Version Changes
Revised. Amendments from Version 1
The majority of reviewers’ recommendations have been effected, and for the others, appropriate explanations/rebuttals have been provided. Details of the cross-sectional sampling in 2012-2013 and 2016-2017 and the genotyping methods used have been included. In addition, an explanation has been provided for the choice of the 2-14 year age group in the study. The data have been disaggregated and reanalyzed separately for the 2012-2013 and 2016-2017 time points. This study depended on archived samples from an erythrocyte invasion study so only samples that were successfully genotyped for any number of mutations were presented in Table 1. Table 1 now compares data from the same time points across study sites while Table 2 now captures frequencies of allele combinations in both pfdhfr and pfdhps. In this study mixed infections were not examined and we have provided an explanation for that in the revised manuscript. A map of Ghana has been included as Figure 1 to show the distance between Sunyani and Kintampo and the use of data for Sunyani to represent Kintampo has been corroborated with relevant literature. The graph on trend analyses is now designated Figure 2. References have been updated to capture extant literature in Ghana and other West and Eastern African countries. The discussion on IPTp and SMC uptake in Ghana and Burkina Faso has been expanded. Further details on the role of ACTs in the selection of mdr1 mutations has been included and relevant references provided therein. The major findings of the study have now been captured in the discussion as recommended. In addition, the use of antifolate drugs for the management of opportunistic infections in HIV has been discussed. The conclusion has been revised to align with the study objectives.
Abstract
Background: The emergence and spread of resistance in Plasmodium falciparum to chloroquine (CQ) necessitated the change from CQ to artemisinin-based combination therapies (ACTs) as first-line drug for the management of uncomplicated malaria in Ghana in 2005. Sulphadoxine-pyrimethamine (SP) which was the second line antimalarial drug in Ghana, was now adopted for intermittent preventive treatment of malaria in pregnancy (IPTp).
Methods: To examine the prevalence of molecular markers associated with CQ and antifolate drug resistance in Ghana, we employed restriction fragment length polymorphism polymerase chain reaction to genotype and compare single nucleotide polymorphisms (SNPs) in the P. falciparum chloroquine resistance transporter ( pfcrt, PF3D7_0709000), multidrug resistance ( pfmdr1, PF3D7_0523000), bifunctional dihydrofolate reductase-thymidylate synthase ( pfdhfr, PF3D7_0417200) and dihydropteroate synthase ( pfdhps, PF3D7_0810800) genes. Parasites were collected from children with malaria reporting to hospitals in three different epidemiological areas of Ghana (Accra, Kintampo and Navrongo) in 2012-2013 and 2016-2017.
Results: The overall prevalence of the CQ resistance-associated pfcrt 76T allele was 8%, whereas pfmdr1 86Y and 184F alleles were present in 10.2% and 65.1% of infections, respectively. The majority of the isolates harboured the antifolate resistance-associated pfdhfr alleles 51I (83.4%), 59R (85.9 %) and 108N (90.5%). Pfdhps 437G and 540E were detected in 90.6% and 0.7% of infections, respectively. We observed no significant difference across the three study sites for all the polymorphisms except for pfdhps 437G , which was more common in Accra compared to Kintampo for the 2016-2017 isolates. Across both pfdhfr and pfdhps genes, a large proportion (61%) of the isolates harboured the quadruple mutant combination ( I 51 R 59 N 108/ G 437).
CQ resistance alleles decreased during the 12 years after CQ withdrawal, but an mediate SP resistance alleles increased.
Conclusion: Surveillance of the prevalence of resistance alleles is necessary in monitoring the efficacy of antimalarial drugs.
Keywords: Drug resistance, Malaria, Antifolates, Chloroquine, Plasmodium falciparum
Introduction
Malaria remains a major global health concern especially in sub Saharan Africa. P. falciparum malaria is considered the most severe and also the leading cause of morbidity and mortality, especially among children under five years ( Schumacher & Spinelli, 2012). In 2016 a global estimate of 216 million malaria cases was reported, which led to about 445,000 deaths ( WHO, 2017). The global malaria mortality rate, however, has reduced by 29% since the year 2010, as a result of increased preventive and control measures ( WHO, 2016).
The use of antimalarial drugs for malaria treatment and prevention has played an integral role in the control of the disease over the decades ( Cui et al., 2015; Gosling et al., 2011; Greenwood, 2004; Schlitzer, 2007). Unfortunately, the emergence and the spread of drug resistant P. falciparum strains militated against the use of antimalarial drugs for the containment of the disease ( Lin et al., 2010). P. falciparum chloroquine (CQ) resistant strains were first reported in the 1950s in Southeast Asia along the Cambodia–Thailand border ( Young et al., 1963) and subsequently reported in other countries globally. Currently, the parasite has been reported to have developed resistance to most available artemisinin monotherapies and this is exhibited by reduced parasite clearance rates and/or treatment failures ( Dondorp et al., 2009). ACTs are now the frontline drugs for treating uncomplicated P. falciparum malaria in almost all countries that are endemic with malaria, including Ghana ( WHO, 2016).
Point mutations in specific genes in the parasite genome are implicated in resistance to specific antimalarial drugs ( Cui et al., 2015; Fidock et al., 2000; Sidhu et al., 2002). A point mutation in the P. falciparum chloroquine resistance transporter gene ( pfcrt, PF3D7_0709000 ) that replaces lysine with threonine at codon 76 had become a common single nucleotide polymorphism (SNP) in parasite populations as it is a critical mediator of resistance to CQ ( Babiker et al., 2001). In addition, mutations in the P. falciparum multidrug resistance gene 1 ( pfmdr1, PF3D7_0523000) that result in amino acid substitutions at positions N86Y and Y184F have been reported to confer parasite resistance to CQ, amodiaquine (AQ) and lumefantrine (L) ( Duraisingh & Cowman, 2005). These mutations are believed to interfere with heme polymerization by preventing the accumulation of active drug within the food vacuole ( Djimdé et al., 2001b).
