Abstract
Pituitary dysfunction with reduced growth hormone (GH) secretion is common in patients following traumatic brain injury (TBI), and these patients often develop chronic symptoms including fatigue and altered cognition. We examined 18 subjects with a history of mild TBI, fatigue, and insufficient GH secretion. Subjects received GH replacement in a year-long, double-blind, placebo-controlled, crossover study, and were assessed for changes in physical performance, body composition, resting energy expenditure, fatigue, sleep, mood, and neuropsychological status. Additionally, magnetic resonance imaging (MRI) was used to assess changes in brain structure and resting state functional connectivity. GH replacement resulted in decreased fatigue, sleep disturbance, and anxiety, as well as increased resting energy expenditure, improved body composition, and altered perception of submaximal effort when performing exercise testing. Associated brain changes included increased frontal cortical thickness and gray matter volume and resting state connectivity changes in regions associated with somatosensory networks. GH replacement altered brain morphology and connectivity and reduced fatigue and related symptoms in mild TBI patients. Additional studies are needed to understand the mechanisms causing TBI-related fatigue and symptom relief with GH replacement.
Keywords: fatigue, GH, TBI
Introduction
Pituitary dysfunction with reduced growth hormone (GH) secretion is a common consequence of traumatic brain injury (TBI).1–4 The combination of brain trauma and GH dysfunction often yields a constellation of symptoms that negatively impacts quality of life. These clustered symptoms include increased fatigue and depression,5 diminished cognition,6 and altered metabolism and body condition.7 The level of fatigue that patients report following TBI appears correlated with other quality-of-life-related symptoms including anxiety, sleep disturbance, and cognitive impairment.8 Although common behavioral and cognitive deficits following mild TBI typically resolve within weeks or months, these deficits persist long term in a subset of patients.9,10 In the course of performing studies on GH replacement in patients following TBI,6,11 our research group has noted these clustered symptoms. Recognizing the comorbidity of these symptoms is of clinical importance, as they may manifest months or even years after the initial trauma and may not be readily recognized as being related to prior TBI.
Our previous studies and initial qualitative clinical experience with GH replacement following TBI indicate a temporal profile of symptom relief. Specifically, patients on GH replacement therapy typically report diminished fatigue after ∼3 months, whereas improvements in cognition often manifest after 4–5 months of continuous treatment. Our previous study suggests that this TBI-related severe fatigue originates centrally in the brain and is not limited to peripheral fatigue.11 Although symptom relief with GH replacement can be profound in responsive subjects, this treatment does not cure the underlying condition, as the symptoms return when treatment has ended. This suggests a persistent or permanent dysfunction in GH secretion in this subset of patients.
Evidence to support the role of central nervous system dysfunction following TBI has been well established using neuroimaging methods such as magnetic resonance imaging (MRI). Following mild TBI, resting state functional connectivity in the brain is altered both acutely and chronically,12,13 with widespread alterations in canonical network connectivity including the default mode network (DMN) and thalamocortical pathways.14,15 Although the underlying mechanisms and pathways involved are not well understood, GH administration has been extensively linked to both neuroprotection and neural repair following damage or disease.16 In the current year-long, double-blind, placebo-controlled crossover study, we sought to characterize the fatigue relief experienced by TBI patients with GH replacement. We assessed physical and behavioral responses to GH replacement including both peripheral measures (body composition, physical performance, resting metabolic rate) and central measures associated with TBI-related fatigue (sleep quality, neurocognition, fatigue, quality of life). By correlating these relevant changes to changes seen in the brain, we can better understand the mechanisms by which GH provides relief of fatigue symptoms following TBI. At baseline, 3 months, 6 months, and 12 months, physical and behavioral measures were assessed, and brain connectivity and morphology were examined to determine their relationship to GH replacement and fatigue relief. Additionally, neurocognitive assessments were conducted at baseline and again at 12 months. We hypothesized that GH replacement would reduce symptom severity, and that symptom relief would be associated with changes in brain morphology and connectivity.
Methods
Study design
This study was conducted in accordance with the principles of the Declaration of Helsinki, approved by the Institutional Review Board at the University of Texas Medical Branch, and registered as a clinical trial before starting subject enrollment (www.clinicaltrials.gov; NCT02114775). We studied the effect of GH replacement on male and female subjects between the ages of 21 and 70 with a history of mild TBI, insufficient GH secretion, and fatigue in a randomized, double-blind, placebo-controlled crossover study. TBI-related eligibility included being at least 6 months post-injury following civilian, non-blast-related mild TBI defined as head trauma followed by <30 min of disorientation, confusion, loss of memory, or loss of consciousness. Participants also met fatigue-related eligibility criteria by scoring ≥3 on any of the three initial fatigue severity questions in the Brief Fatigue Inventory questionnaire.17 Individuals who met the initial inclusionary criteria were further screened for eligibility by measuring peak stimulated growth hormone levels using a glucagon stimulation test by administering 1 mg glucagon to subjects <90 kg and 1.5 mg glucagon to subjects ≥90 kg. This study was powered to select subjects based on symptom manifestation independent of the exact level of GH abnormality. Consistent with our previous studies, subjects were enrolled if peak stimulated GH levels indicated either GH deficiency (< 3 mg*dL−1) or insufficiency (< 8 mg*dL−1),6 and were not stratified based on the degree of their abnormal GH secretion.
