Abstract
Coronary artery disease (CAD) is a serious threat to human health and a major cause of mortality worldwide. Long noncoding RNAs (lncRNAs) affect the occurrence and development of CAD via the regulation of cell proliferation and apoptosis, inflammatory responses and lipid metabolism. Screening methods and therapeutic strategies for CAD have been extensively studied. The present study analyzed clinical indexes of 187 patients with CAD and 150 healthy subjects. The data showed significant differences in diabetes mellitus, hypertension, high-density lipoprotein level and smoking history between the CAD group and the control group. A series of differentially expressed lncRNAs were detected in the plasma samples of three patients with CAD by high-throughput sequencing. Reverse transcription-quantitative (RT-q)PCR data revealed that the expression level of the novel lncRNA ENST00000416361 was ~2.3-fold higher in the plasma of 50 patients with CAD compared with the 50 control subjects. Receiver operating characteristic (ROC) curves were generated, and the area under the ROC curve was 0.7902. Knockdown of ENST00000416361 in human umbilical vein endothelial cells markedly downregulated interleukin-6 and tumor necrosis factor-α levels. In addition, sterol regulatory element binding transcription factor (SREBP)1 and SREBP2 were upregulated in patients with CAD, and they were positively correlated with the expression of ENST00000416361. RT-qPCR further demonstrated that knockdown of ENST00000416361 led to the downregulation of SREBP1 and SREBP2. Overall, the novel lncRNA ENST00000416361 may be associated with CAD-induced inflammation and lipid metabolism, and it may serve as a potential biomarker for CAD.
Keywords: coronary artery disease, long noncoding RNA, ENST00000416361, inflammation, lipid metabolism, biomarker
Introduction
Coronary artery disease (CAD) is a type of heart disease caused by atherosclerotic lesions of coronary arteries, which is characterized by stenosis or obstruction of the vascular cavity that further leads to myocardial ischemia, hypoxia or necrosis (1). Atherosclerosis, the primary cause of cardiovascular disease, is a chronic inflammatory process involving inflammatory plaques, different types of cells with multiple complex interactions and abnormal lipoprotein deposits in the arterial wall (2,3). Hypertension, diabetes mellitus, tobacco consumption, alcohol consumption, sex (male), obesity, dyslipidemia, genetic variance and a lack of physical activity are commonly known risk factors for CAD (4,5).
Long noncoding RNAs (lncRNAs) are a class of noncoding RNAs with a transcript length >200 bp that lack protein-coding potential. Due to their cell and tissue specificity, lncRNAs in blood, plasma or urine can be used as biomarkers and therapeutic targets for cancer and numerous other diseases (6,7). An increasing number of studies have shown that lncRNAs are abnormally expressed in a variety of diseases and have important roles in cell proliferation, apoptosis and invasion (8–10).
It has been demonstrated that lncRNAs are closely associated with the occurrence and development of CAD, including CAD-induced pathological changes such as vascular smooth muscle cell proliferation, apoptosis, lipid metabolism and inflammation (11,12). Various lncRNAs have been considered promising regulatory genes or biomarkers of CAD, such as lncPPARδ, H19 imprinted maternally expressed transcript, maternally expressed 3, antisense noncoding RNA in the INK4 locus (ANRIL), mitochondrially encoded long non-coding cardiac associated RNA, lincRNA-p21, CoroMarker, NONHSAT112178, Novlnc6, metastasis associated lung adenocarcinoma transcript 1, myocardial infarction associated transcript and steroid receptor RNA activator (SRA) (13,14). In atherosclerosis, lincRNA-p21 has been identified to inhibit proliferation and induce apoptosis in vascular smooth muscle cells (15). In addition, the G-A-A-G haplotype of lincRNA-p21 is correlated with a decreased risk of CAD (16).
CoroMarker (lncRNA AC100865.1), abhydrolase domain containing 16A, and IL21R antisense RNA 1 were identified by microarray and confirmed by reverse transcription-quantitative (RT-q) PCR in peripheral blood mononuclear cells (PBMCs) (17). It has been shown that in CAD the CoroMarker is significantly upregulated and has high specificity and sensitivity (17). Studies have also demonstrated that CoroMarker can positively regulate foam cell formation, arteriosclerosis, inflammation and the associated immune responses, and can inhibit cell apoptosis (17,18).
A number of lncRNAs have been shown to be associated with CAD, however key lncRNAs in patients with CAD from different regions and different ethnicities are likely to differ, so further studies are required. In the present study, high-throughput sequencing was adopted to identify differentially expressed lncRNAs and identify a biomarker for CAD according to the local resident (Suzhou, Jiangsu, China). Further study of the mechanism of the lncRNA was performed to identify new targets for the early diagnosis and treatment of clinical CAD. These results also provided a theoretical basis for the further study of the molecular mechanism of CAD.
Materials and methods
Clinical characteristics of patients
A total of 187 patients with CAD, aged 42–85 years, were enrolled in the Department of Cardiology of Nanjing Medical University Affiliated Suzhou Hospital in China between October 2016 and August 2018. The diagnosis of CAD was based on angiographic evidence of at least one segment of a major coronary artery, including the left anterior descending, left circumflex and right coronary arteries, with ≥50% stenosis (19). Exclusion criteria were as follows: i) Patients with severe primary diseases, such as liver, kidney and hematopoietic system; ii) patients with acute infection, trauma or other operations; iii) patients with myocardial infarction, cerebral infarction, cancer and other major diseases; iv) patients with alcoholism and drug addictions; v) patients >85 years; and vi) pregnant patients. During the same period, 150 healthy subjects were selected from patients attending check-up appointments. The routine blood test results (WBC: 4–10×109/L, HGB: 120–170 g/l (male), 110–150 g/l (female), PLT: 100–300×109/l), blood pressure (60-90/90-140 mmHg), blood glucose (4-6.1 mmol/l), blood lipid (total cholesterol: 2.8–5.17 mmol/l, triglycerides: 0.56–1.7 mmol/l, cholesterol lipids: 2.8–5.17 mmol/l) and electrocardiogram (ECG; physician judgment) of those 150 healthy subjects were within the normal ranges. The study was approved by the Ethics Committee of Nanjing Medical University. Informed consent was obtained from the patients and their families.
