Abstract
Phase 0 clinical trials, developed in response to the United States Food and Drug Administration (FDA)’s recent exploratory Investigational New Drug (IND) guidance, are intended to expedite the clinical evaluation of new molecular entities. The exploratory IND supports the performance of first-in-human testing of new investigational agents at subtherapeutic doses based on reduced manufacturing and toxicologic requirements, allowing the demonstration of drug-target effects and assessment of pharmacokinetic–pharmacodynamic relationships in humans earlier in clinical development. The objectives of a phase 0 cancer clinical trial are toestablish at the very earliest opportunity—before large numbers of patients have been accrued and exposed to potential drug-associated toxicity—whether an agent is modulating its target in a tumor, and consequently whether further clinical development is warranted. We review here the fundamental requirements of clinical studies conducted under an exploratory IND and address some common misconceptions regarding oncologic phase 0 trials.
THE EXPLORATORY INVESTIGATIONAL NEW DRUG GUIDANCE
Developing a new anticancer drug is an expensive, long-term, high-risk proposition with a failure rate of more than 90%. More than half of new drugs in oncology fail during later stages of clinical development, adding to the cost and time it takes to make effective therapies available to patients.1,2 To accelerate the discovery and development of new molecular entities, the FDA released an exploratory Investigational New Drug (IND) guidance in 2006 to support clinical evaluation before the dose escalation, safety, and tolerance studies associated with a traditional IND.3 Objectives and endpoints of phase 0 (or pre-phase I) studies conducted under an exploratory IND may include evaluating modulation of a presumed drug target in humans; optimizing target assay methodology using human samples; providing pharmacokinetic (PK) data; assessing PK/pharmacodynamic (PD) relationships; and selecting the most promising lead agent from several chemical entities or formulations.4
A major distinction between phase 0 trials and trials conducted under a traditional IND is that phase 0 trials have no therapeutic intent. Study participants, who can be either patients or healthy volunteers, are administered subtherapeutic but pharmacologically active doses of drug. Participant exposure to the agent is limited, but dose escalation is allowed, provided that the end point is not to establish a safety/toxicity profile. Because the doses and drug exposures are low, significant drug-related adverse events are not anticipated, and the FDA allows more limited (single-dose or short-course) preclinical toxicology studies to be used to establish margin of safety rather than dose-limiting toxicities. Furthermore, because of the modest amount of study drug needed to conduct a phase 0 trial, full-scale, clinical good manufacturing practice-grade commercial manufacturing is not required before trial initiation. Thus, phase 0 trials can be initiated earlier than traditional phase I studies, providing a valuable opportunity to study PK and drug target effects in humans much earlier in the clinical development of an agent. Data obtained from such pilot trials involving small number of patients can guide decisions regarding further clinical development and better inform the design of subsequent trials (Fig. 1). The human PK and PD data will help expedite subsequent trials, such as accelerated (ie, limited dose level) phase I studies, phase I trials combining targeted agents with cytotoxic drugs, or phase I/II trials. In all of these subsequent steps, a traditional IND application must be filed to continue clinical evaluation. In 2007, the first phase 0 clinical trial of a therapeutic agent in oncology was conducted by the authors to evaluate ABT-888, an inhibitor of the DNA repair enzyme poly-ADP ribose polymerase in patients with advanced malignancies.5–7 The potential value of the exploratory IND to expedite the traditional drug development pathway has also been recognized by its growing use in the pharmaceutical industry.8 It is particularly useful in prioritizing potential agents for further study very early in the clinical development process.
FIGURE 1.