P.falciparum resistance to Sulfadoxine-pyrimethamine (SP) has been linked to point mutations in the bifunctional dihydrofolate reductase-thymidylate synthase ( pfdhfr, PF3D7_0417200) and dihydropteroate synthase ( pfdhps, PF3D7_0810800) genes ( Cowman et al., 1988; Triglia et al., 1997; Wang et al., 1997). Resistance to antifolate drugs such as SP is known to be mediated by basal point mutations in these genes that result in amino acid substitutions at positions S108N and A437G in pfdhfr and pfdhps proteins respectively ( Mita et al., 2014). Overall, studies have shown that additional point mutations in these drug resistant genes on top of the basal mutation makes parasites more refractory to the drug ( Mita et al., 2014), and correlates with increased treatment failure ( Plowe et al., 1997; Sibley et al., 2001). Therefore, parasites harbouring haplotypes that include the different SNP alleles in combination have been shown to confer higher resistance to the specific drugs. In this regard, the combined quintuple mutant haplotype ( pfdhfr I 51 R 59 N 108 + pfdhps G 437 E 540) has been correlated with high SP treatment failure in East Africa ( Kublin et al., 2002; Omar et al., 2001).
In Ghana, prior to the withdrawal of CQ a prevalence range of between 46%–98% of the mutant pfcrt 76T was reported across five sentinel sites ( Duah et al., 2007). Interestingly, studies in other settings have shown that the replacement of CQ with ACTs resulted in a decline in the frequency of the mutant alleles and concomitant restoration of CQ susceptibility ( Laufer et al., 2006; Mwai et al., 2009). In a study that was conducted in Tanzania, more than 90% recovery of the sensitive pfcrt K76 allele was reported after 10 years of CQ use being officially discontinued ( Mohammed et al., 2013). Follow -up studies in Ghana have reported a decline in the prevalence of pfcrt 76T and pfmdr1 86Y but an increasing prevalence pfdhfr I 51, R 59, N 108 and 437G resistant alleles from 2003 to 2010 ( Duah et al., 2013; Duah et al., 2012)
This study sought to ascertain the population trends in the prevalence of known drug-resistance-related point mutations in pfcrt, pfmdr1, pfdhfr and pfdhps in clinical isolates from three different malaria-endemic areas in Ghana a decade following the introduction of ACTs.
Methods
Ethical consideration
This study was approved by the Ethics Committees of the Ghana Health Service (GHS-ERC:12/05/12), the Kintampo Health Research Centre (KHRCIEC/FEA/2011-13), the Navrongo Health Research Centre (NHRC-IRB135/08/2012) and the Noguchi Memorial Institute for Medical Research (NMIMR) (NMIMR-IRB CPN 004/11-12). Informed consent of parents or guardians for all participants was obtained. An additional assent was also obtained from children aged 10–14 years prior to recruitment.
Study sites and sample collection
This study leveraged the availability of samples from a concurrent study at the time on erythrocyte invasion mechanisms and whole genome sequencing of the malaria parasites. The appropriate sample collection at the time to meet the erythrocyte invasion studies, the whole genome sequencing of the malaria parasites was adopted. We used the samples so gotten to carry out the drug resistance study. The choice of 2–14 years was premised on development of immunity that was key in the erythrocyte invasion study.
Parasite isolates were obtained from children aged 2–14 years, diagnosed with malaria at Municipal hospitals in Kintampo North Municipality (here after referred as Kintampo; 2012–2013 and 2016–2017), Accra (2016–2017) and Navrongo (2012–2013), in Ghana. Kintampo is a tropical zone in the Brong Ahafo region with all year round high malaria transmission, whereas Navrongo is a savannah zone in the Upper East region where malaria transmission is seasonal and rainfall-dependent ( Owusu-Agyei et al., 2009). Accra lies within the coastal savannah area with low seasonal malaria transmission ( Klinkenberg et al., 2008). Malaria transmission in Accra peaks during the June to August rainy season. The entomological inoculation rate (EIR) in Kintampo, Navrongo and Accra are >250, >100 and <19.2 infectious bites per person per year, respectively ( Kasasa et al., 2013; Klinkenberg et al., 2008). These three regions represent the different malaria transmission intensity zones in the country (Accra<Navrongo<Kintampo), and the study participants have been characterized in greater detail in our previous reports ( Ademolue et al., 2017; Mensah-Brown et al., 2017). Samples were obtained from participants during the rainy seasons at the respective study sites. P. falciparum genomic DNA was analyzed for the prevalence of known antimalarial drug resistance SNPs in pfcrt (K76T), pfmdr1 (N86Y and Y184F), pfdhfr (N51I, C59R and S108N) and pfdhps (A437G and K540E) across the three study sites. Malaria was diagnosed using the first response ®malaria Ag. (HRP2) card test (Premier Medical Corporation, Ltd., Mumbai, India) and confirmed by microscopy. Venous blood samples were obtained and depleted of leucocytes using lymphoprep gradient centrifugation, followed by passage through Plasmodipur filters (EuroProxima, Arnhem, Netherlands), and the resulting infected red blood cells were stored at -20°C until DNA extraction.
Extraction of genomic DNA and nested PCR
Plasmodium gDNA was extracted from the samples using the QIAamp Blood Midi Kit (Qiagen, Manchester, UK) as per manufacturer’s instructions and stored at -20°C. Both outer and nested PCRs were carried out to amplify regions flanking known point mutations in pfcrt (K76T), pfmdr1 (N86Y and Y184F), pfdhfr (N51I, C59R, and S108N) and pfdhps (A437G and K540E) that mediate antimalarial drug resistance. All PCRs were carried out at final volume of 25 µL containing 1X of Maxima Hot Start Green PCR master mix (Thermo Scientific, Waltham, MA, USA) and 250 nM of each of the forward and the reverse primers. Five microlitres of the purified P. falciparum gDNA was used as template in the outer PCR and 1 µL of the resulting products was used as template DNA in the nested PCR. Previously reported primer sets and cycling conditions for both the outer and the nested PCRs were used ( Djimdé et al., 2001a; Duraisingh et al., 1998). Prior to the restriction digest, 5 µL of the nested PCR products were resolved on 2% agarose gel stained with ethidium bromide and images were resolved using the Amersham Imager 600 (General Electric Healthcare Life Sciences, Chicago, IL, USA).
