Abstract
Despite antiretroviral therapy (ART), innate and adaptive immunologic damage persists in the periphery and gut. T memory stem cells (Tscm) and natural killer (NK) cells are pivotal for host defense. Tscm are memory cells capable of antigen response and self-renewal, and circulating and gut NK cell populations may facilitate HIV control. The impact of early ART on circulating and gut Tscm and NK cells is unknown. We enrolled participants who initiated ART during acute versus chronic HIV-1 infection versus no ART in chronic infection. We performed flow cytometry to identify NK and Tscm cells in the blood and rectum and polymerase chain reaction to quantify the HIV-1 reservoir in both sites. We used the Mann–Whitney U-test and Spearman correlation coefficients for analysis. Participants who started ART in acute infection had lower rectal CD56brightCD16dim cell frequencies than participants who started ART in chronic HIV-1 infection and lower CD56bright and CD56brightCD16− cell frequencies than participants with chronic infection without ART. Higher circulating NK cell, CD56−CD16bright, CD56dim, and CD56dimCD16bright frequencies correlated with higher HIV-1 DNA levels in rectal CD4+ T cells, whereas higher circulating CD4+ T cell counts correlated with higher rectal NK, CD56brightCD16dim, and CD56dimCD16bright frequencies. Peripheral CD56brightCD16− cells were inversely associated with rectal CD56−CD16bright cells. Rectal CD8+ Tscm frequencies were higher in participants without ART than participants with chronic infection on ART. Timing of ART initiation determines rectal NK cell populations, and ART may influence rectal Tscm populations. Whether the gut reservoir contributes to NK cell activation requires further study.
Keywords: NK cells, Tscm, HIV, acute HIV, gut
Introduction
HIV-1-induced immunological destruction begins during acute infection and is not completely restored in people with HIV-1 initiating antiretroviral therapy (ART) during chronic infection. However, the initiation of ART during acute infection may delay disease progression, preserve adaptive immune function, decrease viral diversity, and reduce the viral reservoir size in blood and mucosa.1,2 What is less clear from these studies is the effect that early ART may have on innate immunity, which also responds to HIV-1 infection.
T memory stem cells (Tscm) are memory cells that are capable of self-renewal3 and differentiate into central and effector memory cells.4 They can respond to antigen and proliferate rapidly and produce cytokines.3 They are found in significantly lower levels in the rectum compared with the peripheral blood.4 CD4+ Tscm serve as a reservoir for HIV-1 and are decreased in viremic nonprogressors. As these are self-renewing cells with a long half-life, they likely contribute to HIV-1 persistence.3 CD8+ Tscm tend to produce interferon gamma (IFN-γ) and interleukin-2, persist in the absence of antigen, and are less susceptible to apoptosis than other memory cells.4 Moreover, higher frequencies of CD8+ Tscm have been associated with lower plasma HIV-1 RNA levels and less immune activation.5
Natural killer (NK) cells are distributed throughout the body and comprise CD56dim (CD16+ or CD16−) cells, which are cytotoxic; CD56bright cells, which produce cytokines, and have homing receptors, permitting trafficking into tissues; and CD56−, which are dysfunctional, with impaired cytotoxicity and cytokine production, and have been shown to abound in the setting of AIDS.6–8 Circulating NK cells increase in acute HIV-1 infection with increases in CD56dim and decreases in CD56bright subsets.9 Circulating CD56−CD16+ NK cells increase with persistent viral replication. All these changes are reversible with ART.9,10 NK cells are also found in the intraepithelial compartments and lamina propria of the gastrointestinal tract.11 Gut-associated lymphoid tissue NK cells have been shown to increase as viral load decreases with ART.11 Of note, NK cells, particularly CD56bright NK cells, accumulate within atherosclerotic lesions12 and people with unstable coronary artery disease have fewer CD56dim NK cells.13 CD56bright NK cells may contribute to atherosclerosis through IFN-γ production, which can promote matrix metalloproteinase secretion and smooth muscle breakdown.13 As people with HIV have increased risk of cardiovascular disease,14 understanding the evolution of NK cell populations during HIV-1 infection is particularly relevant.
We previously showed that rhesus macaques treated with IFN-α 2a, starting before SIVmac251 inoculation until 4 weeks postinfection, required a greater number of challenges to become infected than placebo animals.15 The treated macaques exhibited higher frequency of circulating CD56+ NK cells at the time of challenge, and higher frequency of circulating CD56+ NK cells predicted that a greater number of challenges would be needed to transmit infection. Ultimately, these animals became resistant to IFN before acquisition and had larger viral reservoirs. These animals subsequently had a higher frequency of rectal CD16+ NK cells 4 weeks after challenge compared with placebo animals. During chronic infection, IFN-treated animals had fewer circulating CD16+ NK cells and accelerated loss of CD4+ T cells compared with placebo animals.
Thus, as people who initiate ART in acute HIV-1 infection have smaller HIV-1 reservoirs,16 we hypothesized people who initiate ART in acute HIV-1 infection would have more peripheral CD16+ NK cells and CD8+ Tscm and fewer CD4+ Tscm and CD16+ NK cells in the rectal mucosa. To test this hypothesis, we compared the effects of initiating ART during acute versus chronic HIV-1 infection versus no ART in chronic infection on NK and Tscm cells and the HIV-1 reservoir in blood and rectal mucosa.