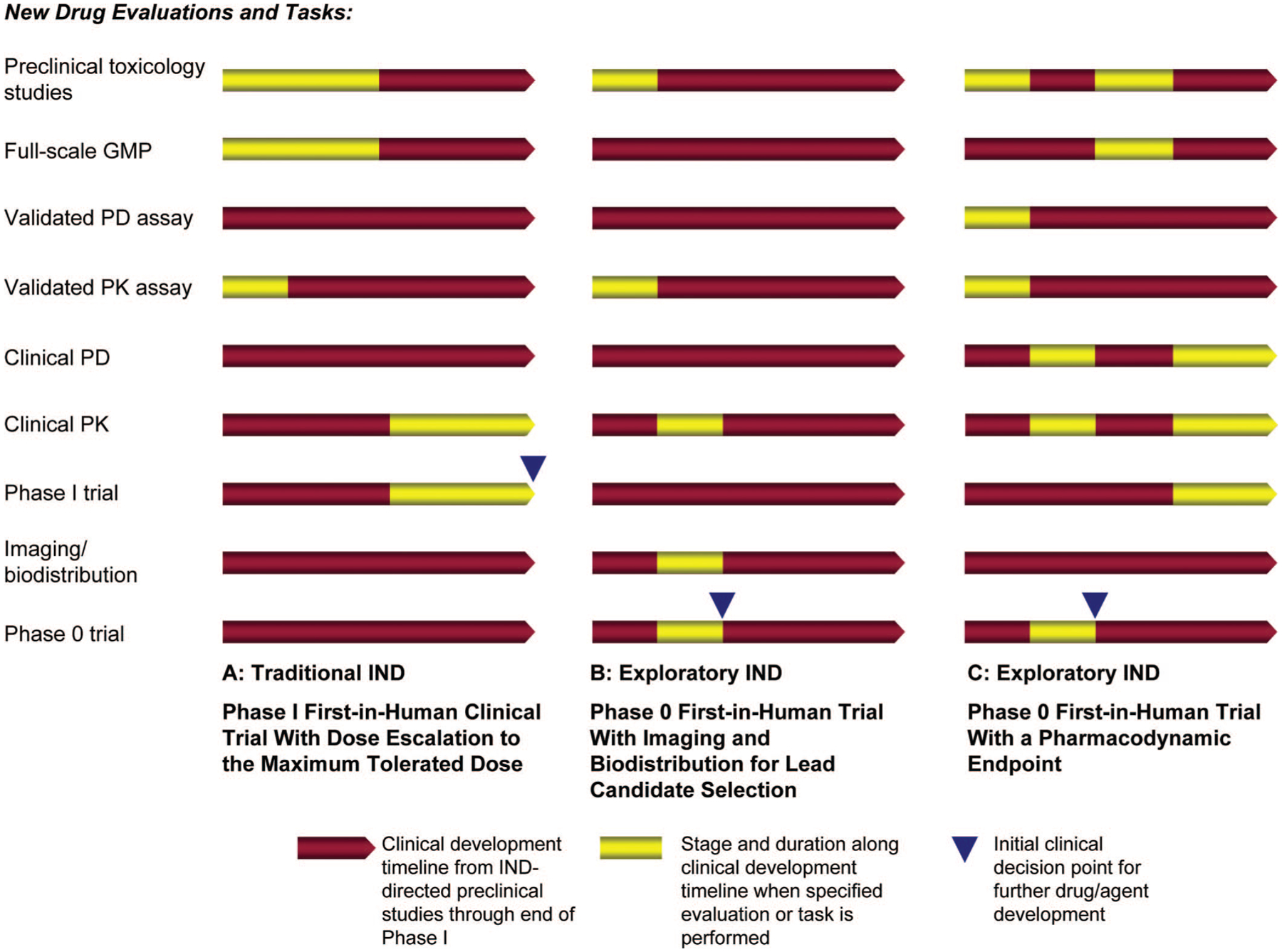
Shortening clinical development timelines with an exploratory Investigational New Drug (IND) guidance. Conducting a phase 0 trial under an exploratory IND can reduce the clinical development time for new agents and inform further clinical decision making. A, Phase I trials under a traditional IND require substantial preclinical toxicology studies and full-scale good manufacturing practice production of the investigational agent before clinical administration. Pharmacodynamic (PD) studies are generally not performed until phase II trials are initiated. B, Phase 0 imaging/biodistribution trials introduce sub-pharmacologic doses of the new agent to patients or healthy volunteers. Results from these trials may be sufficient to establish proof of principle, and no further dose escalation phase I studies may be needed. These imaging studies can be used as correlative studies in subsequent phase II/III trials of therapeutic agents. C, Phase 0 trials with a PD end point must have a validated PD assay before clinical trial accrual. The decision to proceed for further clinical development and conduct accelerated phase I/phase I combination, or phase I/II trials can be made based on whether the PD objective was met in the phase 0 trial.
