polak M, Dechaume A, Cave H, Nimri R, Crosnier H, Sulmont V, de Kerdanet M, Scharfmann R, Lebenthal Y, Froguel P, et al. Heterozygous missense mutations in the insulin gene are linked to permanent diabetes appearing in the neonatal period or in early infancy: a report from the French ND (Neonatal Diabetes) Study Group. Diabetes. 2008;57(4):1115–9. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, and Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 2002;22(11):3864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harding HP, Zhang Y, Bertolotti A, Zeng H, and Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5(5):897–904. [DOI] [PubMed] [Google Scholar]
- 36.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, and Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 2000;25(4):406–9. [DOI] [PubMed] [Google Scholar]
- 37.Oyadomari S, Yun C, Fisher EA, Kreglinger N, Kreibich G, Oyadomari M, Harding HP, Goodman AG, Harant H, Garrison JL, et al. Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell. 2006;126(4):727–39. [DOI] [PubMed] [Google Scholar]
- 38.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, and Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–76. [DOI] [PubMed] [Google Scholar]
- 39.Ladiges WC, Knoblaugh SE, Morton JF, Korth MJ, Sopher BL, Baskin CR, MacAuley A, Goodman AG, LeBoeuf RC, and Katze MG. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54(4):1074–81. [DOI] [PubMed] [Google Scholar]
- 40.Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, and Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab 2006;4(3):245–54. [DOI] [PubMed] [Google Scholar]
- 41.Song B, Scheuner D, Ron D, Pennathur S, and Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. The Journal of clinical investigation. 2008;118(10):3378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee AH, Heidtman K, Hotamisligil GS, and Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A 2011;108(21):8885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fonseca SG, Ishigaki S, Oslowski CM, Lu S, Lipson KL, Ghosh R, Hayashi E, Ishihara H, Oka Y, Permutt MA, et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest 2010;120(3):744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, and Mirmira RG. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61(4):818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marhfour I, Lopez XM, Lefkaditis D, Salmon I, Allagnat F, Richardson SJ, Morgan NG, and Eizirik DL. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55(9):2417–20. [DOI] [PubMed] [Google Scholar]
- 46.Engin F, Yermalovich A, Nguyen T, Hummasti S, Fu W, Eizirik DL, Mathis D, and Hotamisligil GS. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci Transl Med 2013;5(211):211ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engin F, Nguyen T, Yermalovich A, and Hotamisligil GS. Aberrant islet unfolded protein response in type 2 diabetes. Sci Rep 2014;4(4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 2009;15(12):1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, and Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engin F, and Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab 2010;12 Suppl 2(108–15. [DOI] [PubMed] [Google Scholar]
- 51.Bluestone JA, Herold K, and Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]