Restriction digestion of nested PCR amplicons
The resulting nested PCR products for each of the four genes containing the SNP alleles of interest were analyzed by restriction fragment length polymorphism (RFLP). Each of the restriction digestion reactions was set at a final volume of 15 µL containing 5 µL of the nested PCR product, 1X FastDigest Green buffer and 0.3 µL of the appropriate restriction enzyme (Thermo Scientific). The restriction enzymes used, incubation temperature, incubation time as well as the expected band sizes for the wild-type and the mutant alleles of the point mutations were as reported in previous studies ( Djimdé et al., 2001a; Duraisingh et al., 1998). Ten microlitres of the restriction digestion fragments were resolved on 2 % agarose gel stained with ethidium bromide and the resulting image resolved with the Amersham Imager 600 (GE, USA). Purified DNA obtained from laboratory strains of P. falciparum (Dd2, 3D7, FCR3, K1, 7G8 and W2) were used as controls for the sensitive and resistant alleles for each gene.
Data analysis
Data was analyzed using the Stata version 14.2 (Texas, USA), and the GraphPad Prism (Version 6.01). Analysis of contingency tables of frequency distribution of the point mutations between the study sites were analyzed by chi-square test. In addition, allele combination frequency distribution of the 2012–2013 isolates were compared to the 2016–2017 isolates utilizing the Fisher exact test for expected lower cell counts taking each marker as independent. All statistical tests were two-tailed and statistical significance was defined at P < 0.05.
Results
Prevalence of alleles in P. falciparum genes that mediate chloroquine and antifolate drug resistance
The prevalence of antimalarial drug resistance alleles in three different transmission zones were determined and compared across sites and sampling time points.
. We did not observe any significant differences in the distribution of isolates harbouring pfcrt K76T, pfmdr1 N86Y or pfmdr1 Y184F point mutations across the three transmission zones (P > 0.05 for all three SNPs), although all the three mutant alleles were found at a higher prevalence in Navrongo (2012–2013) compared to Kintampo (2012–2013 and 2017) and Accra (2016–2017) ( Table 1). The total prevalence of pfcrt 76T (8%) and pfmdr1 86Y (10.2%) mutant alleles were comparable (P = 0.39). Compared to CQ resistance-associated alleles, higher frequencies were observed in the three study sites for all the antifolate drug resistance-associated alleles, except pfdhps K540E ( Table 1 and Dataset 1). The frequency distribution of isolates harbouring the pfdhfr 51I, 59R and 108N mutant alleles were also comparable across the study sites (P > 0.05 for all the three loci). However, the distribution of pfdhps 437 G mutant allele, was significantly different across the study sites (P = 0.01). Pfdhps 437G was significantly higher in Accra (2016–2017) compared to Navrongo (2012–2013) (P = 0.03), Kintampo (2012–2013) (P = 0.004) and Kintampo (2016–2017) (P = 0.004). The frequency of pfdhps 437G in Navrongo (2012–2013), Kintampo (2012–2013) and Kintampo (2016–2017) were comparable (P > 0.05).
Table 1. The prevalence of pfcrt, pfmdr1, pfdhps and pfdhfr mutant allelles in P. falciparum isolates.
| Gene | Amino acid
a
Position |
Amino
Acid b |
Kintampo
(2012-2013) n (%) |
Navrongo
(2012-2013) n (%) |
Accra
(2016-2017) n (%) |
Kintampo
(2016-2017) n (%) |
Total
c
n (%) |
P-value d |
|---|---|---|---|---|---|---|---|---|
| Pfcrt | K76T | K | 148 (86.3) | 37 (88.1) | 64 (88.9) | 50 (98.0) | 299
(91.7) |
0.243 |
| T | 13 (13.7) | 5 (11.9) | 8 (11.1) | 1 (2.0) | 27
(8.3) |
|||
| Pfmdr1 | N86Y | N | 103 (89.6) | 47 (82.5) | 72 (92.3) | 50 (94.3) | 272
(89.8) |
0.166 |
| Y | 12 (10.4) | 10 (17.5) | 6 (7.7) | 3 (5.7) | 31
(10.2) |
|||
| Y184F | Y | 42 (34.1) | 15 (27.8) | 31 (38.8) | 22 (37.9) | 110
(34.9) |
0.574 | |
| F | 81 (65.9) | 39 (72.2) | 49 (61.2) | 36 (62.1) | 205
(65.1) |
|||
| Pfdhfr | N51I | N | 28 (20.6) | 9 (16.7) | 6 (8.0) | 10 (18.9) | 53
(16.6) |
0.124 |
| I | 108 (79.4) | 46 (83.3) | 69 (92.0) | 43 (81.1) | 266
(83.4) |
|||
| C59R | C | 12 (17.9) | 5 (16.7) | 12 (15.4) | 3 (5.8) | 32
(14.1) |
0.256 | |
| R | 55 (82.1) | 25 (83.3) | 66 (84.6) | 49 (94.2) | 195
(85.9) |
|||
| S108N | S | 17 (13.0) | 5 (9.1) | 4 (5.1) | 4 (7.5) | 30
(9.5) |
0.280 | |
| N | 114 (87.0) | 50 (90.9) | 74 (94.9) | 49 (92.5) | 287
(90.5) |
|||
| Pfdhps | A437G | A | 16 (12.7) | 5 (9.1) | 1 (1.3) | 7 (14.0) | 29
(9.4) |
0.031 |
| G | 110 (87.3) | 50 (90.9) | 77 (98.7) | 43 (86.0) | 280
(90.6) |
|||
| K540E | K | 106 (99.1) | 54 (98.2 | 78 (100) | 52 (100) | 290
(99.3) |
- | |
| E | 1 (0.9) | 1 (1.8) | 0 (0) | 0 (0) | 2 (0.7) |
aMutated amino acid depicted in bold, b P-value based on Pearson chi-Square test or Exact chi-square test for categorical variables.
