Abstract
Background: The emergence and spread of resistance in Plasmodium falciparum to chloroquine (CQ) and the antifolate drug sulfadoxine-pyrimethamine (SP) necessitated the change from CQ to artemisinin-based combination therapies (ACTs) as first-line drug for the management of uncomplicated malaria in Ghana in 2005.
Methods: To examine the prevalence of molecular markers associated with CQ and antifolate drug resistance in Ghana, we genotyped single nucleotide polymorphisms (SNPs) in the P. falciparum chloroquine resistance transporter ( pfcrt, PF3D7_0709000), multidrug resistance ( pfmdr1, PF3D7_0523000), bifunctional dihydrofolate reductase-thymidylate synthase ( pfdhfr, PF3D7_0417200) and dihydropteroate synthase ( pfdhps, PF3D7_0810800) genes in children with malaria reporting to hospitals in three different epidemiological areas of Ghana (Accra, Kintampo and Navrongo) between 2012 and 2017.
Results: The overall prevalence of the CQ resistance-associated pfcrt 76T allele was 8%, whereas pfmdr1 86Y and 184F alleles were present in 10% and 65% of infections respectively. Most of the isolates harboured the antifolate resistance-associated pfdhfr 51I, 59R and 108N alleles, including 68% of them with the triple mutant pfdhfr I 51 R 59 N 108 combination. Pfdhps 437G and 540E were detected in 90.6% and 0.7% of infections, respectively. We observed no significant difference across the three study sites for all the polymorphisms except for pfdhps 437G , which was more common in Accra than at the other sites. Across both pfdhfr and pfdhps genes, a large proportion (61%) of the isolates harboured the quadruple mutant combination ( I 51 R 59 N 108/ G 437).
Conclusion: Comparison of the present results to previously published data shows a significant decrease in the prevalence of CQ resistance alleles during the 12 years after CQ withdrawal, but an increase in the alleles that mediate SP resistance, which could be due to the continuous use of antifolate drugs for prophylaxis.
Keywords: Drug resistance, Malaria, Antifolates, Chloroquine, Plasmodium falciparum
Introduction
Malaria remains a major global health concern especially in Sub Saharan Africa. P. falciparum malaria is considered the most severe and also the leading cause of morbidity and mortality, especially among children under five years ( Schumacher & Spinelli, 2012). In 2016 a global estimate of 216 million malaria cases was reported, which led to about 445,000 deaths ( WHO, 2017). The global malaria mortality rate, however, has reduced by 29% since the year 2010, as a result of increased preventive and control measures ( WHO, 2016).
The use of antimalarial drugs for malaria treatment and prevention has played an integral role in the control of the disease over the decades ( Cui et al., 2015; Gosling et al., 2011; Greenwood, 2004; Schlitzer, 2007). Unfortunately, the emergence and the spread of drug resistant P. falciparum strains militated against the use of antimalarial drugs for the containment of the disease ( Lin et al., 2010). P. falciparum chloroquine (CQ) resistant strains were first reported in the 1950s in Southeast Asia along the Cambodia–Thailand border ( Young et al., 1963) and later reported in other countries globally. Currently, the parasite has been reported to have developed resistance to most available antimalarial monotherapies and this is exhibited by reduced parasite clearance rate and/or treatment failure ( Dondorp et al., 2009). ACTs are now the frontline drugs for treating uncomplicated P. falciparum malaria in almost all countries that are endemic with malaria, including Ghana ( WHO, 2016).
Point mutations in specific genes in the parasite genome are implicated in resistance to specific antimalarial drugs ( Cui et al., 2015; Fidock et al., 2000; Sidhu et al., 2002). A point mutation in the P. falciparum chloroquine resistance transporter gene ( pfcrt, PF3D7_0709000 ) that replaces lysine with threonine at codon 76 had become a common single nucleotide polymorphism (SNP) allele in parasite populations as it is a critical mediator of resistance to CQ ( Babiker et al., 2001). In addition, mutations in the P. falciparum multidrug resistance gene 1 ( pfmdr1, PF3D7_0523000) that result in amino acid substitutions at positions N86Y and Y184F have been reported to confer parasite resistance to CQ, amodiaquine (AQ) and lumefantrine (L) ( Duraisingh & Cowman, 2005). These mutations are believed to interfere with heme polymerization by preventing the accumulation of active drug within the food vacuole ( Djimdé et al., 2001b).