Materials and Methods
This study was approved by the Committee for the Protection of Human Subjects (CPHS), the institutional review board at UTHealth. All participants provided written informed consent before initiating study procedures.
Study design
Participants with chronic HIV-1 infection and CD4+ T cell counts >500 cells/mm3 were recruited in three groups: (1) on ART, started during acute infection, with HIV-1 RNA <50 copies/mL; (2) on ART, started during chronic infection, with HIV-1 RNA <50 copies/mL; and (3) not on ART, in chronic infection, with HIV-1 RNA ≥1,000 copies/mL. Acute infection was defined as detectable HIV-1 RNA or p24 antigen in serum or plasma in the setting of a negative or indeterminate HIV antibody test result. Participants with proctitis or other anorectal sexually transmitted infections including active anal herpes simplex virus infection were excluded. All participants underwent phlebotomy and sigmoidoscopy for rectal biopsies.
Carotid intima media thickness
Subclinical carotid atherosclerosis was evaluated with a Philips IE33 ultrasound system (Amsterdam, the Netherlands). Left and right carotid arteries were imaged from three different angles, and carotid plaque presence was determined by examining the carotid bulb, its bifurcation, the carotid branch arteries, and the common carotid arteries. Carotid wall thickness was measured using the semi-automated carotid analyzer software (Medical Imaging Applications, Coralville, IA). Measurements were made at the R wave of the electrocardiogram on a minimum of two clips on each side and the results were averaged. Carotid plaque was defined as an area of wall thickening that was ≥75th percentile for age and gender or >50% of the thickness of the surrounding wall. The technician was certified by the University of Wisconsin Atherosclerosis Imaging Research Program.
Specimen processing
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using Ficoll density centrifugation. Rectal biopsy samples were placed in RPMI with 10% fetal bovine serum and transported on wet ice. Cells were extracted from rectal samples using digestion with Liberase TM research grade (Roche, Basel, Switzerland) and filtered using 70 μm cell strainers.
Flow cytometry
Flow cytometry was performed on cells from both peripheral blood and rectal samples. Samples were stained with Aqua LIVE/DEAD® Fixable Dead Cell Stain (Invitrogen, Carlsbad, CA) and the following antibodies: CD3 APC-H7, CCR7 PE-Cy7, CD95 PE-Cy5, CD27 PE, CD16 V450 (All BD Biosciences, San Jose, CA); CD4 BV510 (Biolegend, San Diego, CA); CD8 Qdot655, CD56 PE-Cy5.5 (Invitrogen); and CD45RO ECD (Beckman Coulter, Brea, CA). Cells were sorted using a BD Aria, and LIVE/DEAD−CD3+CD4+CD8− T cells were collected for HIV-1 DNA measurement. Data were analyzed using FlowJo (v.10.2 for Mac OS X).
The frequency of Tscm within the live CD3+CD4+ or live CD3+CD8+ population was identified by CD45RO−CCR7+CD27+CD95+.17 NK cells were identified as live CD3−CD4− cells within the lymphocyte population (based on size and scatter) that expressed CD56 and/or CD16. Gating scheme and fluorescence minus one are given in Supplementary Figures S1 and S2, respectively. For the PBMCs, a median of 1,000,000 (range = 843,177–1,000,000) events were collected with median viability by DAPI of 98.7% (range = 95.4%–99%). For the rectal samples, median of 834,000 (range = 260,829–1,226,678) events were collected, with median viability by DAPI of 91.3% (range = 84.5%–95.6%).
Quantification of HIV-1 DNA by real-time polymerase chain reaction
Genomic DNA was isolated from sorted cells using the DNAeasy Blood and Tissue Extraction kit (Qiagen, Hilden, Germany). Quantity of viral DNA in cells of participants was determined by quantitative real-time polymerase chain reaction (PCR) using an ABI One-Step Plus Cycler and a modified protocol of Chun et al. with the described primers (5′-GGTCTCTCTGGTTAGACCAGAT-3′ [5′-primer] and 5′-CTGCTAGAGATTTTCCACACTG-3′ [3′-primer]) and probe (5′-6FAM-AGTAGTGTGTGCCCGTCTGTT-TAMRA-3′) for amplification of an HIV-1 LTR sequence.18 Each 20 μL reaction contained 900 nM of each primer and 250 nM of probe, and 1 × TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA), participant-derived DNA sample, HIV containing standard, or no template control. DNA input was quantified by amplification of RNAseP using the TaqMan Copy Number Reference Assay (VIC-TAMRA; Applied Biosystems). PCR conditions consisted of an initial step at 95°C for 10 min. This step was followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Tenfold serially diluted ACH-2 DNA over a 6-log range were used to generate standard curves for detection of the HIV LTR. Using ACH-2 cells serially diluted in primary human PBMCs, we established the detection limit of this assay to be 2 HIV DNA copies/1 × 105 cells. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) amplification reactions for the HIV LTR were performed using six replicates of each DNA sample.
Statistical analyses
Data were analyzed using GraphPad Prism (v.6.0c for Mac; San Diego, CA). Mann–Whitney U-test was used for comparisons between groups, and Spearman's rank correlation test was used to determine associations among parameters. As this was an exploratory, hypothesis-generating study, there was no correction for multiple comparisons.