Collection and preparation of blood samples
Peripheral blood samples (4–6 ml) were collected from patients with CAD and healthy subjects using EDTA vacuum anticoagulant blood vessels. After incubation for 30 min at 4°C, the blood samples were centrifuged at 200 × g for 10 min (4°C). Then, the plasma was recentrifuged at 1,600 × g for 10 min (4°C) to remove debris. PBMCs were separated from the blood cells with Ficoll-Paque PLUS density gradient media (GE Healthcare) as previously described (20,21).
lncRNA-Seq high-throughput sequencing
Plasma samples from 3 patients with CAD and 3 healthy subjects were subjected to lncRNA-Seq high-throughput sequencing, followed by bioinformatics analysis performed by CloudSeq Biotech. Total RNA (1 µg) was used to remove the ribosomal (r)RNAs using Ribo-Zero rRNA Removal kits (Illumina, Inc.) following the manufacturer's protocols.
Fragments per kilobase million (FPKM) of the expression profiles of lncRNAs and mRNAs was calculated using Cuffdiff software (v2.2.1, part of Cufflinks) (22). Fold change and P-value were calculated based on FPKM to identify the differentially expressed lncRNAs and mRNAs. Gene Ontology (GO) pathway enrichment analysis was performed based on the differentially expressed mRNAs (23,24).
Competing endogenous (ce)RNA analysis of lncRNAs
An RNA transcript with micro (mi)RNA response elements (MREs) may serve as a competing endogenous (ce)RNA. ceRNAs include pseudogene transcripts, lncRNAs, circular RNAs and mRNAs, and these transcripts can compete for the same MREs to exert their biological functions. Potential target miRNAs were predicted with in-house miRNA target prediction software, based on TargetScan and miRanda software (25–31). Cytoscape software (v3.7.0) was used to visualize the ceRNA networks with the target miRNAs (32).
To further analyze the metabolic pathway of lncRNA ENST00000416361, a ceRNA network map was constructed according to the corresponding target miRNAs and mRNAs. The physiological effect of lncRNA ENST00000416361 was studied in depth by analyzing the biological functions of related miRNAs and mRNAs.
Cell culture
HUVECs (human umbilical vein endothelial cells) were purchased from CoWin Biosciences. HUVECs were cultured in DMEM (Thermo Fisher Scientific, Inc.) supplemented with 100 U/ml streptomycin, 100 U/ml penicillin and 10% (v/v) FBS (Gibco; Thermo Fisher Scientific, Inc.) under 37°C and 5% (v/v) CO2 conditions.
Design of small interfering (si)RNAs and cell transfection
The siRNAs were designed and synthesized by Suzhou GenePharma Co., Ltd. The sequences of si-ENST00000416361 were: Sense (5′-3′), CCCAACAGCUCAUUGAGAATT and antisense (5′-3′), UUCUCAAUGAGCUGUUGGGTT. The sequences of siRNA-NC were: Sense (5′-3′), UUCUCCGAACG UGUCACGUTT and antisense (5′-3′), ACGUGACACGUUCGG AGAATT. A total of 100 nM siRNA was used for each transfection. A negative control with fluorescein was used to identify cell transfection efficiency. Cell transfection was performed using Lipofectamine™ 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. Knockdown efficiency and related gene expression were measured after 12 h.
RNA isolation and RT-qPCR
Plasma RNA was extracted by TRIzol™ LS Reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and 1 µg RNA was transcribed into cDNA by 4 µl 5X PrimeScript™ RT Master Mix (RR036A) (Takara Bio, Inc.), up to 20 µl with RNase free dH2O. RNAiso Plus (Takara Bio, Inc.) was used to extract RNA from PBMCs and HUVECs, followed by reverse transcription to cDNA with PrimeScript™ RT Master Mix (RR036A) (Takara Bio, Inc.). RNA quality was analyzed with an ultra-micro nucleic acid analyzer (Hangzhou Allsheng Instruments Co., Ltd.).
RT-qPCR was performed using SYBR® Premix Ex Taq™ II (Takara Bio, Inc.) to detect relative expression using the standard protocols on the LightCycler® 480 Instrument II Real-Time PCR Detection system (Roche Diagnostics). β-actin was used as the internal reference. PCR reaction conditions were: 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 20 sec; 95°C for 0 sec, 65°C for 15 sec and 95°C for 0 sec. The percentage of agarose gel was 2%. The primers for lncRNAs, the lipid-associated genes SREBP1, FAS, ACC1, DGAT2, SREBP2, ApoE, ApoA-I, Cpt1a (33) and β-actin used in the RT-qPCR were synthesized by Genewiz, Inc. The primer sequences are listed in Table I. All measurements were performed in triplicate. Relative expression data were analyzed with the formula 2−∆∆Cq (34). GraphPad Prism 7 (GraphPad Software, Inc.) was used to analyze the differences in expression.
Table I.
The primer sequences.
