Abstract
CD4+ regulatory T (Treg) cells are associated with immune tolerance and antitumor immunosuppression. The aim of the present study was to investigate the role and molecular mechanism of C-C motif chemokine ligand 11 (CCL11) in the regulation of Treg cells from patients with breast cancer (BC) and healthy individuals in vitro, and from tumor-bearing mice in vivo. CD4+ T cells isolated from patients with BC or healthy individuals were incubated with anti-CCL11 neutralizing antibodies or recombinant human CCL11 protein, in the presence or absence of a STAT5 inhibitor. The serum CCL11 level and proportion of Treg cells characterized as CD4+CD25+forkhead box P3+ (Foxp3) among the CD4+ T cells in patients with BC and healthy individuals were analyzed by ELISA and flow cytometry, respectively. CCL11, C-C motif chemokine receptor 3 (CCR3), Foxp3, phosphorylated-STAT5 and STAT5 expression levels were determined by western blotting. The serum CCL11 level and the proportion of CD4+CD25+Foxp3+ Treg cells were significantly increased in patients with BC compared with healthy individuals. CCL11 blockade reduced the proportion of CD4+CD25+Foxp3+ Treg cells, the expression of CCR3 and Foxp3, and the level of STAT5 activation in tumor-associated CD4+ T cells, in a dose-dependent manner. CCL11 blockade also reduced the proportion of CD4+CD25+Foxp3+ Treg cells and the serum levels of interleukin (IL)-2 and transforming growth factor (TGF)-β1 in tumor-bearing mice. The recombinant human CCL11 protein increased the proportion of CD4+CD25+Foxp3+ Treg cells, the expression of CCR3 and Foxp3, and the release of IL-2 and TGF-β1 in non-tumor-associated CD4+ T cells via the STAT5 signaling pathway. The results of the present study may aid in identifying therapeutics that could further modulate the immune system during BC.
Keywords: breast cancer, C-C motif chemokine ligand 11, CD4+CD25+forkhead box P3+ regulatory T cell, STAT5
Introduction
Breast cancer (BC), one of the most common malignancies in women worldwide, remains a major challenge despite improved treatments (1). Based on previous reports, it is estimated that 250,000 new cases of BC occurred in women in 2017 and ~40,000 women will die from breast cancer worldwide each year (2,3). The treatment of BC primarily involves surgical resection, chemotherapy, radiotherapy, targeted therapy and hormone therapy. Nevertheless, patients with advanced BC have highly metastatic and invasive tumors that are difficult to treat with targeted and hormone therapy (4). Therefore, novel treatments could reduce the mortality rate of patients with BC (1). A previous study noted that immunotherapy has become a novel and potentially effective treatment option for solid malignancies, including BC (5). Cellular immune responses and tumor immune escape are two key elements of cancer immunoediting (6). Recruitment of specific immunosuppressive leukocyte populations, including regulatory T (Treg) cells, or inducing the tumor cells to produce immunosuppressive cytokines, including transforming growth factor (TGF)-β, which enhances Treg cell proliferation, contribute to immunosuppression in the tumor microenvironment of patients with BC who did not respond to the immunotherapy treatment (5). These findings suggest that effective tumor immunity can be stimulated in tumor tissues by local or systemic immunosuppression.
Treg cells are an immunosuppressive subset of CD4+ T cells that constitutively express CD25 on their cell surface. The lineage-defining transcription factor, forkhead box P3 (Foxp3), is the most reliable marker of Treg cells and is responsible for the functional maintenance and differentiation of these regulatory cells (7). Therefore, Treg cells can be defined as CD4+CD25+Foxp3+ cells. CD4+CD25+Foxp3+ Treg cells play critical roles in the aggressiveness of disease and cancer by modulating immune responses (8). A number of studies have reported that alterations in the number and function of Treg cells in peripheral blood, as well as alterations to the particular tumor microenvironment, are closely related to the development and progression of various disease settings and different types of cancer (9,10). Evidence that tumor infiltrating lymphocytes and their subsets are parallel to BC progression suggests that these potential interactions with immune cells in the tumor microenvironment are important (11). An increase in the number of Treg cells in a breast tumor biopsy is associated with an invasive phenotype and poor overall and relapse-free survival (12). However, the mechanism by which CD4+CD25+Foxp3+ Treg cells are regulated during the control of autoimmunity and maintenance of immunological tolerance in patients with BC or healthy individuals has not been fully clarified.
In some cases, selective ablation of Treg cells in tumors has been achieved, leading to a strong antitumor response and reduced autoimmune toxicity, suggesting that directly targeting the function of Treg cells in tumors is an effective therapeutic strategy (13). Multiple molecules are involved in Treg cell-mediated immunosuppressive mechanisms, including interleukin (IL)-2 and TGF-β (14). IL-2 is a key cytokine involved in the control of the survival, homeostasis and function of CD4+CD25+Foxp3+ Treg cells (15). In addition, TGF-β1 activates STAT5, which binds to the promoter of Foxp3 to promote the differentiation of Treg cells via IL-2, suggesting the involvement of TGF-β1 in the development of Treg cells and the expression of the transcription factor Foxp3 (16). Although CD4+CD25+Foxp3+ Treg cells are activated by IL-2 and TGF-β1 in vitro and in vivo (17,18), the potential of peripheral Treg cells from healthy individuals and patients with BC as a source of therapeutic cell products generated in vitro has not been clarified.
There is evidence that certain malignant phenotypes of cells release chemokines, including CCL2, CCL5 and CCL22, to attract and activate Treg cells (19,20). CCL11 was reported to limit angiogenesis and cause necrosis in a murine model of osteosarcoma by inducing and attracting eosinophils and reducing the tumor formation ability (21). Increased tissue and serum levels of CCL11 mediate the inhibition of dendritic cell maturation and differentiation in the tumor microenvironment, skewing the microenvironment toward a T helper 2 (Th2) immune response and inhibiting CD8+ T cell-mediated tumor cell lysis (22). Additionally, the loss of CCL11 reduced tumor outgrowth (22). Therefore, the present study hypothesized that CCL11 may promote tumor growth by increasing angiogenesis or inhibiting dendritic cell maturation and the role of CCL11 in regulating tumor growth may vary in different types of cancer. The biological effect of CCL11 is modulated by CC chemokine receptor 3 (CCR3), which is downregulated in Th2 and Treg cells of patients with septic shock (23). However, the potential of CCL11/CCR3 to regulate Treg cells during tumor immunity remains unknown. In the present study, the serum and expression levels of CCL11 within tumors of patients with BC were investigated. Furthermore, the present study evaluated whether CCL11 could regulate non-tumor or BC CD4+CD25+Foxp3+ Treg cells and also aimed to identify the underlying molecular mechanisms.