On the other hand, sulfadoxine-pyrimethamine (SP) resistance has also been linked to point mutations in the bifunctional dihydrofolate reductase-thymidylate synthase ( pfdhfr, PF3D7_0417200) and dihydropteroate synthase ( pfdhps, PF3D7_0810800) genes. Resistance to antifolate drugs such as SP is known to be mediated by basal point mutations in these genes that result in amino acid substitutions at positions S108N and A437G in pfdhfr and pfdhps proteins respectively. Overall, studies have shown that additional point mutations in these drug resistant genes on top of the basal mutation makes parasites more refractory to the drug ( Mita et al., 2014), and correlates with increased treatment failure ( Plowe et al., 1997; Sibley et al., 2001). Therefore, parasites harbouring haplotypes that include the different SNP alleles in combination have been shown to confer higher resistance to the specific drugs. In this regard, the combined quintuple mutant haplotype ( pfdhfr N 108 I 51 R 59 + pfdhps G 437 E 540) has been correlated with high SP treatment failure in East Africa ( Kublin et al., 2002; Omar et al., 2001).
In Ghana, prior to the withdrawal of CQ a prevalence range of between 46%–98% of the mutant pfcrt 76T was reported across five sentinel sites ( Duah et al., 2007). Interestingly, studies in other settings have shown that the replacement of CQ with ACTs resulted in a decline in the frequency of the mutant alleles and concomitant restoration of CQ susceptibility ( Laufer et al., 2006; Mwai et al., 2009). In a study that was conducted in Tanzania, more than 90% recovery of the sensitive pfcrt K76 allele was reported after 10 years of CQ use being officially discontinued ( Mohammed et al., 2013).
This study sought to ascertain the population trends in the prevalence of known drug-resistance-related point mutations in pfcrt, pfmdr1, pfdhfr and pfdhps in clinical isolates from three different malaria-endemic areas in Ghana a decade following the introduction of ACTs.
Methods
Ethical consideration
This study was approved by the Ethics Committees of the Ghana Health Service (GHS-ERC:12/05/12), the Kintampo Health Research Centre (KHRCIEC/FEA/2011-13), the Navrongo Health Research Centre (NHRC-IRB135/08/2012) and the Noguchi Memorial Institute for Medical Research (NMIMR) (NMIMR-IRB CPN 004/11-12). Informed consent of parents or guardians for all participants was obtained. An additional assent was also obtained from children aged 10–14 years prior to recruitment.
Study sites and sample collection
Parasite isolates were obtained from children aged 2–14 years, diagnosed with malaria at Municipal hospitals in Kintampo North Municipality (here after referred as Kintampo; 2012–2013 and 2016–2017), Accra (2016–2017) and Navrongo (2012–2013), in Ghana. Kintampo is a tropical zone in the Brong Ahafo region with all year round high malaria transmission, whereas Navrongo is a savannah zone in the Upper East region where malaria transmission is seasonal and rainfall-dependent ( Owusu-Agyei et al., 2009) and Accra lies within the coastal savannah area with low seasonal malaria transmission ( Klinkenberg et al., 2008). These three regions represent the different malaria transmission intensity zones in the country (Accra<Navrongo<Kintampo), and the study participants have been characterized in greater detail in our previous reports ( Ademolue et al., 2017; Mensah-Brown et al., 2017). P. falciparum genomic DNA was analyzed for the prevalence of known antimalarial drug resistance-associated single nucleotide polymorphisms (SNPs) in pfcrt (K76T), pfmdr1 (N86Y and Y184F), pfdhfr (N51I, C59R and S108N) and pfdhps (A437G and K540E) across the three study sites. Malaria was diagnosed using the first response ®malaria Ag. (HRP2) card test (Premier Medical Corporation, Ltd., Mumbai, India) and confirmed by microscopy. Venous blood samples were obtained and depleted of leucocytes using lymphoprep gradient centrifugation, followed by passage through Plasmodipur filters (EuroProxima, Arnhem, Netherlands), the resulting infected red blood cells were stored at -20°C until DNA extraction.