Trends in the prevalence of antimalarial drug resistance markers in the study populations
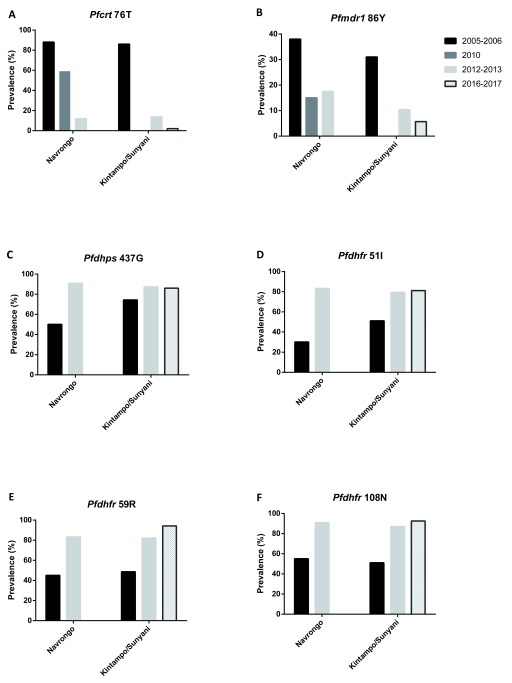
To investigate the dynamics of the drug resistance alleles in the selected areas, we compiled data from previous studies that reported the frequencies of the various mutations in the same or near-by communities. Thus, the current data from Navrongo were compared to previous data from the same area, while data from Kintampo were compared to published data from Sunyani. Kintampo and Sunyani are located in the same region (Brong Ahafo) but approximately 122 Km apart ( Figure 1) Sunyani lies in the Forest Zone whilst Kintampo lies within the Forest Savanah transition zone, however, both sites have similar agricultural practices, housing structure, and land geology, all of which have been reported to influence malaria epidemiology ( Baidjoe et al., 2016; Hu et al., 2016). Generally, a decreasing trend was observed from 2005 to 2017 in the proportions of the alleles associated with CQ resistance in both study sites except for pfmdr1 86 Y in Navrongo ( Figures 2A and B), which decreased from 2005/2006 to 2010 but appeared to plateau between 2012 and 2013. We, however, observed an increasing trend in the proportions of the pyrimethamine and sulfadoxine resistance alleles in pfdhfr and pfdhps respectively at both study sites from 2005 to 2013, with frequencies levelling off subsequently ( Figures 2C–F).
Figure 1. A map of Ghana showing our study sites.
The distance between Sunyani and Kintampo is approximately 122 Km. Housing structures in both localities are largely similar with a slight difference in the vegetation but almost the same geospatial characteristics.
Figure 2. Trends in the prevalence of antimalarial drug resistant alleles from 2005 to 2017.
Summarized data from present study was compared to previous published data from Navrongo and Kintampo/Sunyani, Ghana, for pfmdr1 and pfcrt point mutations ( Duah et al., 2013) and for the antifolate resistance mutations ( Duah et al., 2012). No data was available for the pfcrt 76T and pfmdr1 86Y in 2010 in Kintampo/Sunyani ( Figure 1A–B). Also, no data was available for Navrongo in 2016–2017 in all the analysis. Deep black represents 2005–2006, light black represents 2010, plain grey represents 2012–2013 and crossed grey represents 2016–2017.
Analysis of pfdhfr and pfdhps haplotype combination distributions
We used clinical isolates for which all the pfdhfr and pfdhps SNP alleles of interest were successfully genotyped to survey allele combinations and determine their distribution between study periods. The prevalence of the quadruple allele combination ( I 51 R 59 N 108/ G 437) in Navrongo (2012–2013), Kintampo (2012–2013), Kintampo (2016–2017), and Accra (2016–2017) were 17/25 (68.0), 37/51 (72.6) 43/53 (81.1) and 58/71 (81.7), respectively ( Table 2). The frequency of I 51 R 59 N 108/G 437 was comparable across the study sites (P > 0.05 for all comparisons). Low prevalence (<10%) allele combinations in both pfdhfr and pfdhps were observed for the triple mutant allele combination ( R 59 N 108/ G 437, I 51 R 59/ G 437 and I 51 N 108/ G 437) and these were also comparable across the study sites (P > 0.05 for all haplotypes) ( Table 2).
Table 2. Temporal trends of SP drug resistance haplotypes from 2012 to 2017 by study period.
| Haplotype | Kintampo 2012-2013,
n = 51 (%) |
Navrongo 2012-2013,
n = 25 (%) |
P-value |
|---|---|---|---|
| IRNG | 37 (72.6) | 17 (68.0) | 0.789 |
| RNG | 6 (11.8) | 2 (8.0) | 0.714 |
| IRG | 4 (7.8) | 4 (16.0) | 0.427 |
| ING | 4 (7.8) | 2 (8.0) | 1 |
| Kintampo 2012-2013,
n = 51 (%) |
Kintampo 2016-2017,
n = 53 (%) |
||
| IRNG | 37 (72.6) | 43 (81.1) | 0.356 |
| RNG | 6 (11.8) | 6 (11.3) | 1 |
| IRG | 4 (7.8) | 1 (1.9) | 0.201 |
| ING | 4 (7.8) | 3 (5.7) | 0.713 |
| Kintampo 2016-2017,
n = 53 (%) |
Accra 2016-2017,
n = 71 |
||
| IRNG | 43 (81.1) | 58 (81.7) | 1 |
| RNG | 6 (11.3) | 2 (2.8) | 0.0722 |
| IRG | 1 (1.9) | 1 (1.4) | 1 |
| ING | 3 (5.7) | 10 (14.1) | 0.151 |
Note: Numbers include only isolates that were successfully genotyped for all the four point mutations in pfdhfr and pfdhps. Each haplotype has mutant amino acids shown in bold. aP-value based on Exact chi-square test
Discussion
P. falciparum resistance to antimalarial drugs remains one of the biggest threats to the control and elimination of malaria globally. In Ghana, a change in the use of CQ to ACTs was implemented in 2005 as a result of high rate of malaria treatment failure ( Duah et al., 2007). In this study, we determined the prevalence of alleles associated with CQ and antifolate resistance using clinical isolates from three malaria endemic regions with varying transmission intensities in Ghana. We observed a decreasing prevalence of CQ resistance-associated alleles but an increasing prevalence of SP resistance-associated alleles. The distribution of the alleles across the three study sites were not significant, except for pfdhps 437G which was significantly higher in Accra compared to Navrongo and Kintampo. The frequency of pfdhfr/pfdhps haplotypes in 2012–2013 and 2016–2017 were not significantly different across the three study sites. Both in vitro and molecular surveillance studies have associated CQ resistance mainly with the pfcrt 76T allele, but also with pfmdr1 86Y and 184F alleles. Pfcrt 76T and pfmdr1 86Y mutant alleles have also been reported to decrease P. falciparum susceptibility to amodiaquine but increase parasite sensitivity to dihydroartemisinin, lumefantrine and mefloquine ( Gresty et al., 2014; Veiga et al., 2016). Despite the use of ACTs (artemether-lumefantrine, artesunate-amodiaquine, and dihydroartemisinin-piperaquine) in Ghana since 2005, decreasing