Materials and methods
Patients and specimens
A cohort of 100 women with BC, aged between 18 and 79 years (median, 58 years), who had received mastectomies at the Huangpu Branch, Shanghai Ninth People's Hospital between January 2015 and June 2018 was included in the present study. The healthy group consisted of 50 healthy women, aged between 16 and 83 years (median, 55 years), who had undergone a mammary gland examination prior to sample collection at the Huangpu Branch, Shanghai Ninth People's Hospital between May 2015 and March 2018. Fresh peripheral blood and serum samples (10 ml) were collected from patients with BC and healthy individuals for ELISA and flow cytometry analysis, respectively. Datasets of BC, including 1,041 tumors and 112 adjacent normal tissues, were downloaded from The Cancer Genome Atlas (TCGA) data portal (http://tcga-data.nci.nih.gov). The present study was approved by the Ethics Committee of the Huangpu Branch, Shanghai Ninth People's Hospital. Written informed consent was obtained from all participants.
Cell treatment
The peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood samples collected from each participant in tubes with K2 ethylenediaminetetraacetic acid (EDTA) as anticoagulant as previously described (24). CD4+ T cells were isolated from the PBMCs of patients with BC using the MagCellect Human CD4+ T Cell Isolation kit (cat. no. MAGH102; R&D Systems, Inc.), according to the manufacturer's protocol. The 1×105 CD4+ T cells were treated with different concentrations of anti-CCL11 neutralizing antibodies (0.1, 0.2, 0.5, 1 and 2 µg/ml; cat. no. ab9955; Abcam) or isotype control antibodies (1:1,000 dilution; cat. no. ab171870; Abcam) for 48 h at 4°C. CD4+ T cells were isolated from the PBMCs of healthy individuals and 1×105 CD4+ T cells were treated with different concentrations of recombinant human CCL11 protein (5, 10, 20, 50 and 100 ng/ml; cat. no. ab243753; Abcam; rhCCL11) alone or 50 ng/ml rhCCL11 in the presence or absence of 1 µM STAT5 inhibitor CAS (cat. no. 285986-31-4; EMD Millipore) or vehicle (DMSO) was used as a negative control for 48 h at 4°C.
In vivo xenograft model
Male BALB/c nude mice (age, 6–8 weeks; weight, 18–20 g; n=24) purchased from Vital River Laboratory were housed in a specific pathogen-free environment, with a relative humidity of 60–70%, at a controlled temperature of 25–27°C with 12-h light/dark cycles and were fed a standard diet and water ad libitum. The mice were intravenously injected with 5×106 4T1 breast cancer cells (ATCC) or phosphate buffer saline (PBS; Meilunbio) as control and then intraperitoneally injected with 100 µg anti-CCL11 neutralizing antibodies every 48 h (n=6 per group) (25). Mice were observed over 21 days for tumor formation. At 21 days post-injection, the PBMCs were isolated from peripheral blood samples collected from mice as previously described (26) and CD4+ T cells were isolated from the PBMCs of the mice using the MagCellect Mouse CD4+ T Cell Isolation kit (cat. no. MAGM205; R&D Systems, Inc.), according to the manufacturer's protocol. Mice were monitored until they had reached criteria for predetermined loss of wellness endpoint. These endpoints were defined as tumor burden where any tumor had a diameter of 20 mm, impaired mobility, tumor ulceration, and/or respiratory distress (27). Mice were anesthetized with 1% pentobarbital (45 mg/kg) via intraperitoneal injection and then sacrificed by cervical dislocation. Tissues were fixed in neutral 10% formalin for 10 min at 25°C, dehydrated and embedded in paraffin wax for sectioning (slice thickness of 4 µm) by conventional methods. Sections were stained by routine hematoxylin-eosin processing (hematoxylin for 5 min followed by counterstaining with the eosin for 2 min at 25°C), observed under a BX53 light microscope (magnification, ×100) and photographed with a DP72 camera (Olympus Corporation). The present study was approved by the Ethics Committee for the Care and Use of Laboratory Animals at the Huangpu Branch, Shanghai Ninth People's Hospital.
Evaluation of CD4+CD25+Foxp3+ T cells
CD4+ T cells isolated from PBMCs were stained with either isotype control antibody (cat. no. 553452 for anti-Foxp3 and anti-CD25 antibody; cat. no. 555909 for anti-CD4 antibody, all from BD Biosciences; Becton, Dickinson and Company) or anti-human CD4, anti-human CD25 and anti-Human Foxp3 antibodies, according to the protocol of the Anti-Human Foxp3 Staining kit (cat. no. 560133; BD Biosciences; Becton, Dickinson and Company). The proportion of CD4+CD25+Foxp3+ Treg cells was analyzed using a flow cytometer (BD Accuri C6, software version 1.0.264.21; BD Biosciences; Becton, Dickinson and Company).
Western blotting
Total protein was extracted from CD4+ T cells using RIPA lysis buffer (Applygen Technologies Inc.). The protein concentration was measured using a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.), and absorbance was measured using a microplate reader (SM600 Labsystem; Shanghai Utrao Medical Instrument Co., Ltd.). Equal amounts of protein (25 µg) were separated by 12% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were incubated overnight at 4°C with antibodies targeted against: CCL11 (cat. no. ab133604; 1:2,000; Abcam), CCR3 (cat. no. ab32512; 1:500; Abcam), Foxp3 (cat. no. ab20034; 1:500; Abcam), STAT5 (cat. no. ab230670; 1:500; Abcam), phosphorylated (p)-STAT5 antibody (cat. no. 32364; 1:1,000; Abcam) and GAPDH (cat. no. ab9485; 1:2,000; Abcam). Following the primary incubation, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (cat. nos. A0208 and A0216; 1:1,000; Beyotime Institute of Biotechnology) for 1 h at 37°C. Protein bands were visualized using an ECL detection kit (GE Healthcare), according to the manufacturer's protocol. Signals were quantified by densitometry (Quantity One 4.6.3 software; Bio-Rad Laboratories, Inc.).