Extraction of genomic DNA and nested PCR
Plasmodium gDNA was extracted from the samples using the QIAamp Blood Midi Kit (Qiagen, Manchester, UK) as per manufacturer’s instructions and stored at -20°C. Both outer and nested PCRs were carried out to amplify regions flanking known point mutations in pfcrt (K76T), pfmdr1 (N86Y and Y184F), pfdhfr (N51I, C59R, and S108N) and pfdhps (A437G and K540E) that mediate antimalarial drug resistance. All PCRs were carried out at final volume of 25 µL containing 1X of Maxima Hot Start Green PCR master mix (Thermo Scientific, Waltham, MA, USA) and 250 nM of each of the forward and the reverse primers. Five microlitres of the purified P. falciparum gDNA was used as template in the outer PCR and 1 µL of the resulting products was used as template DNA in the nested PCR. Previously reported primer sets and cycling conditions for both the outer and the nested PCRs were used ( Djimdé et al., 2001a; Duraisingh et al., 1998). Prior to the restriction digest, 5 µL of the nested PCR products were resolved on 2% agarose gel stained with ethidium bromide and images were resolved using the Amersham Imager 600 (General Electric Healthcare Life Sciences, Chicago, IL, USA).
Restriction digestion of nested PCR amplicons
The resulting nested PCR products for each of the four genes containing the SNP alleles of interest were analyzed by restriction fragment length polymorphism (RFLP). Each of the restriction digestion reactions was set at a final volume of 15 µL containing 5 µL of the nested PCR product, 1X FastDigest Green buffer and 0.3 µL of the appropriate restriction enzyme (Thermo Scientific). The restriction enzymes used, incubation temperature, incubation time as well as the expected band sizes for the wild-type and the mutant alleles of the point mutations were as reported in previous studies ( Djimdé et al., 2001a; Duraisingh et al., 1998). Ten microlitres of the restriction digestion fragments were resolved on 2 % agarose gel stained with ethidium bromide and the resulting image resolved with the Amersham Imager 600 (GE, USA). Purified DNA obtained from laboratory strains of P. falciparum (Dd2, 3D7, FCR3, K1, 7G8 and W2) were used as controls for the sensitive and resistant alleles for each gene.
Data analysis
Data was analyzed using the Stata version 14.2 (Texas, USA), and the GraphPad Prism (Version 6.01). Analysis of contingency tables of frequency distribution of the point mutations between the study sites were analyzed by chi-square test. In addition, allele combination frequency distribution of the pooled 2012–2013 isolates was compared to the pooled 2016–2017 isolates utilizing the Fisher exact test for expected lower cell counts taking each marker as independent. All statistical tests were two-tailed and statistical significance was defined at P < 0.05.
Results
Prevalence of alleles in P. falciparum genes that mediate chloroquine and antifolate drug resistance
To survey known point mutations implicated in CQ and SP resistance we carried out RFLP using appropriate primer sets. We did not observe any significant differences in the distribution of isolates harbouring pfcrt K76T, pfmdr1 N86Y or pfmdr1 Y184F point mutations associated with CQ and AQ resistance across the three transmission zones (P > 0.05 for all three SNPs), although all the three mutant alleles were found at a higher prevalence in Navrongo compared to Kintampo and Accra ( Table 1). The total prevalence of pfcrt 76T (8%) and pfmdr1 86Y (10%) mutant alleles were comparable (P = 0.39). Compared to CQ resistance-associated alleles, higher frequencies were observed in the three study sites for all the antifolate drug resistance-associated alleles, except pfdhps K540E ( Table 1 and Dataset 1). The frequency distribution of isolates harbouring the pfdhfr 51I, 59R and 108N mutant alleles were comparable across the study sites (P > 0.05 for all the three loci). The distribution of pfdhps 437 G, which is also associated with sulfadoxine resistance, was significantly different across the study sites (P=0.01).
Table 1. Frequency distribution of pfcrt, pfmdr1, pfdhps and pfdhfr SNPs in P. falciparum isolates.
| Gene | Amino
acid position |
Amino
acid a |
Kintampo | Navrongo | Accra | Total | P-value b |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||||
| Pfcrt | K76T | K | 198 (93.0) | 37 (88.1) | 64 (88.9) | 299 (91.7) | 0.321 |
| T | 14 (7.0) | 5 (11.9) | 8 (11.1) | 27 (8.3) | |||
| Pfmdr1 | N86Y | N | 153 (91) | 47 (82.5) | 72 (92.3) | 272 (89.8) | 0.124 |
| Y | 15 (9.0) | 10 (17.5) | 6 (7.7) | 31 (10.2) | |||
| Y184F | Y | 64 (35.0) | 15 (27.8) | 31 (38.8) | 110 (34.9) | 0.418 | |
| F | 117(65.0) | 39 (72.2) | 49 (61.2) | 205 (65.1) | |||
| Pfdhfr | N51I | N | 38 (20.0) | 9 (16.7) | 6 (8.0) | 53 (16.6) | 0.058 |
| I | 151 (80.0) | 46 (83.3) | 69 (92.0) | 266 (83.4) | |||
| C59R | C | 15 (13.0) | 5 (16.7) | 12 (15.4) | 32 (14.1) | 0.783 | |
| R | 104 (87.0) | 25 (83.3) | 66 (84.6) | 195 (85.9) | |||
| S108N | S | 21 (11.0) | 5 (9.1) | 4 (5.1) | 30 (9.5) | 0.281 | |
| N | 163(89.0) | 50 (90.9) | 74 (94.9) | 287 (90.5) | |||
| Pfdhps | A437G | A | 23 (13.0) | 5 (9.1) | 1 (1.3) | 29 (9.4) | 0.0112 |
| G | 153 (87.0) | 50 (90.9) | 77 (98.7) | 280 (90.6) | |||
| K540E | K | 158 (99.9) | 54 (98.2 | 78 (100) | 290 (99.3) | - | |
| E | 1 (0.1) | 1 (1.8) | 0 (0) | 2 (0.7) |
aMutated amino acid depicted in bold, bP-value based on Pearson chi-Square test or Exact chi-square test for categorical variables.