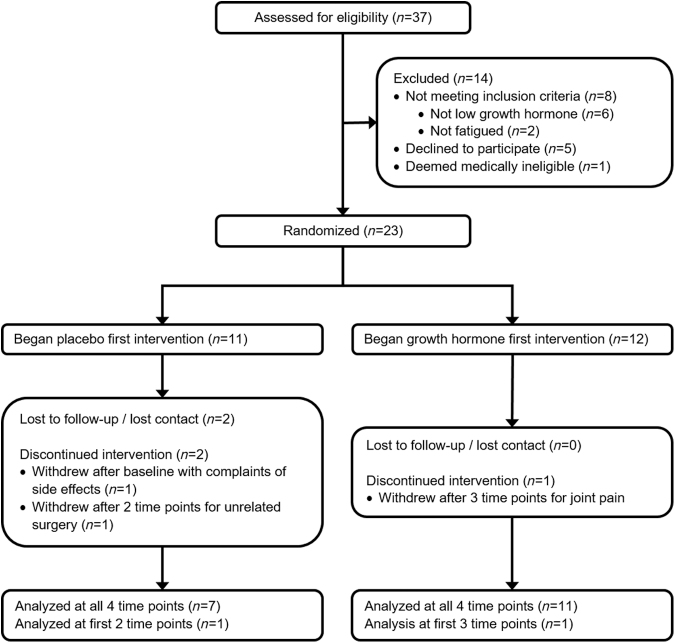
Thirty-seven individuals with a history of mild TBI inquired about participation in the study. Two did not meet the fatigue criteria, and 35 individuals with qualifying history of mild TBI and fatigue were assessed for peak stimulated GH level (Consolidated Standards of Reporting Trials [CONSORT] diagram, Fig. 1). Six otherwise eligible subjects did not qualify to continue in the study because of normal GH stimulation levels, five declined to participate, and one was deemed medically ineligible. Of the 23 qualifying individuals enrolled in the study, 3 subjects dropped out of the study after baseline measures and were not included in the analysis. Twenty subjects completed at least 3 months of study and were included in the analysis. These subjects included 7 males and 13 females with an average age of 48.0 ± 9.2 and 21.2 ± 17.9 years post-injury and an average BMI of 30.0 ± 4.9. Of those subjects, 19 completed 6 months, and 18 completed the entire 12 month study.
FIG. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram depicting subject participation from screening through analysis. Subjects between the ages of 21 and 70 were recruited based on a history of mild traumatic brain injury and complaints of fatigue. Subjects were eligible for participation if peak stimulated growth hormone level tested below 8 mg*dL−1 and they were otherwise medically eligible.
Drug administration
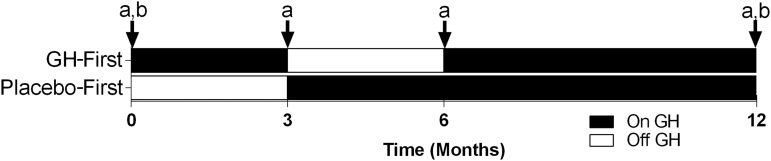
The University of Texas Medical Branch (UTMB) Investigational Drug Service research pharmacy managed study group random assignment and drug dispensing, while the subjects and study personnel remained blinded. The active recombinant human GH and visually identical placebo (Genotropin; Pfizer Inc, New York, NY) were supplied as multi-use cartridges for daily self-administered subcutaneous injections using a Genotropin Pen 5 injection device. Subjects were randomly assigned to receive either recombinant human GH first (GH months 1–3, placebo months 4–6, GH months 7–12) or placebo first (placebo months 1–3, GH months 4–6, GH months 7–12) (Fig. 2) so that all subjects experienced both GH and placebo periods. Similarly to previous studies, subjects underwent a progressive dose increase for each dosing interval.6,11 In the first month of each treatment period, subjects injected the volume equivalent of 0.4 mg of GH or placebo daily and increased to a volume equivalent dose of 0.6 mg of GH for the remainder of the treatment period. There were no reported adverse events associated with the study intervention. Subjects were monitored monthly in person or by phone for clinical changes suggestive of excess GH, including numbness and tingling in the hands. Blood tests were used to confirm that insulin-like growth factor (IGF)-1 serum levels were maintained <400 ng*mL−1 at months 1, 3, 4, 6, and 12. There was no need to reduce GH dose in any subjects, as IGF-1 levels stayed within the normal range and there were no subject complaints of GH side effects.
FIG. 2.
Study timeline for traumatic brain injury patients enrolled in a double-blind, placebo-controlled crossover-designed growth hormone (GH) replacement study. Subjects were clustered into one of two groups receiving either GH first (GH months 1–3, placebo months 4–6, GH months 7–12) or placebo first (placebo months 1–3, GH months 4–6, GH months 7–12). At baseline, 3, 6, and 12 months, assessments included: (a) body composition (dual-energy X-ray absorptiometry [DXA]), physical performance and fatigue, resting energy expenditure, brain morphometry and connectivity (magnetic resonance imaging [MRI]). At baseline and again at 12 months, assessments included: (b) neuropsychological tests, and questionnaires evaluating mood, sleep, and fatigue.
Physical and cognitive assessments
A series of physical, cognitive, and fatigue-related assessments were conducted to evaluate the effect of GH replacement throughout the study (Fig. 2). At baseline, 3, 6, and 12 months, assessments included body composition, physical performance and fatigue, resting energy expenditure, and brain morphometry and connectivity. Additional extensive batteries of neuropsychological tests and questionnaires evaluating mood, sleep, and fatigue were administered at baseline and again at 12 months. Raw test scores were used to assess the change from baseline using paired t tests. Given the large number of neuropsychological tests; questionnaires on mood, sleep, and fatigue; and measures of physical performance, each of these analyses underwent additional correction for multiple comparison using the Benjamini–Hochberg false discovery rate correction value of 0.1.18
A battery of neuropsychological tests was performed on subjects to assess aspects of cognitive function at baseline and again at the end of the 12 month study. These tests included the Hopkins Verbal Learning Test,19 Brief Visuospatial Memory Test,20 Grooved Peg Board Test,21 Color-Word Interference Test,22 Verbal Fluency Test,22 Digit Span Test,23 Beck Depression Inventory-II (BDI),24 and Processing Speed Index25 (for a more detailed description of individual tests see Supplementary Text 1).
Questionnaires evaluating aspects of mood, sleep, and fatigue were administered at baseline and again at the end of the 12 month study for 10 subjects. Average overall scores and individual subtest scores were determined for the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI),26 the Profile of Mood States Standard Form (Multi-Health Systems Inc., North Tonawanda, NY), The Brief Fatigue Inventory,17 and the Pittsburg Sleep Quality Index (PSQI)27 (for more detailed description of the individual tests see supplementary text 2).
Dual-energy X-ray absorptiometry (DXA) was used to assess bone mineral content, fat mass, lean mass, and total body mass at baseline, 3 months, 6 months, and 12 months using a GE Lunar iDXA (GE Healthcare; Chicago, IL). Body composition measures were collected at all time points for 18 subjects. GH-first and placebo-first groups were assessed separately to demonstrate longitudinal change from baseline of body mass, lean mass, and fat mass. Because of variability in body mass, longitudinal changes in body composition were depicted graphically as percent change.