ELISA
CCL11 content of the serum of patients with BC and healthy individuals was measured by ELISA, according to the manufacturer's protocol. The Human Eotaxin ELISA kit (cat. no. ab185985; Abcam) was used to measure CCL11 levels, according to the manufacturer's protocol. The IL-2 and TGF-β1 content of the media of CD4+ T cells from the PBMCs of patients with BC or healthy individuals was measured using the Human IL-2 Quantikine ELISA kit (cat. no. S2050; R&D Systems) and the Human TGF-β1 Quantikine ELISA kit (cat. no. SB100B; R&D Systems), respectively. The CCL11, IL-2 and TGF-β1 content of the serum of tumor-bearing mice was measured using the Mouse CCL11/Eotaxin Quantikine ELISA kit (cat. no. MME00; R&D Systems), the Mouse IL-2 Quantikine ELISA kit (cat. no. SM2000; R&D Systems) and the Mouse/Rat/Porcine/Canine TGF-β1 Quantikine ELISA kit (cat. no. SMB100B; R&D Systems), respectively.
Statistical analysis
Data are presented as the mean ± SD of triplicate experiments. All statistical analyses were performed using SPSS software (version 19.0; IBM Corp.). Data were analyzed using a unpaired two-tailed Student's t-test or ANOVA followed by Tukey's post hoc test. Correlation of CCL11 expression with clinicopathological features of BC in TCGA database and hospital cohort was identified by χ2 test. Pearson's correlation analysis was used to evaluate the relationship between CCL11 level and proportion of CD4+CD25+Foxp3+ Treg cells in BC patients. P<0.05 was considered to indicate a statistically significant difference.
Results
Upregulation of CCL11 correlates with the proportion of CD4+CD25+Foxp3+ Treg cells in tumor-associated CD4+ T cells
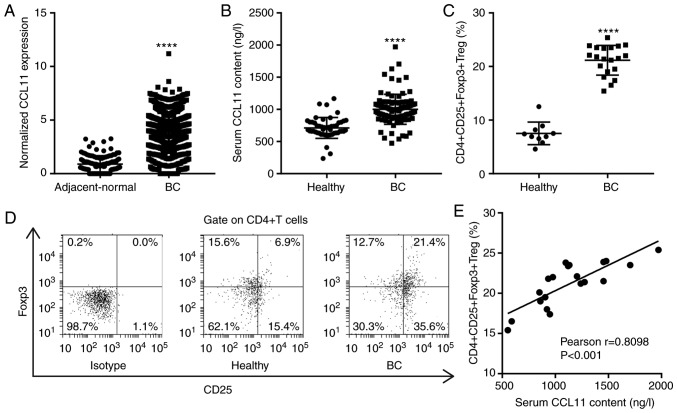
The TCGA BC database revealed that CCL11 was significantly upregulated in BC tissues compared with the adjacent-normal breast tissues (Fig. 1A). The association between CCL11 and the clinicopathological features of BC in the TCGA database suggested that CCL11 expression was associated with tumor stage, histologic grade and estrogen receptor (ER) status, but not with the other clinicopathological features (Table I). To further define CCL11 expression patterns in BC, the serum CCL11 levels in healthy individuals and patients with BC were analyzed. The serum CCL11 levels were significantly increased in patients with BC (n=100) compared with healthy individuals (n=50; Fig. 1B). The association of serum CCL11 levels with the clinicopathological features of BC in the hospital cohort suggested that serum CCL11 levels were associated with tumor diameter, tumor stage and ER status but not with the other clinicopathological features (Table II). Treg cells are a T cell subset with regulatory functions that can inhibit tumor immunity. Foxp3 is the most specific marker of CD4+CD25+ Treg cells and is critical for the regulation of Treg cells and the maintenance of immune tolerance in patients with cancer (7). To further study the regulation of Treg cells in BC, the proportion of CD4+CD25+Foxp3+ Treg cells among the CD4+ T cells was measured by flow cytometry. The percentage of CD4+CD25+Foxp3+ Treg cells was significantly increased in patients with BC (n=20) compared with healthy individuals (n=10; Fig. 1C and D). Furthermore, the serum CCL11 levels were positively correlated with the proportion of CD4+CD25+Foxp3+ Treg cells in patients with BC, by Pearson's correlation analysis (Fig. 1E). The results suggested that the increase in CCL11 in patients with BC contributed to an increase in the proportion of peripheral CD4+CD25+Foxp3+ Treg cells.
Figure 1.
CCL11 is upregulated in patients with BC and is correlated with the proportion of CD4+CD25+Foxp3+ Treg cells. (A) Normalized CCL11 expression in TCGA BC tumors (n=1,041) and adjacent-normal breast tissues (n=112). (B) Serum content of CCL11 in patients with BC (n=100) and healthy individuals (n=50), measured by ELISA. (C) The CD4+CD25+Foxp3+ Treg cell proportion among CD4+ T cells from peripheral blood mononuclear cells of healthy individuals (n=10) and patients with BC (n=20), measured by flow cytometry. (D) Representative flow cytometry plots of the proportion of CD4+CD25+Foxp3+ Treg cells. (E) Correlation between the serum level of CCL11 and the proportion of CD4+CD25+Foxp3+ Treg cells in patients with BC (n=20). ****P<0.0001 vs. adjacent-normal or healthy controls. CCL11, C-C motif chemokine ligand 11; BC, breast cancer; Foxp3, forkhead box P3; Treg, T regulatory; TCGA, The Cancer Genome Atlas.
Table I.