| Gene | Primer sequence |
|---|---|
| ENST00000416361 | 5′-CTCATTGAGAACGGGCCATG-3′ |
| 5′-CATGTTTCAGAGAGCACCGG-3′ | |
| SREBP1 | 5′-GGAGGGGTAGGGCCAACGGCCT-3′ |
| 5′-CATGTCTTCGAAAGTGCAATCC-3′ | |
| SREBP2 | 5′-CAAGATGCACAAGTCTGGCG-3′ |
| 5′-GCTTCAGCACCATGTTCTCCTG-3′ | |
| FAS | 5′-ACAGGGACAACCTGGAGTTCT-3′ |
| 5′-CTGTGGTCCCACTTGATGAGT-3′ | |
| ACC1 | 5′-GTTGCACAAAAGGATTTCAG-3′ |
| 5′-CGCATTACCATGCTCCGCAC-3′ | |
| DGAT2 | 5′-CGGTCCCCAATCACCTCATC-3′ |
| 5′-GGGATGTTCCAGTTCTGCCA-3′ | |
| ApoE | 5′-CCCAGGTCACCCAGGAACT-3′ |
| 5′-TTCCGATTTGTAGGCCTTCAA-3′ | |
| ApoA-I | 5′-ATCGAGTGAAGGACCTGGC-3′ |
| 5′-AGCTTGCTGAAGGTGGAGGT-3′ | |
| Cpt1a | 5′-TGCTTTACAGGCGCAAACTG-3′ |
| 5′-TGGAATCGTGGATCCCAAA-3′ | |
| β-actin | 5′-CACGAAACTACCTTCAACTCC-3′ |
| 5′-CATACTCCTGCTTGCTGATC-3′ |
Cytokine detection
The human Th1/Th2 subgroup test kit (CellGene Biotech Co., Ltd.) was used to detect the levels of the inflammatory cytokines interleukin (IL)-2, IL-4, IL-6, IL-10, interferon-γ and tumor necrosis factor-α (TNF-α). The standard solution and standard curves were prepared according to the manufacturer's protocols. The mixed microsphere solution was centrifuged at 200 × g for 5 min (4°C). Subsequently, the same volume of microsphere buffer solution was added and incubated for 30 min in the dark at room temperature. For each tube, 25 µl microsphere mixture, 25 µl sample and 25 µl fluorescent detection reagent was added, and the solution was incubated for 2.5 h at room temperature. After washing with 1 ml of PBS, the mixture was centrifuged at 200 × g for 5 min at room temperature, and the precipitate was diluted in 100 µl of PBS solution. Finally, cytokine detection was performed using BD FACSCalibur™ (Becton-Dickinson and Company).
Statistical analysis
GraphPad Prism 7 software was used for statistical analysis. All data were obtained from at least 3 independent experiments and are expressed as the mean ± SD. One-way ANOVA followed by Dunnett's test was performed to analyze multiple group comparisons of quantitative data and Student's t-test was performed for analyzing two-group comparisons of quantitative data. A Chi-square test was used for category data. Correlation was confirmed by Pearson's correlation analysis. P<0.05 was considered statistically significant.
Results
Pathological differences between patients with CAD and healthy subjects
A total of 187 patients with CAD (diagnosed with at least one vascular ≥50% stenosis) and 150 healthy subjects were selected for the experiment. The clinical characteristics of the enrolled patients and healthy controls are presented in Table II. The data showed that 82.9% of patients with CAD had hypertension, 28.9% had diabetes, 45% had a history of smoking and were male, all of which were significantly increased compared with those in the control group. In addition, high-density lipoprotein (HDL) levels were significantly decreased in patients with CAD relative to the controls.
Table II.
Clinical characteristics of patients with CAD and controls.
| Sequencing samples | All clinical samples collected | ||||
|---|---|---|---|---|---|
| Characteristics | CAD (n=3) | CON (n=3) | CAD (n=187) | CON (n=150) | P-value |
| Age, years | 60.33 | 57.78 | 67.5±17.5 | 62.06±18.06 | >0.050 |
| Sex, male (%) | 2 (66.7) | 2 (66.7) | 115 (61.5) | 78 (52) | 0.083 |
| BMI (kg/m2) | 24.32 | 23.99 | 25.13 | 25.68 | >0.050 |
| Diabetes mellitus (%) | 0 (0) | 0 (0) | 54 (28.9) | 22 (14.7) | 0.002 |
| Hypertension (%) | 1 (33.4) | 0 (0) | 155 (82.9) | 58 (38.7) | <0.001 |
| TC (mmol/l) | 3.56 | 3.69 | 4.14 | 4.589 | 0.066 |
| TG (mmol/l) | 1.57 | 1.77 | 1.7 | 1.56 | 0.679 |
| HDL (mmol/l) | 0.98 | 1.08 | 1.02 | 1.19 | 0.002 |
| LDL (mmol/l) | 2.13 | 2.21 | 2.56 | 2.89 | 0.171 |
| VLDL (mmol/l) | 0.34 | 0.5 | 0.56 | 0.53 | 0.425 |
| ApoA (g/l) | 1.32 | 1.11 | 1.21 | 1.25 | 0.776 |
| ApoB (g/l) | 0.71 | 0.69 | 0.82 | 0.83 | 0.421 |
| Lipoprotein A | 200.67 | 162.09 | 207.24 | 177 | 0.051 |
| CHE | 8.41 | 8.74 | 7.8 | 8.04 | 0.773 |
| TBA | 5.23 | 5.15 | 5.77 | 5.56 | 0.894 |
| Smoking (%) | 0 (0) | 0 (0) | 84 (45) | 40 (26.7) | 0.001 |
| Drinking (%) | 0 (0) | 0 (0) | 37 (19.8) | 20 (13.3) | 0.116 |
P<0.05 was regarded as significant. Chi-square test and unpaired t-test were used. CAD, coronary artery disease; CON, control; BMI, body mass index; n, number of patients; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein; ApoA, apolipoprotein A1; ApoB, apolipoprotein B; CHE, cholinesterase; TBA, total bile acid.
Differential expression profiles of lncRNAs
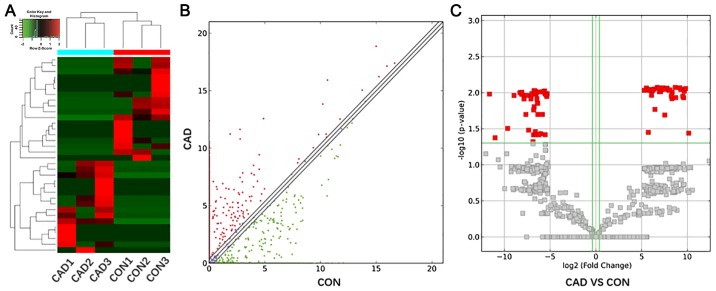
Plasma samples from 3 patients with CAD and 3 healthy controls were examined via high-throughput sequencing methods. The 3 patients with CAD were screened based on their clinical records, in order to exclude the interference of other diseases as well as to avoid including the effects of smoking and drinking. In the 3 healthy controls, all the physical examination indexes were in the normal range, and the interference of other diseases was excluded. All the physical examination indexes were in the normal range, and the interference of other diseases was excluded of the normal group. By analyzing FPKM, fold changes and P-values using the Cuffdiff software, 66 upregulated and 86 downregulated lncRNAs were identified. Cluster analysis, scatter diagram and volcano plots show the differentially expressed lncRNAs with a P≤0.05 and fold change ≥2.0 (Fig. 1).