Results
Study population
We enrolled five participants who started ART during acute HIV-1 infection, five who started ART during chronic HIV infection, and five with chronic HIV-1 infection who were not taking ART (Table 1). The median age of all participants was 40 years (31 in the acute group, 45 in the chronic on ART group, and 26 in the chronic no ART group). Seven black non-Hispanic, three white non-Hispanic, and five Hispanic people were enrolled. The racial and ethnic distribution of the groups differed (p = .03 by chi-square), with Hispanic people dominating the acute group and black people dominating the chronic no ART group. The median CD4+ T cell count at the time of recruitment was 823 (range = 555–1,051) cells/mm3 with no significant differences among groups (826 [555–1,051] in acute, 819 [640–970] in chronic on ART, and 782 [583–1,004] cells/mm3 in chronic not on ART). The HIV-1 RNA levels at entry were <20 copies/mL in the acute and chronic on ART groups and 8,960 (4,250–127,000) copies/mL in the chronically infected without ART group. Median duration of ART was 6 (4–28) months in the acute group and 82 (11–142) months in the chronic group. Antiretroviral regimens are given in Table 1.
Table 1.
Characteristics
| Overall | Acute on ART | Chronic on ART | Chronic no ART | |
|---|---|---|---|---|
| Age, years, median (range) | 40 (19–55) | 31 (26–47) | 45 (40–52) | 26 (19–55) |
| Race/ethnicity (N) | ||||
| White non-Hispanic | 3 | 1 | 2 | 0 |
| White Hispanic | 5 | 4 | 0 | 1 |
| Black non-Hispanic | 7 | 0 | 3 | 4 |
| CD4, cells/mm3, median (range) | 823 (555–1,051) | 826 (555–1,051) | 819 (640–970) | 782 (583–1,004) |
| HIV-1 RNA, copies/mL, median (range) | <20 | <20 | <20 | 8,960 (4,250–127,000) |
| Time since diagnosis, years, median (range) | 1.8 (0.03–26.0) | 1.7 (0.6–2.5) | 9.2 (3.1–26.0) | 0.46 (0.03–18.2) |
| ART | ||||
| NRTI | 9 | 5 | 4 | 0 |
| NNRTI | 4 | 1 | 3 | 0 |
| INSTI | 6 | 3 | 3 | 0 |
| PI | 2 | 1 | 1 | 0 |
| CIMT, mm | 0.61 (0.54–1.18) | 0.57 (0.55–0.91) | 0.70 (0.59–0.83) | 0.65 (0.54–1.18) |
ART, antiretroviral therapy; CIMT, carotid intima media thickness.
Circulating CD4, Tscm, and NK cells
First, we aimed to determine whether early ART affected CD4+ Tscm and NK cells in circulation. We found no significant differences among groups in frequencies of CD4+ T cells (CD3+CD4+), CD4+ Tscm, or CD8+ Tscm (Supplementary Table S1). In addition, we found no significant differences among groups in the frequencies of NK cells overall (CD3−CD4− lymphocytes that express CD56 and/or CD16) as a percentage of live cells or, within this population, in the frequencies of CD56−CD16bright, CD56bright, CD56brightCD16dim, CD56brightCD16−, CD56dim, or CD56dimCD16bright cells (Supplementary Table S1).
Rectal NK cells
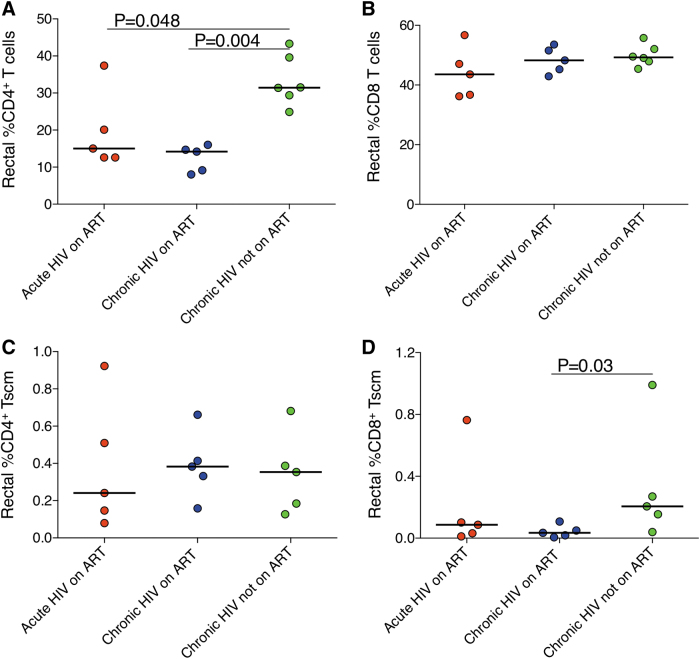
Next, we evaluated whether early ART affected CD4+ T cells, CD4+ Tscm, and NK cells in the rectum. We found, unexpectedly, a significantly lower frequency of rectal CD4+ T cells in the group that started ART during acute infection (15.0%, p = .048) or chronic infection (14.2%, p = .004) compared with the group not on ART (31.5%; Fig. 1A), but no differences in rectal CD8+ T cells (Fig. 1B). We found no differences among groups in the frequencies of CD4+ Tscm (Fig. 1C). In contrast, we found that the group not on ART had a higher frequency of rectal CD8+ Tscm (0.21%) compared with the group that started ART in chronic infection (0.03%, p = .03; Fig. 1D).
