Abstract
Total saponins extracted from Dioscorea collettii (TSD), extracts of the Chinese herb Dioscorea, are thought to exhibit therapeutic benefit in gouty arthritis. However, its exact mechanism remains unclear. The current study aimed to elucidate the underlying mechanisms by investigating the effects of TSD on the inflammation induced by monosodium urate (MSU) crystals in THP-1 macrophages. The viability of THP-1 macrophages was examined using the MTT assay and the levels of inflammatory cytokines, including interleukin (IL)-1β, IL-18 and tumor necrosis factor (TNF)-α, released by the cells were quantitatively measured using ELISA kits. The results revealed that the protein level of cluster of differentiation 11b increased in THP-1 cells treated with 100 ng/ml phorbol ester, suggesting that monocytic THP-1 cells were successfully differentiated into macrophages. TSD decreased the levels of inflammatory cytokines, including TNF-α, IL-18 and IL-1β, secreted by THP-1 macrophages. As the release of IL-1β and IL-18 is dependent on the NLR family pyrin domain containing 3 (NALP3) inflammasome and caspase-1, the current study investigated the effect of TSD on the aforementioned proteins. The results revealed that TSD decreased the protein levels of NALP3 and apoptosis-associated speck-like, which serve important roles in the assembly of the NALP3 inflammasome. Furthermore, NALP3 inflammasome-related proteins were also decreased by TSD in rotenone induced THP-1 macrophages, TSD inhibited the activation of caspase-1 and rotenone-induced NALP3 inflammasome activation in THP-1 macrophages. The results obtained in the current study revealed that TSD attenuated MSU crystal-induced inflammation by inhibiting rotenone-induced activation of the NALP3 inflammasome and caspase-1, suggesting that these two proteins may be novel targets for the treatment of gouty arthritis.
Keywords: total saponin of Dioscorea collettii, inflammatory cytokines, NALP3 inflammasome, caspase-1, THP-1 macrophages, monosodium urate
Introduction
Gout, a type of inflammation caused by the disturbance of purine metabolism and deposition of monosodium urate (MSU) crystals in synovial fluid and other tissues, is one of the most common forms of inflammatory arthritis, usually in the presence of prolonged hyperuricemia. A longitudinal study reported that the prevalence of gout and hyperuricemia in China were 2.8 and 18.1%, respectively (1). The incidence of gout and hyperuricemia are increasing due to obesity, insulin resistance, metabolic syndrome, renal impairment, cardiovascular disease and hypertension (2).
Dioscorea collettii is a traditional Chinese medicine that been used for the treatment of inflammatory conditions, such as gouty arthritis and hyperuricemia, in China for several years (3). Total saponin from Dioscorea collettii (TSD) extracted from Dioscorea has been reported to have significant anti-inflammatory, analgesic and anti-hyperuricemia effects. A previous study in rats with chronic hyperuricemia revealed that TSD exhibited its effects through the downregulation of solute carrier family 22 member 12 and solute carrier family 2 member 6 and the upregulation of solute carrier family 22 members 6 and 8 (4). A previous study (5) suggested that the monosodium urate (MSU)-induced inflammatory response is dependent on the inflammatory cytokine interleukin (IL)-1β. The IL-1-dependent innate inflammatory phenotype relies on the formation of the macromolecular NLR family pyrin domain containing 3 (NALP3) inflammasome complex in response to the MSU ‘danger signal’ (6). Therefore, the NALP3 inflammasome may be a potential target of TSD in gouty arthritis. The present demonstrated that TSD inhibited the secretion of inflammatory cytokines, including IL-1β, IL-18 and tumor necrosis factor (TNF)-α, in THP-1 macrophages treated with MSU crystals. Furthermore, the present study revealed that TSD inhibited the assembly of the NALP3 inflammasome and the activation of caspase-1.
Materials and methods
Drug and reagents
Dioscorea rhizomes were purchased from The First Affiliated Hospital of Anhui University of Chinese Medicine. According to the literature, the saponins were extracted from Dioscorea (4). The total content of TSD in the extract of Dioscorea was 53.1% (4). Urate sodium was purchased from Sigma-Aldrich (Merck KGaA). Colchicine and rotenone were purchased from Shanghai Aladdin Biochem Technology Co., Ltd. ELISA kits for IL-1β (cat. no. F0179A), IL-18 (cat. no. F0138A) and TNF-α (cat. no. F0121A) were purchased from Shanghai Fankewei Technology Industry Co., Ltd (www.shfksc.com).
Preparation of MSU crystals
MSU was prepared according to the method of Huang el al's study (7). Briefly, 1 g uric acid was dissolved in 200 ml boiling water and the solution pH was adjusted to 7.2 with 1N NaOH. The solution was cooled gradually by stirring at room temperature. The crystals were collected by centrifugation at 3,000 × g at 4°C for 2 min and settled at 4°C for 6 h. The crystals were evaporated and sterilized by heating at 180°C for 2 h and stored in a sterile environment until use. The crystals were suspended in PBS at a concentration of 50 mg/ml and sonicated 10 min in 40 kHz at room temperature. 10 min to obtain rod-shaped crystals with uniform sizes (5–25 µm in length). A Limulus amebocyte cell lysate assay (cat. no. L00350; GenScript) was used to verify the absence of endotoxin in the preparation. The assay was performed according to the manufacturer's protocol.