prevalence of pfcrt 76T and pfmdr1 86Y mutant alleles were observed in this study when compared to study by Duah and colleagues in 2013 ( Duah et al., 2013). This shows a gradual decline in the frequencies of these alleles since the discontinuation of CQ as an antimalarial in Ghana, this observation is consistent with findings in other malaria endemic populations in east Africa such as Tanzania, Malawi , Kenya and Zambia where artemether lumefantrine is the first-line drug for uncomplicated malaria ( Mohammed et al., 2013; Mwai et al., 2009; Mwanza et al., 2016). A study in Kenya posits that the K76 is preferential selection by Artemeter Lumefantrine(AL) ( Achieng et al., 2015) The fitness cost of harbouring the mutant alleles is thought to select against them in favour of the non-resistant background alleles ( Babiker et al., 2009; Kiarie et al., 2015). Unlike pfcrt 76T and pfmdr1 86Y, the prevalence of pfmdr1 184F mutant allele (65%) appears to have not varied so much from 2005 to 2017 when compared to the 43% to 69% prevalence reported from 2005 to 2010 ( Duah et al., 2013). Contrary to this observation, a study in Tanzania reported an increasing prevalence of pfmdr1 N86 and 184F following the introduction of artemether-lumefantrine ( Thomsen et al., 2011). Notably, parasites that have a combination of pfmdr1 mutant alleles (N86, 184F and D1246) are reported to have reduced sensitivity to artemether-lumefantrine treatment ( Baliraine & Rosenthal, 2011; Happi et al., 2009; Kavishe et al., 2014). Other studies have also linked duplication of pfmdr1 to resistance to partner drugs of ACTs (Rodrigues, Henriques et ( Borges et al., 2011; Rodrigues et al., 2010).
Although high frequencies of the point mutations implicated in the development of resistance to antifolates were reported before the change in malaria treatment guidelines in 2005 in Ghana, the drug is still in use for intermittent preventive treatment of malaria in pregnancy (IPTp) and also recommended for seasonal malaria chemotherapy (SMC) among children under five in areas of high but seasonal malaria transmission. The percentages of the pfdhfr 51I (81%), 59R (82%), 108N (88%) and pfdhps 437G (88%) mutant alleles reported in this study are relatively higher when compared to the 71%, 42%, 64% and 80% prevalence reported in a recent study in a neighbouring country, Burkina Faso, using samples obtained in 2010 ( Cisse et al., 2017). SP was used as a second-line treatment for uncomplicated malaria in both countries until 2005 when its usage was restricted for IPTp ( Koram et al., 2005; Tahita et al., 2015). In Burkina Faso, resource persons are engaged at the community level to promote IPTp uptake and referrals to antenatal clinics (ANCs) whereas IPTp in Ghana is taken at the ANCs and health care centres ( Gies et al., 2009; Hill et al., 2013). With the aforementioned strategies there is likely increased compliance in Burkina Faso compared to Ghana and this may explain the low resistance in the former. Furthermore, since drug resistance evolution is spatiotemporal the differences in periods of sampling could also account for the differences observed. The high prevalence may be due to SP intervention in groups such as pregnant women and young children acting as reservoirs of infections with resistance alleles as a direct consequence of continuous use of SP in IPTp and SMC campaigns that fuel transmission of these alleles in the general population. Another important factor may be the unauthorized use of SP for self-medication as it is readily available at health centres and pharmacy shops in the study areas ( Abuaku et al., 2004), particularly because it is a single dose drug with very minimal to no adverse reactions. Co-trimoxazole is used in Ghana ( Fadeyi et al., 2015), however, there is limited data on its usage in the three study sites. Besides the prevalence of HIV in Ghana is only 3 % ( Ghana AIDs Commission, 2017) and therefore the use of antifolate drugs such as cotrimazole for the management of opportunistic infections is not as widespread as the use of antifolate antimalarial drugs. Higher SP treatment failure has been correlated with the pfdhfr/pfdhps quintuple ( pfdhfr/pfdhps I 51R 59N 108/G 437E 540) haplotypes ( Kublin et al., 2002; Triglia et al., 1997). Parasites habouring pfdhfr/pfdhps I 51R 59N 108/G 437 and I 51R 59N 108/G 437E 540 haplotypes have been described as “partial” and “full” SP resistance, respectively ( Naidoo & Roper, 2013). In this study, no isolate was observed to carry the full resistance allele combination. This is consistent with other studies which show that though the variant quintuple mutant allele is almost fixed in east Africa, it is largely absent from West Africa ( Naidoo & Roper, 2013; Roper et al., 2004). However, our data show an increased prevalence of parasite isolates that harbour other SP resistance haplotype in the 2012–2013 and 2016–2017 study periods. This suggests that selection by SP in our study settings is still continuing. These findings are corroborated by previous studies in Ghana (25%–69%) ( Duah et al., 2012), Cameroon (47%) ( Chauvin et al., 2015) and Equatorial Guinea (54%) ( Berzosa et al., 2017), which suggests a high prevalence of variant quadruple mutant alleles in West to Central Africa. The pfdhps K540E point mutation, which is a surrogate for high level resistance to SP was found in a very low proportion of the clinical isolates (1%) in this study. This is consistent with reports in other countries in the sub-region including Mali and Burkina Faso ( Cisse et al., 2017; Coulibaly et al., 2014), and suggests that if selection is increased it might eventually lead to a higher level of SP resistance in West Africa.