Physical performance and fatigue were assessed at baseline, 3 months, 6 months, and 12 months using leg extension exercises, hand grip strength, and a modified 6 min walk test. Data from 17 subjects were used in the analysis, as one subject sustained a back injury prior to the final time point and was unable to perform the final physical performance measures. Three and 6 month time point values are depicted graphically but not included in the statistical analysis.
A Biodex System 4 Pro dynamometer (Biodex Medical Systems; Shirley, NY) was used to measure isometric and isokinetic leg extension torque at baseline and again at 12 months. Before beginning torque measures, the subject performed a warm-up set consisting of 30 isokinetic leg extensions with maximum angular velocity set to 500 degrees/sec to provide minimal resistance. Isometric leg extension torque was measured over three contractions of 5 sec each against a fixed angle of 90 degrees. The three contractions were performed at subject-determined estimates of 25%, 50%, and 100% effort respectively with a 15 sec rest period between each contraction. The set of three isometric contractions was performed in triplicate, and torque for each contraction was measured as the average over a 3 sec window centered on peak torque. Following a 2 min rest period, peak isokinetic torque was similarly measured over three contractions at an angular velocity of 120 degrees/sec. The three contractions were performed at subject-determined 25%, 50%, and 100% efforts respectively with ∼5 sec between contractions. The set of three isokinetic contractions was performed in triplicate with a rest period of 15 sec between sets. Torque for each contraction was measured as the average over a 100 ms window centered on peak torque. The first set of contractions for each set was used to anchor perceived effort and the second and third sets were used to determine average torque measures for 25%, 50%, and 100% effort.
Following torque measures, subjects performed a fatigue protocol consisting of a series of 50 maximum effort isokinetic leg extensions with a cadence of one extension every second at a maximum angular velocity of 120 degrees/sec. Torque was assessed for each contraction, as the average over a 100 ms window centered on peak torque. Immediately after completing the 50 contraction fatigue protocol, subjects repeated a set of isometric contractions at 100%, 50%, and 25% subject-perceived effort. This series of recovery isometric contractions was then repeated after 3 min and again after 5 min of recovery. A best-fit power line was fitted to the declining torque and power output over the 50 contraction protocol to estimate the contraction number that declined to 50% of protocol maximum. The estimated 50% contraction number was compared for baseline and 12 months using paired t tests. Isometric torque at 0, 3, and 5 min of post-fatigue recovery were assessed as the percent of pre-fatigue torque, and recovery was compared between baseline and 12 months using paired t tests. Subjects were asked to subjectively rank their whole-body overall fatigue level on a scale of 0–10 both before and again immediately after leg extension testing, as a measure of fatigue.
Hand-grip strength and fatigue were assessed using a set of two Smedley hand dynamometers (model 56380; Stoelting Co., Wood Dale, IL) calibrated before each use. The dynamometers were connected via a bridge amp (model FE221, ADInstruments, Bella Vista, NSW, Australia) and data acquisition hardware (PowerLab 16/35, ADInstruments) to a laptop computer for data collection and processing (LabChart 7 Pro V 7.3.8, ADInstruments). To assess the maximum grip strength and perceived force production, subjects were asked to squeeze three times with their dominant hand for ∼5 sec each at perceived efforts of 25%, 50%, and then 100% exertion. After the first set with the dominant hand, the subject repeated the same procedure with the non-dominant hand. The set of three grip measures was recorded in triplicate for both dominant and non-dominant hands, with the average of each repeated measure used for analysis.
A modified 6 min walk incorporating self-pacing based on perceived effort was conducted at baseline, 3 months, 6 months, and 12 months as a measure of performance and fatigue during a routine activity. During the modified 6 min walk test, subjects walked for 2 min at a perceived effort of 25%, 2 min at a perceived effort of 50%, and the last two min at 100% effort walking pace. Total distance and distance traversed for each 2 min interval was recorded and compared with baseline.
Resting energy expenditure (REE) was measured at baseline, 3 months, 6 months, and 12 months using indirect calorimetry. Following overnight fasting, subjects were asked to rest quietly for 15 min in a supine position. Using a draped acrylic gas collection canopy, oxygen consumption and carbon dioxide production were monitored for 30 min using a metabolic cart (Vmax Encore 29, CareFusion, San Diego, CA) calibrated prior to use. The final 25 min of data collection were used to calculate REE. REE was collected at all time points for 18 subjects. To demonstrate the longitudinal change in REE among the two crossover groups, the average REE was assessed separately for the GH-first and placebo-first groups over the duration of the study.
MRI: Image acquisition
MRIs were acquired using a Siemens Magnetom Skyra 3-Tesla MRI at the University of Texas Medical Branch at Galveston. MRI was conducted as the first measure on each study day (6:00–7:00 a.m. start time) following overnight fasting. High-resolution anatomical images covering the whole brain including the cerebellum were acquired using a T1-weighted gradient-echo (GRE) pulse sequence with the following parameters: 3D T1 axial (repetition time [TR] = 1900 ms, echo time [TE] = 2.49 ms, flip angle = 9 degrees, field of view [FOV] = 270 x 270 mm, voxel size = 0.9 x 0.9 x 0.9 mm, 192 slices, matrix = 288 × 288). Resting state functional connectivity MRI data were collected for 10 min using a single-shot GRE planar imaging sequence to acquire 164 T2-weighted blood oxygenation level dependent (BOLD) images (TR = 3660 ms, TE = 39 ms, flip angle = 90 degrees, FOV = 240 x 240 mm, voxel size = 2.6 x 2.6 x 4 mm, 36 axial slices, matrix = 94 x 94). Participants were instructed to keep their eyes open and to not think about anything in particular during the resting state scan.
MRI: Pre-processing
Structural and functional images were preprocessed in the CONN functional connectivity toolbox (17.f)28 using the default volume-based pre-processing pipeline. Functional images underwent realignment and unwarping for motion correction, slice-timing correction, registration and normalization to Montreal Neurological Institute (MNI) 152 space, outlier detection, band-pass filtration between 0.008 and 0.09 Hz, and spatial smoothing using an 8 mm full width at half maximum (FWHM) Gaussian kernel. Additionally, structural images underwent realignment; segmentation of gray matter (GM), white matter, and cerebrospinal fluid; bias correction; and normalization to MNI space.