Trends in the prevalence of antimalarial drug resistance markers in the study populations
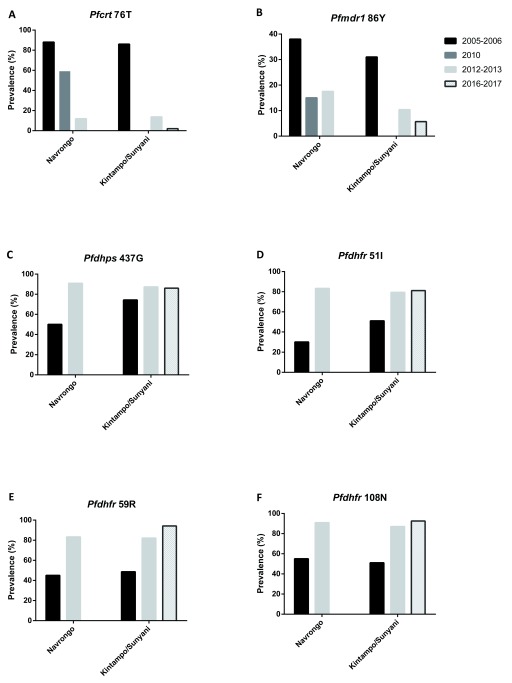
To investigate the dynamics of the drug resistance alleles in the selected areas, we compiled data from previous studies that reported the frequencies of the various mutations in the same or near-by communities. Thus, the current data from Navrongo were compared to previous data from the same area, while data from Kintampo were compared to published data from Sunyani, which is a town located in the same region (Brong Ahafo) as Kintampo. Generally, a decreasing trend was observed from 2005 to 2017 in the proportions of the alleles associated with CQ resistance in both study sites except for pfmdr1 86 Y in Navrongo ( Figures 1A and B), which decreased from 2005/2006 to 2010 but appeared to plateau between 2012 and 2013. We, however, observed an increasing trend in the proportions of the pyrimethamine and sulfadoxine resistance alleles in pfdhfr and pfdhps respectively at both study sites from 2005 to 2013, with frequencies levelling off subsequently ( Figures 1C–F).
Figure 1. Trends in the prevalence of antimalarial drug resistant alleles from 2005 to 2017.
Summarized data from present study was compared to previous published data from Navrongo and Kintampo/Sunyani, Ghana, for pfmdr1 and pfcrt point mutations ( Duah et al., 2013) and for the antifolate resistance mutations ( Duah et al., 2012). No data was available for the pfcrt 76T and pfmdr1 86Y in 2010 in Kintampo/Sunyani ( Figure 1A–B). Also, no data was available for Navrongo in 2016–2017 in all the analysis. Deep black represents 2005–2006, light black represents 2010, plain grey represents 2012–2013 and crossed grey represents 2016–2017.