The study indicates that CQ sensitive parasites have again become more common since the replacement of CQ with a variety of ACTs as first-line treatments of uncomplicated malaria in Ghana. This notwithstanding, our findings also show that between 5% to 14% of clinical infections may still carry CQ resistant parasites, which suggest that ACT partner drugs such as AQ that are widely used in Ghana may still be maintaining significant selection pressure on the pfcrt locus. In addition, the increasing prevalence of the pfdhfr/pfdhps partial SP resistance haplotypes could result in the fixation of these alleles within the parasite population. The continuous use of SP for IPTp and SMC may result in emergence of the “full” SP resistance haplotype and compromise the use of SP IPTp and SMC are the two significant sources of SP drug pressure on the parasite population in pregnant women and young children, respectively. Recent studies have shown that these interventions have contributed to reduction in maternal and child morbidity and mortality ( Coldiron et al., 2017). Undoubtedly, these interventions are critical ( York, 2017), but could easily be undermined by rising resistance in these populations. Therefore, it is very important to closely monitor the prevalence of molecular markers of resistance associated with antifolate antimalarial drugs to guide policies on the continuous use of these drugs in Ghana and other African countries. There are probably other factors that contribute to the evolution of resistance markers as SP has been shown to be efficacious even in the face of fixation of SP resistant alleles ( Iriemenam et al., 2012).
Conclusion
This study reports an increasing prevalence of CQ sensitive clinical isolates after 12 years of CQ withdrawal at three different study sites that capture the eco-epidemiology of malaria in Ghana. The prevalence of the antifolate drug resistant alleles remain relatively high across the study sites. Besides, there is an increasing trend in the frequency of SP-resistance associated alleles at all sites. Taken together, these observations point to the need for a robust antimalarial drug discovery strategy to provide a vast array of alternatives for chemotherapy in readiness for the likelihood of future poor parasite response to the use of SP for prevention of malaria in pregnant women and for SMC in children. However, it is pre-mature to recommend the discontinuation of SP use due to the high prevalence of antifolate drug resistance alleles since the drug can be efficacious where there is fixation of these alleles.
Data availability
The data supporting this article is available online at Open Science Framework: Dataset 1. Prevalence of chloroquine and antifolate drug resistance alleles in Plasmodium falciparum clinical isolates from three areas in Ghana. http://dx.doi.org/10.17605/OSF.IO/N2GZF ( Abugri et al., 2018) under a CC0 1.0 Universal licence.
Funding Statement
This work was supported by the African Academy of Science through the Developing Excellence in Leadership, Training and Science (DELTAS) programme [DEL-15-007] (GA). This work was also supported by the Wellcome Trust [107755/Z/15/Z] (GA), and World Bank Group [ACE02: WACCBIP] (GA).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- Abuaku BK, Koram KA, Binka FN: Antimalarial drug use among caregivers in Ghana. Afr Health Sci. 2004;4(3):171–7. [PMC free article] [PubMed] [Google Scholar]
- Abugri J, Ansah F, Asante KP, et al. : Raw_data_for_antimalarial_drug_resistant_alleles_Navrongo_Kintampo_Accra.2018. 10.17605/OSF.IO/N2GZF [DOI] [Google Scholar]
- Achieng AO, Muiruri P, Ingasia LA, et al. : Temporal trends in prevalence of Plasmodium falciparum molecular markers selected for by artemether-lumefantrine treatment in pre-ACT and post-ACT parasites in western Kenya. Int J Parasitol Drugs Drug Resist. 2015;5(3):92–9. 10.1016/j.ijpddr.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ademolue TW, Aniweh Y, Kusi KA, et al. : Patterns of inflammatory responses and parasite tolerance vary with malaria transmission intensity. Malar J. 2017;16(1):145. 10.1186/s12936-017-1796-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker HA, Hastings IM, Swedberg G: Impaired fitness of drug-resistant malaria parasites: evidence and implication on drug-deployment policies. Expert Rev Anti Infect Ther. 2009;7(5):581–93. 10.1586/eri.09.29 [DOI] [PubMed] [Google Scholar]
- Babiker HA, Pringle SJ, Abdel-Muhsin A, et al. : High-level chloroquine resistance in Sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance Gene pfmdr1. J Infect Dis. 2001;183(10):1535–8. 10.1086/320195 [DOI] [PubMed] [Google Scholar]
- Baidjoe AY, Stevenson J, Knight P, et al. : Factors associated with high heterogeneity of malaria at fine spatial scale in the Western Kenyan highlands. Malaria J. 2016;15(1):307. 10.1186/s12936-016-1362-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliraine FN, Rosenthal PJ: Prolonged selection of pfmdr1 polymorphisms after treatment of falciparum malaria with artemether-lumefantrine in Uganda. J Infect Dis. 2011;204(7):1120–1124. 10.1093/infdis/jir486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzosa P, Esteban-Cantos A, García L, et al. : Profile of molecular mutations in pfdhfr, pfdhps, pfmdr1, and pfcrt genes of Plasmodium falciparum related to resistance to different anti-malarial drugs in the Bata District (Equatorial Guinea). Malar J. 2017;16(1):28. 10.1186/s12936-016-1672-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges S, Cravo P, Creasey A, et al. : Genomewide scan reveals amplification of mdr1 as a common denominator of resistance to mefloquine, lumefantrine, and artemisinin in Plasmodium chabaudi malaria parasites. Antimicrob Agents Chemother. 2011;55(10):4858–65. 10.1128/AAC.01748-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin P, Menard S, Iriart X, et al. : Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaoundé, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles. J Antimicrob Chemother. 2015;70(9):2566–71. 10.1093/jac/dkv160 [DOI] [PubMed] [Google Scholar]