Figure 1.
Differentially expressed lncRNAs in CAD and control groups identified by high-throughput sequencing. (A) Cluster analysis of differentially expressed lncRNAs in the CAD (CAD1, CAD2 and CAD3) and control groups (CON1, CON2 and CON3). Upregulated genes are represented as red and downregulated ones are represented as green. (B) Scatter diagram of two groups was generated according to the expression values. Red indicates upregulation and green indicates downregulation. The default fold change threshold was 2.0. (C) Volcano plots were generated using fold-change and P-value data, and the red rectangles represent the expression differences of lncRNAs. lncRNA, long noncoding RNA; CAD, coronary artery disease; CON, control.
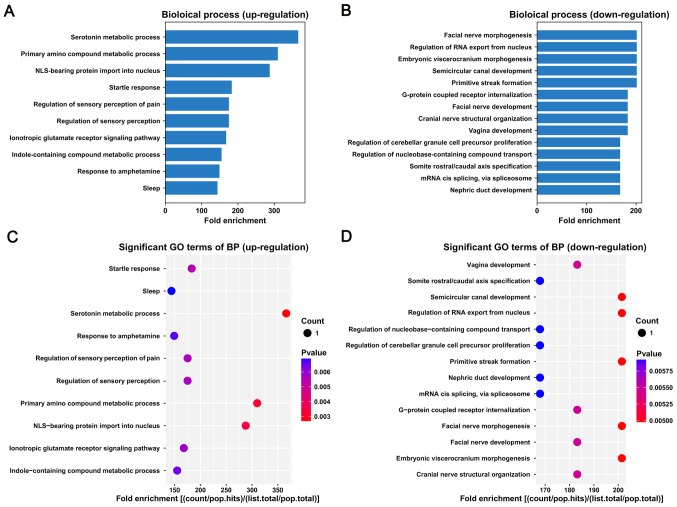
GO pathway analysis of differentially expressed lncRNAs
Target genes of lncRNAs were predicted based on their proximity to the lncRNAs, and GO pathway analysis of biological processes was performed based on the identified target genes. The bar plot shows the top fold enrichment value of the significantly enriched terms (Fig. 2). The target genes of upregulated lncRNAs were associated with serotonin metabolic process, primary amino compound metabolic process and nuclear localization signal (NLS)-bearing protein import into nucleus (Fig. 2A and C). The target genes of the downregulated lncRNAs were mainly involved in primitive streak formation, semicircular canal development, embryonic viscerocranium morphogenesis, regulation of RNA export from nucleus and facial nerve morphogenesis (Fig. 2B and D).
Figure 2.
GO pathway analysis of target genes. (A and C) GO pathway analysis of upregulated lncRNAs. (B and D) GO pathway analysis of downregulated lncRNAs. GO, Gene Ontology; lncRNA, long noncoding RNA.
Target lncRNA selection
The top 5 upregulated and downregulated lncRNAs with P<0.01 were selected, and overlapping sequences were excluded (Table III).
Table III.
Target lncRNA selection.
| Transcript_ID | Fold change | P-value | FDR | Regulation | LncRNA_source | LncRNA_length (bp) | Relationship |
|---|---|---|---|---|---|---|---|
| ENST00000416361 | inf | 0.009 | 0.400733 | Up | Ensembl | 2,102 | Intergenic |
| ENST00000565257 | inf | 0.010 | 0.400733 | Up | Ensembl | 1,406 | Intergenic |
| ENST00000568324 | inf | 0.010 | 0.400733 | Up | Ensembl | 3,102 | Intergenic |
| uc003pxg.1 | inf | 0.009 | 0.400733 | Up | UCSC knowngene | 2,183 | Nidirectional |
| ENST00000607001 | inf | 0.010 | 0.400733 | Up | Ensembl | 744 | Nidirectional |
| ENST00000418006 | -inf | 0.009 | 0.400733 | Down | Ensembl | 2,350 | Intergenic |
| ENST00000564481 | -inf | 0.010 | 0.400733 | Down | Ensembl | 992 | Nidirectional |
| ENST00000537921 | -inf | 0.011 | 0.400733 | Down | Ensembl | 477 | Nidirectional |
| ENST00000554522 | -inf | 0.009 | 0.400733 | Down | Ensembl | 1,701 | Intergenic |
| ENST00000425077 | -inf | 0.010 | 0.400733 | Down | Ensembl | 422 | Intergenic |
P<0.05 was considered to indicate significance. FDR, false discovery rate; lncRNA, long noncoding RNA.
lncRNA ENST00000416361 is significantly upregulated in the plasma of patients with CAD
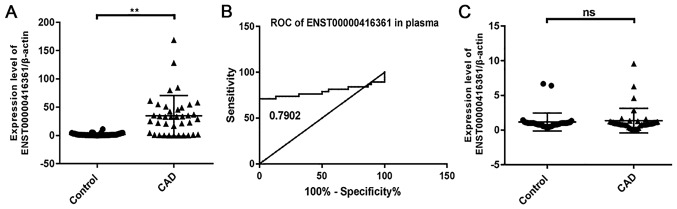
A total of 3 pairs of primers were designed for each lncRNA, and the results showed that ENST00000416361 was upregulated in the peripheral blood plasma samples of patients with CAD (Fig. 3A). However, the upregulation trend of ENST00000416361 was not obvious in PBMCs (Fig. 3C). The relative expression levels of these lncRNAs in plasma and PBMC samples from 50 patients with CAD and 50 healthy controls were verified by RT-qPCR. Then, 50 samples per group were randomly selected from the 187 patients with CAD and 150 controls enrolled in the study. The results indicated that the expression level of ENST00000416361 in patients with CAD was 2.3-fold higher compared with that in the normal group, and P<0.01 was used as a cut-off value to indicate a statistically significant difference. A ROC curve was generated for analyzing the early-stage screening and diagnostic potential of ENST00000416361 in CAD (Fig. 3B). The area under the curve was 0.7902, indicating that the plasma level of ENST00000416361 may represent a useful clinical diagnostic biomarker for CAD.