MRI: Assessing longitudinal changes
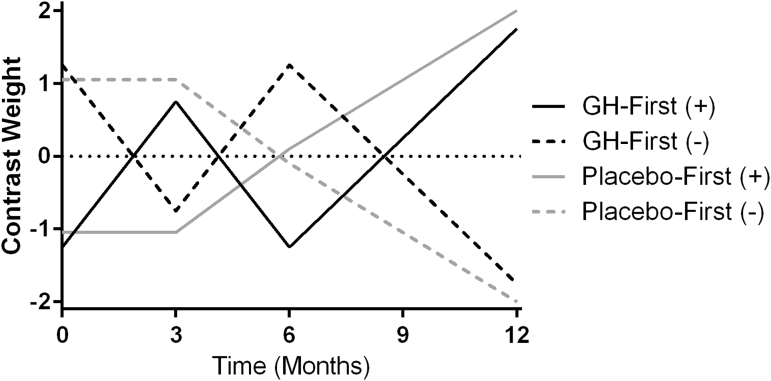
A flexible factorial model was established to assess functional and morphological brain changes throughout the study, including crossover periods on and off GH replacement (Fig. 3). Subjects were grouped based on the order in which they received treatment into GH-first or placebo-first groups for analysis. The modeled contrast timeline was based on our clinical experience of adult patients receiving GH administration. In our experience, patients typically experience modest symptom reduction after ∼3 months of treatment, with more pronounced symptom reduction following 6 months to 1 year of treatment. After discontinuing GH treatment, patients tend to exhibit a gradual return of symptoms over ∼3 months. The weighted contrasts tested fit to a model of brain changes while on GH and return to baseline while on placebo. The GH-first and placebo-first group-weighted models were defined for each time point as (see study design and Fig. 2 for timing) -1.25, 0.75, -1.25, 1.75 and -1.05, -1.05, 0.1, 2, respectively to test for longitudinal increases in brain morphology or connectivity with GH treatment (Fig. 3). The inverse of those model weights (1.25, -0.75, 1.25, -1.75 and 1.05, 1.05, -0.1, -2 respectively) were used to assess longitudinal decreases in morphology or connectivity with GH treatment.
FIG. 3.
This placebo-controlled crossover designed study assessed the effects of growth hormone (GH) replacement on mild traumatic brain injury (TBI) patients. Weighted contrast models were used to test fit to a model of changes in brain connectivity and morphometry while subjects were on GH, with no change or reversal of GH-induced changes while on placebo. Separately weighted contrasts were created to test for increased (+) or decreased (−) cortical thickness, gray matter volume, or functional connectivity with GH replacement in both the GH-first and placebo-first groups.
MRI: Functional connectivity
Using SPM12 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK) we implemented the flexible factorial model described previously to conduct a hypothesis-free intrinsic connectivity (IC) analysis to assess voxelwise whole-brain changes in connectivity over the course of the study. First-level voxel to voxel beta files were calculated for each subject at each time point with interacting factors of group assignment and session. In addition, 10 a priori selected regions of interest (ROI) were chosen for similar analysis based on the current literature as regions associated with altered connectivity following TBI or associated with fatigue (Table S1). The selected ROIs include: the posterior cingulate cortex as a region representing the default mode network,29,30 the right frontal eye field as a region representing the dorsal attention network (as contrast to default mode network), left and right thalamus,14,15 the left post-central gyrus as somatosensory cortex,14 the left pre-central gyrus as primary motor cortex,14 the right cerebellar lobules IV/V (lobules IV and V were unified in pre-processing using CONN) and lobule VIII,31 the left nucleus accumbens as a region linked to motivation and fatigue,32,33 and the left supplementary motor area (SMA).34 First-level voxelwise seed ROI-specific beta files were calculated for each subject at each time point with interacting factors of group assignment and session for each ROI. Significantly altered connectivity was determined by peak-level familywise error corrected pFWE < 0.05; clusters ≥8 contiguous voxels are reported.
MRI: Correlations with altered connectivity
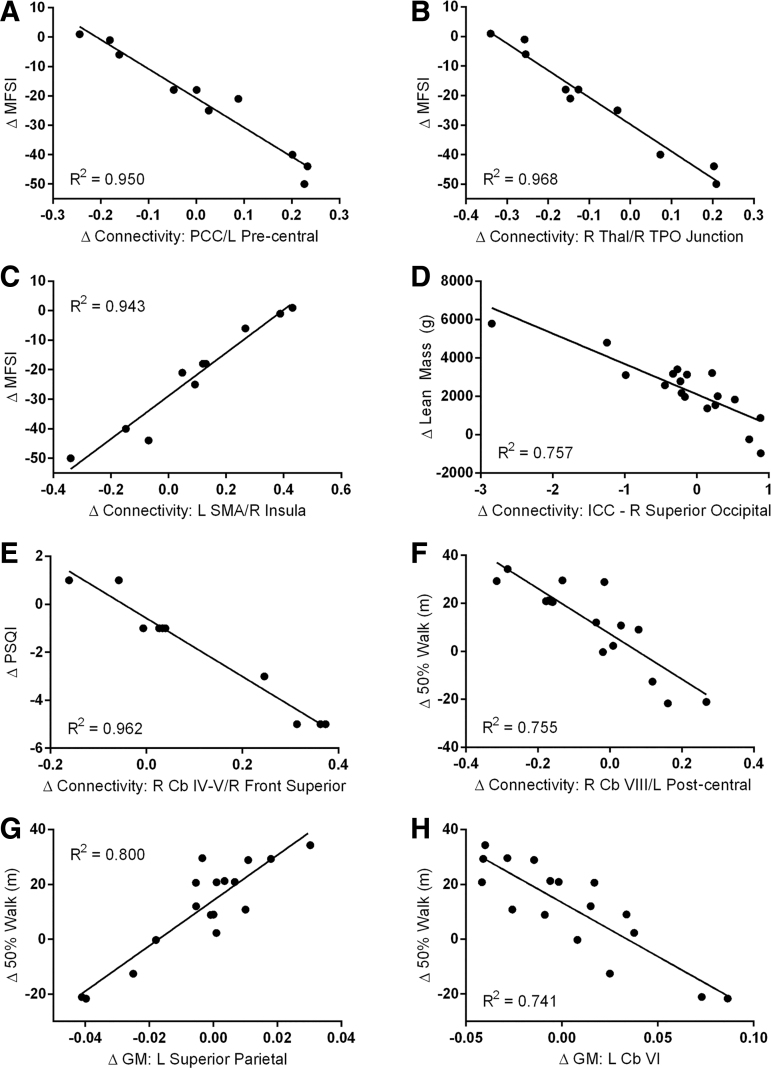
An exploratory analysis was conducted to identify potential associations between physical and behavioral measures of interest (MOI) and changes in brain connectivity and morphology. Five MOIs were selected a posteriori that were both significantly altered over the course of the study and deemed by the research team as being the most relevant unique measures. The five selected MOIs for correlation analysis included MFSI total score, PSQI, Beck Depression Inventory score, lean mass, and 50% effort modified walk distance. Pre- and post-treatment measures of MFSI and PSQI were complete for 10 subjects. Pre- and post-treatment measures of 50% effort modified walk and Beck Depression Inventory were available for 17 subjects. Change in lean mass was available for 18 subjects.