- Cisse M, Awandare GA, Soulama A, et al. : Recent uptake of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine is associated with increased prevalence of Pfdhfr mutations in Bobo-Dioulasso, Burkina Faso. Malar J. 2017;16(1):38. 10.1186/s12936-017-1695-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldiron ME, Von Seidlein L, Grais RF: Seasonal malaria chemoprevention: successes and missed opportunities. Malar J. 2017;16(1):481. 10.1186/s12936-017-2132-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly SO, Kayentao K, Taylor S, et al. : Parasite clearance following treatment with sulphadoxine-pyrimethamine for intermittent preventive treatment in Burkina-Faso and Mali: 42-day in vivo follow-up study. Malar J. 2014;13:41. 10.1186/1475-2875-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman AF, Morry MJ, Biggs BA, et al. : Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988;85(23):9109–13. 10.1073/pnas.85.23.9109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Mharakurwa S, Ndiaye D, et al. : Antimalarial Drug Resistance: Literature Review and Activities and Findings of the ICEMR Network. Am J Trop Med Hyg. 2015;93(3 Suppl):57–68. 10.4269/ajtmh.15-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djimdé A, Doumbo OK, Cortese JF, et al. : A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001a;344(4):257–63. 10.1056/NEJM200101253440403 [DOI] [PubMed] [Google Scholar]
- Djimdé A, Doumbo OK, Steketee RW, et al. : Application of a molecular marker for surveillance of chloroquine-resistant falciparum malaria. Lancet. 2001b;358(9285):890–1. 10.1016/S0140-6736(01)06040-8 [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, et al. : Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. 10.1056/NEJMoa0808859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah NO, Matrevi SA, de Souza DK, et al. : Increased pfmdr1 gene copy number and the decline in pfcrt and pfmdr1 resistance alleles in Ghanaian Plasmodium falciparum isolates after the change of anti-malarial drug treatment policy. Malar J. 2013;12:377. 10.1186/1475-2875-12-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah NO, Quashie NB, Abuaku BK, et al. : Surveillance of molecular markers of Plasmodium falciparum resistance to sulphadoxine-pyrimethamine 5 years after the change of malaria treatment policy in Ghana. Am J Trop Med Hyg. 2012;87(6):996–1003. 10.4269/ajtmh.2012.12-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah NO, Wilson MD, Ghansah A, et al. : Mutations in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance genes, and treatment outcomes in Ghanaian children with uncomplicated malaria. J Trop Pediatr. 2007;53(1):27–31. 10.1093/tropej/fml076 [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Cowman AF: Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Trop. 2005;94(3):181–90. 10.1016/j.actatropica.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Curtis J, Warhurst DC: Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes by PCR and restriction digestion. Exp Parasitol. 1998;89(1):1–8. 10.1006/expr.1998.4274 [DOI] [PubMed] [Google Scholar]
- Fadeyi I, Lalani M, Mailk N, et al. : Quality of the antibiotics--amoxicillin and co-trimoxazole from Ghana, Nigeria, and the United Kingdom. Am J Trop Med Hyg. 2015;92(6 Suppl):87–94. 10.4269/ajtmh.14-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock DA, Nomura T, Talley AK, et al. : Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6(4):861–71. 10.1016/S1097-2765(05)00077-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghana AIDS Commission: Summary of the 2017 HIV Sentinel Survey Report.2017; Accessed on 8th November, 2018. Reference Source [Google Scholar]
- Gies S, Coulibaly SO, Ouattara FT, et al. : Individual efficacy of intermittent preventive treatment with sulfadoxine-pyrimethamine in primi- and secundigravidae in rural Burkina Faso: impact on parasitaemia, anaemia and birth weight. Trop Med Int Health. 2009;14(2):174–82. 10.1111/j.1365-3156.2008.02215.x [DOI] [PubMed] [Google Scholar]
- Gosling RD, Okell L, Mosha J, et al. : The role of antimalarial treatment in the elimination of malaria. Clin Microbiol Infect. 2011;17(11):1617–23. 10.1111/j.1469-0691.2011.03660.x [DOI] [PubMed] [Google Scholar]
- Greenwood B: The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am J Trop Med Hyg. 2004;70(1):1–7. [PubMed] [Google Scholar]
- Gresty KJ, Gray KA, Bobogare A, et al. : Genetic mutations in pfcrt and pfmdr1 at the time of artemisinin combination therapy introduction in South Pacific islands of Vanuatu and Solomon Islands. Malar J. 2014;13:406. 10.1186/1475-2875-13-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happi CT, Gbotosho GO, Folarin OA, et al. : Selection of Plasmodium falciparum multidrug resistance gene 1 alleles in asexual stages and gametocytes by artemether-lumefantrine in Nigerian children with uncomplicated falciparum malaria. Antimicrob Agents Chemother. 2009;53(3):888–895. 10.1128/AAC.00968-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Hoyt J, van Eijk AM, et al. : Factors affecting the delivery, access, and use of interventions to prevent malaria in pregnancy in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med. 2013;10(7):e1001488. 10.1371/journal.pmed.1001488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zhou G, Ruan Y, et al. : Seasonal dynamics and microgeographical spatial heterogeneity of malaria along the China-Myanmar border. Acta Trop. 2016;157:12–19. 10.1016/j.actatropica.2016.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriemenam NC, Shah M, Gatei W, et al. : Temporal trends of sulphadoxine-pyrimethamine (SP) drug-resistance molecular markers in Plasmodium falciparum parasites from pregnant women in western Kenya. Malar J. 2012;11:134. 10.1186/1475-2875-11-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasasa S, Asoala V, Gosoniu L, et al. : Spatio-temporal malaria transmission patterns in Navrongo demographic surveillance site, northern Ghana. Malar J. 2013;12:63. 10.1186/1475-2875-12-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavishe RA, Paulo P, Kaaya RD, et al. : Surveillance of artemether-lumefantrine associated Plasmodium falciparum multidrug resistance protein-1 gene polymorphisms in Tanzania. Malar J. 2014;13:264. 10.1186/1475-2875-13-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie WC, Wangai L, Agola E, et al. : Chloroquine sensitivity: diminished prevalence of chloroquine-resistant gene marker pfcrt-76 13 years after cessation of chloroquine use in Msambweni, Kenya. Malar J. 2015;14:328. 10.1186/s12936-015-0850-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg E, McCall P, Wilson MD, et al. : Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar J. 2008;7:151. 10.1186/1475-2875-7-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koram KA, Abuaku B, Duah N, et al. : Comparative efficacy of antimalarial drugs including ACTs in the treatment of uncomplicated malaria among children under 5 years in Ghana. Acta Trop. 2005;95(3):194–203. 10.1016/j.actatropica.2005.06.018 [DOI] [PubMed] [Google Scholar]