Analysis of pfdhfr and pfdhps haplotype combination distributions
We used clinical isolates for which all the pfdhfr and pfdhps SNP alleles of interest were successfully genotyped to survey allele combinations and determine their distribution between study periods. The pfdhfr triple mutant allele combination ( I 51 R 59 N 108) was found at a prevalence of 53% in 2012/2013 and 79% in 2016/2017 (P<0.0001, Table 2). Across both genes, the prevalence of the quadruple allele combination ( I 51 R 59 N 108/ G 437) increased from 43% to 73% from 2012/2013 to 2016/2017 study periods and the difference was statistically significant (P<0.0001). Double mutant allele combinations of pfdhfr ( I 51 N 108, R 59 N 108 and I 51 R 59) had comparably low frequencies. In addition, other generally low prevalence (<10%) allele combinations in both pfdhfr and pfdhps included the triple mutant allele combination ( R 59 N 108/ G 437, I 51 R 59/ G 437 and I 51 N 108/ G 437) and the double mutant allele combination ( R 59/ G 437, N 108/ G 437 and I 51/ G 437) for both the 2012/2013 and the 2016/2017 periods ( Table 2). A comparison of allele combination distribution by malaria transmission intensity revealed a significantly higher prevalence of the mutant allele combination in pfdhfr ( I 51 R 59 N 108) and in both genes I 51 R 59 N 108/ G 437 in the lowest transmission area, Accra, compared to Kintampo (P=0.002 and P=0.04, respectively).
Table 2. Temporal trends of SP drug resistance haplotypes from 2012 to 2017 by study period.
| pfdhfr | pfdhps | 2012–2013 | 2016–2017 | Total | P-value a | ||
|---|---|---|---|---|---|---|---|
| n = 95 (%) | n =128 (%) | n=223 (%) | |||||
| N51I | C59R | S108N | A437G | ||||
| I | R | N | G/A | 50 (52.6) | 101(79) | 151(67.7) | <0.0001 |
| N | R | N | G | 10 (10.5) | 8 (6.3) | 18 (8.1) | 0.321 |
| I | R | S | G | 8 (8.4) | 2 (1.6) | 10 (4.5) | 0.020 |
| I | C | N | G | 7 (7.4) | 12 (9.4) | 19 (8.5) | 0.637 |
| N | R | S | G | 5 (5.3) | 2 (1.6) | 7(3.1) | 0.140 |
| I | C | N | A | 4 (4.2) | 0 (0) | 4 (1.8) | 0.032 |
| N | C | N | G | 3 (3.2) | 1 (0.8) | 4 (1.8) | 0.315 |
| N | R | N | A | 3 (3.2) | 1 (0.8) | 4 (1.8) | 0.315 |
Note: Numbers include only isolates that were successfully genotyped for all the four point mutations in pfdhfr and pfdhps.
Each haplotype has mutant amino acids shown in bold. aP-value based on Exact chi-square test
Discussion
P. falciparum resistance to antimalarial drugs remains one of the biggest threats to the control and elimination of malaria globally. In Ghana, a change in the use of CQ to ACTs was implemented in 2005 as a result of high rate of malaria treatment failure ( Duah et al., 2007). Mutant alleles in some key genes of the parasite are clearly linked to CQ and antifolate drug resistance. Recent reports have shown that some of these point mutations might also modulate the efficacy of the currently used ACTs ( Gresty et al., 2014; Veiga et al., 2016). Thus, the availability of data on the prevalence of antimalarial drug resistance-associated alleles will be important for informing national policies on malaria treatment. Hence, in this study we determined the prevalence of alleles associated with CQ and antifolate resistance using clinical isolates from three malaria endemic regions in Ghana that have varying transmission intensities.
Both in vitro and molecular surveillance studies have associated CQ resistance mainly with the pfcrt 76T allele, but also with pfmdr1 86Y and 184F alleles. Data presented here show a gradual decline in frequency of the pfcrt 76T resistance-associated allele over the years since the official discontinuation of CQ as an antimalarial in Ghana, which is consistent with findings in other malaria endemic populations in east Africa such as Tanzania, Malawi and Kenya ( Mohammed et al., 2013; Mwai et al., 2009). The fitness cost of harbouring the mutant alleles is thought to select against them in favour of the non-resistant background alleles ( Babiker et al., 2009; Kiarie et al., 2015). Unlike pfcrt 76T and pfmdr1 86Y, the prevalence of pfmdr1 184F mutant allele (65%) appears to have not varied so much from 2005 to 2017 when compared to the 43% to 69% prevalence reported from 2005 to 2010 ( Duah et al., 2013).
