Abstract
Epigallocatechin-3-gallate (EGCG) is beneficial for inhibiting dyslipidemia and reducing hyperlipidemic risk. The purpose of the present study was to investigate the glycolipid regulatory effects and potential mechanisms of EGCG in a high-fat diet and streptozotocin-induced type 2 diabetes mellitus (T2DM) mouse model. The results demonstrated that EGCG can decrease blood glucose levels and increase insulin resistance in T2DM mice. In addition, EGCG can regulate serum lipid levels, including those of total cholesterol, triglyceride and low-density lipoprotein receptor (LDL-r), and reduce lipid deposition in vascular endothelial cells in a dose-dependent manner. In addition, the gene and protein expression of related scavenger receptors, including cluster of differentiation 36, sterol regulatory element binding protein 2 (SREBP), SREBP cleavage-activating protein and LDL-r, were downregulated in a dose-dependent manner. The present study noted that EGCG possesses potential as a natural product for preventing and treating metabolic hyperlipidemia syndrome, probably by reducing the blood lipid levels, alleviating vascular endothelial cell damage, maintaining normal lipid metabolism in blood vessels and ameliorating glycolipid disorders.
Keywords: epigallocatechin-3-gallate, diabetic, deposition, sterol regulatory element binding protein 2, sterol regulatory element binding protein 2 cleavage-activating protein, low-density lipoprotein receptor
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic, systemic metabolic disease related to a variety of genetic and environmental factors (1). Glycolipid metabolism disorder is a consequence of T2DM (2). Normally, glycolipid metabolism is maintained in a stable state to provide energy, but disorders of glycolipid metabolism play a primary role in obesity and can increase the risk of various diseases, such as T2DM and vascular endothelial dysfunction, which are closely related to cardiovascular diseases (3). Additionally, endothelial dysfunction can accelerate the pathological process of atherosclerosis, hypertension, T2DM and cerebrovascular disease (4). Lipoprotein levels are also disturbed or complicated by glycolipid metabolism disorders, accompanied by a reduction in high-density lipoprotein (HDL) levels and an increase in total cholesterol (TC), triglyceride (TG) and low-density lipoprotein (LDL) levels (5). Glycolipid metabolism disorders lead to insulin resistance and hyperlipidemia in T2DM mice (2). Hyperlipidemia is classified as one of the major risk factors leading to cardiovascular disease (6). In recent years, the impact of proinsulin C-peptide has been highlighted in the development of vascular disease. It plays an important role in both type 1 DM and T2DM (7). A study among T2DM patients has shown that with the progression of diabetic retinopathy, there are lower levels of fasting and 2 h postprandial C-peptide compared with healthy individuals (8). T2DM may progress to a late stage due to pancreatic β-cell demise and C-peptide deficiency (9).
Cluster of differentiation (CD)36, a member of the scavenger receptor B (SRB) family, is the key transcription factor responsible for fatty acid synthesis, lipid uptake and catabolism, and is expressed predominantly by vascular cells (10). CD36 has increasingly been considered as a possible biomarker for obesity, metabolic syndrome and T2DM (11). CD36 protein serves a role in lipid metabolism and insulin resistance, and the expression of CD36 is inducible in obesity (12). Studies in CD36-deficient humans and spontaneously hypertensive rats suggested that CD36 is responsible for hyperlipidemia and insulin resistance (13,14). Inhibition of vascular endothelial cell-specific CD36 directly contributes to a reduction in lipid levels that correlates with improvement in the lipid markers LDL-r and TG (15,16). CD36 not only has been implicated in mediating lipid internalization, but also can bind modified forms of LDL r and increase excessive cholesterol accumulation in macrophages (17). CD36 is the main cause of atherosclerotic plaque formation induced by vascular lipid deposition (18). However, the mechanism whereby CD36 controls glycolipid metabolism associated with T2DM remains to be elucidated.
Sterol regulatory element binding protein 2 (SREBP-2) is a main regulator that controls cholesterol biosynthesis gene expression (19). CD36 can regulate SREBP-2 expression and subsequently stimulate the transcription of various lipogenic genes (20). It has been reported that the SREBP cleavage-activating protein (SCAP)/SREBP2/LDL-r pathway plays a crucial role in regulating lipid droplets in numerous diseases (21). This pathway regulates the transduction of a number of factors related to insulin sensitivity and glycolipid metabolism (21). Lipid accumulation can be due to the upregulation of SREBP2, its target gene LDL-r and its regulatory gene scavenger receptor A(SRA), the subtype SRA1 (22) was examined in the present study.
Epigallocatechin-3-gallate (EGCG) is a major polyphenol found in green tea. Preclinical studies in animals, and studies in cell line models and humans have reported the beneficial effects of EGCG (23) on obesity-related parameters, including decreases in body weight (24–26), adipose mass (24), food intake (27) and total lipid, TC and TG levels in the liver and plasma, and improvement of glucose homeostasis (24,25,28). Treatment of C57BL/6J mice with 0.32% dietary EGCG for 16 weeks has been shown to reduce body weight gain and markers of T2DM induced by a high-fat diet (HFD) (24). EGCG reduces the reactive oxygen species content of the serum of obese KK-ay mice, as well as that of 3T3-L1 adipose cells, whilst also decreasing glucose levels and increasing glucose tolerance in animals (29). Research has shown beneficial effects of the anti-lipid deposition activity of EGCG, and indicated that it may affect SREBP and CD36 in blood vessels (30). Chen et al (31), found that EGCG reduced oxidized LDL (ox-LDL) levels, decreased SRA expression (but not that of CD36), and suppressed ox-LDL uptake and foam cell formation in human macrophages in a dose-dependent manner. Furthermore, there are other mechanisms involved in the effects of EGCG on endothelial dysfunction. This includes prevention of oxidative injury in cultured endothelial cell lines via the downregulation of intercellular adhesion molecule 1 expression and 4-hydroxynonenal accumulation, and restoration of nitrite/nitrate levels and nitric oxide synthase 3 activity. EGCG can also protect endothelial cells via the downregulation of transforming growth factor-β and the Bax/Bcl-2/caspase 3 signaling pathway in the progression of diabetic nephropathy in rats (32,33).
Therefore, the present study investigated the role of EGCG in regulating a macrovascular glycolipid metabolic disorder in T2DM, and the results may provide improved understanding of T2DM and its mechanisms.