FIG. 1.
(A) Frequency of rectal CD4+ T cells. (B) Frequency of rectal CD8+ T cells. (C) Frequency of rectal CD4+ Tscm. (D) Frequency of rectal CD8+ Tscm. Tscm, T memory stem cells.
In the rectum, there were no significant differences among groups in the frequencies of NK cells overall (Fig. 2A) or CD56−CD16dim cells (Fig. 2C). Compared with the group with chronic HIV-1 not on ART, the group that started ART in chronic infection had lower rectal CD56−CD16bright cell frequencies (0.006% vs. 0.002%, p = .056; Fig. 2B), CD56bright cells (1.26% vs. 0.49%, p = .04; Fig. 2E), and CD56brightCD16−cells (1.04% vs. 0.38%, p = .03; Fig. 2G), whereas the group that started ART in acute infection had lower CD56bright cell (1.26% vs. 0.39%, p = .016; Fig. 2E) and CD56brightCD16− cell (1.04% vs. 0.33%; p = .016; Fig. 2G) frequencies. The group that started ART in acute infection also had lower CD56dim (4.26% vs. 6.30%, p = .095; Fig. 2D) and CD56brightCD16dim (0.014% vs. 0.087%, p = .02; Fig. 2F) populations, although the former did not reach statistical significance.
FIG. 2.
Frequency of rectal NK cell populations. (A) Frequency of rectal NK cells as a percentage of live cells. (B) Frequency of rectal CD56−CD16bright cells as a percentage of live CD3−CD4− cells. (C) Frequency of rectal CD56−CD16dim cells. (D) Frequency of rectal CD56dim cells. (E) Frequency of rectal CD56bright cells. (F) Frequency of rectal CD56brightCD16dim cells. (G) Frequency of rectal CD56brightCD16− cells. NK, natural killer.
HIV-1 DNA+ in CD4+ T cells
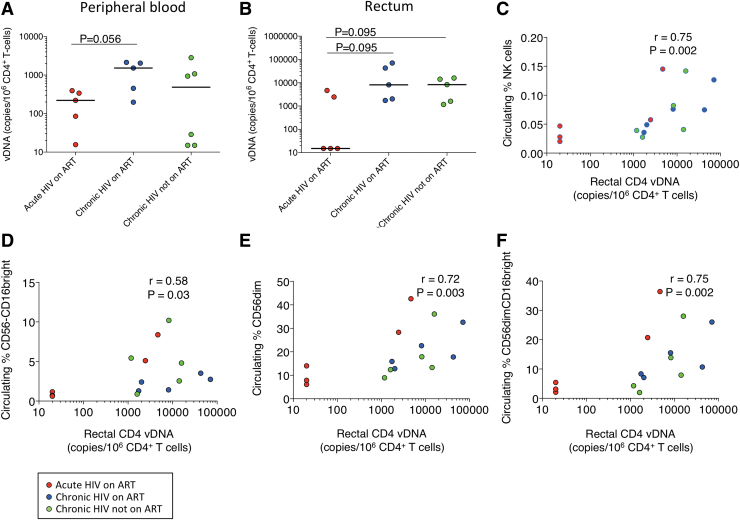
We examined whether early ART affected the amount of HIV-1 DNA in circulating and rectal CD4+ T cells. We found that HIV-1 DNA levels were lower in circulating CD4+ T cells in the group that started ART in acute infection compared with chronic on ART (221 vs. 1,527 copies/106 CD4+ T cells; p = .056; Fig. 3A) with similar findings in the rectum (<20 vs. 8,133 copies/106 CD4+ T cells; p = .095; Fig. 3B). In addition, HIV-1 DNA levels were lower in rectal CD4+ T cells in the group that started ART in acute infection compared with chronic without ART (<20 vs. 8,257 copies/106 CD4+ T cells; p = .095; Fig. 3B). Thus, early initiation of ART may decrease the HIV-1 DNA levels in both peripheral blood and gut.
FIG. 3.
Cell-associated HIV-1 DNA. (A) HIV-1 DNA levels in circulating CD4+ T cells. (B) HIV-1 DNA levels in rectal CD4+ T cells. (C) Association of circulating NK cells with rectal HIV-1 DNA in CD4+ T cells. (D) Association of circulating CD56−CD16bright cells with rectal HIV-1 DNA in CD4+ T cells. (E) Association of circulating CD56dim cells with rectal HIV-1 DNA in CD4+ T cells. (F) Association of CD56dimCD16bright cells with rectal HIV-1 DNA in CD4+ T cells. Red circles represent participants who started ART in acute HIV infection, blue circles represent participants who started ART in chronic HIV infection, and green circles represent participants with chronic HIV infection not taking ART. ART, antiretroviral therapy.