- Kublin JG, Dzinjalamala FK, Kamwendo DD, et al. : Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185(3):380–8. 10.1086/338566 [DOI] [PubMed] [Google Scholar]
- Laufer MK, Thesing PC, Eddington ND, et al. : Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355(19):1959–66. 10.1056/NEJMoa062032 [DOI] [PubMed] [Google Scholar]
- Lin JT, Juliano JJ, Wongsrichanalai C: Drug-Resistant Malaria: The Era of ACT. Curr Infect Dis Rep. 2010;12(3):165–73. 10.1007/s11908-010-0099-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah-Brown HE, Abugri J, Asante KP, et al. : Assessing the impact of differences in malaria transmission intensity on clinical and haematological indices in children with malaria. Malar J. 2017;16(1):96. 10.1186/s12936-017-1745-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita T, Ohashi J, Venkatesan M, et al. : Ordered accumulation of mutations conferring resistance to sulfadoxine-pyrimethamine in the Plasmodium falciparum parasite. J Infect Dis. 2014;209(1):130–9. 10.1093/infdis/jit415 [DOI] [PubMed] [Google Scholar]
- Mohammed A, Ndaro A, Kalinga A, et al. : Trends in chloroquine resistance marker, Pfcrt-K76T mutation ten years after chloroquine withdrawal in Tanzania. Malar J. 2013;12:415. 10.1186/1475-2875-12-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwai L, Ochong E, Abdirahman A, et al. : Chloroquine resistance before and after its withdrawal in Kenya. Malar J. 2009;8:106. 10.1186/1475-2875-8-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanza S, Joshi S, Nambozi M, et al. : The return of chloroquine-susceptible Plasmodium falciparum malaria in Zambia. Malar J. 2016;15(1):584. 10.1186/s12936-016-1637-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo I, Roper C: Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitol. 2013;29(10):505–15. 10.1016/j.pt.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Omar SA, Adagu IS, Warhurst DC: Can pretreatment screening for dhps and dhfr point mutations in Plasmodium falciparum infections be used to predict sulfadoxine-pyrimethamine treatment failure? Trans R Soc Trop Med Hyg. 2001;95(3):315–9. 10.1016/S0035-9203(01)90250-0 [DOI] [PubMed] [Google Scholar]
- Owusu-Agyei S, Asante KP, Adjuik M, et al. : Epidemiology of malaria in the forest-savanna transitional zone of Ghana. Malar J. 2009;8:220. 10.1186/1475-2875-8-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowe CV, Cortese JF, Djimde A, et al. : Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J Infect Dis. 1997;176(6):1590–6. 10.1086/514159 [DOI] [PubMed] [Google Scholar]
- Rodrigues LA, Henriques G, Borges ST, et al. : Experimental evolution of resistance to artemisinin combination therapy results in amplification of the mdr1 gene in a rodent malaria parasite. PLoS One. 2010;5(7):e11593. 10.1371/journal.pone.0011593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper C, Pearce R, Nair S, et al. : Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305(5687):1124. 10.1126/science.1098876 [DOI] [PubMed] [Google Scholar]
- Schlitzer M: Malaria chemotherapeutics part I: History of antimalarial drug development, currently used therapeutics, and drugs in clinical development. ChemMedChem. 2007;2(7):944–86. 10.1002/cmdc.200600240 [DOI] [PubMed] [Google Scholar]
- Schumacher RF, Spinelli E: Malaria in children. Mediterr J Hematol Infect Dis. 2012;4(1):e2012073. 10.4084/MJHID.2012.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley CH, Hyde JE, Sims PF, et al. : Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 2001;17(12):582–8. 10.1016/S1471-4922(01)02085-2 [DOI] [PubMed] [Google Scholar]
- Sidhu AB, Verdier-pinard D, Fidock DA: Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 2002;298(5591):210–3. 10.1126/science.1074045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahita MC, Tinto H, Erhart A, et al. : Prevalence of the dhfr and dhps Mutations among Pregnant Women in Rural Burkina Faso Five Years after the Introduction of Intermittent Preventive Treatment with Sulfadoxine-Pyrimethamine. PLoS One. 2015;10(9):e0137440. 10.1371/journal.pone.0137440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen TT, Ishengoma DS, Mmbando BP, et al. : Prevalence of single nucleotide polymorphisms in the Plasmodium falciparum multidrug resistance gene ( Pfmdr-1) in Korogwe District in Tanzania before and after introduction of artemisinin-based combination therapy. Am J Trop Med Hyg. 2011;85(6):979–983. 10.4269/ajtmh.2011.11-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T, Menting JG, Wilson C, et al. : Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1997;94(25):13944–9. 10.1073/pnas.94.25.13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga MI, Dhingra SK, Henrich PP, et al. : Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat Commun. 2016;7: 11553. 10.1038/ncomms11553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Sims PF, Hyde JE: A modified in vitro sulfadoxine susceptibility assay for Plasmodium falciparum suitable for investigating Fansidar resistance. Parasitology. 1997;115(Pt 3):223–30. 10.1017/S0031182097001431 [DOI] [PubMed] [Google Scholar]
- WHO: World malaria report 2016. Geneva: World Health Organization;2016. Reference Source [Google Scholar]
- WHO: World malaria report 2017. Geneva: World Health Organization;2017. Reference Source [Google Scholar]
- York A: Seasonal malaria chemoprevention in the Sahel. Lancet Infect Dis. 2017;17(6):588. 10.1016/S1473-3099(17)30255-4 [DOI] [PubMed] [Google Scholar]
- Young MD, Contacos PG, Stitcher JE, et al. : Drug Resistance in Plasmodium Falciparum from Thailand. Am J Trop Med Hyg. 1963;12:305–14. 10.4269/ajtmh.1963.12.305 [DOI] [PubMed] [Google Scholar]