Figure 3.
Expression level and ROC curve analysis of lncRNA ENST00000416361. (A) Expression level of ENST00000416361 in peripheral blood plasma samples (n=50). **P<0.01. (B) ROC curve of ENST00000416361 expression in peripheral blood plasma samples. (C) Expression level of ENST00000416361 in peripheral blood mononuclear cells (n=50). ROC, receiver operating characteristic; lncRNA, long noncoding RNA; CAD, coronary artery disease; n.s., no significant difference.
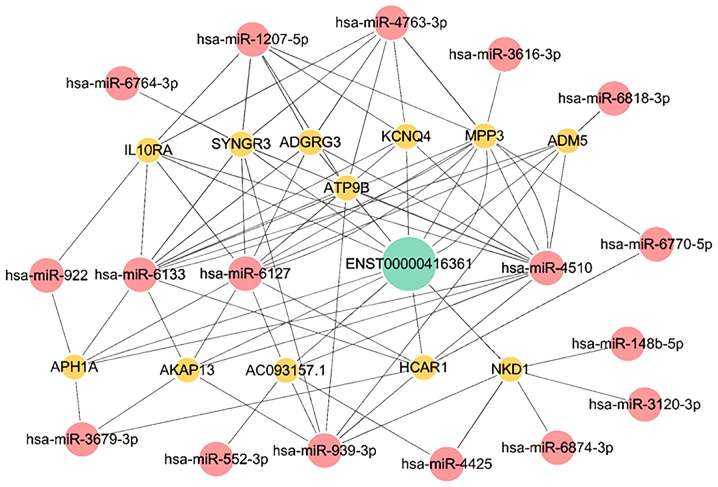
lncRNA ENST00000416361 serves as a ceRNA
Bioinformatics analysis was conducted to explore the gene metabolic pathway of lncRNA ENST00000416361. A ceRNA network map was constructed with the relevant miRNAs and mRNAs (Fig. 4).
Figure 4.
CeRNA network map of lncRNA ENST00000416361-associated miRNAs and mRNAs. The ceRNA network map was constructed using Cytoscape software. The green circle is lncRNA, the red circles are miRNAs and the yellow circles are mRNAs. ceRNA, competing endogenous RNA; lncRNA, long noncoding RNA; miRNA/miR, microRNA.
Among these related mRNAs, synaptogyrin 3, membrane palmitoylated protein 3, adhesion G protein-coupled receptor G3, aph-1 homolog A, γ-secretase subunit, hydroxycarboxylic acid receptor 1, ATPase phospholipid transporting 9B and potassium voltage-gated channel subfamily Q member 4 were associated with membrane structures.
The miRNA-939 expression is downregulated in patients with CAD. miRNA-939 inhibits angiogenesis by targeting human-catenin proteins, thus destroying the integrity of blood vessels. Overexpression of miRNA-939 in HUVECs can significantly inhibit cell proliferation, adhesion and angiogenesis but increase cell migration (35).
Inflammatory factors IL-6 and TNF-α are significantly downregulated following lncRNA ENST00000416361 knockdown
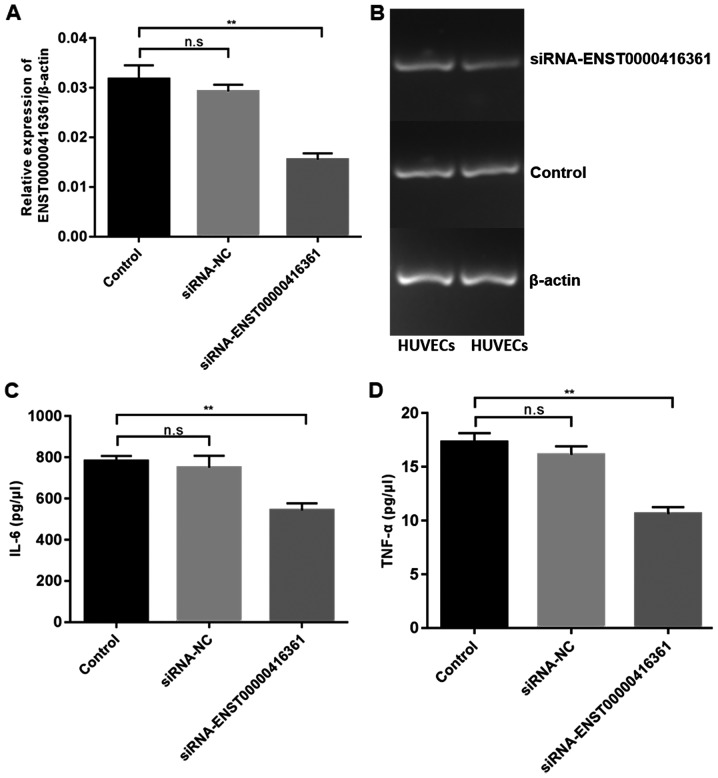
Transfection of si-ENST00000416361 in HUVECs exhibited a 50% knockdown efficiency (Fig. 5A and B). Furthermore, transfection of si-ENST00000416361 led to the downregulation of IL-6 (Fig. 5C) and TNF-α (Fig. 5D).
Figure 5.
Transfection of si-ENST00000416361 downregulated inflammatory factors. (A and B) Transfection efficacy of si-ENST00000416361 in HUVECs determined by reverse transcription-quantitative PCR. β-actin was used as the internal reference. (C and D) Inflammatory cytokines IL-6 and TNF-α in the cell supernatant were measured by the human Th1/Th2 subgroup test kit. **P<0.01. HUVECs, human umbilical vein endothelial cells; IL, interleukin; TNF-α, tumor necrosis factor-α; siRNA, small interfering RNA; n.s., no significant difference; NC, negative control.