The FDAs exploratory IND guidance provides 3 general examples of early-phase clinical trials that address (1) PK or imaging, (2) pharmacologically relevant doses, and (3) evaluation of an agent’s mechanism of action. In the first example provided by the FDA, studies are designed to obtain PK data but use drug doses that do not have pharmacologic effects; this example introduces the concept of “microdosing.” Microdoses are defined in the guidance as less than 1/100th of that calculated in preclinical animal toxicology studies to have a pharmacologic effect, up to a limit of 100 mg (or no more than 30 nmol for protein products). In practice, preclinical toxicology studies conducted to support the exploratory IND should demonstrate that a dose 100 times greater than the proposed clinical dose does not cause adverse events. In comparison, the starting dose for a first-in-human oncology study conducted under a traditional IND might be 1/10th of the dose that resulted in severe toxicity or death in 10% of the rodents tested.9 Microdosing studies, also called trace-dose human absorption, distribution, metabolism, and excretion screens, involve administration of a single subpharmacological dose of an isotopically labeled drug for analysis by “ultrasensitive” accelerator mass spectrometry or positron emission tomography. One major concern about microdose studies is that extrapolation to therapeutic doses may be difficult because of the presence of nonlinear PK; in such circumstances, the PK determined with a microdose study are not predictive of the agent’s PK at clinical dose levels.10–12
It is important to differentiate studies administering microdoses from those administering pharmacologically active but subtherapeutic doses. The former studies measure drug PK parameters, such as binding affinity and absorption, distribution, metabolism, and excretion. The latter, addressed in the second and third FDA guidance examples, assesses specific, predefined PK, and PD endpoints of special interest for oncologic drug development. For instance, in the second FDA guidance example, phase 0 studies of pharmacologically relevant doses can establish the PK parameters (such as oral bioavailability) of one or more investigational agents, assessing suitability for further development at doses carrying minimal risk of drug-associated toxicity. The starting dose is defined as 1/50th of the no observed adverse effect level determined in a rodent 2-week toxicology study. If the non-rodent is the most sensitive species, the candidate agent should be excluded from the exploratory IND. Dose escalation for a desired drug exposure or target modulation is allowed, but limited in the guidance to several maximum dose criteria, for example, the dose at which the pharmacological effect or target modulation is first measured, or clinically equivalent to 1/4 of the no observed adverse effect level in a 2-week rodent toxicology study, or 1/2 the area under the curve of the most sensitive species, whichever is lowest.
The third FDA guidance example covers phase 0 studies to evaluate an agent’s mechanism of action. These studies incorporate a PD end point that reflects drug activity, such as inhibition of a target enzyme in surrogate or tumor tissue samples. The starting dose for these studies is consistent with those for studies measuring PK and PD endpoints and is based on efficacy in animal models. The guidance allows considerable flexibility in study design; a recent review describes how the FDA allowed a pharmaceutical company to conduct a phase 0 trial with a longer dosing period than the maximum 7 days described in the guidance.8
Because the emphasis of phase 0 trials is on proof-of-concept rather than identification of a dose to take to phase II testing based on toxicity, the number of participants needed is smaller than for a phase I trial, typically only 10 to 15. Phase 0 study designs must therefore address the statistical limitations of small sample size clinical studies, the analytical performance of the PD assay to be employed, intrapatient variability, and interpatient molecular and histologic heterogeneity when measuring PK/PD effects as the primary endpoints. The issue of intrapatient variability is of particular concern when the primary end point is derived from invasive tumor biopsies that by their nature do not allow frequent tissue sampling. In this case, posttreatment effects must be measured against the pretreatment end point variability that can be examined across patients, rather than within an individual patient, making achievement of statistical significance substantially more difficult because interpatient end point variability is, by definition, greater (often much greater) than intrapatient variability.