Cell culture and drug treatments
The human THP-1 cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences. THP-1 cells were cultured in RPMI-1640 medium (Hyclone; GE Healthcare), containing 10% FBS (Zhejiang Tianhang Biotechnology Co., Ltd.). The air in the cell incubator was humidified and contained 5% CO2 and 95% air at 37°C. The medium was changed every 2 days. In order to certify the effect of macrophages on MSU crystals, THP-1 cells were induced into macrophage-like cells. THP-1 cells (2×106 cells/well) were seeded in six-well culture plates and incubated with phorbol 12-myristate acetate (PMA) from 25–200 ng/ml for 24 h, then cells were washed by PBS and observed the morphology under an inverted light microscope at ×200 magnification. Images were captured of each well in at least 5 random fields, the result was calculated by the ratio of adhered or pseudopodia-formed THP-1 cells to the total cells. The cells were identified by morphology and cluster of differentiation (CD)11b protein level was characteristic of macrophages.
Viability assays
To evaluate the effects of MSU crystals or TSD on the viability of THP-1 macrophages, THP-1 macrophages were treated with MSU (0, 25, 50, 100, 200, 300 and 400 µg/ml) or TSD (0, 0.1, 0.3, 1, 3, 10 and 30 µg/ml) for 24 h. The viability of THP-1 macrophages was examined by MTT assay and the formazan was dissolved by DMSO (≥99.7%; Sigma-Aldrich; Merck KGaA). Every well was measured at a wavelength of 490 nm (optical density at 490) with the Thermo Varioskan Flash (Thermo Fisher Scientific, Inc.). Cell viability was expressed as a percentage of control cells, which were defined as 100% viable. All the assays were performed in triplicate.
Inflammatory cytokine ELISAs
In order to investigate the most appropriate MSU crystals concentration in THP-1 macrophages, cells were treated with MSU crystals at different concentrations (0, 50, 100, 200, 300 and 400 µg/ml). In follow-up experiments, THP-1 macrophages were treated with TSD (0.3, 1.0 and 3.0 µg/ml) or colchicines (0.5 and 5 µM) for 24 h prior to the stimulation with MSU crystals (400 µg/ml) or rotenone (80 µM). The level of the inflammatory cytokines, such as IL-1β, IL-18 and TNF-α in the supernatants of media were quantitatively measured with ELISA kits as listed above. The ELISA plates were measured using a Thermo Varioskan Flash (Thermo Fisher Scientific, Inc.).
Western blot analysis
THP-1 macrophages grouping was the same as above. Cells were lysed in RIPA (Beyotime Institute of Biotechnology) buffer containing 1 mM PMSF (Beyotime Institute of Biotechnology). Protein samples (30 µg/lane) extracted from cell lysate was separated by 10% SDS-PAGE. The separated proteins in the gel were transferred onto a polyvinylidene difluoride membrane (EMD Millipore), blocked with 2.5% TBST milk [10 mM Tris HCl (pH 8.0), 150 mM NaCl, 0.5% Tween-20, 2.5% skim milk] for 2 h at room temperature and probed with anti-CD11b antibody (cat. no. ab133357; Abcam), anti-NALP3 antibody (D2P5E; cat. no. 13158; Cell Signaling Technology, Inc.), anti-ASC antibody (AW5459; Abgent, Inc.) anti-caspase-1 p20 antibody (D57A2; cat. no. 4199; Cell Signaling Technology, Inc.), anti-caspase-1 antibody (cat. no. sc-2225; Santa Cruz Biotechnology, Inc.), or anti-actin antibody (8H10D10; cat. no. 3700; Cell Signaling Technology, Inc.), respectively. All antibodies were used at 1:1,000 dilution at 4°C overnight. All antibodies were diluted with TBST milk. The secondary antibodies, 1:10,000, (Alexa Fluor® 790 goat anti-rabbit IgG, cat. no. 111-655-144 and Alex Fluor® 680 goat anti-mouse IgG; cat. no. 115-625-146) were incubated with the membranes for 2 h at room temperature and detected with an Odyssey® CLx Infrared Imaging System (LI-COR Biosciences), and the grey value were analyzed with ImageJ 1.52a (National Institutes of Health). All the assays were performed in triplicate.
Assay of mRNA expression level of NALP3 with reverse transcription (RT)-PCR
The THP-1 macrophage grouping was the same as above. The mRNA expression levels of NALP3 gene were determined by RT-PCR. Total RNA was extracted from THP-1 macrophages with different treatments using the total RNA extraction kit. RT-PCR was performed using SuperScript™ IV One-Step RT-PCR system (Thermo Fisher Scientific, Inc.). The β-actin gene was used as an internal reference for normalization of expression levels. The following primer sequences were used: NALP3 (NALP3 forward, 5′-TTCTCTGATGAGGCCCAAG-3′ and NALP3 reverse, 5′-GGATCTTCATGAGGTAGTCAG-3′); and β-actin (β-actin forward, 5′-GAGACCTTCAACACCCCAGCC-3′ and β-actin reverse, 5′-GGATCTTCATGAGGTAGTCAG-3′). RT-PCR amplification (Thermo Fisher Scientific, Inc.) was performed using a protocol of 50°C for 2 min and 95°C for 10 min, then 95°C for 15 sec followed by 53°C for 1 min for 40 cycles. The analysis method of RT-PCR was carried out using the 2−ΔΔCq method (8).