Correlation of C-C motif chemokine ligand 11 expression with clinicopathological features of breast cancer in The Cancer Genome Atlas database.
| Serum CCL11 levels | |||
|---|---|---|---|
| Clinicopathological feature | High (n=521) | Low (n=520) | P-value |
| Age (years) | 0.1074 | ||
| <58 | 215 | 179 | |
| ≥58 | 207 | 216 | |
| Missing: 224 | |||
| Tumor stage | 0.0030 | ||
| I | 123 | 87 | |
| II | 239 | 229 | |
| III | 30 | 49 | |
| IV | 10 | 20 | |
| Missing: 254 | |||
| Histologic grade | 0.0210 | ||
| I | 96 | 71 | |
| II | 274 | 292 | |
| III | 111 | 109 | |
| IV | 3 | 12 | |
| Missing: 73 | |||
| Histology | 0.9002 | ||
| Ductal | 176 | 196 | |
| Lobular | 19 | 17 | |
| Mix | 8 | 7 | |
| Other | 4 | 4 | |
| Missing: 610 | |||
| ER status | 0.0091 | ||
| Positive | 309 | 291 | |
| Indeterminate | 15 | 2 | |
| Negative | 99 | 80 | |
| Missing: 245 | |||
| PR status | 0.5448 | ||
| Positive | 274 | 247 | |
| Indeterminate | 1 | 3 | |
| Negative | 133 | 122 | |
| Missing: 261 | |||
| HER2 status | 0.3407 | ||
| Positive | 65 | 49 | |
| Negative | 329 | 322 | |
| Equivocal | 4 | 6 | |
| Missing: 266 | |||
The χ2 test was used to identify differences between the groups. ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type 2.
Table II.
Correlation of serum C-C motif chemokine ligand 11 levels with clinicopathological features of breast cancer in the hospital cohort.
| CCL11 expression | |||
|---|---|---|---|
| Clinicopathological feature | High (n=50) | Low (n=50) | P-value |
| Age (years) | 0.6853 | ||
| <58 | 30 | 28 | |
| ≥58 | 20 | 22 | |
| Tumor diameter (cm) | 0.0394 | ||
| <2 | 26 | 36 | |
| ≥2 | 24 | 14 | |
| Tumor stage | 0.0302 | ||
| I | 9 | 20 | |
| II | 30 | 27 | |
| III | 8 | 2 | |
| IV | 3 | 1 | |
| Histologic grade | 0.5860 | ||
| I | 7 | 11 | |
| II | 25 | 26 | |
| III | 14 | 11 | |
| IV | 4 | 2 | |
| Histology | 0.6701 | ||
| Ductal | 40 | 43 | |
| Lobular | 8 | 5 | |
| Other | 2 | 2 | |
| ER status | 0.0211 | ||
| Positive | 38 | 27 | |
| Negative | 12 | 23 | |
| PR status | 0.8399 | ||
| Positive | 29 | 28 | |
| Negative | 21 | 22 | |
| HER2 status | 0.8595 | ||
| Positive | 25 | 20 | |
| Negative | 25 | 30 | |
The χ2 test was used to identify differences between the groups. ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type 2.
CCL11 blockade decreases the proportion of CD4+CD25+Foxp3+ Treg cells and the level of STAT5 activation in tumor-associated CD4+ T cells
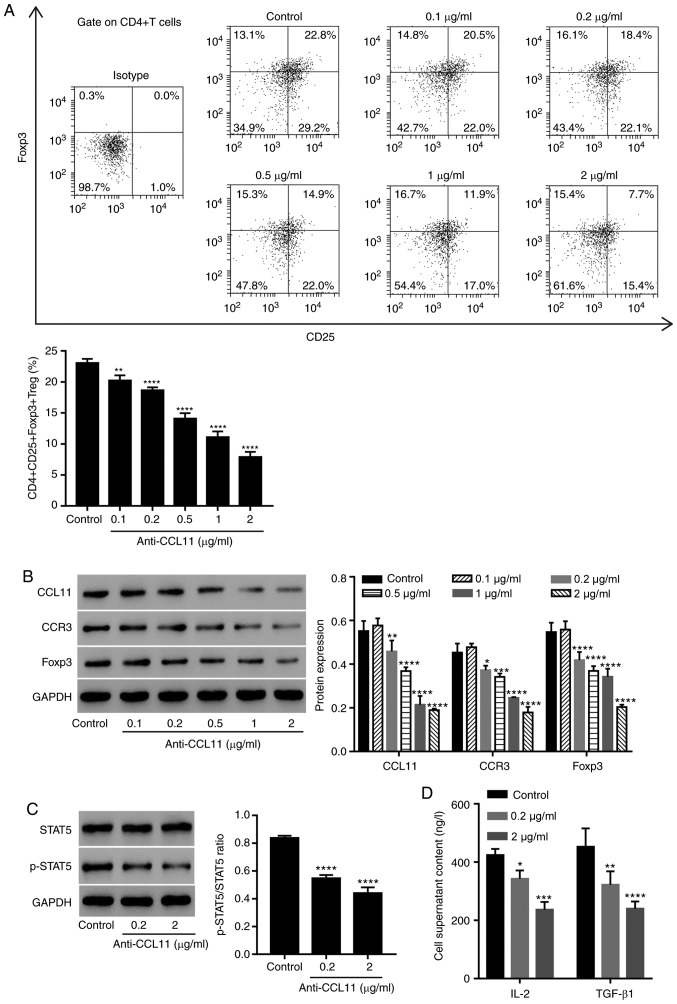
To assess the role of CCL11 in regulating the proportion of Treg cells, CD4+ T cells were isolated from patients with BC and treated with anti-CCL11 neutralizing antibody. The anti-CCL11 neutralizing antibody significantly reduced the proportion of CD4+CD25+Foxp3+ Treg cells in a dose-dependent manner, compared with the control group (Fig. 2A). Moreover, the expression of CCL11, CCR3 and Foxp3 in CD4+ T cells was also measured. The anti-CCL11 neutralizing antibody decreased the expression of CCL11, CCR3 and Foxp3 in a dose-dependent manner, compared with the control group (Fig. 2B).
Figure 2.
CCL11 blockade reduces the proportion of CD4+CD25+Foxp3+ Treg cells and the level of STAT5 activation in tumor-associated CD4+ T cells. CD4+ T cells collected from the peripheral blood mononuclear cells of patients with BC were incubated with anti-CCL11 neutralizing antibodies (0.1, 0.2, 0.5, 1 and 2 µg/ml; n=3 per group). (A) Proportion of CD4+CD25+Foxp3+ Treg cells, measured by flow cytometry. (B) CCL11, CCR3 and Foxp3 expression, measured by western blotting. CD4+ T cells were incubated with anti-CCL11 neutralizing antibody (0.2 and 2 µg/ml), and the expression levels of (C) p-STAT5 and STAT5, as well as (D) the serum content of IL-2 and TGF-β1, were measured by western blotting and ELISA, respectively (n=3 per group). *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs. the control group. CCL11, C-C motif chemokine ligand 11; Foxp3, forkhead box P3; Treg, T regulatory; BC, breast cancer; CCR3, C-C motif chemokine receptor 3; p, phosphorylated; IL, interleukin; TGF, transforming growth factor.