We tested for potential correlations between regional changes in connectivity and changes in MOIs from baseline to 12 months. The change in connectivity from baseline to post-study was plotted by generating a delta image (post-study beta image minus baseline beta image) using the SPM IMcalc function for whole-brain IC analysis and separately for the 10 pre-selected ROIs. The resulting delta images were used to examine correlations between regional changes in connectivity and changes in MOIs. Correlations were evaluated with SPM using single-sample t tests of delta connectivity with the change in score for each MOI as a covariate. Separate contrasts were used to determine negative and positive correlations. For this exploratory analysis, the threshold for significance was set as a peak voxel uncorrected p < 0.00001; clusters >8 voxels are reported. Plots were generated to assess the correlative relationship between changes in connectivity and changes in MOIs. For clusters with significant correlations, the change in connectivity (post-study beta value – pre-study beta value) at the peak coordinates were recorded and plotted against the change in MOI.
MRI: Brain morphometry
Voxel-based morphometry was used to assess changes in regional brain volumes and to estimate changes in cortical thickness using the SPM CAT12 toolbox35 running on Matlab. Structural images were pre-processed using the CAT12 processing pipeline for segmenting longitudinal data. These protocols compensate for non-linear registration by performing intra-subject realignment, bias correction, segmentation, and normalization. Within this protocol, individual images were Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) registered to MNI space and intra-subject mean segmented images were generated to apply intra-subject deformations for assessing volume changes. During pre-processing, additional cortical thickness (CT) estimation was performed for later CT analysis and total intracranial volume was estimated for each subject as a nuisance covariate for GM analysis.
MRI: GM volume
The change in GM volume from baseline to post-study was assessed using the previously described flexible factorial model. Modulated GM volumes were smoothed with a spherical FWHM Gaussian smoothing kernel of 8 mm using CAT12 and assigned for flexible factorial analysis with interacting factors of group assignment and session. Total intracranial volume was assigned as a nuisance covariate to remove the confounding factor of total brain size. Significant changes in GM volume were determined using peak-level pFWE < 0.05; clusters ≥8 voxels are reported.
MRI: Correlations with altered GM volume
An exploratory analysis was conducted to assess correlations between changes in GM volume and changes in selected MOIs from baseline to post-study. Delta images were generated for each subject using the extracted GM volumes from morphometry analysis (post-study GM map minus baseline GM map) using the SPM IMcalc function. Correlations were assessed with SPM using single-sample t tests of delta GM volume, with the change in score for each MOI as a covariate. Separate contrasts were used to determine negative and positive correlations. Using small volume correction, an additional focused correlative analysis was applied for the four a posteriori regions shown by the longitudinal flexible factorial analysis to have significant change in GM volume (Table S4). These regions were explored using a 10 mm sphere centered on the peak of each of the four significant clusters. Separate contrasts were used to determine negative and positive correlations. For this exploratory analysis, the threshold for significance was set as a peak uncorrected p < 0.00001; clusters >8 voxels are reported. Plots were generated to assess the correlative relationship between changes in GM volume and changes in MOIs. For clusters with significant correlations, the change in GM volume (post-study value – pre-study value) at the peak cluster coordinates were recorded and plotted against the change in MOI.
MRI: Cortical thickness
During initial CAT12 voxel-based morphometry longitudinal segmentation, surface and thickness estimation were performed using projection-based thickness for cortical thickness estimation36 for left and right hemispheres. Cortical thickness estimates were resampled to a 32 k mesh and smoothed with a 15 mm filter size. Changes in regional cortical thickness throughout the study were assessed using CAT12 with the previously described flexible factorial model using merged left and right hemisphere cortical thickness estimates for each subject. The model was then estimated using CAT12 surface model estimation. Significant change in cortical thickness was determined using peak-level pFWE < 0.05; clusters ≥8 voxels are reported.
MRI: Correlations with altered cortical thickness
The correlation between changes in cortical thickness and selected MOIs from baseline to post-study were examined using CAT12. Pre- and post-study smoothed cortical thickness images were assessed using a paired t test with selected measures of interest from each time point as a covariate. For this exploratory analysis, the threshold for significance was set as a peak uncorrected p < 0.00001; clusters with >8 voxels are reported.
Results
Neuropsychological testing
Baseline and 12 month measures were compared using combined data from the GH-first and placebo-first groups to assess the effects of GH treatment on neuropsychological measures (Table 1). None of the neuropsychological measures demonstrated significant changes with GH treatment following Benjamini–Hochberg false discovery rate correction for multiple testing, although the initial paired t tests indicated a less favorable score for the Brief Visuospatial Memory Delayed Recall subtest, along with improvements in the Color-Word Interference subtest CW4, and reduced depressive symptoms as measured by the Beck Depression Inventory.
Table 1.