Statistical analysis
Data were expressed as the mean ± standard deviation. The results were analyzed for statistical significance using one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference. All data analysis was performed with SPSS 17.0 software (IBM Corp.).
Results
THP-1 cells are successfully differentiated into macrophages by PMA
THP-1 cells were exposed to 25–200 ng/ml PMA for 24 h and examined under an inverted microscope (9,10). As shown in Fig. 1A, THP-1 cells treated with PMA at concentrations >25 ng/ml exhibited cell adhesion, spreading and formed pseudopodia, suggesting that the cells successfully differentiated into macrophages (11,12). Furthermore, the level of CD11b, a macrophage surface marker (13), was analyzed in the THP-1 macrophages. There was a significant increase in the CD11b protein level in cells treated with PMA at concentrations exceeding 100 ng/ml (P<0.05; Fig. 1B). Therefore, 100 ng/ml PMA was used in subsequent experiments.
Figure 1.
Induction of THP-1 differentiation by PMA at different concentrations. (A) Following treatment with PMA at the indicated concentrations for 24 h, THP-1 cells adhered to the culture flask and differentiated into macrophages (magnification, ×200). Black arrows indicate differenciated cells to macrophages. (B) In cells treated with PMA at concentrations of 100–200 ng/ml, the expression levels of CD11b were significantly increased. These data are representative of three independent experiments. *P<0.05 vs. control, generated using one-way analysis of variance. PMA, phorbol 12-myristate acetate; CD, cluster of differentiation.
Cytotoxic effect of MSU and TSD on THP-1 macrophages
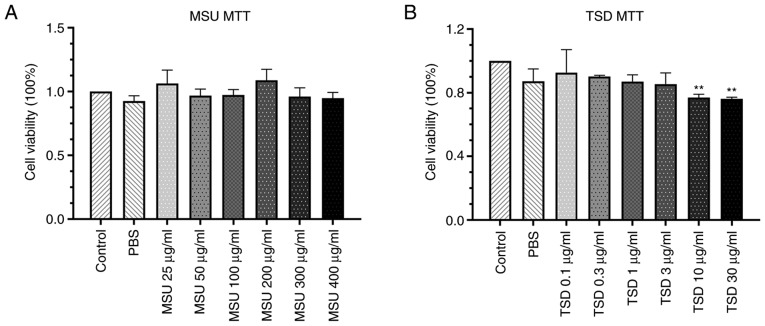
The cytotoxic effect of MSU and TSD on differentiated THP-1 cells was measured using the MTT assay. As shown in Fig. 2, no significant changes in cell viability were observed in THP-1 macrophages treated with MSU at concentrations up to 400 µg/ml or TSD at concentrations up to 3 µg/ml for 24 h (48 or 72 h-treatment showed the similar results with 24 h, Fig. S1). Based on these results, MSU at a concentration of 400 µg/ml and TSD at a concentration range of 0.1–3 µg/ml were used for further experimentation.
Figure 2.
Cytotoxicity of MSU or TSD on THP-1 macrophages. Cells were treated with (A) MSU at concentrations of 25–400 µg/ml and (B) TSD at concentrations of 0.1–30 µg/ml for 24 h. MSU and TSD were dissolved in PBS. Cell viability was measured using the MTT assay. Data are expressed as the mean ± standard deviation. These data are representative of three independent experiments. **P<0.01 vs. non-treated control cells (generated using one-way analysis of variance). MSU, monosodium urate; TSD, total saponins extracted from Dioscorea collettii.
MSU induces THP-1 macrophages to secrete IL-1β
Previous studies reported that macrophages respond to MSU crystals during the progression of the gout inflammatory response (14–16). MSU crystals subsequently lead to the production of inflammatory cytokines such as IL-1β, which is a key regulatory cytokine in gout (6). The present study investigated whether MSU induces the secretion of IL-1β in THP-1 macrophages. As shown in Fig. 3, the level of IL-1β in the cell culture medium increased ~1.79- and 1.92-fold following treatment with MSU at concentrations of 300 and 400 µg/ml, respectively. Based on these results, MSU at a concentration of 400 µg/ml was selected for subsequent experimentation.
Figure 3.

Effects of MSU on IL-1β secretion in THP-1 macrophages. THP-1 macrophages were treated with 0–400 µg/ml MSU for 6 h. The cell culture medium was collected and analyzed for the levels of inflammatory cytokines. The levels of IL-1β were significantly increased by MSU at 300 and 400 µg/ml, and the highest levels of IL-1β were obtained at a concentration of 400 µg/ml. These data are representative of three independent experiments. *P<0.05 vs. control, generated using one-way analysis of variance. MSU, monosodium urate; IL, interleukin.