Lipid-associated genes SREBP1 and SREBP2 are positively correlated with the expression of ENST00000416361
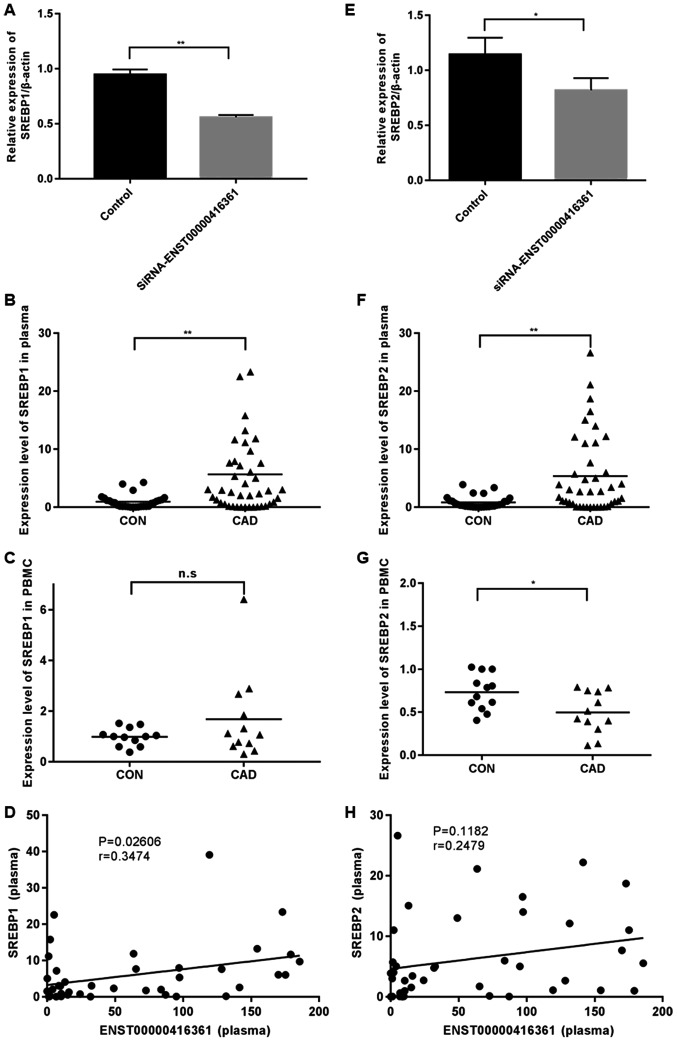
It was identified that SREBP1 and SREBP2 were significantly upregulated in plasma samples of patients with CAD (Fig. 6B and F), but not in PBMCs (Fig. 6C and G). Knockdown of ENST00000416361 led to the significant downregulation of SREBP1 and SREBP2 (Fig. 6A and E). Pearson's correlation scatter plots were generated based on the plasma levels of ENST00000416361 and SREBP, and it was indicated that ENST00000416361 could affect the occurrence and development of CAD by interacting with SREBP. The levels of SREBP1 (P=0.02606, r=0.3474) and SREBP2 (P=0.1182, r=0.2479) were both positively correlated with the expression of ENST00000416361, which was more pronounced in the former gene (Fig. 6D and H).
Figure 6.
Expression levels of SREBP1 and SREBP2 in HUVECs and plasma samples. (A and E) Relative expression levels of (A) SREBP1 and (E) SREBP2 were detected by RT-qPCR following ENST00000416361 knockdown in HUVECs. (B and F) Plasma levels of (B) SREBP1 and (F) SREBP2 in patients with CAD and control subjects determined by RT-qPCR (CAD=42 cases, CON=28 cases). **P<0.01. (C and G) Relative expression of (C) SREBP1 and (G) SREBP2 in peripheral blood mononuclear cells determined by RT-qPCR (CAD=12 cases, CON=12 cases). *P<0.05. (D and H) Pearson's correlation scatter plots revealed the correlation between plasma levels of (D) ENST00000416361 and (H) SREBP1/SREBP2 analyzed with GraphPad Prism 7.0 (n=41). SREBP, sterol regulatory element binding transcription factor; HUVECs, human umbilical vein endothelial cells; RT-qPCR, reverse transcription-quantitative PCR; CAD, coronary artery disease; CON, control; n.s, no significant difference.
Discussion
lncRNAs have been identified to be closely associated with the occurrence and development of CAD (36). These differentially expressed lncRNAs have attracted increasing attention due to their value in disease diagnosis and prognosis. In the present study, high-throughput sequencing and pathway analysis were conducted to find the differentially expressed lncRNAs in the peripheral blood of patients with CAD. The top upregulated lncRNAs were primarily associated with serotonin metabolic process, primary amino compound metabolic process and NLS-bearing protein import into nucleus. By analyzing clinical indexes of patients with CAD and healthy subjects, it was identified that HDL levels were significantly decreased in patients with CAD. The expression pattern of lncRNA ENST00000416361 was determined in plasma samples and PBMCs, indicating that the plasma level of lncRNA ENST00000416361 may be a potential biomarker for clinical CAD screening.
Knockdown of ENST00000416361 significantly decreased the levels of the inflammatory factors IL-6 and TNF-α. A growing number of studies have shown that IL-6 and TNF-α are closely related to CAD progression. It has been demonstrated that the serum levels of IL-6 and TNF-α are increased in patients with CAD and are positively correlated with miR-342-5p expression (37). Decreased serum levels of C1q/TNF-α related protein 12 (CTRP12) have been identified in patients with CAD compared to controls, and IL-6 and TNF-α are negatively correlated with CTRP12 in patients with CAD (38). It has been suggested that inflammatory cytokines, particularly IL-6, may be elicited in response to an acute inflammatory stressor, thus contributing to the progression of CAD (39,40). The C allele of rs1800795 is located on the IL-6 gene promoter, and IL-6 rs1800795-tobacco smoking and IL-6 rs1800795-alcohol consumption interactions have been associated with increased CAD risk (41). lncRNA ANRIL affects the progression of CAD by targeting miR-181b, activating the NF-κB signal pathway and inducing the release of inflammatory factors such as IL-6 and TNF-α (42).