Since STAT5 plays a role in the maintenance of normal immune function and homeostasis (28), especially in the function and development of Treg cells, and induces Foxp3 transcription by binding to multiple regulatory regions in the Foxp3 gene (29), the effect of CCL11 on the STAT5 signaling pathway was assessed. The anti-CCL11 neutralizing antibody significantly inhibited the level of phosphorylated-STAT5, but not STAT5 compared with the control group, suggesting the inactivation of STAT5 signaling (Fig. 2C). To further investigate the regulation of CCL11 in the STAT5 signaling pathway, the expression of IL-2 and TGF-β1, which are important for IL-2-driven STAT5 phosphorylation in Treg cells, was also measured (16,30). The anti-CCL11 neutralizing antibody significantly inhibited the level of IL-2 and TGF-β1 protein expression in the media of CD4+ T cells compared with the control group (Fig. 2D). The data suggested that CCL11 increased the proportion of BC-associated CD4+CD25+Foxp3+ Treg cells and therefore may have a role during BC immunity.
CCL11 blockade reduces the proportion of CD4+CD25+Foxp3+ Treg cells and the serum levels of IL-2 and TGF-β1 in tumor-bearing mice
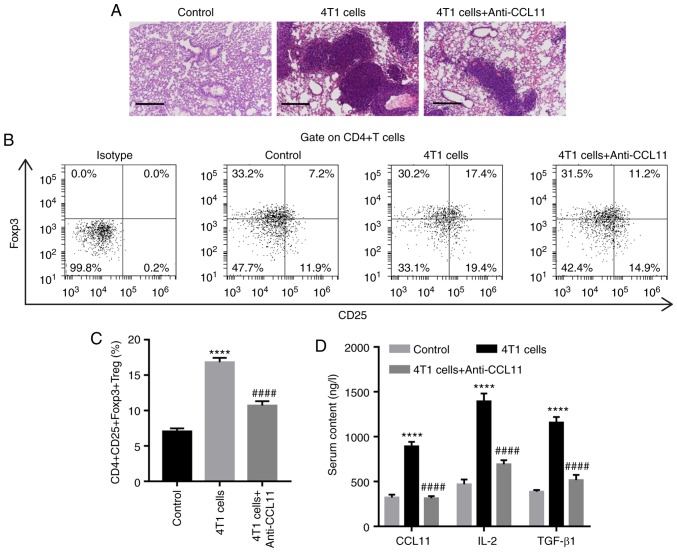
The aforementioned in vitro studies indicated that CCL11 increases the proportion of CD4+CD25+Foxp3+ Treg cells and the release of IL-2 and TGF-β1 by CD4+ T cells. Thus, whether CCL11 affected the proportion of CD4+CD25+Foxp3+ Treg cells and the release of IL-2 and TGF-β1 in vivo was investigated. Mice were simultaneously treated with 4T1 breast cancer cells and the anti-CCL11 neutralizing antibody or its isotype antibody, as indicated. At 28 days post-treatment, lung tissues displayed increased microscopic lesions, the proportion of CD4+CD25+Foxp3+ Treg cells and serum levels of IL-2 and TGF-β1 compared with the control group (Fig. 3). However, CCL11 blockade decreased microscopic lesions, the proportion of CD4+CD25+Foxp3+ Treg cells and the serum levels of IL-2 and TGF-β1 in mice, compared with mice treated with 4T1 cells alone (Fig. 3). Taken together, these results suggested that CCL11 also increased the proportion of BC-associated CD4+CD25+Foxp3+ Treg cells in vivo.
Figure 3.
CCL11 blockade inhibits the 4T1 cell-induced proportion of CD4+CD25+Foxp3+ Treg cells and the release of IL-2 and TGF-β1 in vivo. BALB/c nude mice were intravenously injected with 4T1 cells and intraperitoneally injected with anti-CCL11 neutralizing (100 µg) every 48 h (n=6 per group). (A) Representative images of the histological inspection of mouse lungs at 21 days post-treatment. Scale bar, 100 µm. (B) Representative plots and (C) quantification of the proportion of CD4+CD25+Foxp3+ Treg cells, measured by flow cytometry. (D) Serum levels of CCL11, IL-2 and TGF-β1, measured by ELISA. ****P<0.0001 vs. the control group; ####P<0.0001 vs. the 4T1 cell group. CCL11, C-C motif chemokine ligand 11; Foxp3, forkhead box P3; Treg, T regulatory; IL, interleukin; TGF, transforming growth factor.
CCL11 upregulation increases the proportion of CD4+CD25+Foxp3+ Treg cells in non-tumor-associated CD4+ T cells via the STAT5 signaling pathway
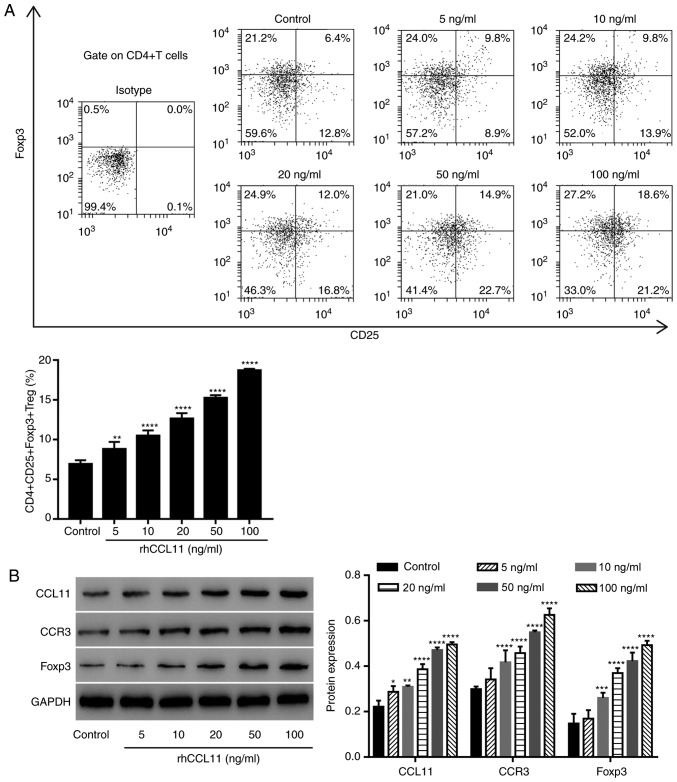
To assess whether the effects of CCL11 on the proportion of CD4+CD25+Foxp3+ Treg cells were also present in non-tumor-associated CD4+ T cells, CD4+ T cells isolated from healthy individuals were treated with or without rhCCL11, at the indicated doses. RhCCL11 treatment significantly increased the proportion of CD4+CD25+Foxp3+ Treg cells and the protein expression levels of CCL11, CCR3 and Foxp3 in CD4+ T cells in a dose-dependent manner, compared with the control group (Fig. 4A and B).