TSD significantly inhibits MSU-induced secretion of inflammatory cytokines in THP-1 macrophages
In order to investigate whether TSD inhibits the production of inflammatory cytokines in THP-1 macrophages treated with MSU crystals, cells were treated with or without TSD for 24 h prior to stimulation with 400 µg/ml MSU. Cells treated with colchicine (0.5 or 5 µM) served as the positive controls. As shown in Fig. 4, treatment with MSU crystals significantly increased the protein levels of IL-1β, IL-18 and TNF-α in THP-1 macrophages (P<0.05). However, the levels of IL-1β decreased to ~72.2 and 71.2% in cells treated with 1 and 3 µg/ml TSD, respectively (P<0.01; Fig. 4A). The levels of IL-18 significantly decreased to ~57.0 and 66.6% in cells treated with 1 and 3 µg/ml TSD, respectively (P<0.01; Fig. 4B). The levels of TNF-α decreased to ~50.1% in cells treated with 3 µg/ml TSD (P<0.01; Fig. 4C). Cells treated with colchicine exhibited similar results. These data indicated that TSD decreased the secretion of IL-1β, IL-18 and TNF-α from THP-1 macrophages.
Figure 4.
TSD inhibits MSU-induced cytokine production. THP-1 macrophages were pretreated with TSD at a concentration of 0–0.3 µg/ml for 24 h and subsequently stimulated with MSU at 400 µg/ml for 6 h. The levels of inflammatory cytokines in the cultured media were analyzed: (A) IL-1β, (B) IL-18 and (C) TNF-α. Secreted levels of IL-1β and IL-18 were significantly inhibited by TSD at 1 and 3 µg/ml. The secreted levels of TNF-α were significantly inhibited by TSD at 3 µg/ml. These data are representative of three independent experiments. ▲P<0.05 and ▲▲P<0.01 vs. control and *P<0.05 and **P<0.01 vs. MSU (generated using one-way analysis of variance). TSD, total saponins extracted from Dioscorea collettii; MSU, monosodium urate; IL, interleukin; TNF-α, tumor necrosis factor-α.
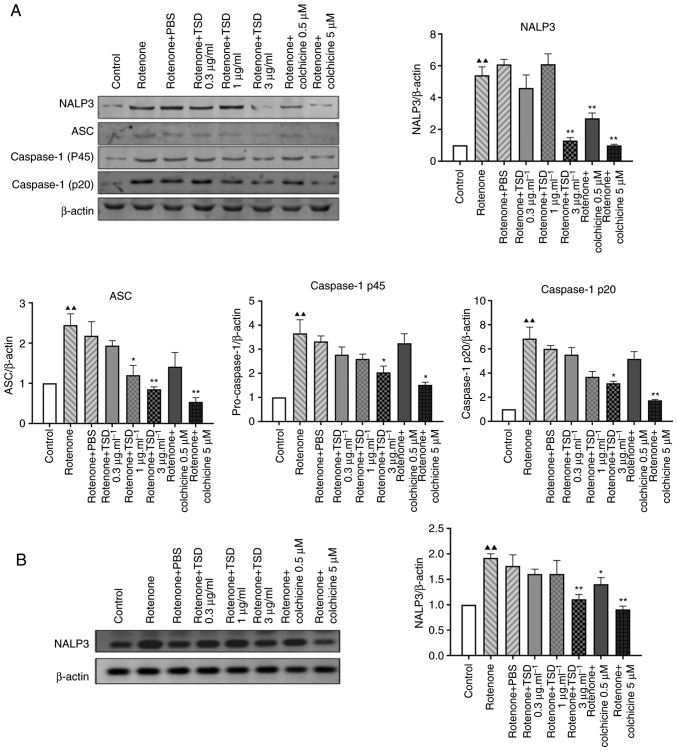
TSD inhibits the activation of the NALP3 inflammasome
The release of IL-1β and IL-18 is dependent on the NALP3 inflammasome and caspase-1 (17–19). The present study therefore investigated whether TSD inhibited the secretion of IL-1β via the NALP3 inflammasome. As NALP3 and apoptosis-associated speck-like (ASC) serve an important role in NALP3 inflammasome assembly (20,21), the protein levels of NALP3 and ASC in TSD-treated THP-1 macrophages were measured in the current study. As shown in Fig. 5A, the protein level of NALP3 decreased to ~64.5 and 38.8% in cells treated with 1 and 3 µg/ml TSD compared with the MSU + PBS (control) group, respectively (P<0.05). The protein level of ASC decreased to ~66.3, 63.2 and 42.9% in cells treated with 0.3, 1 and 3 µg/ml TSD, respectively (P<0.05; Fig. 5A). Additionally, the protein levels of caspase-1 p45 and p20, which are activated by the NALP3 inflammasome, were investigated. As presented in Fig. 5A, the protein levels of caspase-1 p45 and p20 significantly decreased to ~64.4 and 88.1% in cells treated with 3 µg/ml TSD compared with the MSU + PBS (control) group, respectively (P<0.05). The same trend was observed in cells treated with colchicine (Fig. 5A). However, there were no significant differences in the protein levels of NALP3, ASC, caspase-1 p45 and p20 in cells treated with MSU + PBS compared with the MSU group (Fig. 5A).
Figure 5.