Carotid intima media thickness
To determine whether cardiovascular risk was affected by the timing of ART initiation or by these cell populations, we quantified carotid intima media thickness (CIMT). We found no differences across groups in CIMT (0.57 mm in acute on ART, 0.70 mm in chronic on ART, 0.65 mm in chronic without ART; Table 1), suggesting early ART does not affect cardiovascular risk based on this small group of participants.
Associations
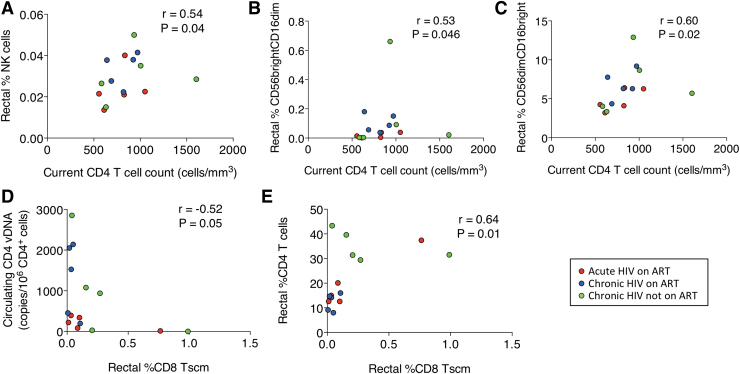
To explore the relevance of these findings, we determined correlations among the cell populations, HIV-1 DNA reservoir and CD4+ T cells. We found that higher amounts of HIV-1 DNA in rectal CD4+ T cells were associated with higher frequencies of circulating NK (r = 0.75, p = .002; Fig. 3C), CD56−CD16bright (r = 0.58, p = .03; Fig. 3D), CD56dim (r = 0.72, p = .003; Fig. 3E), and CD56dimCD16bright (r = 0.75, p = .002; Fig. 3F) cells. We found that higher current CD4+ T cell counts were associated with higher frequencies of rectal NK cells (r = 0.54, p = .04; Fig. 4A) and rectal CD56brightCD16dim (r = 0.53, p = .046; Fig. 4B) and CD56dimCD16bright (r = 0.60, p = .02; Fig. 4C) cells. We also found that higher circulating nadir CD4+ T cell counts were associated with higher frequencies of rectal CD56bright (r = 0.71, p = .008; Supplementary Fig. S3A) and CD56brightCD16− cells (r = 0.66, p = .017; Supplementary Fig. S3B).
FIG. 4.
Association of circulating CD4+ T cells with (A) rectal NK cells. (B) CD56brightCD16dim cells. (C) CD56dimCD16bright cells. Association of rectal CD8+ Tscm with (D) circulating CD4-associated HIV-1 DNA and (E) rectal CD4+ T cells. Red circles represent participants who started ART in acute HIV infection, blue circles represent participants who started ART in chronic HIV infection, and green circles represent participants with chronic HIV infection not taking ART.
Higher circulating CD4+ Tscm frequencies were associated with higher frequencies of circulating CD8+ Tscm (r = 0.61, p = .018; Supplementary Fig. S4A) and rectal CD4+ Tscm (r = 0.63, p = .01; Supplementary Fig. S4B) and lower frequencies of circulating NK cells (r = −0.69, p = .006; Supplementary Fig. S4C) but not with CD4+ HIV-1 DNA levels. Higher circulating CD8+ Tscm frequencies were also associated with lower circulating NK cell (r = −0.56, p = .03; Supplementary Fig. S4D) and higher rectal CD4 Tscm (r = 0.61, p = .017; Supplementary Fig. S4E) frequencies. Higher frequencies of rectal CD4+ Tscm were associated with lower frequencies of circulating CD56dim (r = −0.57, p = .03; Supplementary Fig. S5A) and CD56dimCD16bright (r = −0.53, p = .047; Supplementary Fig. S5B) cells and higher rectal CD8+ Tscm frequencies (r = 0.57, p = .03; Supplementary Fig. S5C). Higher frequencies of rectal CD8+ Tscm were associated with lower circulating CD4-associated vDNA levels (r = −0.52, p = .05; Fig. 4C) and higher frequencies of rectal CD4+ T cells (r = 0.64, p = .01; Fig. 4D) and rectal CD56bright (r = 0.58, p = .02; Supplementary Fig. S5D), CD56brightCD16− (r = 0.58, p = .03; Supplementary Fig. S5E), and CD56−CD16bright (r = 0.56, p = .03; Supplementary Fig. S5F) cells.
Older age was associated with higher frequencies of rectal CD56brightCD16dim (r = 0.78, p = .001; Supplementary Fig. S6A) and CD56dimCD16bright (r = 0.62, p = 0.015; Supplementary Fig. S6B) cells and higher CIMT values (r = 0.91, p < .0001; Supplementary Fig. S6C), and higher CIMT values were also associated with higher frequencies of rectal CD56brightCD16dim cells (r = 0.69, p = .008; Supplementary Fig. S6D) and rectal CD56dimCD16bright cells (r = 0.56, p = .04; Supplementary Fig. S6E). In summary, a larger rectal HIV reservoir was associated with greater frequencies of circulating NK cells; higher current CD4+ T cell counts were associated with higher frequencies of rectal NK cells; rectal CD56brightCD16dim and CD56dimCD16bright NK cells were associated with higher cardiovascular risk; and rectal CD8+ Tscm were associated with a smaller circulating reservoir and more rectal CD4+ T cells.