Average (± SD) Neuropsychological Scores for Mild TBI Subjects before and after One Year Including 9 Months of GH Therapy
| Baseline | 12 Months | p | |
|---|---|---|---|
| Hopkins Verbal Learning Test-Revised | |||
| Total Recalla | 26.6 ± 3.9 | 27.6 ± 3.1 | 0.251 |
| Delayed Recalla | 9.8 ± 1.8 | 10.2 ± 1.7 | 0.280 |
| Recognition Discriminationa | 11.1 ± 0.8 | 11.0 ± 0.8 | 0.791 |
| Brief Visuospatial Memory Test | |||
| Total Recalla | 25.8 ± 4.7 | 24.5 ± 7.1 | 0.365 |
| Delayed Recalla | 10.6 ± 2.0 | 9.3 ± 2.5 | 0.037 |
| Recognition Discriminationa | 5.5 ± 1.0 | 5.8 ± 0.8 | 0.482 |
| Grooved Peg Board | |||
| Dominant Handb | 72.1 ± 25.8 | 65.9 ± 10.2 | 0.182 |
| Non-Dominant Handb | 78.8 ± 26.1 | 73.3 ± 12.4 | 0.287 |
| Color-Word (CW) Interference Test | |||
| CW1b | 31.8 ± 10.4 | 29.5 ± 5.1 | 0.251 |
| CW2b | 24.1 ± 11.5 | 21.6 ± 3.9 | 0.375 |
| CW3b | 56.8 ± 15.8 | 51.3 ± 9.7 | 0.190 |
| CW4b | 61.5 ± 19.9 | 52.5 ± 7.6 | 0.037 |
| Verbal Fluency Test | |||
| Phenomica | 39.8 ± 12.9 | 39.4 ± 12.3 | 0.895 |
| Semantica | 43.8 ± 7.7 | 44.1 ± 7.6 | 0.922 |
| Digit Spana | 27.2 ± 3.9 | 28.0 ± 5.5 | 0.441 |
| Beck Depression Inventoryb | 14.9 ± 9.0 | 8.9 ± 8.1 | 0.028 |
| Processing Speed Indexa | 101.8 ± 16.7 | 104.2 ± 13.4 | 0.200 |
Baseline and 12 month measures were compared using paired t tests. Although three tests (shown in italics) had p values <0.05, there were no significant differences based on Benjamini–Hochberg false discovery rate correction of 0.1.
Higher score indicates a more favorable outcome.
Lower score indicates a more favorable outcome.
SD, standard deviation; TBI, traumatic brain injury.
Fatigue, sleep, and mood
GH treatment was associated with significant improvements in subject-rated fatigue, sleep, and mood (Table 2). Over the course of 1 year, total MFSI fatigue score decreased significantly with GH treatment (p = 0.002) as did individual subtest scores for general fatigue (p = 0.020), physical fatigue (p = 0.003), emotional fatigue (p = 0.015), and mental fatigue (p = 0.005). Fatigue as measured by Brief Fatigue Inventory total score was also improved (p = 0.009). Subject-perceived whole-body fatigue following exercise testing was reduced with GH administration (p = 0.026), and although pre-exercise ranking of whole-body fatigue trended toward a decline, it did not reach statistical significance (p = 0.060). Although the Profile of Mood States Total Mood Disturbance score was not significantly reduced with GH treatment (p = 0.110; Table 3), there was a significant improvement in scores on subtests examining tension/anxiety (p = 0.036) and fatigue (p = 0.031). In addition, sleep quality as measured by PSQI was also improved with treatment (p = 0.025).
Table 2.
Average (± SD) Response Scores Related to Mood, Sleep, and Fatigue for Mild TBI Subjects before and after One Year Including Nine Months of GH Therapy
| Baseline | 12 Months | p | |
|---|---|---|---|
| Multidimensional Fatigue Symptom Inventory-Short Form | |||
| General Fatigue | 17.2 ± 4.4 | 11.0 ± 7.8 | 0.011* |
| Physical Fatigue | 7.2 ± 3.4 | 3.3 ± 3.4 | 0.003* |
| Emotional Fatigue | 8.4 ± 5.5 | 3.7 ± 5.7 | 0.030* |
| Mental Fatigue | 13.1 ± 7.8 | 8.2 ± 5.9 | 0.005* |
| Vigor | 9.1 ± 4.3 | 11.6 ± 5.6 | 0.138 |
| Total Score | 36.8 ± 21.2 | 14.6 ± 24.0 | 0.003* |
| Profile of Mood States | |||
| Tension/Anxiety | 11.0 ± 10.4 | 4.8 ± 6.7 | 0.036* |
| Depression | 9.0 ± 15.5 | 6.8 ± 13.8 | 0.635 |
| Anger/Hostility | 7.0 ± 10.2 | 2.8 ± 5.4 | 0.127 |
| Vigor | 10.1 ± 5.4 | 10.9 ± 8.5 | 0.773 |
| Fatigue | 14.8 ± 6.7 | 10.3 ± 7.6 | 0.031* |
| Confusion | 11.5 ± 8.9 | 7.7 ± 7.1 | 0.056 |
| Total Mood Disturbance | 43.2 ± 51.9 | 21.5 ± 43.0 | 0.110 |
| Brief Fatigue Inventory | 5.8 ± 2.0 | 3.5 ± 2.7 | 0.009* |
| Whole-Body Fatigue Before and After Exercise | |||
| Pre-Exercise | 3.2 ± 2.5 | 1.9 ± 2.6 | 0.060 |
| Post-Exercise | 5.2 ± 2.6 | 3.2 ± 2.5 | 0.026* |
| Pittsburg Sleep Quality Index | 11.8 ± 3.7 | 9.8 ± 4.3 | 0.025* |
Baseline and 12 month measures were compared using paired t tests. For vigor scores, a higher score indicates a more favorable outcome. For all other measures a lower score indicates a more favorable outcome.
Statistical significance based on p < 0.05 and Benjamini–Hochberg false discovery rate correction of 0.1.
SD, standard deviation; TBI, traumatic brain injury; GH, growth hormone.
Table 3.