TSD inhibits the expression of proteins involved in MSU-induced NALP3 inflammasome activation. THP-1 cells were pre-treated with TSD for 24 h, followed by treatment with 400 µg/ml MSU crystals for 6 h. (A) Western blot analysis for the expression levels of NALP3, ASC, pro caspase-1 and caspase-1 p20 in cell lysates from THP-1 cells treated with MSU crystals revealed activation of the NALP3 inflammasome, which was attenuated by TSD. (B) mRNA levels of NALP3 were analyzed by reverse transcription-quantitative polymerase chain reaction. NALP3 upregulation by MSU crystals was attenuated by TSD at 3 µg/ml. Data are representative of three independent experiments. ▲P<0.05 and ▲▲P<0.01 vs. the control and *P<0.05 and **P<0.01 vs. MSU (generated using one-way analysis of variance). TSD, total saponins extracted from Dioscorea collettii; MSU, monosodium urate; NALP3, NLR family pyrin domain containing 3; ASC, apoptosis-associated speck-like.
NALP3 is a critical inflammasome component which recognize numerous exogenous and host ligands (14). Once activated, NALP3 recruits the adapter ASC, which in turn recruits pro-caspase-1 (5,17). It was reported that MSU crystals directly increased intercellular NALP3 mRNA expression in human FLS cells (22). Additionally a previous study showed that NALP3 transcript levels are increased after treatment of macrophages for 6 h with MSU (23). Therefore it was investigated whether TSD could inhibit the transcriptional level of NALP3. As shown in Fig. 5B, the level of NALP3 decreased in cells treated with 3 µg/ml TSD. However, no significant difference in the level of NALP3 was observed in cells treated with colchicine.
Mitochondrial reactive oxygen species (ROS) may be a major trigger for the activation of the NLRP3 inflammasome and production of inflammatory cytokines (6). The current study investigated the effects of TSD in rotenone-induced THP-1 macrophages. Rotenone is a respiration chain complex I inhibitor and induces mitochondrial ROS production. As shown in Fig. 6, THP-1 macrophages NALP3 inflammasome-associated protein levels were decreased in THP-1 macrophages which were incubated with TSD before and after incubation with rotenone for 6 h. The protein levels of NALP3, caspase-1 p45 and caspase-1 p20 significantly decreased ~78.7, 38.7 and 47.4% in cells treated with 3 µg/ml TSD, respectively, compared with rotenone + PBS-treated cells (P<0.05). The protein level of ASC decreased ~45.0 and 61% in cells treated with 1 and 3 µg/ml TSD, respectively.
Figure 6.
TSD inhibits expression of proteins involved in rotenone-induced NALP3 inflammasome activation. THP-1 cells were pre-treated with TSD for 24 h, followed by treatment with (80 µM) rotenone for 6 h. (A) Western blot analysis for the expression levels of NALP3, ASC, pro caspase-1 and caspase-1 p20 in cell lysates from THP-1 cells treated with MSU crystals revealed activation of the NALP3 inflammasome, which was attenuated by TSD. (B) mRNA levels of NALP3 were analyzed by reverse-transcription quantitative polymerase chain reaction. The upregulation of the NALP3 gene by rotenone was attenuated by TSD at 3 µg/ml. Data are representative of three independent experiments. ▲▲P<0.01 vs. the control and *P<0.05 and **P<0.01 vs. MSU (generated using one-way analysis of variance). TSD, total saponins extracted from Dioscorea collettii; NALP3, NLR family pyrin domain containing 3; MSU, monosodium urate; ASC, apoptosis-associated speck-like.
Discussion
MSU deposition is the major source of inflammation in gouty arthritis (24–26). Current treatment strategies for gout remain suboptimal. Clinical studies have revealed that a number of novel drugs, including IL-1R antagonists, may reduce the pain and inflammation associated with gout (27–30). Furthermore, several trials demonstrated that IL-1 inhibitors prevent flares during the initial stages of allopurinol therapy (31).
Dioscorea collettii has been widely used for the treatment of gouty arthritis in traditional Chinese medicine. TSD, the extract prepared from Dioscorea collettii, is primarily used for the treatment of gout and hyperuricemia in rats (32). However, mechanisms by which TSD exerts its effects in gout have not been fully elucidated. The present study therefore investigated the mechanism of TSD in gouty arthritis.
Previous studies demonstrated that MSU crystals recruit monocytes and promote their differentiation into proinflammatory M1-like macrophages (33–35). The resulting inflammation is likely to serve a role in the deposition of MSU crystals in gout (36). A growing body of evidence suggests that the MSU crystal-induced differentiation of monocytes into macrophages is associated with the initiation, progression and resolution of acute gouty inflammation (5,37). In addition, it was reported that MSU crystals increase the secretion of cytokines and chemokines, including TNF-α, IL-1β, IL-18, IL-6, and IL-8, by macrophages and monocytes (38–40). IL-1β and TNF-α have been implicated in the development of gout flare-ups (41). A previous study demonstrated increased levels of NALP3 mRNA and protein in MSU-treated human fibroblast-like synoviocytes 6 to 48 h after treatment (42). Additionally, MSU crystals induced a significant increase in IL-1β in the culture medium with a peak concentration at 6 h following MSU treatment (21). The authors' previous study revealed that 400 µg/ml MSU significantly increased the level of IL-1β and NALP3 inflammasome activation in THP-1 macrophages 6 h following treatment (43). Similar results were obtained in the current study. Additionally, the present study demonstrated that TSD inhibited the secretion of inflammatory cytokines, including IL-1β, IL-18 and TNF-α, from THP-1 macrophages treated with MSU crystals, suggesting that TSD may exert a therapeutic effect in gout by decreasing the inflammation induced by MSU.