Figure 4.
CCL11 upregulation increases the proportion of CD4+CD25+Foxp3+ Treg cells in non-tumor associated CD4+ T cells. CD4+ T cells collected from peripheral blood mononuclear cells of healthy individuals were treated with or without rhCCL11 (5, 10, 20, 50 and 100 ng/ml; n=3 per group). (A) Proportion of CD4+CD25+Foxp3+ Treg cells, measured by flow cytometry. (B) CCL11, CCR3 and Foxp3 protein expression levels, measured by western blotting. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs. the control group. CCL11, C-C motif chemokine ligand 11; Foxp3, forkhead box P3; Treg, T regulatory; rhCCL11, recombinant human CCL11; CCR3, C-C motif chemokine receptor 3.
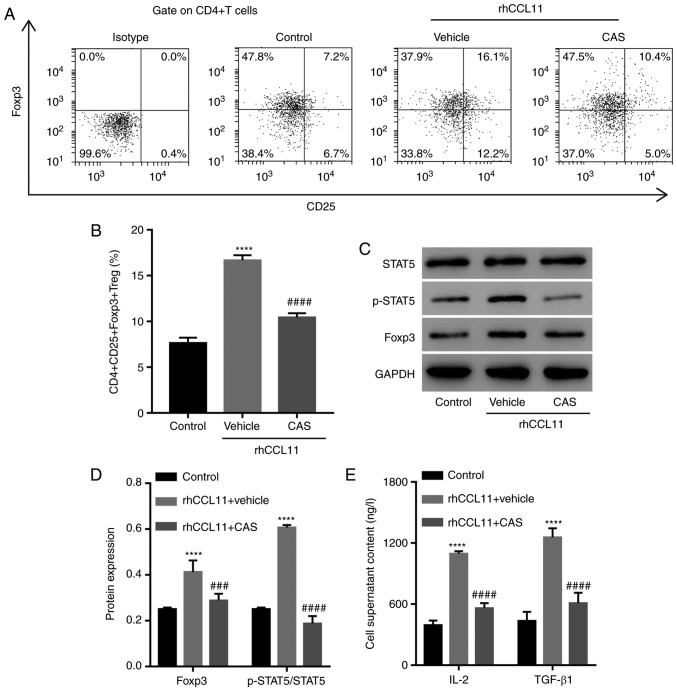
To further assess whether the role of CCL11 in regulating the proportion of CD4+CD25+Foxp3+ Treg cells was dependent on the STAT5 signaling pathway, CD4+ T cells were treated with rhCCL11 and the STAT5 inhibitor CAS. A vehicle was used as a negative control. RhCCL11 treatment significantly increased the proportion of CD4+CD25+Foxp3+ Treg cells and the levels of Foxp3 expression, STAT5 activation and release of IL-2 and TGF-β1 by CD4+ T cells, compared with the control group (Fig. 5). However, the STAT5 inhibitor CAS significantly limited the RhCCL11-induced effects (Fig. 5). These data suggested that CCL11 increased the proportion of CD4+CD25+Foxp3+ Treg cells via the STAT5 signaling pathway.
Figure 5.
Involvement of the STAT5 signaling pathway in the function of CCL11. CD4+ T cells collected from peripheral blood mononuclear cells of healthy individuals were treated with rhCCL11 (50 ng/ml) in the presence or absence of the STAT5 inhibitor CAS (1 µM) or vehicle (n=3 per group). (A) Representative plots and (B) quantification of the proportion of CD4+CD25+Foxp3+ Treg cells, measured by flow cytometry. (C) Western blots and (D) quantification of p-STAT5, STAT5 and Foxp3 protein expression levels, measured by western blotting. (E) IL-2 and TGF-β1 levels in the cell media, measured by ELISA. ****P<0.0001 vs. the control group; ###P<0.001 and ####P<0.0001 vs. the rhCCL11 + vehicle group. CCL11, C-C motif chemokine ligand 11; rhCCL11, recombinant human CCL11; Foxp3, forkhead box P3; Treg, T regulatory; p, phosphorylated; IL, interleukin; TGF, transforming growth factor.
Discussion
CD4+CD25+Foxp3+ Treg cells in patients with solid tumors have been reported to be attracted to and activated by chemokines (19,20,31). Increased infiltration of CD4+CD25+Foxp3+ Treg cells was observed in BC tissues and was associated with high histologic grade, negative estrogen receptor and progesterone receptor status, and human epidermal growth factor receptor type 2 overexpression, as well as decreased overall and progression-free survival (32). However, patients with triple-negative breast cancer and a high number of intratumoural CD4+CD25+Foxp3+ Treg cells displayed a higher tumor grade, lymph node status and improved prognosis (33). These data indicate that the presence of tumor-infiltrating lymphocytes is not enough to reliably predict their effects. Therefore, the present study focused on the peripheral proportion of CD4+CD25+Foxp3+ Treg cells and the regulatory mechanism of these Treg cells in patients with BC and healthy individuals. Patients with BC displayed altered serum CCL11 levels and proportions of CD4+CD25+Foxp3+ Treg cells in PBMCs compared with healthy individuals. A positive correlation between CCL11 expression and the proportion of CD4+CD25+Foxp3+ Treg cells was identified, which is required for the pathogenesis and development of BC (31). The present study found that CD4+CD25+Foxp3+ Treg cells were induced by CCL11 and higher CCL11 expression and proportions of CD4+CD25+Foxp3+ Treg cells were associated with an increased probability of BC occurring. Similarly, CCL11 levels are increased in the serum and tissues of patients with melanoma and are involved in tumorigenesis by regulating the function of CD8+ T cells and Th2 cells (22). However, in gastric cancer, the frequency of CD4+CD25+Foxp3+ Treg cells was significantly increased in tumor-infiltrating lymphocytes but not in tumor-associated peripheral blood lymphocytes compared with healthy individuals (7).