In addition, SREBP1 and SREBP2, which were significantly upregulated in CAD plasma samples, were downregulated following the knockdown of lncRNA ENST00000416361. SREBPs are important molecules in lipid synthesis and are encoded by 2 SREBP genes, SREBP1 and SREBP2 (43). SREBP1 is primarily distributed in the liver and adrenal gland and it is closely associated with genes involved in fatty acid metabolism (44). SREBP2 is widely expressed and participates in cholesterol biosynthesis and metabolism (44,45). Increasing studies have focused on the correlation between SREBP and CAD. SREBP is upregulated in patients with CAD and is a cardiovascular risk factor for the severity of CAD and poor lipid control (46). Another study identified that the recessive allele (TT vs. CT+CC) of SREBP1 rs9902941 is highly expressed in patients with CAD compared with the normal control (47). miRNA-33a and miRNA-33b are intron miRNAs encoded by the intron regions of SREBP1 and SREBP2 genes (48). miRNA-122 affects CAD by regulating genes associated with lipid synthesis and oxidation, including SREBP, microsomal triglyceride transfer protein, Krüppel-like factor 6 and cationic amino acid transporter 1 (49). Silencing of miRNA-122 leads to a decrease in total cholesterol and triglyceride in plasma (49). At present, lncRNAs have emerged as critical factors in the promotion of hepatic gluconeogenesis and cholesterol biosynthesis via the SREBP pathway (46). However, their functions in CAD remain unknown, which highlights the importance of the results of the present study.
In conclusion, a novel lncRNA ENST00000416361 was first identified to be significantly upregulated in patients with CAD by high-throughput sequencing in the present study. lncRNA ENST00000416361 has potential as a biomarker for clinical screening and diagnosis of CAD. The experimental results indicated that ENST00000416361 had an association with 2 inflammatory cytokines (IL-6 and TNF-α) and the lipid metabolism-associated genes (SREBP1 and SREBP2). These results suggested that ENST00000416361 may be related to the pathogenesis of atherosclerosis, such as inflammation and lipid metabolism. Nevertheless, CAD is often accompanied by hypertension, and its occurrence and development are also affected by numerous environmental factors. Although these parameters are markedly associated with CAD, it is difficult to independently evaluate the effect of each factor, therefore this should be investigated in future studies. Therefore, the specific mechanism underlying ENST00000416361 in the progression of CAD has not been elucidated, which requires further exploration.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the Special Diagnosis Techniques for Clinical Key Diseases of Suzhou Municipal Health and Family Planning Commission (grant no. LCZX201610), Science and Technology Development Fund of Nanjing Medical University (grant no. NMUB2018219) and the Suzhou Basic Research in Medical and Health Application Grant (grant no. sys2018090).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KS designed and funded this study. PL was the principal experimenter and drafted the manuscript. XY assisted the experiment and provided statistical analysis. QC designed the study and revising this manuscript critically for important intellectual content. GX, JW, and ZP performed the collection and interpretation of data. JY, ML and LY conducted literature search and data interpretation. All authors reviewed the results and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Nanjing Medical University. Informed consent was obtained from the patients and their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pagliaro BR, Cannata F, Stefanini GG, Bolognese L. Myocardial ischemia and coronary disease in heart failure. Heart Fail Rev. 2020;25:53–65. doi: 10.1007/s10741-019-09831-z. [DOI] [PubMed] [Google Scholar]
- 2.Sayols-Baixeras S, Luis-Ganella C, Lucas G, Elosua R. Pathogenesis of coronary artery disease: focus on genetic risk factors and identification of genetic variants. Appl Clin Genet. 2014;7:15–32. doi: 10.2147/TACG.S35301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/S0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 4.Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: Coronary heart disease. The American journal of medicine. 2014;127:807–812. doi: 10.1016/j.amjmed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Dai X, Wiernek S, Evans JP, Runge MS. Genetics of coronary artery disease and myocardial infarction. World J Cardiol. 2016;8:1–23. doi: 10.4330/wjc.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meseure D, Drak Alsibai K, Nicolas A, Bieche I, Morillon A. Long noncoding RNAs as new architects in cancer epigenetics, prognostic biomarkers, and potential therapeutic targets. Biomed Res Int. 2015;2015:320214. doi: 10.1155/2015/320214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 8.Hueso M, Cruzado JM, Torras J, Navarro E. ALUminating the path of atherosclerosis progression: Chaos theory suggests a role for Alu repeats in the development of atherosclerotic vascular disease. Int J Mol Sci. 2018;19:E1734. doi: 10.3390/ijms19061734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139:437–445. doi: 10.1007/s00432-012-1324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Leung A, Natarajan R. Noncoding RNAs in vascular disease. Curr Opin Cardiol. 2014;29:199–206. doi: 10.1097/HCO.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitarafan S, Yari M, Broumand MA, Ghaderian SM, Rahimi M, Mirfakhraie R, Azizi F, Omrani MD. Association of increased levels of lncRNA H19 in PBMCs with risk of coronary artery disease. Cell J. 2019;20:564–568. doi: 10.22074/cellj.2019.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Gao W, Long QQ, Zhang J, Li YF, Liu DC, Yan JJ, Yang ZJ, Wang LS. Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci Rep. 2017;7:7491. doi: 10.1038/s41598-017-07611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu D, Tang C, Liu P, Qian W, Sheng L. Withdrawal notice: Targeting lncRNAs for cardiovascular therapeutics in coronary artery disease. Curr Pharm Des. 2018 Jan 8; doi: 10.2174/1381612824666180108120727. (Epub ahead of print). doi: 10.2174/1381612824666180108120727. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130:1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang SS, Cheng J, Cai MY, Yang XL, Liu XG, Zheng BY, Xiong XD. Association of lincRNA-p21 haplotype with coronary artery disease in a Chinese Han population. Dis Markers. 2016;2016:9109743. doi: 10.1155/2016/9109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Y, Yang Y, Chen X, Wu G, Zhang X, Liu Y, Yu J, Wang X, Fu J, Li C, et al. Circulating ‘lncRNA OTTHUMT00000387022’ from monocytes as a novel biomarker for coronary artery disease. Cardiovasc Res. 2016;112:714–724. doi: 10.1093/cvr/cvw022. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Cai Y, Wu G, Chen X, Liu Y, Wang X, Yu J, Li C, Chen X, Jose PA, et al. Plasma long non-coding RNA, CoroMarker, a novel biomarker for diagnosis of coronary artery disease. Clin Sci (Lond) 2015;129:675–685. doi: 10.1042/CS20150121. [DOI] [PubMed] [Google Scholar]