A previous study reported that innate immunity is involved in the pathogenesis of gout (44), suggesting that MSU crystals may be recognized by pattern-recognition receptors (PRRs), such as nucleotide-binding oligomerization domain-like receptors (NLRs) and toll-like receptors (TLRs). NALP3 is a member of the NLR family, intracellular PRRs that recognize pathogen-associated molecular patterns and danger-associated molecular patterns (14).
MSU crystal-induced inflammation relies on the activation of the NALP3 inflammasome, which consists of NALP3, ASC and caspase-1 (17,45,46). Following activation by MSU crystals, NALP3 assembles with the adaptor protein ASC to form a protein-complex termed the NALP3 inflammasome. The NALP3 inflammasome subsequently cleaves caspase-1 to the active enzyme form. The activated caspase-1 cleaves pro-IL-1β to IL-1β (47–49). Several studies have indicated that the aberrant activation of the NALP3 inflammasome is associated with the pathogenesis of autoimmune and chronic inflammatory and metabolic diseases, including atherosclerosis, type 2 diabetes and gout (50–53). A previous study revealed that macrophages deficient of the NALP3 inflammasome components caspase-1 and ASC exhibited significantly reduced MSU crystals-induced inflammatory responses, and did not produce IL-1β. This suggests that the NALP3 inflammasome and activated caspase-1 play an important role in MSU crystal-induced inflammation (14). The current study revealed that TSD decreased the protein levels of NALP3 and ASC in MSU-treated THP-1 macrophages. Additionally, the present study demonstrated that TSD inhibited the activation of caspase-1 in THP-1 macrophages treated with MSU crystals. The aforementioned results suggested that TSD may attenuate MSU-induced inflammation by inhibiting the activation of the NALP3 inflammasome and caspase-1. Zhou et al (54) reported that the Chinese herbal medicine Dioscorea nipponica decreased the activities of β-galactosidase, β-N acetyl glucosamine, β-glucuronidase, acid phosphatase and malonaldehyde and decreased the levels of TNF-α, IL-1β and IL-8 in rats with gouty arthritis, suggesting that Dioscorea nipponica decreased the extent of the self-limiting responses by the NALP3 inflammasome.
ROS are major mediators of the NALP3/IL-1β signaling pathway. Production of pro-inflammatory cytokines is associated with the generation of ROS (55). Mitochondria-and NADPH oxidase-derived ROS have been implicated in pro-inflammatory microglia activation (56). As the abnormal activation of the NALP3 inflammasome is linked to the pathogenesis of autoimmune and chronic inflammatory and metabolic diseases, including atherosclerosis, and type 2 diabetes and gout (50–52), regulating the NALP3 inflammasome may prevent unwanted host damage and excessive inflammation. Dysfunction of the mitochondrial respiratory chain (MRC) is associated with the activation of the NALP3 inflammasome partially due to the release of mitochondrial ROS and DNA. Impairment of the MRC by rotenone confers a selective priming signal for the activation of the NALP3 inflammasome (57).
Ma et al (24) demonstrated that intracerebral hemorrhage-induced inflammatory activation was associated with the activation of the NALP3 inflammasome and revealed that rotenone induced ROS production as well as NALP3 inflammasome activation. Similarly, the present study demonstrated that rotenone induced the activation of the NALP3 inflammasome, which was subsequently suppressed by TSD. The aforementioned results suggested that ROS may trigger the activation of the NALP3 inflammasome and that the NALP3 inflammasome is a potential therapeutic target of TSD in gouty arthritis.
While the present study suggested that TSD may exert a therapeutic effect in gouty arthritis, future in vivo experimental models are required to substantiate the results obtained. Additionally, the effects of TSD on the expression of TLRs and the pro-IL-1β or NALP3 signaling pathway warrant further investigation, and these are the main areas of interest for the next study.
In summary, the current study demonstrated that TSD attenuated MSU-induced production of inflammatory cytokines, such as TNF-α, IL-1β and IL-18, via inhibition of the NALP3 inflammasome and caspase-1 in THP-1 macrophages. The NALP3 inflammasome and caspase-1 may therefore serve as therapeutic targets for TSD in gouty arthritis.
Supplementary Material
Acknowledgements
The authors would like to thank Ms. Guoying Li (College of Integrative Medicine, Anhui University of Chinese Medicine) for her help with extracting Dioscorea collettii.
Glossary
Abbreviations
- TSD
total saponins of Dioscorea collettii
- MSU
monosodium urate
- MRC
mitochondrial respiratory chain
Funding
The current study was supported by the Fund of the National Natural Science Foundation of China (grant no. 81573670).