CCL11 signaling is involved in the regulation of the tumor microenvironment and cancer progression. The CCL11/CCR3 signaling pathway potently stimulates cell proliferation, invasion and migration in ovarian carcinoma (34), prostate cancer (35) and lymphoma (36), and regulates necrosis and angiogenesis via the induction and attraction of eosinophils in murine osteosarcoma (21). These data suggest that the CCL11/CCR3 signaling pathway broadly contributes to pathological and physiological processes of cancer. In the present study, blockade of CCL11 by administration of an anti-CCL11 neutralizing antibody, in tumor-associated CD4+ T cells, resulted in decreased expression levels of CCR3 and Foxp3 and the proportion of CD4+CD25+Foxp3+ Treg cells in a dose-dependent manner. Furthermore, a decreased proportion of CD4+CD25+Foxp3+ Treg cells was also observed in tumor-bearing mice in the presence of the anti-CCL11 neutralizing antibody. Foxp3 is crucial for immunosuppression by Treg cells. Foxp3 undergoes methylation or ubiquitination results in aberrant perinuclear accumulation and disrupted regulatory function in Treg cells (37,38). Moreover, upregulation of CCL11 in non-tumor-associated CD4+ T cells by administration of rhCCL11 resulted in increased expression levels of CCR3 and Foxp3 and the proportion of CD4+CD25+Foxp3+ Treg cells in a dose-dependent manner. These data suggested a role for the CCL11/CCR3 signaling pathway in the regulation of CD4+CD25+Foxp3+ Treg cells among tumor and non-tumor-associated CD4+ T cells. Similarly, hepatocellular carcinoma cells recruit CD4+CD25+Foxp3+ Treg cells to promote angiogenesis under hypoxic conditions and regulate Foxp3 expression by CCL28 upregulation (39).
The present study also further suggested that STAT5 activation is involved in the control of Foxp3, TGF-β1 and IL-2 expression levels and the increased proportion of CD4+CD25+Foxp3+ Treg cells in healthy individuals in the presence of rhCCL11. Previous studies have reported that TGF-β1 and IL-2 regulate Foxp3 expression in human CD4+CD25+ Treg cells via the STAT5 signaling pathway, and are involved in the generation, function and stabilization of peripheral Treg cells (40–42). Moreover, TGF-β1 activates the transcription factor STAT5 via IL-2 (16). The present study suggested that CCL11 may regulate the proportion of CD4+CD25+Foxp3+ Treg cells via a positive feedback loop involving the STAT5 signaling pathway, IL-2 and TGF-β1. However, CCL11 inhibits granulocyte-macrophage colony stimulating factor-mediated STAT5 activation in a range of hematopoietic cells (43). Therefore, further investigation is required to clarify the alternative mechanism by which the TGF-β1/IL-2/STAT5 signaling pathway regulates the CCL11-mediated effects on the proportion of CD4+CD25+Foxp3+ Treg cells in non-tumor or BC-associated CD4+ T cells.
To the best of our knowledge, the present study is the first to report that CCL11 increased the proportion of CD4+CD25+Foxp3+ Treg cells and the production of IL-2 and TGF-β1 via the STAT5 signaling pathway. The results of the present study may aid in the development of new therapeutics for BC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
RW and KH conceived the study, collected and analyzed the data, performed the experiments and drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Huangpu Branch, Shanghai Ninth People's Hospital. All participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mansoori B, Mohammadi A, Ghasabi M, Shirjang S, Dehghan R, Montazeri V, Holmskov U, Kazemi T, Duijf P, Gjerstorff M, Baradaran B. miR-142-3p as tumor suppressor miRNA in the regulation of tumorigenicity, invasion and migration of human breast cancer by targeting Bach-1 expression. J Cell Physiol. 2019;234:9816–9825. doi: 10.1002/jcp.27670. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Giovannelli P, Di Donato M, Galasso G, Di Zazzo E, Bilancio A, Migliaccio A. The androgen receptor in breast cancer. Front Endocrinol (Lausanne) 2018;9:492. doi: 10.3389/fendo.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domschke C, Schneeweiss A, Stefanovic S, Wallwiener M, Heil J, Rom J, Sohn C, Beckhove P, Schuetz F. Cellular immune responses and immune escape mechanisms in breast cancer: Determinants of immunotherapy. Breast Care (Basel) 2016;11:102–107. doi: 10.1159/000446061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Workman CJ, Vignali DA. Targeting regulatory T cells in tumors. FEBS J. 2016;283:2731–2748. doi: 10.1111/febs.13656. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Liu X, Wang W, Wang S, Zhang J, Jiang S, Wang Y, Li L, Li J, Zhang Y, Huang H. Low-dose IL-2 expands CD4+ regulatory T cells with a suppressive function in vitro via the STAT5-dependent pathway in patients with chronic kidney diseases. Ren Fail. 2018;40:280–288. doi: 10.1080/0886022X.2018.1456462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol. 2015;33:101–111. doi: 10.1016/j.coi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 11.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, Chudakov DM, Rudensky AY. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 16.Yang TT, Song SJ, Xue HB, Shi DF, Liu CM, Liu H. Regulatory T cells in the pathogenesis of type 2 diabetes mellitus retinopathy by miR-155. Eur Rev Med Pharmacol Sci. 2015;19:2010–2015. [PubMed] [Google Scholar]