Although high frequencies of the point mutations implicated in the development of resistance to antifolates were reported before the change in malaria treatment guidelines in 2005 in Ghana, the drug is still in use for intermittent preventive treatment of malaria in pregnancy (IPTp) and also recommended for seasonal malaria chemotherapy (SMC) among children under five in areas of high but seasonal malaria transmission. The percentages of the pfdhfr 51I (81%), 59R (82%), 108N (88%) and pfdhps 437G (88%) mutant alleles reported in this study are relatively higher when compared to the 71%, 42%, 64% and 80% prevalence reported in a recent study in a neighbouring country, Burkina Faso ( Cisse et al., 2017), this could be due to differences in the uptake of IPT P in both countries. Unlike the mutations associated with CQ resistance which decreased, an increasing trend in the prevalence of the antifolate resistance-associated mutant alleles was observed from 2005 to 2017. This high prevalence may be due to SP intervention in groups such as pregnant women and young children acting as reservoirs of infections with resistance alleles as a direct consequence of continuous use of SP in IPTp and SMC campaigns that fuel transmission of these alleles in the general population. Another important factor may be the unauthorized use of SP for self-medication as it is readily available at health centres and pharmacy shops in the study areas ( Abuaku et al., 2004), particularly because it is a single dose drug with very minimal to no adverse reactions. Higher SP treatment failure have been correlated with the pfdhfr/pfdhps quintuple ( pfdhfr/pfdhps I 51R 59N 108/G 437E 540) haplotypes ( Kublin et al., 2002; Triglia et al., 1997). In this study, however, no isolate was observed to carry the quintuple mutant allele. This is consistent with other studies which show that though the variant quintuple mutant allele is almost fixed in east Africa, it is largely absent from West Africa ( Naidoo & Roper, 2013; Roper et al., 2004). However, our data shows a significant increased prevalence of parasite isolates that harbour the quadruple mutant allele combination involving both genes (I 51R 59N 108/G 437) in the 2012–2013 and 2016–2017 study periods. This suggests that selection by SP in our study settings is still continuing. These findings are corroborated by previous studies in Ghana (25%–69%) ( Duah et al., 2012), Cameroon (47%) ( Chauvin et al., 2015) and Equatorial Guinea (54%) ( Berzosa et al., 2017), which suggests a high prevalence of variant quadruple mutant alleles in West to Central Africa. The pfdhps K540E point mutation, which is a surrogate for high level resistance to SP was found in a very low proportion of the clinical isolates (1 %) in this study. This is consistent with reports in other countries in the sub-region including Mali and Burkina Faso ( Cisse et al., 2017; Coulibaly et al., 2014), and suggests that if selection is increased it might eventually lead to a higher level of SP resistance in West Africa.
The increasing trend in the prevalence of the SP resistance-associated alleles is of concern and highlights the need for monitoring in populations receiving IPTp, and SMC, and for trials of other drug combinations. The study indicates that CQ sensitive parasites have again become more common since the replacement of CQ with a variety of ACTs as first-line treatments of uncomplicated malaria in Ghana. This notwithstanding, our findings also show that between 5% to 14% of clinical infections may still carry CQ resistant parasites, which suggest that ACT partner drugs such as AQ that is widely used in Ghana may still be maintaining significant selection pressure on the pfcrt locus. In addition, the increasing prevalence of pfdhfr haplotypes known to correlate with SP treatment failure calls for continuous monitoring of parasite populations across the different eco-epidemiological areas of Ghana to gather data for assessing the public health implications.
Conclusion
This study reports an increasing prevalence of CQ sensitive clinical isolates after 12 years of CQ withdrawal at three diverse study sites in Ghana. On the contrary, if an increasing trend in the frequency of SP-resistance associated alleles continues, it may in the future undermine use of SP for prevention of malaria in pregnant women and children.
Data availability
The data supporting this article is available online at Open Science Framework: Dataset 1. Prevalence of chloroquine and antifolate drug resistance alleles in Plasmodium falciparum clinical isolates from three areas in Ghana. http://dx.doi.org/10.17605/OSF.IO/N2GZF ( Abugri et al., 2018) under a CC0 1.0 Universal licence.