Materials and methods
Animals
A total of 67 C57BL6/J mice (male; 18–22 g; 4 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. The mice were raised under controlled temperature (23±1°C) and humidity (50±5%) conditions with a 12 h light/dark cycle. The animals were allowed free access to drinking water and food. The animals were acclimated to the housing conditions for 7 days before the experiments. The health and behavior of each group of mice were observed daily. All experiments were approved by the Ethics Review Committee for Animal Experimentation of the Institute of Clinical Pharmacology, Kunming Medical University.
Materials
The levels of TC (cat. no. CS0005; Sigma-Aldrich; Merck KGaA), TG (cat. no. MAK266; Sigma-Aldrich; Merck KGaA) and LDL-r (cat. no. RAB0707; Sigma-Aldrich; Merck KGaA) were measured using ELISA kits. An Oil Red O staining kit (cat. no. ab150678; Abcam) was also employed. The primary antibodies were anti-β-actin (cat. no. ab8227; 1:4,000; Abcam), anti-SRA1 (1:500; cat. no. sc-166139; Santa Cruz Biotechnology, Inc.), anti-CD36 (1:3,500; cat. no. ab133625; Abcam), anti-SREBP2 (1:3,000; cat. no. ab30682; Abcam) and anti-LDL-r (1:4,000; cat. no. ab52818; Abcam). The secondary antibodies used were Goat IgG horseradish peroxidase (HRP)-conjugated antibody (1:2,000; cat. no. HAF017; R&D Systems, Inc.) and Rabbit IgG HRP-conjugated antibody (1:2,000; cat. no. HAF008; R&D Systems, Inc.).
Drugs
EGCG, a white powder with >95% purity, was provided by the School of Pharmaceutical Sciences and Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University. Pravastatin was obtained as a white power with >97% purity from Sigma-Aldrich; Merck KGaA (cat. no. P4498).
Experimental design
Diabetes was induced in 4-week-old male C57BL/6J mice as described previously (12). Male mice were fed a HFD containing 22% fat, 48% carbohydrates and 20% protein blended with standard laboratory chow consisting of 5% fat, 53% carbohydrates and 23% protein for 4 weeks. After the period of dietary manipulation, animals were deprived of food for 12 h, and then the mice were injected intraperitoneally with a low dose of streptozotocin (STZ; 45 mg/kg; cat. no. 572201; Sigma-Aldrich; Merck KGaA), which was dissolved in 50 nm Na citrate buffer (pH 4.5). Thereafter, the animals had free access to water and standard food (34,35). After 72 h of STZ administration, fasting blood sugar and insulin levels were measured with a reagent kit (cat. no. P006-1-1; Nanjing Jiancheng Bioengineering Institute Co., Ltd.). Mice were deprived for food for 6 h, and then fasting blood glucose levels were assessed, blood was drawn from the tail and body weight was measured weekly. Mice with fasting blood glucose ≥16.7 mmol/l were considered diabetic (36). The diabetic mice were randomly divided into 5 groups (n=12/group): A diabetic model group, a pravastatin group (40 mg/kg body weight administered daily) and 3 EGCG groups. A total of 20 mice had succumbed to STZ toxicity during modeling (37) so they were euthanized after reaching a humane endpoint, leaving only 8 in each diabetic model group. This level of mortality was accounted for in the ethical approval granted for this study. The health and behavior of each group of mice were observed daily.
EGCG was suspended in a 0.5% sodium carboxymethyl cellulose (CMC-Na) solution. Normal mice (n=7) and mice in the diabetic model groups (n=8/group) received CMC-Na. EGCG-treated diabetic mice received EGCG at 50, 100, or 200 mg/kg/day. All substances were given by oral gavage once daily for 6 weeks. Mouse weights were measured every 3 days. The duration of the animal experiment was 10 weeks. The longer the diabetic model mice were kept, the clearer their symptoms were (poor mental state, inactivity, reduced activity capacity, dull hair, eating more and urinating more), and the more evident their distress. The setting of the sampling time point was based on the relevant literature of EGCG (38,39), and according to our previous preliminary experiments (data not shown), in order to ensure that the effects of EGCG could be evaluated while minimizing the distress of the experimental mice. All the procedures of the experiments in the present study were designed to reduce the suffering of experimental animals as much as possible in order to guarantee the stability of experimental results. At the end of the experiments, the animals were anesthetized with 1% sodium pentobarbital (50 mg/kg) via intraperitoneal injection. Blood was collected from the inferior vena cava into heparinized syringes, followed by centrifugation (1,300 × g, 20 min, 4°C). The serum was collected and stored in a deep freezer before analysis. Blood was taken from the inferior vena cava under anesthesia, and the hearts stopped beating and the mice succumbed after the blood was collected. The total volume of blood obtained from each mouse was 0.6±0.2 ml/mouse. After the blood was collected, each mouse experienced a 4–6% reduction in body weight. Additionally, respiratory arrest and cardiac arrest were monitored for 30 min to assess animal mortality. The abdominal aortas from each group were also obtained. All samples were kept at −80°C for mRNA, protein and oil red O staining analysis.
Insulin tolerance test (ITT)
After 10 weeks of EGCG treatment, mice underwent an oral ITT (40). Mice were deprived of food for 4 h, and the ITT was performed after intraperitoneal injection of insulin (0.75 UI/kg). Blood glucose concentrations were measured at 0 min, and then at 15, 30, 60, 90 and 120 min after insulin injection using a portable Contour® glucose monitor (Bayer AG) and test strips. The concentration of insulin C-peptide in blood serum at 120 min was measured using a mouse insulin C-peptide radioimmunoassay kit (cat. no. DIC P00; R&D Systems, Inc.), according to the manufacturers protocols.
Serum biochemical analysis
Serum was obtained from blood samples by centrifugation (1,350 × g, 4°C, 10 min) and stored at 80°C. Serum TG, TC and LDL-r levels were investigated with commercial assay kits and an automated Mindray BS-200 biochemistry analyzer instrument (Mindray Bio-Medical Electronics Co,. Ltd.).
Reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted from the tissue of each abdominal aorta using TRIzol® (Thermo Fisher Scientific, Inc.). cDNA was synthesized from RevertAid First Strand cDNA Synthesis kit (cat. no. K1621; Thermo Fisher Scientific, Inc.) according to the manufacturers instructions. RT-qPCR was performed using SYBR Green PCR master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling conditions included, initial denaturation, 95°C for 10 min; followed by 40 cycles of 95°C for 5 sec and 60°C for 35 sec. The following primer sequences: CD36 forward, 5 CTGCTGTTCTTTGCCACGTC-3 and reverse, 5 CTGCTGTTCTTTGCCACGTC-3; SRA1 forward, 5 CCCCAGGTTCTTCACTACGCC-3 and reverse, 5 TCCTTATCCTGGGAGCCCTT-3; SREBP2 forward, 5 AGCATACCGCAAGGTGTTCC-3 and reverse, 5 CCAGGTGTCTACTTCTCCGTGT-3; LDL-r forward, 5 CAGCGTATCTGTGGCTGACA-3 and reverse, 5 AGTGTCGACTTCTCTAGGCT-3; β-actin forward, 5 GGCTGTGCTATCCCTGTACG-3 and reverse, 5 TTGATCTTCATTGTGCTGGGTG-3. Expression was analyzed with the 2−∆∆Cq method (41) and gene expression was presented as expression relative to that of the reference gene β-actin.
Oil red O staining
Fresh-frozen optimal cutting temperature compound-embedded tissue sections were stained after 8-µm slices were obtained on a cryostat. The slides were placed in propylene glycol for 2 min and then incubated in oil red O solution for 6 min at room temperature. A mixture of 85% propylene glycol in distilled water was prepared, and the tissue sections were incubated in this mixture for 1 min at room temperature. The slides were rinsed in 2 changes of distilled water and then thoroughly rinsed in tap water at room temperature. For oil red O quantification, the slides were dried, and 250 µl of isopropanol was added, followed by incubation for 3 min at room temperature; the eluted solution was read at 510 nm using a Varioskan LUX multimode microplate reader (Thermo Fisher Scientific, Inc.).
Western blot analysis
Protein from all frozen abdominal aorta samples was extracted with 1 ml of RIPA buffer (150 mM sodium chloride 1.0% NP40 or Triton X-100 Tris, pH 8.0). Protease and phosphatase inhibitor cocktails (cat. no. 78439; Thermo Fisher Scientific, Inc.) were added to the homogenization buffer. The concentration of protein in the supernatant of each sample was determined by using a Pierce™ Bradford assay kit (cat. no. 23200; Thermo Fisher Scientific, Inc.). The protein was then loaded using loading dye (40 µg protein/well) and separated in 8–12% SDS-PAGE gels. PVDF membranes were blocked with 0.5% BSA (cat. no. 17879-45-7; Guidechem), washed and incubated with the primary antibodies (1:1,000) described in the Materials section at 4°C overnight. The membranes were then blotted with the corresponding secondary antibody (1:2,000; cat. nos. HAF017 and HAF008; R&D Systems, Inc.) at room temperature for 1 h, and antigen-antibody interactions were detected by using Pierce ECL reagents (Thermo Fisher Scientific, Inc.) and quantified using a C-DiGit blot scanner (LI-COR Biosciences) and in-built Image Studio DiGit software (Flow max 2.82). Protein expression was presented as expression relative to that of the reference protein β-actin.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, Inc.) and P<0.05 was considered to indicate a statistically significant difference. Students t-test was used to compare the two groups. One-way ANOVA followed by Tukeys post hoc test or repeated measures ANOVA was used to determine the significance of the differences between groups. The data are presented as the means ± standard error of the means unless otherwise stated.
Results
EGCG increases insulin sensitivity and decreases blood sugar levels in T2DM mice
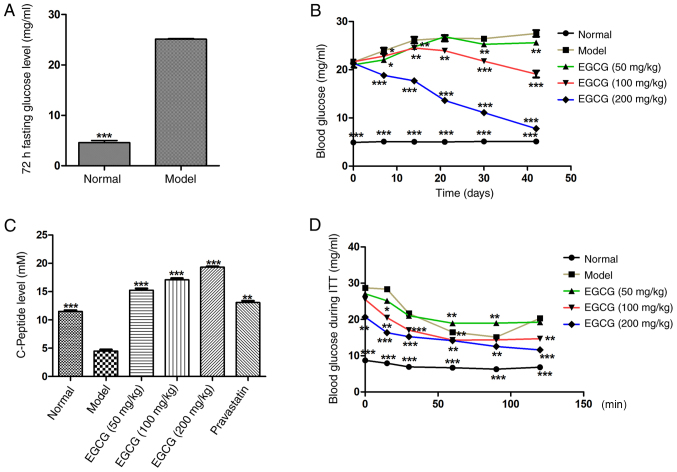
To investigate whether EGCG reversed insulin resistance and blood sugar in T2DM mice, EGCG was administered to T2DM mice for 6 weeks; fasting blood glucose was measured and ITTs were performed. It was found that 72 h fasting blood glucose (normal 5.12±0.29 mg/ml vs. model 25.01±1.22 mg/ml; P<0.001; Fig. 1A) was significantly increased in the model group after treatment with EGCG for 6 weeks. Blood glucose in the EGCG (200 mg/kg) group was significantly lower than in the model group at each tested time point (P<0.01; Fig 1B), and the decrease occurred in a dose-dependent manner [glucose in the model group (26.17±1.53 mg/ml) vs. the EGCG (200 mg/kg) group (7.76±1.09 mg/ml)]. The results demonstrated that EGCG could decrease glucose levels in T2DM mice in a dose-dependent manner (Fig. 1B).
Figure 1.
Effects of EGCG on the concentration of blood glucose and insulin resistance. (A) Effects of EGCG on the 72-h fasting glucose level. (B) Effects of EGCG on blood glucose after 6 weeks treatment with EGCG. EGCG (200 mg/kg) decreased blood glucose. (C) EGCG increased the C-peptide levels in T2DM mice at 120 min following insulin injection. (D) Blood glucose levels of all groups at 0, 15, 30, 60, 90 and 120 min following insulin injection. Comparisons for (A) were assessed with Student's t-test. Comparisons of (C) were assessed with one-way ANOVA followed by Tukey's post hoc test. **P<0.01, ***P<0.001 vs. model group. Comparisons of (B and D) were assessed with repeated measures ANOVA. *P<0.05, **P<0.01, ***P<0.001 vs. model at the respective time points. EGCG, epigallocatechin-3-gallate; T2DM, type 2 diabetes mellitus.