Discussion
In this study, we aimed to determine how peripheral blood and gut NK cells, Tscm cells, and HIV-1 DNA varied across people who started ART in acute infection, people who started ART in chronic infection, and people not taking ART. We found that (1) the group that started ART in acute infection had significantly lower rectal CD56brightCD16dim cell frequencies compared with the group that started on ART in chronic HIV infection and lower rectal CD56bright and CD56brightCD16− cell frequencies compared with the group with chronic HIV infection not on ART; (2) the group that was not taking ART had higher rectal CD56−CD16bright, CD56bright, and CD56brightCD16− cell frequencies than the group with chronic HIV on ART; (3) HIV-1 DNA levels tended to be lower in circulating and rectal CD4+ T cells in the group that started ART in acute infection compared with the group that started ART in chronic infection; (4) higher levels of HIV-1 DNA in rectal CD4+ T cells were associated with higher frequencies of circulating NK cells, including CD56−CD16bright, CD56dim, and CD56dimCD16bright NK cells; and (5) rectal CD8+ Tscm frequencies were higher in the group not on ART compared with the chronic group on ART with no differences in circulating or rectal CD4+ Tscm populations. Thus, the timing of ART initiation determines rectal NK cell populations, and the use of ART can influence rectal CD8+ Tscm populations.
In acute HIV-1 infection, NK cells are activated and proliferate.19 HIV-infected cells stimulate NK cell-mediated lysis by downregulating inhibitory receptors on NK cells and stimulating the activating NKG2D receptor; the ensuing lysis of the infected cell thereby inhibits viral replication and the chemokine production blocks virus entry by blocking CCR5.20 NK cells are essential for HIV-1 control.20
The majority of circulating NK cells are CD56dim NK cells, which express perforin and granzyme B and are cytotoxic.9,21 CD56bright NK cells have limited cytotoxic capacity but produce copious cytokines such as IFN-γ and tumor necrosis factor.22 CD56bright NK cells contract in peripheral blood and expand in the gut and CD56dimCD16+ cells expand in the periphery in acute HIV-1 infection.9,20,23 In addition, a subset of CD56dimCD16+ cells expressing CD11b and CD161 but not CD57 or Siglec-7 may contribute to virologic control, potentially by a higher frequency of degranulation, as they are more common in elite controllers than viremic persons.24 The correlation of higher frequencies of CD56dimCD16bright cells in the rectal tissue with higher circulating CD4+ T cell counts in our study would be consistent with this hypothesis. As this population also correlates with a higher rectal HIV-1 reservoir, it is tempting to speculate that these populations are responding to the increased reservoir. Thus, in acute infection, NK cell subsets that may control viral replication expand. Recent data indicate that CD56bright NK cells in tissues can be antigen specific, raising the question of whether the expansion of this population in the gut may be responding to HIV and inducing local inflammation.25 Indeed, the frequency of rectal CD56bright NK cells was higher in the group that was not taking ART compared with the groups taking ART, suggesting that their presence may be stimulated by ongoing HIV replication.
Of note, perforin and Ki67 expression are higher in lymph node NK cells in acute simian immunodeficiency virus (SIV) compared with chronic SIV infection, suggesting increased degranulation and turnover in acute infection.20 CD56dimCD16+ and CD56bright cells subsequently decrease in chronic HIV-1 infection,19,21 whereas CD56−CD16+ cells emerge and accumulate in the periphery and the lymph node.10,19,20,26
Indeed, CD56−CD16bright cells are rare in the absence of disease,10,22 but CD56−CD16+ NK cells are increased in viremic persons compared with HIV-negative controls.24 Consistent with this observation, we found that this population tended to be higher in the rectum of people who were not taking ART compared with those who initiated ART in chronic HIV-1 infection.