Availability of data and materials
All data generated or analyzed during this study are included in this published article
Authors' contributions
GC and LL conceived and designed the study. LW, LZ and CD performed the experiments and data analysis. LW, LZ and LL wrote the manuscript. All authors reviewed, edited and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lu X, Li X, Zhao Y, Zheng Z, Guan S, Chan P. Contemporary epidemiology of gout and hyperuricemia in community elderly in Beijing. Int J Rheum Dis. 2014;17:400–407. doi: 10.1111/1756-185X.12156. [DOI] [PubMed] [Google Scholar]
- 2.Stamp LK, Chapman PT. Gout and its comorbidities: Implications for therapy. Rheumatology (Oxford) 2013;52:34–44. doi: 10.1093/rheumatology/kes211. [DOI] [PubMed] [Google Scholar]
- 3.Pan BQ, Pan JK, Liu J, Yang JY. Analysis on medication rule in herbal prescriptions for gout based on apriori and clustering algorithm. China J Trad Chin Med Pharmacy. 2014;29:2040–2043. (In Chinese) [Google Scholar]
- 4.Zhu L, Dong Y, Na S, Han R, Wei C, Chen G. Saponins extracted from Dioscorea collettii rhizomes regulate the expression of urate transporters in chronic hyperuricemia rats. Biomed Pharmacother. 2017;93:88–94. doi: 10.1016/j.biopha.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Kingsbury SR, Conaghan PG, McDermott MF. The role of the NLRP3 inflammasome in gout. J Inflamm Res. 2011;4:39–49. doi: 10.2147/JIR.S11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 7.Huang HG, Sun YF, Hu M, Yuan GS, Wang Q, Liu YX. Characteristics of monosodium urate monohydrate crystal-induced acute arthritis in rats that mimicked human gouty arthritis. Bulletin of the Academy of Military (Medicinal Sciences) 2005;29:538–542. [Google Scholar]
- 8.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami A, Tani M, Chiba T, Yui K, Shinozaki S, Nakajima K, Tanaka A, Shimokado K, Yoshida M. Pitavastatin inhibits remnant lipoprotein-induced macrophage foam cell formation through ApoB48 receptor-dependent mechanism. Arterioscler Thromb Vasc Biol. 2005;25:424–429. doi: 10.1161/01.ATV.0000152632.48937.2d. [DOI] [PubMed] [Google Scholar]
- 10.Park EK, Jung HS, Yang HI, Yoo MC, Kim C, Kim KS. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res. 2007;56:45–50. doi: 10.1007/s00011-007-6115-5. [DOI] [PubMed] [Google Scholar]
- 11.Xian H, Wang P, Jing H, Chen GQ, Cheng DF, Ji F, Song S, Zhang L. Comparative study of components and anti-oxidative effects between sulfated polysaccharide and its iron complex. Int J Biol Macromol. 2018;118:1303–1309. doi: 10.1016/j.ijbiomac.2018.04.177. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Zheng J, Yu Y, Wang L. Panax notoginseng saponins regulate macrophage polarization under hyperglycemic condition via NF-κB signaling pathway. Biomed Res Int. 2018;2018:9239354. doi: 10.1155/2018/9239354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol. 1996;59:555–561. doi: 10.1002/jlb.59.4.555. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 15.Chhana A, Pool B, Callon KE, Tay ML, Musson D, Naot D, McCarthy G, McGlashan S, Cornish J, Dalbeth N. Monosodium urate crystals reduce osteocyte viability and indirectly promote a shift in osteocyte function towards a proinflammatory and proresorptive state. Arthritis Res Ther. 2018;20:208. doi: 10.1186/s13075-018-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sil P, Wicklum H, Surell C, Rada B. Macrophage-derived IL-1 beta enhances monosodium urate crystal-triggered NET formation. Inflamm Res. 2017;66:227–237. doi: 10.1007/s00011-016-1008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Mankan AK, Dau T, Jenne D, Hornung V. The NLRP3/ASC/Caspase-1 axis regulates IL-1β processing in neutrophils. Eur J Immunol. 2012;42:710–715. doi: 10.1002/eji.201141921. [DOI] [PubMed] [Google Scholar]
- 20.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010;184:5743–5754. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng SC, Zhu XX, Xue Y, Zhang LH, Zou HJ, Qiu JH, Liu Q. Role of the NLRP3 inflammasome in the transient release of IL-1β induced by monosodium urate crystals in human fibroblast-like synoviocytes. J Inflamm (Lond) 2015;12:30. doi: 10.1186/s12950-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gicquel T, Robert S, Loyer P, Victoni T, Bodin A, Ribault C, Gleonnec F, Couillin I, Boichot E, Lagente V. IL-1β production is dependent on the activation of purinergic receptors and NLRP3 pathway in human macrophages. FASEB J. 2015;29:4162–4173. doi: 10.1096/fj.14-267393. [DOI] [PubMed] [Google Scholar]
- 24.Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75:209–219. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landis RC, Haskard DO. Pathogenesis of crystal-induced inflammation. Curr Rheumatol Rep. 2001;3:36–41. doi: 10.1007/s11926-001-0049-7. [DOI] [PubMed] [Google Scholar]
- 27.Lamprecht P, Till A, Kabelitz D. New aspects of the pathogenesis of gout. Danger signals, autoinflammation and beyond. Z Rheumatol. 2008;67:151–156. doi: 10.1007/s00393-007-0254-5. (In German) [DOI] [PubMed] [Google Scholar]