The response to extrinsic insulin in the mice was investigated by an ITT
The results demonstrated that C-peptide levels in the EGCG group were significantly higher than in the model group at the 120-min time point (P<0.001; Fig. 1C), and this increase occurred in a dose-dependent manner. The blood glucose levels at the 120 min after insulin injection in the EGCG (100 and 200 mg/kg) group were significantly lower than in the model group (P<0.001; Fig. 1D). The results demonstrated that EGCG could decrease insulin resistance and ameliorate insulin sensitivity in T2DM mice.
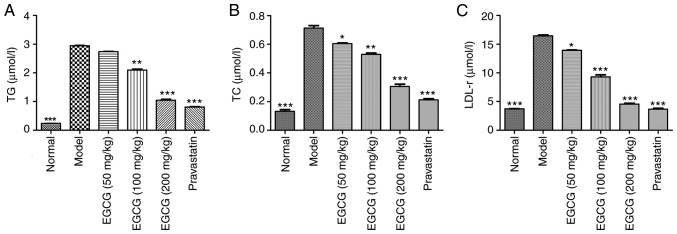
EGCG decreases the contents of TG, TC and LDL-r in T2DM mice
STZ-induced diabetic mice exhibited significantly increased blood serum levels of TG, TC and LDL r compared with normal mice (P<0.001). EGCG (100, 200 mg/kg) decreased serum levels of TG, TC and LDL-r, as did pravastatin (P<0.05; Fig. 2A-C).
Figure 2.
Effects of EGCG on TG and LDL-r levels in serum. (A) Effects of EGCG on the concentration of TG. (B) Effects of EGCG on the concentration of TC. (C) Effects of EGCG on the concentration of LDL-r. The levels of serum TG and LDL-r were significantly increased in diabetic mice. Comparisons were assessed with one-way ANOVA followed by Tukey's post hoc test. *P<0.05, **P<0.01, ***P<0.001 vs. model group. EGCG, epigallocatechin-3-gallate; TG, triglyceride; TC, total cholesterol; LDL-r, low-density lipoprotein receptor.
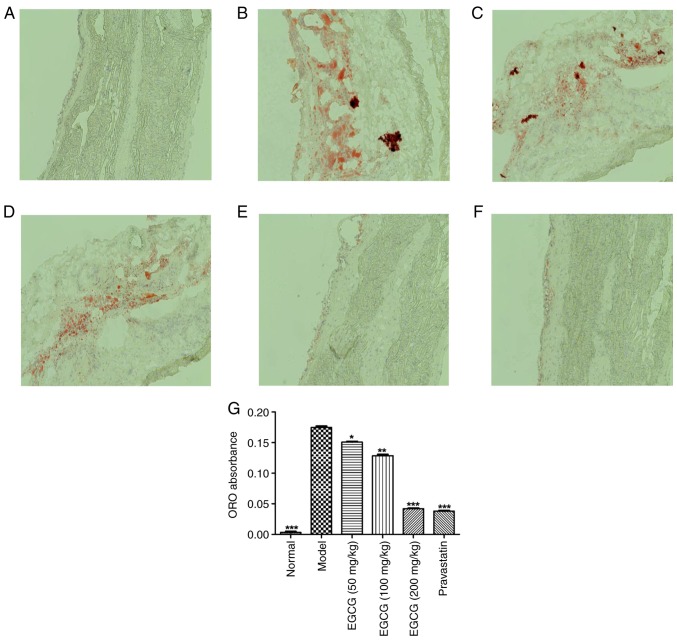
EGCG controls lipid deposition in the blood vessels of T2DM mice
The effects of EGCG on lipid deposition in blood vessels were investigated, as shown in Fig. 3. The lipid droplets in the blood vessels of the model group were irregular and larger, and they were significantly greater in number than those observed in the normal group (P<0.001). EGCG (200 mg/kg) decreased the number of lipid droplets to a similar extent as pravastatin (P>0.05).
Figure 3.
EGCG repression controls lipid deposition in blood vessels of diabetic mice. Photomicrographs of oil red O-stained blood vessels in (A) the normal group, (B) the model group, (C) the EGCG (50 mg/kg) group, (D) the EGCG (100 mg/kg) group, (E) the EGCG (200 mg/kg) group and (F) the pravastatin group; magnification, 200×. (G) Quantification of oil red O-stained blood vessels for all groups. Comparisons were assessed with one-way ANOVA followed by Tukey's post hoc test. *P<0.05, **P<0.01, ***P<0.001 vs. model group. EGCG, epigallocatechin-3-gallate.
EGCG reduces the gene expression of SRA1, CD36, SREBP2 and LDL-r in the blood vessels of T2DM mice
All mRNA products of all tested genes were within normal values (Cq=16-30). The levels of the loading control and β-actin were constant in all tested samples. Significant increases in the relative mRNA expression of SRA1, CD36, SREBP2 and LDL-r were observed in the blood vessel samples obtained from STZ-induced mice compared with the normal mice. However, the EGCG group exhibited reduced gene expression of SRA1, CD36, SREBP2 and LDL r in blood vessels compared with the model group (P<0.01 or P<0.001; Fig. 4).
Figure 4.
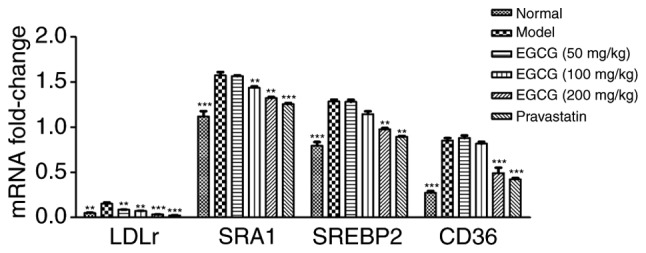
Relative mRNA expression of SRA1, SREBP2, LDL-r and CD36 in the blood vessels of STZ-induced treated mice. Significant increases in mRNA relative expression of SRA1 and CD36, SREBP2 and LDL-r were observed in the blood vessels from STZ-induced treated mice, compared with the normal mice. However, EGCG (100 mg/kg, 200 mg/kg) was able to reduce the gene expression of SRA1 and CD36, SREBP2 and LDL-r in blood vessels. Comparisons were assessed with one-way ANOVA followed by Tukey's post hoc test. **P<0.01, ***P<0.001 vs. model group. SRA, scavenger receptor A; CD, cluster of differentiation; SREBP2, sterol regulatory element binding protein 2; LDL-r, low-density lipoprotein receptor; STZ, streptozotocin; EGCG, epigallocatechin-3-gallate.