Patient eligibility for phase 0 and phase I oncology trials is similar in that patients’ tumors are likely to be refractory to FDA-approved therapies; however, phase 0 studies, because of their limited duration, may also include patients with indolent diseases such as chronic lymphocytic leukemia or follicular lymphomas for which standard therapy may not be indicated. Choosing to participate in a phase 0 rather than a phase I first-in-human trial requires understanding by the patient that, in the case of the phase 0 study, there is no possibility of therapeutic benefit.
MOLECULAR TARGETS AND PHARMACODYNAMIC ASSAYS
The decision to evaluate a new investigational agent under an exploratory rather than traditional IND depends on several factors. For the agent, these factors include low toxicity and a wide therapeutic index in animal models allowing demonstration of target modulation in the absence of significant side effects.4 For phase 0 trials evaluating mechanism of action, considerable preexisting information on the molecular pharmacology of the drug is required, as is the availability of a PD assay that can reliably measure drug effect on target, either directly in tumor or in a surrogate tissue. Therefore, one barrier to the conduct of a phase 0 trial is the availability of the resources to develop an assay that is sufficiently sensitive, robust, and reliable to obtain significant results from a small study population.13 The assay must also be clinically feasible in that the target effect being investigated can be observed in accessible tissue. Standard operating procedures for handling and processing of clinical specimens also need to be optimized in preclinical models before trial initiation.14 In short, the clinical qualification of the PD assay to be employed is essential; the assay must be capable of providing a high degree of confidence that the drug’s effect on its intended target can be measured accurately, and it must be possible to use assay results to reliably support clinical development decisions.
Some of the additional challenges associated with using a PD end point as the primary objective in a phase 0 trial include the small number of patients involved, intra- and interpatient tumor and surrogate tissue sampling variability, varied tumor histologies in the clinical trial sample, and molecular heterogeneity within a tumor type, all of which can limit the possibility of demonstrating a statistically significant PD effect in the tumor target or surrogate tissue.14,15
ETHICAL ISSUES
A common criticism of phase 0 trials is that they are experiments in people—specifically, patients with terminal cancer—that are unethical because they offer no possibility of direct therapeutic benefit. Phase I and phase 0 oncology studies both accrue patients with advanced malignancies that are refractory to standard therapies. The ethical issues surrounding phase I trials, including the appropriateness and voluntary nature of the informed consent obtained, the scientific validity of the study, and risk/benefit perception and assessment, have been the subject of considerable discussion.16–19 Phase 0 trials have yet to be subjected to the same level of scrutiny, but their inherent lack of therapeutic intent is an obvious ethical issue.20–22
As with all trials involving human subjects, potential risks must be carefully evaluated before being granted protocol approval from an Institutional Review Board, and patient safety is of paramount importance. The Institutional Review Board must ensure that, in addition to minimizing risks, the “risks to subjects are reasonable in relation to anticipated benefits, if any, and the importance of the knowledge that may reasonably be expected to result” (45CFR.46.111).23 Evaluating the ratio of potential risks to potential benefits when there is no direct benefit to patients is therefore challenging. Even with low doses and limited dosing schedules, the risks are not negligible and include those associated with biopsy procedures. In our experience, during the development of the protocol and consent document, discussions with bioethicists about the study and associated risks are helpful. The consent document should clearly state the lack of therapeutic intent and the requirement and associated risks of tumor biopsies. Additionally, patients should be made to verbalize their understanding of these elements before signing the consent document. It is worth stating, however, that patients with incurable diseases do appreciate information regarding both the risks associated with the trial and the value of the knowledge to be gained from their participation.24 In the authors’ experience, most patients have participated in multiple clinical trials before considering the phase 0 study and are thus familiar with the concepts of clinical research and research biopsies. A recent analysis of phase I oncology trial participants revealed no cognitive, health, or demographic factors consistent with a reduced ability to make informed decisions.25 Furthermore, unlike the ethical concerns raised for obtaining research biopsies in phase I and II trials, a patient’s decision to participate and provide biopsy samples for research purposes as part of a phase 0 trial is not clouded by any perception of direct medical benefit.26,27 A trial which has as its primary objective providing evidence of a drug’s effect on its intended target cannot meet this objective without an assay capable of measuring these effects. Therefore, it is essential to ensure that there is a reliable PD assay that could help answer the scientific question with a high level of confidence before asking patients to undergo invasive biopsy procedures that have known risks.26–28
Patient willingness to collaborate in a study designed solely to obtain generalizable knowledge is remarkable and stems from a desire to help future cancer patients. Therefore, it is important to keep patients informed of study results and how these have had an impact on the further development of the agent. It is also essential to ensure that participation in a phase 0 trial neither delays nor excludes patients from participating in other clinical trials that do offer the possibility of direct benefit. This can be accomplished by limiting the washout period from prior therapy (eg, no more than 2 weeks) both for enrolling in a phase 0 trial and after completion of the study before enrolling on another trial. Also, participation in a phase 0 trial should not exclude patients from participating in a subsequent, later stage trial of that agent; there is now common language in multiple NCI phase I and II protocols specifically addressing this issue. It is our hope that this language will be widely adopted by other cancer research centers.