“Dysfunctional” or “exhausted” CD56−CD16+/bright cells, which correlated with rectal CD4-associated HIV-1 DNA, expand in the setting of continued HIV-1 replication,9 and their frequency is three times higher in SIV-infected compared with SIV-uninfected rhesus macaques.20 CD56−CD16+ cells are terminally differentiated cells21 that have fewer activating and more inhibitory receptors.22 Thus, CD56−CD16+ cells are ineffective, or at least less effective, at cytotoxicity and producing cytokines and chemokines including CCR5 ligands (CCL3, CCL4, and CCL5),9,27 and some propose that their expansion, replacing more functional cells, may facilitate HIV-1 infection of CD4+ T cells.9 Of note, CD56−CD16+ NK cells can suppress IFN-γ production by CD8+ T cells.28 Indeed, high plasma viral load is associated with higher frequencies of CD56−CD16+ cells.29
On the contrary, the presence of these cells may reflect recent degranulation of more functional cells. CD56−CD16+ cells express less granzyme B and perforin compared with other NK cells, and CD95 expression is upregulated to a greater extent on CD56−CD16+ cells compared with CD56+CD16+ cells in people with HIV, suggesting recent target cell engagement.22 CD56−CD16+ cells from people with HIV-1 produce more IFN-γ than people without HIV at baseline, suggesting they are activated, but do not respond significantly to stimulation.22
The decreased frequency of CD56bright cells, the major cytokine producers, in people who were taking ART, may result in less local cytokine production, which could limit NK and CD8+ T cell activation and compromise virus control if ART were stopped.30,31 Alternatively, the decreased cytokine production could lead to less local inflammation to stimulate HIV-1 replication and help preserve CD4+ T cells.30–32 As noted previously, NK cells, particularly CD56bright NK cells, accumulate within atherosclerotic lesions,12 and participants with higher CIMT in our study had higher frequencies of rectal CD56brightCD16dim cells. The rectal cells may contribute to higher CIMT, or they could reflect an overall increase in this population in tissues, including the vasculature. We did not have sufficient samples to further characterize CD56bright cells, such as by evaluating NK cell receptors or surface markers such as CD11b and CD161 that distinguish subsets of CD56bright cells that differentiate viremic from treated people with HIV.24
The inverse association of rectal CD4 Tscm with circulating cytotoxic NK cells raises the question of whether these CD4 Tscm persist because of insufficient control by NK cells. In contrast, the direct association of rectal CD8 Tscm with rectal cytokine-producing and cytotoxic NK cells may reflect an overall environment supporting HIV-1 control. The association of higher rectal CD8+ Tscm frequencies with lower amounts of HIV-1 DNA in CD4+ T cells is consistent with this postulation. Higher frequencies of rectal CD8+ Tscm in the group not on ART could reflect that this population was relatively healthy as they retained a median CD4+ T cell count of 782 cells/mm3 despite the absence of ART. Four of these five participants had been diagnosed in the previous year (three of whom were 26 years of age or younger) and may be in the early stages of infection; the fifth participant was diagnosed more than 18 years before enrollment and had HIV-1 RNA of 4,250 copies/mL, suggesting the participant was a long-term nonprogressor. A larger number of participants may be needed to elucidate whether there are differences across these three populations.
Previous studies observed lower HIV-1 DNA levels in blood and rectal CD4+ T cells of participants starting ART in early/acute infection compared with those starting ART in chronic infection.33,34 Furthermore, the level of HIV-1 DNA in rectal CD4+ T cells tended to be higher than in CD4+ T cells from blood.33–35 Our results are largely in agreement with these studies, and others16,33,34,36,37 for participants who initiated ART during acute infection. Differences may be because of the small study size and the relative timing of ART initiation during acute/early infection.
This study has several limitations. First, the sample size in each group was small, which may have prevented us from discovering other differences across groups. Second, the cell yield of rectal biopsies did not permit more than one flow cytometry panel to be performed, limiting the degree to which we could characterize the cells. Thus, by simultaneously quantifying NK cell and T cell markers on the limited samples we had, we could not thoroughly characterize either cell type. Third, the groups were not matched on age, sex, race, duration of infection, and for the treated groups, duration of ART, which may have confounded our results. Fourth, the chronic HIV not on ART group may not have progressed to the same stage of disease as the chronic on ART group. Fifth, we performed multiple comparisons on a small sample size, increasing our chances of a type I error.
In summary, we found that rectal NK cell populations differed based on whether ART was initiated in acute or chronic infection, or not at all, and correlated with circulating CD4+ T cell count, whereas higher circulating NK cell frequencies were associated with higher levels of HIV-1 DNA in rectal CD4+ T cells. Together, these findings suggest an evolution of NK cell populations during the course of infection, but residual HIV-1 in the gut may contribute to systemic NK cell activation.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Funding Information
NIH grant AI1116167 to J.T.K and NIH grant 5P30AI036211-18 to K.J.V. This project was supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the CPRIT Core Facility Support Award (CPRIT-RP180672), the NIH (CA125123 and RR024574) and the assistance of Joel M. Sederstrom.