EGCG inhibits the expression of SRA1, CD36, SREBP2, and LDL-r in diabetic mice
As shown in Fig. 5, the protein levels of SRA1, SREBP2 and LDL-r were increased in the diabetic mice compared with the normal group (P<0.001). EGCG reduced the expression of SRA1 (P<0.05), CD36 (P<0.001), SREBP2 (P<0.001) and LDL-r (P<0.001) in a dose-dependent manner; EGCG downregulated the levels of SREBP2 and CD36 in a similar manner to pravastatin (P>0.05).
Figure 5.
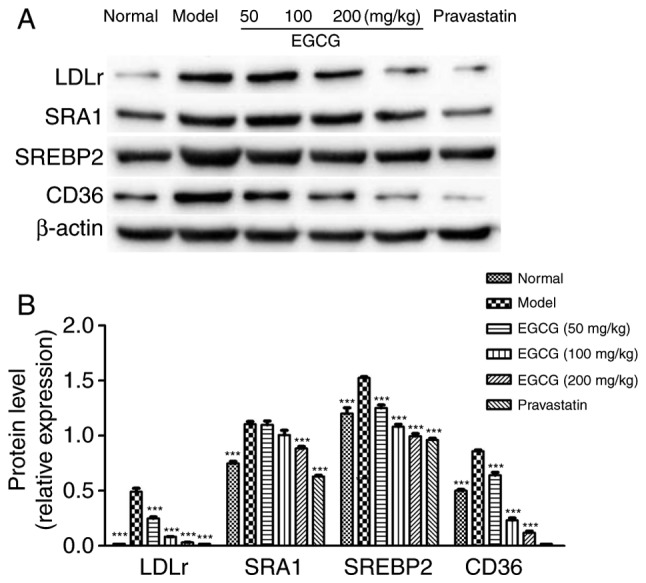
Protein levels of SRA1, CD36, SREBP2 and LDL-r. (A) Western blot analysis of SRA1, CD36, SREBP2 and LDL-r. (B) Quantification of the target protein level. Comparisons were assessed with one-way ANOVA followed by Tukey's post hoc test. **P<0.01, ***P<0.001 vs. model group. SRA1, scavenger receptor A; CD, cluster of differentiation; SREBP2, sterol regulatory element binding protein 2; LDL-r, low-density lipoprotein receptor.
Discussion
Lipid metabolic dysfunction is associated with the development and progression of high-mortality diseases, including T2DM, as well as the subsequent associated cardiovascular and cerebrovascular pathologies (42). Lipoprotein levels are also disturbed or complicated by lipid metabolism disorders, accompanied by a reduction in HDL levels, and an increase in TC, TG and LDL-r levels (43). The present study identified that the levels of TC, TG and LDL were increased in HFD and STZ-induced T2DM mice. The serum TC, TG and LDL levels of the T2DM group were significantly higher compared with the normal group, showing that a hyperlipidemia T2DM mouse model had been successfully established. A previous clinical trial revealed that the serum levels of TG and LDL-r are significantly higher in T2DM patients with vascular lesions compared with T2DM patients without such complications (44). The HFD and STZ-induced diabetic mice also demonstrated dyslipidemia, as evidenced by elevated levels of TC, LDL-r and TG, with a concomitant decrease in HDL, as observed in human T2DM (45), which was consistent with the findings of the present study. Additionally, decreased expression of LDL-r and TG can reduce lipid accumulation in vascular lesions (46). To observe this effect, lipid deposition in the vascular endothelium was measured by oil red O staining, and it was found that the lipid droplets in the blood vessels of the T2DM group were irregular and larger compared with those observed in the normal group.
The present study observed increased levels of TG and LDL-r in the serum of HFD/STZ-induced T2DM mice, as well as large amounts of lipid droplets deposited in vascular endothelial cells. The model mice treated with HFD/STZ had the same genetic background as the normal mice. This model involved the combination of HFD feeding to induce hyperlipidemia, hyperinsulinemia and glucose intolerance, and subsequent treatment with STZ (47–49), which resulted in a reduction in the number of functional β-cells. HFD/STZ-induced mice are designed to mimic the pathology of T2DM but on a shorter time scale than in humans. As pravastatin is not soluble in water, a suspension was made in 0.5% CMC-Na. In order to achieve solvent consistency, 0.5% CMC-Na was also used to dissolve the EGCG. CMC-Na is recognized as a solvent without any effect in pharmacological research.
In recent years, drugs used for the treatment of patients with T2DM, such as rosiglitazone and metformin, have been found to induce various side effects; in addition, they are unable to prevent angiopathy (50). Due to the lack of effective and applicable pharmacological treatments for T2DM with hyperlipidemia and related vascular lesion dysfunctions, an increasing number of researchers are now studying traditional medicines. The investigation of the therapeutic functions of traditional medicinal herbs in T2DM presents promising potential.
TC, TG and LDL-r are the main markers of T2DM with angiopathy; a previous study indicated that they directly increase the risk of hyperlipidemia (51). To investigate the protective mechanisms of EGCG, the expression of TC, TG and LDL-r in serum was tested. The results demonstrated that compared with the T2DM group, the EGCG treatment (200 mg/kg) group exhibited marked reductions in serum TC, TG and LDL levels. C-peptide is not affected by exogenous insulin, nor is it degraded by the liver (52). It can directly reflect the secretion of endogenous insulin and the function of islet β-cells (52). The ITT results of the present study demonstrated that EGCG (100 and 200 mg/kg) reduced blood glucose levels and promoted C-peptide levels. These results indicated that EGCG ameliorated the degree of fatty degeneration in vascular endothelial cells and modulated the expression levels of lipid metabolism genes in a dose-dependent manner.