- 17.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-β and retinoic acid. PLoS One. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Wang SH, Chen SC, Chen CY, Lin TM. Zoledronic acid blocks the interaction between breast cancer cells and regulatory T-cells. BMC Cancer. 2019;19:176. doi: 10.1186/s12885-019-5379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higuchi T, Matsuo K, Hashida Y, Kitahata K, Ujihara T, Taniguchi A, Yoshie O, Nakayama T, Daibata M. Epstein-Barr virus-positive pyothorax-associated lymphoma expresses CCL17 and CCL22 chemokines that attract CCR4-expressing regulatory T cells. Cancer Lett. 2019;453:184–192. doi: 10.1016/j.canlet.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 21.Xing Y, Tian Y, Kurosawa T, Matsui S, Touma M, Yanai T, Wu Q, Sugimoto K. CCL11-induced eosinophils inhibit the formation of blood vessels and cause tumor necrosis. Genes Cells. 2016;21:624–638. doi: 10.1111/gtc.12371. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Hawkins OE, Barham W, Gilchuk P, Boothby M, Ayers GD, Joyce S, Karin M, Yull FE, Richmond A. Myeloid IKKβ promotes antitumor immunity by modulating CCL11 and the innate immune response. Cancer Res. 2014;74:7274–7284. doi: 10.1158/0008-5472.CAN-14-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venet F, Lepape A, Debard AL, Bienvenu J, Bohe J, Monneret G. The Th2 response as monitored by CRTH2 or CCR3 expression is severely decreased during septic shock. Clin Immunol. 2004;113:278–284. doi: 10.1016/j.clim.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez Rivas E, Ximenez C, Nieves-Ramirez ME, Moran Silva P, Partida-Rodríguez O, Hernandez EH, Rojas Velázquez L, Serrano Vázquez A, Magaña Nuñez U. Entamoeba histolytica calreticulin induces the expression of cytokines in peripheral blood mononuclear cells isolated from patients with amebic liver abscess. Front Cell Infect Microbiol. 2018;8:358. doi: 10.3389/fcimb.2018.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinho V, Oliveira SH, Souza DG, Vasconcelos D, Alessandri AL, Lukacs NW, Teixeira MM. The role of CCL22 (MDC) for the recruitment of eosinophils during allergic pleurisy in mice. J Leukocyte Biol. 2003;73:356–362. doi: 10.1189/jlb.0502243. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Liu M, Ding N, Li Y, Shao J, Zhu M, Xie Z, Sun K. Vaccine based on antibody-dependent cell-mediated cytotoxicity epitope on the H1N1 influenza virus increases mortality in vaccinated mice. Biochem Biophys Res Commun. 2018;503:1874–1879. doi: 10.1016/j.bbrc.2018.07.129. [DOI] [PubMed] [Google Scholar]
- 27.Clark-Knowles KV, Dewar-Darch D, Jardine KE, McBurney MW. SIRT1 catalytic activity has little effect on tumor formation and metastases in a mouse model of breast cancer. PLoS One. 2013;8:e82106. doi: 10.1371/journal.pone.0082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rani A, Murphy JJ. STAT5 in cancer and immunity. J Interferon Cytokine Res. 2016;36:226–237. doi: 10.1089/jir.2015.0054. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein JD, Burlion A, Zaragoza B, Sendeyo K, Polansky JK, Huehn J, Piaggio E, Salomon BL, Marodon G. Inhibition of the JAK/STAT signaling pathway in regulatory T cells reveals a very dynamic regulation of Foxp3 expression. PLoS One. 2016;11:e0153682. doi: 10.1371/journal.pone.0153682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen E, Kumari S, Tohme M, Ullas S, Barrera V, Tas JM, Castillo-Rama M, Bronson RT, Usmani SM, Irvine DJ, et al. DOCK8 enforces immunological tolerance by promoting IL-2 signaling and immune synapse formation in Tregs. JCI insight. 2017;2(pii):94298. doi: 10.1172/jci.insight.94298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos RN, Chin LS, Dos Santos AP, Bergami-Santos PC, Laginha F, Barbuto JA. Monocyte-derived dendritic cells from breast cancer patients are biased to induce CD4+CD25+Foxp3+ regulatory T cells. J Leukocyte Biol. 2012;92:673–682. doi: 10.1189/jlb.0112048. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, Yin D, Gu F, Yao Z, Fu L. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130:645–655. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 33.Yeong J, Thike AA, Lim JC, Lee B, Li H, Wong SC, Hue SS, Tan PH, Iqbal J. Higher densities of Foxp3+ regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Res Treat. 2017;163:21–35. doi: 10.1007/s10549-017-4161-4. [DOI] [PubMed] [Google Scholar]
- 34.Levina V, Nolen BM, Marrangoni AM, Cheng P, Marks JR, Szczepanski MJ, Szajnik ME, Gorelik E, Lokshin AE. Role of eotaxin-1 signaling in ovarian cancer. Clin Cancer Res. 2009;15:2647–2656. doi: 10.1158/1078-0432.CCR-08-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu F, Liu P, Li J, Zhang Y. Eotaxin-1 promotes prostate cancer cell invasion via activation of the CCR3-ERK pathway and upregulation of MMP-3 expression. Oncol Rep. 2014;31:2049–2054. doi: 10.3892/or.2014.3060. [DOI] [PubMed] [Google Scholar]
- 36.Miyagaki T, Sugaya M, Murakami T, Asano Y, Tada Y, Kadono T, Okochi H, Tamaki K, Sato S. CCL11-CCR3 interactions promote survival of anaplastic large cell lymphoma cells via ERK1/2 activation. Cancer Res. 2011;71:2056–2065. doi: 10.1158/0008-5472.CAN-10-3764. [DOI] [PubMed] [Google Scholar]
- 37.Ni X, Kou W, Gu J, Wei P, Wu X, Peng H, Tao J, Yan W, Yang X, Lebid A, et al. TRAF6 directs FOXP3 localization and facilitates regulatory T-cell function through K63-linked ubiquitination. EMBO J. 2019;38(pii):e99766. doi: 10.15252/embj.201899766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kagoya Y, Saijo H, Matsunaga Y, Guo T, Saso K, Anczurowski M, Wang CH, Sugata K, Murata K, Butler MO, et al. Arginine methylation of FOXP3 is crucial for the suppressive function of regulatory T cells. J Autoimmun. 2019;97:10–21. doi: 10.1016/j.jaut.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Ren L, Yu Y, Wang L, Zhu Z, Lu R, Yao Z. Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget. 2016;7:75763–75773. doi: 10.18632/oncotarget.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahmud SA, Manlove LS, Farrar MA. Interleukin-2 and STAT5 in regulatory T cell development and function. JAKSTAT. 2013;2:e23154. doi: 10.4161/jkst.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. Eur J Immunol. 2008;38:912–915. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 42.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevenson NJ, Addley MR, Ryan EJ, Boyd CR, Carroll HP, Paunovic V, Bursill CA, Miller HC, Channon KM, McClurg AE, et al. CCL11 blocks IL-4 and GM-CSF signaling in hematopoietic cells and hinders dendritic cell differentiation via suppressor of cytokine signaling expression. J Leukocyte Biol. 2009;85:289–297. doi: 10.1189/jlb.0708394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.