CONCLUSION AND PERSPECTIVES
Phase 0 trials may help to address some of the most challenging issues for new drug development in oncology, by helping to prioritize potential agents for future study, reducing development timelines, and demonstrating proof-of-concept target inhibition. For example, results from a phase 0 imaging study may be sufficient to establish proof of principle and eliminate the need for a phase I dose escalation trial; imaging can instead be included as a correlative study in subsequent phase II/III therapeutic agent evaluations. A phase 0 trial with a PD end point that meets its objective can support the decision to proceed to accelerated phase I, phase I combination, or phase I/II trials. It is important to emphasize that phase 0 trials will not replace the phase I trials conducted under a traditional IND to establish the maximum tolerated dose and drug toxicity profile. Not all investigational agents are suitable for phase 0 evaluation. Considerable investment of time and resources are needed to develop suitable PD assays and sample handling procedures. Furthermore, investigators may have difficulty developing the resources for phase 0 studies because nontherapeutic clinical trials are not covered by most third-party payors. The ethical considerations required to conduct a phase 0 trial are not minor, but frank and open discussion with participants before, during, and after the trial will be of mutual benefit.
Phase 0 trials do offer an option to evaluate PK and confirm an agent’s effect on its intended molecular target in human specimens much earlier in clinical development. Experience to date with studies conducted under exploratory INDs is limited, but positive.8 If drug effect on a target can be evaluated earlier in the drug development cycle and requires fewer patients than a traditional IND, it follows that clinical trials will be smaller, and development timelines may be compressed.
ACKNOWLEDGMENTS
The authors thank Dr. Guilio Draetta for providing the inspiration for Figure 1 and Ms. Gina Uhlenbrauck, SAIC-Frederick, Inc., for editorial assistance in the preparation of this manuscript.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
REFERENCES
- 1.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–715. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services, Food and Drug Administration. Innovation or Stagnation? Challenge and Opportunity on the Critical Path to New Medical Products. March 2004. Available at: http://www.fda.gov/oc/initiatives/criticalpath/whitepaper.pdf. Accessed February 21, 2008.
- 3.US Department of Health and Human Services, Food and Drug Administration. Guidance for Industry, Investigators, and Reviewers: Exploratory IND Studies. January 2006. Available at: http://www.fda.gov/cder/guidance/7086fnl.pdf. Accessed February 21, 2008.