To further confirm the protective mechanism of EGCG against vascular endothelial cell injury caused by lipid metabolism disorder in HFD and STZ-induced T2DM mice, gene and protein expression levels in vascular endothelial cells were detected. It was found that EGCG downregulated the expression of CD36 in a dose-dependent manner. CD36 has been found to act as a scavenger receptor for ox-LDL and as a cellular transporter of long-chain fatty acids (53), and it is expressed on diverse cell types, including adipocytes, cardiac and skeletal myocytes, retinal pigment epithelial cells, platelets, mononuclear phagocytes and the microvascular endothelium (54). In addition, a CD36 inhibitor can be used to lower lipid levels and reduce cholesterol accumulation in vivo (55).
Yang et al (56), identified enriched genes through Gene Ontology enrichment analyses. These genes were mainly related to biological processes such as steroid metabolism and lipid metabolism; their results indicated that CD36, SCAP, SREBP2 and LDL-r gene expression in the arterial endothelium may be a target of the lipid-reducing action of EGCG, which is consistent with the results of the present study. CD36 is a type of SRB, and scavenger receptor genes are closely related to lipid deposition in vascular endothelial cells and hyperlipidemia. The present study identified that the expression of CD36 was higher in the model group compared with the normal group at the mRNA level and the protein level, and that CD36 upregulated the expression of SREBP2 in the T2DM group. The oil red O staining revealed that the lipid droplets in the blood vessels of mice in the EGCG groups were fewer in number and smaller compared with the model group, particularly in the 200 mg/kg group. In addition, EGCG inhibited the expression of CD36, downregulated the expression of SREBP2 and decreased LDL-r levels.
At present, EGCG is widely used in research. A number of trials of patients with clinical tumors have proved that EGCG is safe and reliable, and oral administration is the main method of drug administration. For example, no adverse reactions were found following 1-year oral EGCG (300 mg/day) in patients with highly differentiated prostate cancer (57). In the study on the safety of EGCG supplementation in postmenopausal women at high risk for breast cancer, only a few subjects who took 1,315 mg/day of green tea extract (with an EGCG content of 843 mg) experienced mild, transient adverse reactions (58). The adverse reactions of EGCG were found to be mild in the dose expansion test of patients with estrogen receptor-negative breast cancer, and the main adverse reactions included nausea, heartburn, abdominal pain, headache, dizziness and myalgia (59). In stage III non-small cell lung cancer treated with radiation and chemotherapy, and patients with breast cancer with neoadjuvant radiotherapy, giving EGCG was reported to be safe, and EGCG prevented radiation and chemotherapy injury, playing a positive role in promoting tissue repair (60,61). These clinical trials of EGCG have shown its low toxicity and wide margin of safety.
Overall, the present study demonstrated that CD36 is a treatment target of EGCG in T2DM mice. EGCG downregulated glucose levels and upregulated C-peptide levels in T2DM mice, which may be associated with the protection of β-cell in the pancreas. In addition, EGCG ameliorates lipid metabolism disorders, perhaps by regulating the SREBP2/SCAP/LDL-r pathway, which is an effective way to control serum cholesterol synthesis levels. It was hypothesized that EGCG could regulate lipid metabolism in T2DM mice through CD36/SRA1/SREBP2/LDL-r pathways, and then reduce blood lipid deposition. Together, the results of the present work suggested that EGCG might be a promising natural hypoglycemic product for the prevention and treatment of lipid regulation disorders and the protection of vascular endothelial cells from lipid injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from Yunnan Applied Basic Research Projects-Joint Special Project [grant no. 2018FE001(−210)] and Yunnan Provincial Education Department Project (grant nos. 2017ZDX157 and 2020J0151).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors contributions
ZR and HY conceived and designed the study. ZR, ZY, YL and RZ performed the experiments. ZY, YL, RZ and HY analyzed the data. ZR, ZY and HY wrote the manuscript. ZR and HY reviewed and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The animal experiments were approved by the Animals Ethics Committee of Kunming Medical University and the Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tanaka S, Tanaka S, Iimuro S, Yamashita H, Katayama S, Ohashi Y, Akanuma Y, Yamada N, Sone H, Japan Diabetes Complications Study Group Cohort profile: The Japan diabetes complications study: a long-term follow-up of a randomised lifestyle intervention study of type 2 diabetes. Int J Epidemiol. 2014;43:1054–1062. doi: 10.1093/ije/dyt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry RJ, Samuel VT, Petersen KF, Shulman GI, RJ P The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 5.Torimoto K, Okada Y, Tanaka Y. Type 2 diabetes and vascular endothelial dysfunction. J UOEH. 2018;40:65–75. doi: 10.7888/juoeh.40.65. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 6.Smith SA. Peroxisome proliferator-activated receptors and the regulation of mammalian lipid metabolism. Biochem Soc Trans. 2002;30:1086–1090. doi: 10.1042/bst0301086. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Lin P, Ma A, Zheng H, Wang K, Li W, Wang C, Zhao R, Liang K, Liu F, et al. C-Peptide is independently associated with an increased risk of coronary artery disease in T2DM subjects: A cross-sectional study. PLoS One. 2015;10:e0127112. doi: 10.1371/journal.pone.0127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai X, Han X, Zhang S, Luo Y, Chen Y, Ji L. Age at diagnosis and C-peptide level are associated with diabetic retinopathy in Chinese. PLoS One. 2014;9:e91174. doi: 10.1371/journal.pone.0091174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massi-Benedetti M, Orsini-Federici M. Treatment of type 2 diabetes with combined therapy: what are the pros and cons? Diabetes Care. 2008;31:S131–135. doi: 10.2337/dc08-s233. [DOI] [PubMed] [Google Scholar]