- 28.Janssen CA, Oude Voshaar MAH, Vonkeman HE, Jansen TLTA, Janssen M, Kok MR, Radovits B, van Durme C, Baan H, van de Laar MAFJ. Anakinra for the treatment of acute gout flares: A randomized, double-blind, placebo-controlled, active-comparator, non-inferiority trial. Rheumatology (Oxford) 2019 doi: 10.1093/rheumatology/key402. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 29.Sundy JS, Schumacher HR, Kivitz A, Weinstein SP, Wu R, King-Davis S, Evans RR. Rilonacept for gout flare prevention in patients receiving uric acid-lowering therapy: results of RESURGE, a phase III, international safety study. J Rheumatol. 2014;41:1703–1711. doi: 10.3899/jrheum.131226. [DOI] [PubMed] [Google Scholar]
- 30.Schlesinger N, Alten RE, Bardin T, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: Results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis. 2012;71:1839–1848. doi: 10.1136/annrheumdis-2011-200908. [DOI] [PubMed] [Google Scholar]
- 31.Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, Coyfish M, Guillo S, Jansen TL, Janssens H, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 32.Chen GL, Wei W, Xu SY. Effect and mechanism of total saponin of dioscorea on animal experimental hyperuricemia. Am J Chin Med. 2006;34:77–85. doi: 10.1142/S0192415X06003655. [DOI] [PubMed] [Google Scholar]
- 33.Martin WJ, Walton M, Harper J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 2009;60:281–289. doi: 10.1002/art.24185. [DOI] [PubMed] [Google Scholar]
- 34.Pope RM, Tschopp J. The role of interleukin-1 and the inflammasome in gout: Implications for therapy. Arthritis Rheum. 2007;56:3183–3188. doi: 10.1002/art.22938. [DOI] [PubMed] [Google Scholar]
- 35.Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol. 2006;177:6370–6378. doi: 10.4049/jimmunol.177.9.6370. [DOI] [PubMed] [Google Scholar]
- 36.Martin WJ, Shaw O, Liu X, Steiger S, Harper JL. Monosodium urate monohydrate crystal-recruited noninflammatory monocytes differentiate into M1-like proinflammatory macrophages in a peritoneal murine model of gout. Arthritis Rheum. 2011;63:1322–1332. doi: 10.1002/art.30249. [DOI] [PubMed] [Google Scholar]
- 37.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: A sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 38.Choe JY, Jung HY, Park KY, Kim SK. Enhanced p62 expression through impaired proteasomal degradation is involved in caspase-1 activation in monosodium urate crystal-induced interleukin-1β expression. Rheumatology (Oxford) 2014;53:1043–1053. doi: 10.1093/rheumatology/ket474. [DOI] [PubMed] [Google Scholar]
- 39.Terkeltaub R, Zachariae C, Santoro D, Martin J, Peveri P, Matsushima K. Monocyte-derived neutrophil chemotactic factor/interleukin-8 is a potential mediator of crystal-induced inflammation. Arthritis Rheum. 1991;34:894–903. doi: 10.1002/art.1780340716. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, Zhang D, Hu D, Zhou X, Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol. 2018;103:115–124. doi: 10.1016/j.molimm.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Punzi L, Scanu A, Ramonda R, Oliviero F. Gout as autoinflammatory disease: New mechanisms for more appropriated treatment targets. Autoimmun Rev. 2012;12:66–71. doi: 10.1016/j.autrev.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Zheng SC, Zhu XX, Xue Y, Zhang LH, Zou HJ, Qiu JH, Liu Q. Role of the NLRP3 inflammasome in the transient release of IL-1 β induced by monosodium urate crystals in human fibroblast-like synoviocytes. J Inflamm (Lond) 2015;12:30. doi: 10.1186/s12950-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu W, Sha N, Guang-liang C. Effect of total saponin of Dioscorea on NALP3 inflammasome signaling pathway with acute gouty in rats. Chinese Pharmacological Bulletin. 2017;33:354–360. [Google Scholar]
- 44.Blomgran R, Patcha Brodin V, Verma D, Bergström I, Söderkvist P, Sjöwall C, Eriksson P, Lerm M, Stendahl O, Särndahl E. Common genetic variations in the NALP3 inflammasome are associated with delayed apoptosis of human neutrophils. PLoS One. 2012;7:e31326. doi: 10.1371/journal.pone.0031326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 46.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J Inflamm Res. 2015;8:15–27. doi: 10.2147/JIR.S51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menu P, Vince JE. The NLRP3 inflammasome in health and disease: The good, the bad and the ugly. Clin Exp Immunol. 2011;166:1–15. doi: 10.1111/j.1365-2249.2011.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mason DR, Beck PL, Muruve DA. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of non-microbial inflammation and diseases. J Innate Immun. 2012;4:16–30. doi: 10.1159/000334247. [DOI] [PubMed] [Google Scholar]
- 53.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: First line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Q, Yu DH, Zhang N, Liu SM. Anti-inflammatory effect of total saponin fraction from Dioscorea nipponica Makino on gouty arthritis and its influence on NALP3 inflammasome. Chin J Integr Med. 2017;25:663–670. doi: 10.1007/s11655-016-2741-5. [DOI] [PubMed] [Google Scholar]
- 55.Yang D, Elner SG, Bian ZM, Till GO, Petty HR, Elner VM. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85:462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bordt EA, Polster BM. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic Biol Med. 2014;76:34–46. doi: 10.1016/j.freeradbiomed.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Won JH, Park S, Hong S, Son S, Yu JW. Rotenone-induced impairment of mitochondrial electron transport chain confers a selective priming signal for NLRP3 inflammasome activation. J Biol Chem. 2015;290:27425–27437. doi: 10.1074/jbc.M115.667063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article