Funding Statement
This work was supported by the African Academy of Science through the Developing Excellence in Leadership, Training and Science (DELTAS) programme [DEL-15-007] (GA). This work was also supported by the Wellcome Trust [107755/Z/15/Z] (GA), and World Bank Group [ACE02: WACCBIP] (GA).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 1 approved, 1 approved with reservations]
References
- Abuaku BK, Koram KA, Binka FN: Antimalarial drug use among caregivers in Ghana. Afr Health Sci. 2004;4(3):171–7. [PMC free article] [PubMed] [Google Scholar]
- Abugri J, Ansah F, Asante KP, et al. : Raw_data_for_antimalarial_drug_resistant_alleles_Navrongo_Kintampo_Accra.2018. 10.17605/OSF.IO/N2GZF [DOI] [Google Scholar]
- Ademolue TW, Aniweh Y, Kusi KA, et al. : Patterns of inflammatory responses and parasite tolerance vary with malaria transmission intensity. Malar J. 2017;16(1):145. 10.1186/s12936-017-1796-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker HA, Hastings IM, Swedberg G: Impaired fitness of drug-resistant malaria parasites: evidence and implication on drug-deployment policies. Expert Rev Anti Infect Ther. 2009;7(5):581–93. 10.1586/eri.09.29 [DOI] [PubMed] [Google Scholar]
- Babiker HA, Pringle SJ, Abdel-Muhsin A, et al. : High-level chloroquine resistance in Sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance Gene pfmdr1. J Infect Dis. 2001;183(10):1535–8. 10.1086/320195 [DOI] [PubMed] [Google Scholar]
- Berzosa P, Esteban-Cantos A, García L, et al. : Profile of molecular mutations in pfdhfr, pfdhps, pfmdr1, and pfcrt genes of Plasmodium falciparum related to resistance to different anti-malarial drugs in the Bata District (Equatorial Guinea). Malar J. 2017;16(1):28. 10.1186/s12936-016-1672-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin P, Menard S, Iriart X, et al. : Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaoundé, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles. J Antimicrob Chemother. 2015;70(9):2566–71. 10.1093/jac/dkv160 [DOI] [PubMed] [Google Scholar]
- Cisse M, Awandare GA, Soulama A, et al. : Recent uptake of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine is associated with increased prevalence of Pfdhfr mutations in Bobo-Dioulasso, Burkina Faso. Malar J. 2017;16(1):38. 10.1186/s12936-017-1695-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly SO, Kayentao K, Taylor S, et al. : Parasite clearance following treatment with sulphadoxine-pyrimethamine for intermittent preventive treatment in Burkina-Faso and Mali: 42-day in vivo follow-up study. Malar J. 2014;13:41. 10.1186/1475-2875-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Mharakurwa S, Ndiaye D, et al. : Antimalarial Drug Resistance: Literature Review and Activities and Findings of the ICEMR Network. Am J Trop Med Hyg. 2015;93(3 Suppl):57–68. 10.4269/ajtmh.15-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djimdé A, Doumbo OK, Cortese JF, et al. : A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001a;344(4):257–63. 10.1056/NEJM200101253440403 [DOI] [PubMed] [Google Scholar]
- Djimdé A, Doumbo OK, Steketee RW, et al. : Application of a molecular marker for surveillance of chloroquine-resistant falciparum malaria. Lancet. 2001b;358(9285):890–1. 10.1016/S0140-6736(01)06040-8 [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, et al. : Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. 10.1056/NEJMoa0808859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah NO, Matrevi SA, de Souza DK, et al. : Increased pfmdr1 gene copy number and the decline in pfcrt and pfmdr1 resistance alleles in Ghanaian Plasmodium falciparum isolates after the change of anti-malarial drug treatment policy. Malar J. 2013;12:377. 10.1186/1475-2875-12-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah NO, Quashie NB, Abuaku BK, et al. : Surveillance of molecular markers of Plasmodium falciparum resistance to sulphadoxine-pyrimethamine 5 years after the change of malaria treatment policy in Ghana. Am J Trop Med Hyg. 2012;87(6):996–1003. 10.4269/ajtmh.2012.12-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah NO, Wilson MD, Ghansah A, et al. : Mutations in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance genes, and treatment outcomes in Ghanaian children with uncomplicated malaria. J Trop Pediatr. 2007;53(1):27–31. 10.1093/tropej/fml076 [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Cowman AF: Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Trop. 2005;94(3):181–90. 10.1016/j.actatropica.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Curtis J, Warhurst DC: Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes by PCR and restriction digestion. Exp Parasitol. 1998;89(1):1–8. 10.1006/expr.1998.4274 [DOI] [PubMed] [Google Scholar]
- Fidock DA, Nomura T, Talley AK, et al. : Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6(4):861–71. 10.1016/S1097-2765(05)00077-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling RD, Okell L, Mosha J, et al. : The role of antimalarial treatment in the elimination of malaria. Clin Microbiol Infect. 2011;17(11):1617–23. 10.1111/j.1469-0691.2011.03660.x [DOI] [PubMed] [Google Scholar]