- 10.Febbraio M, Hajjar DP, Silverstein RL. CD36: A class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Susztak K, Ciccone E, McCue P, Sharma K, Böttinger EP. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med. 2005;2:e45. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautam S, Pirabu L, Agrawal CG, Banerjee M. CD36 gene variants and their association with type 2 diabetes in an Indian population. Diabetes Technol Ther. 2013;15:680–687. doi: 10.1089/dia.2012.0326. [DOI] [PubMed] [Google Scholar]
- 13.Pravenec M, Landa V, Zidek V, Musilova A, Kren V, Kazdova L, Aitman TJ, Glazier AM, Ibrahimi A, Abumrad NA, et al. Transgenic rescue of defective Cd36 ameliorates insulin resistance in spontaneously hypertensive rats. Nat Genet. 2001;27:156–158. doi: 10.1038/84777. [DOI] [PubMed] [Google Scholar]
- 14.Miyaoka K, Kuwasako T, Hirano K, Nozaki S, Yamashita S, Matsuzawa Y. CD36 deficiency associated with insulin resistance. Lancet. 2001;357:686–687. doi: 10.1016/S0140-6736(00)04138-6. [DOI] [PubMed] [Google Scholar]
- 15.Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 16.Martin CA, Longman E, Wooding C, Hoosdally SJ, Ali S, Aitman TJ, Gutmann DAP, Freemont PS, Byrne B, Linton KJ. Cd36, a class B scavenger receptor, functions as a monomer to bind acetylated and oxidized low-density lipoproteins. Protein Sci. 2007;16:2531–2541. doi: 10.1110/ps.073007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med. 2014;46:e99. doi: 10.1038/emm.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trent CM, Yu S, Hu Y, Skoller N, Huggins LA, Homma S, Goldberg IJ. Lipoprotein lipase activity is required for cardiac lipid droplet production. J Lipid Res. 2014;55:645–658. doi: 10.1194/jlr.M043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moslehi A, Hamidi-zad Z. Role of SREBPs in liver diseases: A mini-review. J Clin Transl Hepatol. 2018;28:332–338. doi: 10.14218/JCTH.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi Q, Liang L, Doria A, Hu FB, Qi L. Genetic predisposition to dyslipidemia and type 2 diabetes risk in two prospective cohorts. Diabetes. 2012;61:745–752. doi: 10.2337/db11-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee EJ, Oh H, Kang BG, Kang MK, Kim DY, Kim YH, Lee JY, Ji JG, Lim SS, Kang YH. Lipid-lowering effects of medium-chain triglyceride-enriched coconut oil in combination with licorice extracts in experimental hyperlipidemic mice. J Agric Food Chem. 2018;66:10447–10457. doi: 10.1021/acs.jafc.8b04080. [DOI] [PubMed] [Google Scholar]
- 22.Mysliwiec P, Choromanska B, Winnicka MM, Kaminski K, Mysliwiec H, Dadan J, Supruniuk E, Chabowski A. Interleukin-6 deficiency modifies the effect of high fat diet on myocardial expression of fatty acid transporters and myocardial lipids. J Physiol Pharmacol. 2018;69:69. doi: 10.26402/jpp.2018.4.11. [DOI] [PubMed] [Google Scholar]
- 23.Yoon JY, Kwon HH, Min SU, Thiboutot DM, Suh DH. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J Invest Dermatol. 2013;133:429–440. doi: 10.1038/jid.2012.292. [DOI] [PubMed] [Google Scholar]
- 24.Song X, Du J, Zhao W, Guo Z. Epigallocatechin-3-gallate(EGCG): Mechanisms and the combined applications. Comb Chem High Throughput Screen. doi: 10.2174/1386207321666171218115850. doi:10.2174/1386207321666171218115850. [DOI] [PubMed] [Google Scholar]
- 25.Xiao J, Ho CT, Liong EC, Nanji AA, Leung TM, Lau TY, Fung ML, Tipoe GL. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 2014;53:187–199. doi: 10.1007/s00394-013-0516-8. [DOI] [PubMed] [Google Scholar]
- 26.Annaba F, Kumar P, Dudeja AK, Saksena S, Gill RK, Alrefai WA. Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT. Am J Physiol Gastrointest Liver Physiol. 2010;298:G467–G473. doi: 10.1152/ajpgi.00360.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P, Yu JF, Zhao CG, Sui FX, Teng X, Wu YB. Therapeutic potential of EGCG on acute renal damage in a rat model of obstructive nephropathy. Mol Med Rep. 2013;7:1096–1102. doi: 10.3892/mmr.2013.1296. [DOI] [PubMed] [Google Scholar]
- 28.Farabegoli F, Papi A, Orlandi M. (−)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9 and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci Rep. 2011;31:99–108. doi: 10.1042/BSR20090143. [DOI] [PubMed] [Google Scholar]
- 29.Yan J, Zhao Y, Suo S, Liu Y, Zhao B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic Biol Med. 2012;52:1648–1657. doi: 10.1016/j.freeradbiomed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Baselga-Escudero L, Blade C, Ribas-Latre A, Casanova E, Suárez M, Torres JL, Salvadó MJ, Arola L, Arola-Arnal A. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells. Nucleic Acids Res. 2014;42:882–892. doi: 10.1093/nar/gkt1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen SJ, Kao YH, Jing L, Chuang YP, Wu WL, Liu ST, Huang SM, Lai JH, Ho LJ, Tsai MC, et al. Epigallocatechin-3-gallate reduces scavenger receptor A expression and foam cell formation in human macrophages. J Agric Food Chem. 2017;65:3141–3150. doi: 10.1021/acs.jafc.6b05832. [DOI] [PubMed] [Google Scholar]
- 32.Chang HH, Chien CY, Chen KH, Huang SC, Chien CT. Catechins blunt the effects of oxLDL and its primary metabolite phosphatidylcholine hydroperoxide on endothelial dysfunction through inhibition of oxidative stress and restoration of eNOS in rats. Kidney Blood Press Res. 2017;42:919–932. doi: 10.1159/000485082. [DOI] [PubMed] [Google Scholar]
- 33.Mohan T, Velusamy P, Chakrapani LN, Srinivasan AK, Singh A, Johnson T, Periandavan K. Impact of EGCG supplementation on the progression of diabetic nephropathy in rats: An insight into fibrosis and apoptosis. J Agric Food Chem. 2017;65:8028–8036. doi: 10.1021/acs.jafc.7b03301. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. J Diabetes Res. 2008;2008:704045. doi: 10.1155/2008/704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian C, Zhu C, Yu W, Jiang X, Zhang F. High-fat diet/low-dose streptozotocin-induced type 2 diabetes in rats impacts osteogenesis and wnt signaling in bone marrow stromal cells. PLoS One. 2015;10:e0136390. doi: 10.1371/journal.pone.0136390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Algandaby MM, Alghamdi HA, Ashour OM, Abdel-Naim AB, Ghareib SA, Abdel-Sattar EA, Hajar AS. Mechanisms of the antihyperglycemic activity of Retama raetam in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2010;48:2448–2453. doi: 10.1016/j.fct.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Estil·les E, Téllez N, Nacher M, Montanya E. A model for human islet transplantation to immunodeficient streptozotocin-induced diabetic mice. Cell Transplant. 2018;27:1684–1691. doi: 10.1177/0963689718801006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grove KA, Sae-tan S, Kennett MJ, Lambert JD. (−)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity (Silver Spring) 2012;20:2311–2313. doi: 10.1038/oby.2011.139. [DOI] [PubMed] [Google Scholar]