Supplementary Material
References
- 1. Krebs SJ, Ananworanich J: Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr Opin HIV AIDS 2016;11:163–172 [DOI] [PubMed] [Google Scholar]
- 2. Robb ML, Ananworanich J: Lessons from acute HIV infection. Curr Opin HIV AIDS 2016;11:555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gattinoni L, Speiser DE, Lichterfeld M, Bonini C: T memory stem cells in health and disease. Nat Med 2017;23:18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lugli E, Dominguez MH, Gattinoni L, et al. : Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 2013;123:594–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ribeiro SP, Milush JM, Cunha-Neto E, et al. : The CD8(+) memory stem T cell (T(SCM)) subset is associated with improved prognosis in chronic HIV-1 infection. J Virol 2014;88:13836–13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taborda NA, Gonzalez SM, Alvarez CM, Correa LA, Montoya CJ, Rugeles MT: Higher frequency of NK and CD4+ T-cells in mucosa and potent cytotoxic response in HIV controllers. PLoS One 2015;10:e0136292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meier UC, Owen RE, Taylor E, et al. : Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol 2005;79:12365–12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smyth MJ, Nutt SL: IL-7 and the thymus dictate the NK cell ‘labor market’. Nat Immunol 2006;7:1134–1136 [DOI] [PubMed] [Google Scholar]
- 9. Mikulak J, Oriolo F, Zaghi E, Di Vito C, Mavilio D: Natural killer cells in HIV-1 infection and therapy. AIDS 2017;31:2317–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scully E, Alter G: NK cells in HIV disease. Curr HIV/AIDS Rep 2016;13:85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sips M, Sciaranghella G, Diefenbach T, et al. : Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol 2012;5:30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonaccorsi I, Spinelli D, Cantoni C, et al. : Symptomatic carotid atherosclerotic plaques are associated with increased infiltration of natural killer (NK) cells and higher serum levels of NK activating receptor ligands. Front Immunol 2019;10:1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parisi L, Bassani B, Tremolati M, Gini E, Farronato G, Bruno A: Natural killer cells in the orchestration of chronic inflammatory diseases. J Immunol Res 2017;2017:4218254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah ASV, Stelzle D, Lee KK, et al. : Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation 2018;138:1100–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandler NG, Bosinger SE, Estes JD, et al. : Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 2014;511:601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ananworanich J, Chomont N, Eller LA, et al. : HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine 2016;11:68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chahroudi A, Silvestri G, Lichterfeld M: T memory stem cells and HIV: A long-term relationship. Curr HIV/AIDS Rep 2015;12:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chun TW, Murray D, Justement JS, et al. : Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis 2011;204:135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nabatanzi R, Cose S, Joloba M, Jones SR, Nakanjako D: Effects of HIV infection and ART on phenotype and function of circulating monocytes, natural killer, and innate lymphoid cells. AIDS Res Ther 2018;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schafer JL, Li H, Evans TI, Estes JD, Reeves RK: Accumulation of cytotoxic CD16+ NK cells in simian immunodeficiency virus-infected lymph nodes associated with in situ differentiation and functional anergy. J Virol 2015;89:6887–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hong HS, Ahmad F, Eberhard JM, et al. : Loss of CCR7 expression on CD56(bright) NK cells is associated with a CD56(dim)CD16(+) NK cell-like phenotype and correlates with HIV viral load. PLoS One 2012;7:e44820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Milush JM, Long BR, Snyder-Cappione JE, et al. : Functionally distinct subsets of human NK cells and monocyte/DC-like cells identified by coexpression of CD56, CD7, and CD4. Blood 2009;114:4823–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holzemer A, Garcia-Beltran WF, Altfeld M: Natural killer cell interactions with classical and non-classical human leukocyte antigen class I in HIV-1 infection. Front Immunol 2017;8:1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pohlmeyer CW, Gonzalez VD, Irrinki A, et al. : Identification of NK cell subpopulations that differentiate HIV-infected subject cohorts with diverse levels of virus control. J Virol 2019;93:e01790-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nikzad R, Angelo LS, Aviles-Padilla K, et al. : Human natural killer cells mediate adaptive immunity to viral antigens. Sci Immunol 2019;4:eaat8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Florez-Alvarez L, Hernandez JC, Zapata W: NK cells in HIV-1 infection: From basic science to vaccine strategies. Front Immunol 2018;9:2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Voigt J, Malone DFG, Dias J, et al. : Proteome analysis of human CD56(neg) NK cells reveals a homogeneous phenotype surprisingly similar to CD56(dim) NK cells. Eur J Immunol 2018;48:1456–1469 [DOI] [PubMed] [Google Scholar]
- 28. Ma M, Yin X, Zhao X, et al. : CD56(-) CD16(+) NK cells from HIV-infected individuals negatively regulate IFN-gamma production by autologous CD8(+) T cells. J Leukoc Biol 2019;106:1313–1323 [DOI] [PubMed] [Google Scholar]
- 29. Gregson JN, Kuri-Cervantes L, Mela CM, Gazzard BG, Bower M, Goodier MR: Short communication: NKG2C+ NK cells contribute to increases in CD16+CD56- cells in HIV type 1+ individuals with high plasma viral load. AIDS Res Hum Retroviruses 2013;29:84–88 [DOI] [PubMed] [Google Scholar]
- 30. Roff SR, Noon-Song EN, Yamamoto JK: The significance of interferon-gamma in HIV-1 pathogenesis, therapy, and prophylaxis. Front Immunol 2014;4:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pasquereau S, Kumar A, Herbein G: Targeting TNF and TNF receptor pathway in HIV-1 infection: From immune activation to viral reservoirs. Viruses 2017;9:E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tabb B, Morcock DR, Trubey CM, et al. : Reduced inflammation and lymphoid tissue immunopathology in rhesus macaques receiving anti-tumor necrosis factor treatment during primary simian immunodeficiency virus infection. J Infect Dis 2013;207:880–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eriksson S, Graf EH, Dahl V, et al. : Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 2013;9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Josefsson L, von Stockenstrom S, Faria NR, et al. : The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc Natl Acad Sci U S A 2013;110:E4987–E4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khoury G, Fromentin R, Solomon A, et al. : Human immunodeficiency virus persistence and T-cell activation in blood, rectal, and lymph node tissue in human immunodeficiency virus-infected individuals receiving suppressive antiretroviral therapy. J Infect Dis 2017;215:911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jain V, Hartogensis W, Bacchetti P, et al. : Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013;208:1202–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo L, Wang N, Yue Y, et al. : The effects of antiretroviral therapy initiation time on HIV reservoir size in Chinese chronically HIV infected patients: A prospective, multi-site cohort study. BMC Infect Dis 2019;19:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.