Measures of Physical Performance Including Average (±SD) Leg Extension Torque Production, Fatigue, and Recovery as Well as Grip Strength and Modified Six Minute Walk Distance for Mild TBI Subjects before and after One Year Including Nine Months of GH Therapy
| Baseline | 12 Months | p | |
|---|---|---|---|
| Leg Extension Isometric Torque (Nm) | |||
| 100% Effort | 138.5 ± 66.0 | 128.8 ± 60.5 | 0.146 |
| 50% Effort | 65.6 ± 33.1 | 62.5 ± 36.5 | 0.570 |
| 25% Effort | 45.8 ± 22.4 | 40.0 ± 25.2 | 0.260 |
| Leg Extension Isokinetic Torque (Nm) | |||
| 100% Effort | 95.5 ± 51.3 | 103.2 ± 47.1 | 0.144 |
| 50% Effort | 60.0 ± 40.9 | 74.9 ± 45.0 | 0.038* |
| 25% Effort | 32.9 ± 28.0 | 41.3 ± 34.2 | 0.238 |
| Leg Extension Fatigue (no. of kicks to 50% max. isokinetic torque) | |||
| 31.9 ± 7.7 | 29.6 ± 6.0 | 0.217 | |
| Leg Extension Recovery Isometric Torque (% of baseline max.) | |||
| 0 min | 54.4 ± 11.2 | 60.6 ± 9.6 | 0.023* |
| 3 min | 80.6 ± 19.8 | 84.9 ± 15.0 | 0.232 |
| 5 min | 90.6 ± 13.1 | 88.8 ± 10.9 | 0.552 |
| Grip Force - Dominant Hand (Kg) | |||
| 100% Effort | 32.1 ± 12.3 | 29.4 ± 11.7 | 0.063 |
| 50% Effort | 18.2 ± 8.5 | 14.9 ± 5.5 | 0.009* |
| 25% Effort | 13.1 ± 6.4 | 9.9 ± 4.2 | 0.002* |
| Grip Force - Non-dominant Hand (Kg) | |||
| 100% Effort | 30.3 ± 12.3 | 28.5 ± 13.8 | 0.235 |
| 50% Effort | 17.5 ± 8.9 | 13.8 ± 6.6 | 0.006* |
| 25% Effort | 12.4 ± 6.8 | 8.6 ± 4.6 | 0.001* |
| Modified 6 Minute Walk Distance (m) | |||
| Total Distance | 496.1 ± 75.5 | 519.5 ± 56.7 | 0.060 |
| 2 min at 100% | 209.6 ± 35.7 | 209.1 ± 25.7 | 0.942 |
| 2 min at 50% | 156.9 ± 26.4 | 168.3 ± 18.6 | 0.016* |
| 2 min at 25% | 129.5 ± 27.6 | 142.1 ± 18.4 | 0.018* |
Leg extension fatigue is the number of contractions required to fatigue to 50% of maximum pre-fatigue torque. Baseline and 12 month measures were compared using paired t tests.
Statistical significance based on p < 0.05 and Benjamini–Hochberg false discovery rate correction of 0.1.
SD, standard deviation; TBI, traumatic brain injury; GH, growth hormone.
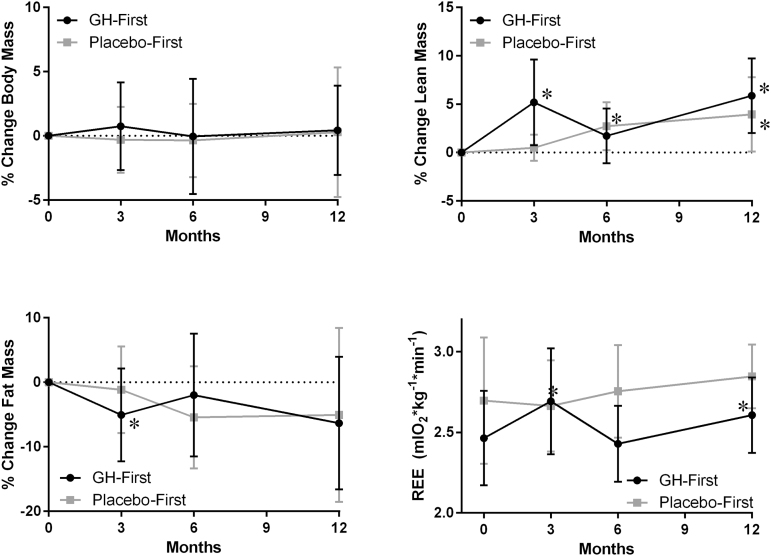
Body composition
Although overall body mass did not change with GH treatment (p = 0.507), body composition was altered, with a significant increase in lean mass (p < 0.001) and a trend toward a loss of fat mass (p = 0.054) (Fig. 4 and Table S2). GH treatment was also associated with a small but significant decrease in bone mineral content (p < 0.001). Over the course of the study, gains in lean mass and losses in fat mass occurred during periods of GH treatment, and this shift in body composition was reversed during periods of placebo administration (Fig. 4).
FIG. 4.
Change in body composition and resting energy expenditure (REE) for mild traumatic brain injury (TBI) subjects enrolled in a double-blind crossover designed growth hormone (GH) replacement protocol. Subjects were clustered into GH-first (GH months 0–3, placebo months 3–6, GH months 6–12) or placebo-first (placebo months 0–3, GH months 3–6, GH months 6–12) groups. *Significant difference in absolute change from baseline determined by paired t test.
REE
Over the course of the study, REE increased significantly with GH treatment from an average baseline rate of 2.53 ± 0.34 mL O2*kg−1*min−1 to a month 12 average rate of 2.70 ± 0.25 mL O2*kg−1*min−1 (p = 0.018) (Table S2). Within the GH-first group, REE increased significantly during the initial 3 months of treatment (Fig. 4). This increase in REE was reversed during the following 3 months of placebo administration. Once GH treatment was resumed at 6 months, this increase in REE returned. In the placebo-first group, the average REE trended toward an increase when on GH treatment but was not significantly different from baseline.
Leg extension
GH treatment was not associated with an increase in leg extension isokinetic torque at maximum effort, but was associated with a significant increase in torque production at subject-determined 50% effort (Table 3) (p = 0.038). In addition, GH treatment was not associated with altered number of repetitions to fatigue during the 50 contraction isokinetic fatigue protocol.
Hand grip
Over the course of the study, average grip strength measured at maximal volitional force did not change significantly with GH treatment (Table 3). However, at subject-perceived efforts of 25% and 50%, force declined significantly for both dominant (p = 0.002 at 25%, p = 0.009 at 50%) and non-dominant (p = 0.001 at 25%, p = 0.006 at 50%) hands.
Six minute walk
GH treatment was not associated with changes in the total distance traversed during the modified 6 min walk test (Table 3) (p = 0.060) or the subset of 2 min of walking at 100% effort (p = 0.94). However, there was a significant increase in the distance traversed during 2 min of walking at subject-perceived 25% effort (p = 0.018) and 50% effort (p = 0.016).