- 39.Uchiyama Y, Suzuki T, Mochizuki K, Goda T. Dietary supplementation with a low dose of (−)-epigallocatechin-3-gallate reduces pro-inflammatory responses in peripheral leukocytes of non-obese type 2 diabetic GK rats. J Nutr Sci Vitaminol (Tokyo) 2013;59:541–547. doi: 10.3177/jnsv.59.541. [DOI] [PubMed] [Google Scholar]
- 40.Zhang YY, Li C, Yao GF, Du LJ, Liu Y, Zheng XJ, Yan S, Sun JY, Liu Y, Liu MZ, et al. Deletion of macrophage mineralocorticoid receptor protects hepatic steatosis and insulin resistance through ERα/HGF/Met pathway. Diabetes. 2017;66:1535–1547. doi: 10.2337/db16-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Δ Δ C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Parhofer KG. The treatment of disorders of lipid metabolism. Dtsch Arztebl Int. 2016;113:261–268. doi: 10.3238/arztebl.2016.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. doi: 10.1038/s41580-019-0190-7. doi:10.1038/s41580-019-0190-7. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Ojeda FJ, Anguita-Ruiz A, Rupérez AI, Gomez-Llorente C, Olza J, Vázquez-Cobela R, Gil-Campos M, Bueno G, Leis R, Cañete R, et al. Effects of X-chromosome tenomodulin genetic variants on obesity in a childrens cohort and implications of the gene in adipocyte metabolism. Sci Rep. 2019;9:3979. doi: 10.1038/s41598-019-40482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahangari N, Ghayour Mobarhan M, Sahebkar A, Pasdar A. Molecular aspects of hypercholesterolemia treatment: Current perspectives and hopes. Ann Med. 2018;50:303–311. doi: 10.1080/07853890.2018.1457795. [DOI] [PubMed] [Google Scholar]
- 46.Hammad RH, El-Madbouly AA, Kotb HG, Zarad MS. Frequency of circulating B1a and B2 B-cell subsets in Egyptian patients with type 2 diabetes mellitus. Egypt J Immunol. 2018;25:71–80. [PubMed] [Google Scholar]
- 47.Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horváth B, Mukhopadhyay B, Becker L, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–2125. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, Mellen N, Kong M, Gu J, Tan Y, et al. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol. 2014;77:42–52. doi: 10.1016/j.yjmcc.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol. 2005;25:2498–2510. doi: 10.1128/MCB.25.6.2498-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su N, Zhao N, Wang G, Wang L, Zhang Y, Li R, Liu Y, Yang X, Li C, Hou M. The effects of adiponectin and adiponectin receptor 1 levels on macrovascular complications among patients with type 2 diabetes mellitus. Cell Physiol Biochem. 2019;52:225–231. doi: 10.33594/000000016. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, He K, Yang C, Lin X, Zhang X, Wang Y, Liu G, Xian X. Dietary cholesterol is highly associated with severity of hyperlipidemia and atherosclerotic lesions in heterozygous LDLR-deficient hamsters. Int J Mol Sci. 2019;20:20. doi: 10.3390/ijms20143515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walcher D, Marx N, D W, N M. Advanced glycation end products and C-peptide-modulators in diabetic vasculopathy and atherogenesis. Semin Immunopathol. 2009;31:103–111. doi: 10.1007/s00281-009-0144-9. [DOI] [PubMed] [Google Scholar]
- 53.Chien KL, Hsu HC, Liu PH, Lin HJ, Chen MF. Common sequence variants in CD36 gene and the levels of triglyceride and high-density lipoprotein cholesterol among ethnic Chinese in Taiwan. Lipids Health Dis. 2012;11:174. doi: 10.1186/1476-511X-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maréchal L Maximilien Laviolette M, Rodrigue-Way A, Sow B, Brochu M, Caron V, Tremblay A. The CD36-PPARγ pathway in metabolic disorders. Int J Mol Sci. 2018;19:1529. doi: 10.3390/ijms19051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farook VS, Puppala S, Schneider J, Fowler SP, Chittoor G, Dyer TD, Allayee H, Cole SA, Arya R, Black MH, et al. Metabolic syndrome is linked to chromosome 7q21 and associated with genetic variants in CD36 and GNAT3 in Mexican Americans. Obesity (Silver Spring) 2012;20:2083–2092. doi: 10.1038/oby.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang D, Hu C, Deng X, Bai Y, Cao H, Guo J, Su Z. Therapeutic effect of chitooligosaccharide tablets on lipids in high-fat diets induced hyperlipidemic rats. Molecules. 2019;24:24. doi: 10.3390/molecules24030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar NB, Pow-Sang J, Spiess PE, Park J, Salup R, Williams CR, Parnes H, Schell MJ. Randomized, placebo-controlled trial evaluating the safety of one-year administration of green tea catechins. Oncotarget. 2016;7:70794–70802. doi: 10.18632/oncotarget.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dostal AM, Samavat H, Bedell S, Torkelson C, Wang R, Swenson K, Le C, Wu AH, Ursin G, Yuan J-M, Kurzer MS. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: Results of the Minnesota green tea trial. Food Chem Toxicol. 2015;83:26–35. doi: 10.1016/j.fct.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crew KD, Brown P, Greenlee H, Bevers TB, Arun B, Hudis C, McArthur HL, Chang J, Rimawi M, Vornik L, et al. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev Res (Phila) 2012;5:1144–1154. doi: 10.1158/1940-6207.CAPR-12-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu W, Jia L, Chen G, Zhao H, Sun X, Meng X, Zhao X, Xing L, Yu J, Zheng M. Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget. 2016;7:48607–48613. doi: 10.18632/oncotarget.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun X, Yu J, Xing L. A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer. Radiother Oncol. 2014;110:132–136. doi: 10.1016/j.radonc.2013.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.