- Greenwood B: The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am J Trop Med Hyg. 2004;70(1):1–7. [PubMed] [Google Scholar]
- Gresty KJ, Gray KA, Bobogare A, et al. : Genetic mutations in pfcrt and pfmdr1 at the time of artemisinin combination therapy introduction in South Pacific islands of Vanuatu and Solomon Islands. Malar J. 2014;13:406. 10.1186/1475-2875-13-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie WC, Wangai L, Agola E, et al. : Chloroquine sensitivity: diminished prevalence of chloroquine-resistant gene marker pfcrt-76 13 years after cessation of chloroquine use in Msambweni, Kenya. Malar J. 2015;14:328. 10.1186/s12936-015-0850-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg E, McCall P, Wilson MD, et al. : Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar J. 2008;7:151. 10.1186/1475-2875-7-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublin JG, Dzinjalamala FK, Kamwendo DD, et al. : Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185(3):380–8. 10.1086/338566 [DOI] [PubMed] [Google Scholar]
- Laufer MK, Thesing PC, Eddington ND, et al. : Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355(19):1959–66. 10.1056/NEJMoa062032 [DOI] [PubMed] [Google Scholar]
- Lin JT, Juliano JJ, Wongsrichanalai C: Drug-Resistant Malaria: The Era of ACT. Curr Infect Dis Rep. 2010;12(3):165–73. 10.1007/s11908-010-0099-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah-brown HE, Abugri J, Asante KP, et al. : Assessing the impact of differences in malaria transmission intensity on clinical and haematological indices in children with malaria. Malar J. 2017;16(1):96. 10.1186/s12936-017-1745-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita T, Ohashi J, Venkatesan M, et al. : Ordered accumulation of mutations conferring resistance to sulfadoxine-pyrimethamine in the Plasmodium falciparum parasite. J Infect Dis. 2014;209(1):130–9. 10.1093/infdis/jit415 [DOI] [PubMed] [Google Scholar]
- Mohammed A, Ndaro A, Kalinga A, et al. : Trends in chloroquine resistance marker, Pfcrt-K76T mutation ten years after chloroquine withdrawal in Tanzania. Malar J. 2013;12:415. 10.1186/1475-2875-12-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwai L, Ochong E, Abdirahman A, et al. : Chloroquine resistance before and after its withdrawal in Kenya. Malar J. 2009;8:106. 10.1186/1475-2875-8-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo I, Roper C: Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitol. 2013;29(10):505–15. 10.1016/j.pt.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Omar SA, Adagu IS, Warhurst DC: Can pretreatment screening for dhps and dhfr point mutations in Plasmodium falciparum infections be used to predict sulfadoxine-pyrimethamine treatment failure? Trans R Soc Trop Med Hyg. 2001;95(3):315–9. 10.1016/S0035-9203(01)90250-0 [DOI] [PubMed] [Google Scholar]
- Owusu-Agyei S, Asante KP, Adjuik M, et al. : Epidemiology of malaria in the forest-savanna transitional zone of Ghana. Malar J. 2009;8:220. 10.1186/1475-2875-8-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowe CV, Cortese JF, Djimde A, et al. : Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J Infect Dis. 1997;176(6):1590–6. 10.1086/514159 [DOI] [PubMed] [Google Scholar]
- Roper C, Pearce R, Nair S, et al. : Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305(5687):1124. 10.1126/science.1098876 [DOI] [PubMed] [Google Scholar]
- Schlitzer M: Malaria chemotherapeutics part I: History of antimalarial drug development, currently used therapeutics, and drugs in clinical development. ChemMedChem. 2007;2(7):944–86. 10.1002/cmdc.200600240 [DOI] [PubMed] [Google Scholar]
- Schumacher RF, Spinelli E: Malaria in children. Mediterr J Hematol Infect Dis. 2012;4(1):e2012073. 10.4084/MJHID.2012.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley CH, Hyde JE, Sims PF, et al. : Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 2001;17(12):582–8. 10.1016/S1471-4922(01)02085-2 [DOI] [PubMed] [Google Scholar]
- Sidhu AB, Verdier-pinard D, Fidock DA: Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 2002;298(5591):210–3. 10.1126/science.1074045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T, Menting JG, Wilson C, et al. : Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1997;94(25):13944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga MI, Dhingra SK, Henrich PP, et al. : Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat Commun. 2016;7: 11553. 10.1038/ncomms11553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO: World malaria report 2016. Geneva: World Health Organization;2016. Reference Source [Google Scholar]
- WHO: World malaria report 2017. Geneva: World Health Organization;2017. Reference Source [Google Scholar]
- Young MD, Contacos PG, Stitcher JE, et al. : Drug Resistance in Plasmodium Falciparum from Thailand. Am J Trop Med Hyg. 1963;12:305–14. 10.4269/ajtmh.1963.12.305 [DOI] [PubMed] [Google Scholar]