- 4.Kummar S, Kinders R, Rubinstein L, et al. Compressing drug development timelines in oncology using phase ‘0’ trials. Nat Rev Cancer. 2007;7:131–139. [DOI] [PubMed] [Google Scholar]
- 5.Kummar S, Kinders R, Gutierrez M, et al. Inhibition of poly(ADP ribose) polymerase (PARP) by ABT-888 in patients with advanced malignancies: results of a phase 0 trial [abstract]. J Clin Oncol. 2007; 25:3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. [DOI] [PubMed] [Google Scholar]
- 7.Ratnam K, Low JA. Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clin Cancer Res. 2007;13: 1383–1388. [DOI] [PubMed] [Google Scholar]
- 8.Robinson WT. Innovative early development regulatory approaches: expIND, expCTA, microdosing. Clin Pharmacol Ther. 2008;83:358–360. [DOI] [PubMed] [Google Scholar]
- 9.DeGeorge JJ, Ahn CH, Andrews PA, et al. Regulatory considerations for preclinical development of anticancer drugs. Cancer Chemother Pharmacol. 1998;41:173–185. [DOI] [PubMed] [Google Scholar]
- 10.Lappin G, Garner RC. Big physics, small doses: the use of AMS and PET in human microdosing of development drugs. Nat Rev Drug Discov. 2003;2:233–240. [DOI] [PubMed] [Google Scholar]
- 11.Lappin G, Kuhnz W, Jochemsen R, et al. Use of microdosing to predict pharmacokinetics at the therapeutic dose: experience with 5 drugs. Clin Pharmacol Ther. 2006;80:203–215. [DOI] [PubMed] [Google Scholar]
- 12.Boyd RA, Lalonde RL. Nontraditional approaches to first-in-human studies to increase efficiency of drug development: will microdose studies make a significant impact? Clin Pharmacol Ther. 2007;81: 24–26. [DOI] [PubMed] [Google Scholar]
- 13.Steps to consider in pharmacodynamic assay development. National Cancer Institute Developmental Therapeutics Program Web Site. Available at: http://dtp.nci.nih.gov/docs/phase0/PharmacoDynamicAssaydeveloment.html. Accessed February 21, 2008.
- 14.Kinders RJ, Hollingshead M, Parchment RE, et al. Preclinical modeling of a phase 0 clinical trial protocol [abstract]. J Clin Oncol. 2007;25: 14058. [Google Scholar]
- 15.Betensky RA, Louis DN, Cairncross JG, et al. Influence of unrecognized molecular heterogeneity on randomized clinical trials. J Clin Oncol. 2002;20:2495–2499. [DOI] [PubMed] [Google Scholar]
- 16.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283:2701–2711. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal M, Emanuel EJ. Ethics of phase I oncology studies: reexamining the arguments and data. JAMA. 2003;290:1075–1082. [DOI] [PubMed] [Google Scholar]
- 18.Joffe S, Miller FG. Rethinking risk-benefit assessment for phase I cancer trials. J Clin Oncol. 2006;24:2987–2990. [DOI] [PubMed] [Google Scholar]
- 19.Koyfman SA, Agrawal M, Garrett-Mayer E, et al. Risks and benefits associated with novel phase 1 oncology trial designs. Cancer. 2007;110: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 20.Kimmelman J Ethics at phase 0: clarifying the issues. J Law Med Ethics. 2007;35:727–733. [DOI] [PubMed] [Google Scholar]
- 21.Hill TP. Phase 0 trials: are they ethically challenged? Clin Cancer Res. 2007;13:783–784. [DOI] [PubMed] [Google Scholar]
- 22.Marchetti S, Schellens JHM. The impact of FDA and EMEA guidelines on drug development in relation to Phase 0 trials. Br J Cancer. 2007; 97:577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services. Code of Federal Regulations Title 45—Public Welfare, Department of Health and Human Services, Part 46: Protection of Human Subjects. Revised June 23, 2005. Available at: http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm. Accessed February 21, 2008.
- 24.Agrawal M Voluntariness in clinical research at the end of life. J Pain Symptom Manage. 2003;25:S25–S32. [DOI] [PubMed] [Google Scholar]
- 25.Seidenfeld J, Horstmann E, Emanuel EJ, et al. Participants in phase 1 oncology research trials: are they vulnerable? Arch Intern Med. 2008; 168:16–20. [DOI] [PubMed] [Google Scholar]
- 26.Agulnik M, Oza AM, Pond GR, et al. Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. J Clin Oncol. 2006;24:4801–4807. [DOI] [PubMed] [Google Scholar]
- 27.Helft PR, Daugherty CK. Are we taking without giving in return? The ethics of research-related biopsies and the benefits of clinical trial participation. J Clin Oncol. 2006;24:4793–4795. [DOI] [PubMed] [Google Scholar]
- 28.Stadler WM, Ratain MJ. Development of target-based antineoplastic agents. Invest New Drugs. 2000;18:7–16. [DOI] [PubMed] [Google Scholar]