- 19.Wenger NK. 2011 ACCF/AHA focused update of the guidelines for the management of patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 Guideline): highlights for the clinician. Clin Cardiol. 2012;35:3–8. doi: 10.1002/clc.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaatinen T, Laine J. Isolation of mononuclear cells from human cord blood by Ficoll-Paque density gradient. Curr Protoc Stem Cell Biol. 2007 Jun 1; doi: 10.1002/9780470151808.sc02a01s1. (Epub ahead of print). doi: org/10.1002/9780470151808.sc02a01s1. [DOI] [PubMed] [Google Scholar]
- 21.Jia Y, Xu H, Li Y, Wei C, Guo R, Wang F, Wu Y, Liu J, Jia J, Yan J, et al. A Modified Ficoll-Paque gradient method for isolating mononuclear cells from the peripheral and umbilical cord blood of humans for biobanks and clinical laboratories. Biopreserv Biobank. 2018;16:82–91. doi: 10.1089/bio.2017.0082. [DOI] [PubMed] [Google Scholar]
- 22.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Gene Ontology Consortium, corp-author. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–D338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang M, Zhong Z, Lv M, Shu J, Tian Q, Chen J. Comprehensive analysis of differentially expressed profiles of lncRNAs and circRNAs with associated co-expression and ceRNA networks in bladder carcinoma. Oncotarget. 2016;7:47186–47200. doi: 10.18632/oncotarget.9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol. 2011;18:1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimson A, Farh KK-H, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 31.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363–e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iliopoulos D, Drosatos K, Hiyama Y, Goldberg IJ, Zannis VI. MicroRNA-370 controls the expression of microRNA-122 and Cpt1alpha and affects lipid metabolism. J Lipid Res. 2010;51:1513–1523. doi: 10.1194/jlr.M004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Hou S, Fang M, Zhu Q, Liu Y, Liu L, Li X. MicroRNA-939 governs vascular integrity and angiogenesis through targeting gamma-catenin in endothelial cells. Biochem Biophys Res Commun. 2017;484:27–33. doi: 10.1016/j.bbrc.2017.01.085. [DOI] [PubMed] [Google Scholar]
- 36.Jaé N, Heumüller AW, Fouani Y, Dimmeler S. Long non-coding RNAs in vascular biology and disease. Vascul Pharmacol. 2019;114:13–22. doi: 10.1016/j.vph.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Ahmadi R, Heidarian E, Fadaei R, Moradi N, Malek M, Fallah S. miR-342-5p Expression levels in coronary artery disease patients and its association with inflammatory cytokines. Clin Lab. 2018;64:603–609. doi: 10.7754/Clin.Lab.2017.171208. [DOI] [PubMed] [Google Scholar]
- 38.Fadaei R, Moradi N, Kazemi T, Chamani E, Azdaki N, Moezibady SA, Shahmohamadnejad S, Fallah S, et al. Decreased serum levels of CTRP12/adipolin in patients with coronary artery disease in relation to inflammatory cytokines and insulin resistance. Cytokine. 2019;113:326–331. doi: 10.1016/j.cyto.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–1879. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammadah M, Sullivan S, Pearce B, Mheid IA, Wilmot K, Ramadan R, Tahhan AS, O'Neal WT, Obideen M, Alkhoder A, et al. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav Immun. 2018;68:90–97. doi: 10.1016/j.bbi.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H, Ding S, Liu X, Wu Y, Wu X. Association of interleukin-6 genetic polymorphisms and environment factors interactions with coronary artery disease in a Chinese Han population. Clin Exp Hypertens. 2018;40:514–517. doi: 10.1080/10641963.2017.1403618. [DOI] [PubMed] [Google Scholar]
- 42.Guo F, Tang C, Li Y, Liu Y, Lv P, Wang W, Mu Y. The interplay of LncRNA ANRIL and miR-181b on the inflammation-relevant coronary artery disease through mediating NF-kappaB signalling pathway. J Cell Mol Med. 2018;22:5062–5075. doi: 10.1111/jcmm.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuan YC, Hashidume T, Shibata T, Uchida K, Shimizu M, Inoue J, Sato R. Heat shock protein 90 modulates lipid homeostasis by regulating the stability and function of sterol regulatory element-binding protein (SREBP) and SREBP cleavage-activating protein. J Biol Chem. 2017;292:3016–3028. doi: 10.1074/jbc.M116.767277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/S0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 45.Sato R. Sterol metabolism and SREBP activation. Arch Biochem Biophys. 2010;501:177–181. doi: 10.1016/j.abb.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Belmonte LM, Moreno-Santos I, Cabrera-Bueno F, Sánchez-Espín G, Castellano D, Such M, Crespo-Leiro MG, Carrasco-Chinchilla F, Alonso-Pulpón L, López-Garrido M, et al. Expression of Sterol Regulatory Element-Binding Proteins in epicardial adipose tissue in patients with coronary artery disease and diabetes mellitus: Preliminary study. Int J Med Sci. 2017;14:268–274. doi: 10.7150/ijms.17821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abudesimu A, Adi D, Siti D, Xie X, Yang YN, Li XM, Wang YH, Wang YT, Meng YJ, Liu F, et al. Association of genetic variations in the lipid regulatory pathway genes FBXW7 and SREBPs with coronary artery disease among Han Chinese and Uygur Chinese populations in Xinjiang, China. Oncotarget. 2017;8:88199–88210. doi: 10.18632/oncotarget.21082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price NL, Singh AK, Rotllan N, Goedeke L, Wing A, Canfrán-Duque A, Diaz-Ruiz A, Araldi E, Baldán A, Camporez JP, et al. Genetic ablation of miR-33 increases food intake, enhances adipose tissue expansion, and promotes obesity and insulin resistance. Cell Rep. 2018;22:2133–2145. doi: 10.1016/j.celrep.2018.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong J, Liang YZ, Zhang J, Wu LJ, Wang S, Hua Q, Yan YX. Potential role of lipometabolism-related MicroRNAs in peripheral blood mononuclear cells as biomarkers for coronary artery disease. J Atheroscler Thromb. 2017;24:430–441. doi: 10.5551/jat.35923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.