Altered brain connectivity
Using the flexible factorial model, the hypothesis-free whole brain IC analysis did not reveal significant changes in connectivity with GH treatment. However, GH treatment was associated with altered connectivity for seed regions when examining the 10 a priori ROI seed regions selected as potentially relevant to mild TBI and fatigue (Fig. 5; Table S3). Altered connectivity was primarily observed in brain somatosensory networks, including the left post-central gyrus. The left post-central gyrus increased connectivity with the left hippocampus (pFWE = 0.003) and left superior temporal gyrus (pFWE = 0.006). Connectivity to the left post-central gyrus was altered when seed regions of the right frontal eye field (pFWE < 0.001), and the left thalamus (pFWE = 0.001) were assessed. In addition, GH treatment was associated with altered connectivity in motor control regions including reduced connectivity between the posterior cingulate cortex and the left cerebellar lobule VIIb (pFWE < 0.001).
FIG. 5.
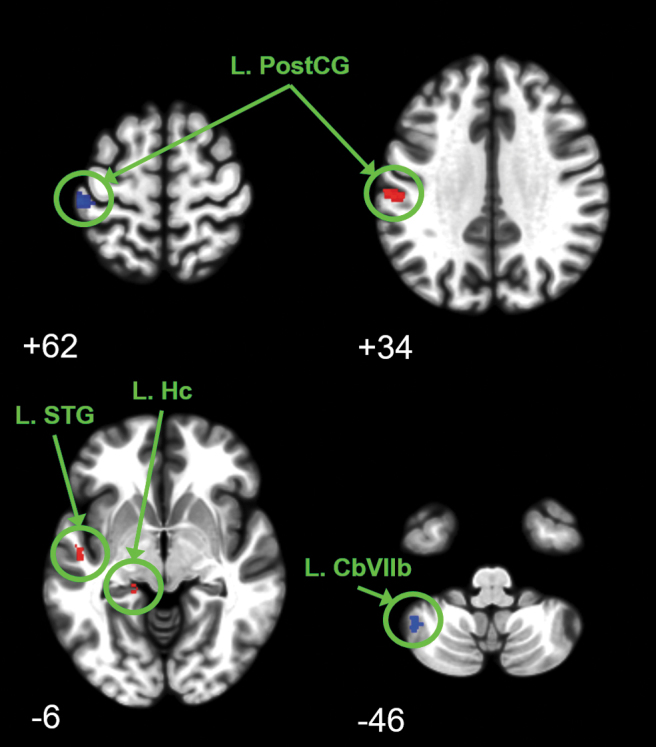
Regions of altered brain connectivity for mild traumatic brain injury (TBI) subjects over 1 year including 9 months of growth hormone (GH) replacement. Significant change in connectivity was determined with flexible factorial analysis using peak-level familywise error (FWE) corrected p < 0.05. Clusters ≥8 voxels are reported with blue clusters depicting decreased connectivity and red clusters depicting increased connectivity. Images are depicted at Montreal Neurological Institute (MNI) oriented axial slice number. Connectivity was decreased between the right frontal eye field and the left post-central gyrus (upper left image) whereas connectivity increased between the left thalamus and the left post-central gyrus (upper right image). Connectivity was increased between the left post-central gyrus and the left superior temporal gyrus and left hippocampus (lower left image). Connectivity was decreased between the posterior cingulate and the left cerebellar (Cb) lobule VIIb (lower right image).
Correlations with altered brain connectivity
In our exploratory analysis, correlations were found between changes in connectivity associated with GH treatment for the 10 a priori selected ROIs and changes in the a posteriori selected MOIs. Subjects with the greatest reduction in MFSI fatigue total score had the greatest increase in connectivity between the posterior cingulate cortex and the left pre-central gyrus (Fig. 6A). Subjects with the greatest reduction in MFSI fatigue total score also had the greatest increase in connectivity between the right thalamus and the right temporo-parieto-occipital (TPO) junction (Fig. 6B). In addition, subjects with the greatest reduction in MFSI fatigue had decreased connectivity between the left supplemental motor area and the right insula (Fig. 6C).
FIG. 6.
Regions where changes in measures of interest were correlated with changes in functional connectivity and gray matter (GM) volume were correlated with changes in measures of interest for mild traumatic brain injury (TBI) subjects over 1 year including 9 months of growth hormone (GH) therapy. In the exploratory analysis, correlation was established at uncorrected p < 0.00001 with an extent threshold of 8 voxels. Decreased fatigue (Multidimensional Fatigue Symptom Inventory [MFSI]) was correlated with increased connectivity between the posterior cingulate cortex (PCC) and the left pre-central gyrus (A; Montreal Neurological Institute [MNI] x y z = -44 0 10), increased connectivity between the right thalamus and right temporo-parieto-occipital (TPO) junction (B; MNI x y z = 54 -68 28), and reduced connectivity between the left supplemental motor area (SMA) and right insula (C; MNI x y z = 34 28 6). Increased lean mass was correlated with reduced whole-brain connectivity (via intrinsic connectivity [IC]) in the right superior occipital region (D; MNI x y z = 22 -82 34). Improved sleep quality (Pittsburg Sleep Quality Index [PSQI]) was correlated with reduced connectivity between right cerebellar (Cb) lobules IV-V and right frontal superior cortex (E; MNI x y z = 16 66 2). Increased distance at 50% walk effort was correlated with increased connectivity between the right Cb lobule VIII and the left post-central gyrus (F; MNI x y z = -38 -32 70), increased GM volume in the left superior parietal cortex (G; MNI x y z = -42 -59 48) and reduced GM volume in the left Cb lobule VI (H; MNI x y z = -33 60 -20).
IC analysis demonstrated correlations between the change in lean mass and whole-brain connectivity; subjects with the greatest increase in lean mass had decreased whole-brain connectivity with the right superior occipital lobe (Fig. 6D).
PSQI sleep quality score was also correlated with significantly altered regional connectivity. Subjects with the greatest improvement in sleep quality had increased connectivity between the right cerebellar lobules IV/V and the right frontal superior gyrus (Fig. 6E).
The 50% effort walk score was correlated with altered connectivity between the right cerebellar lobule VIII and the left post-central gyrus, and subjects with the greatest increase in 50% walk distance had the greatest decrease in connectivity between these regions (Fig. 6F).
Altered GM volume
Using flexible factorial longitudinal analysis, voxel-based morphometry demonstrated GH associated changes in GM volume (Fig. 7; Table S4) with increased volume in regions located in the left mid-cingulate, and left mid-frontal region. Within the left orbital inferior frontal gyrus there was an apparent shift in GM volume, with a significant increase in one region and a decrease in another.
FIG. 7.
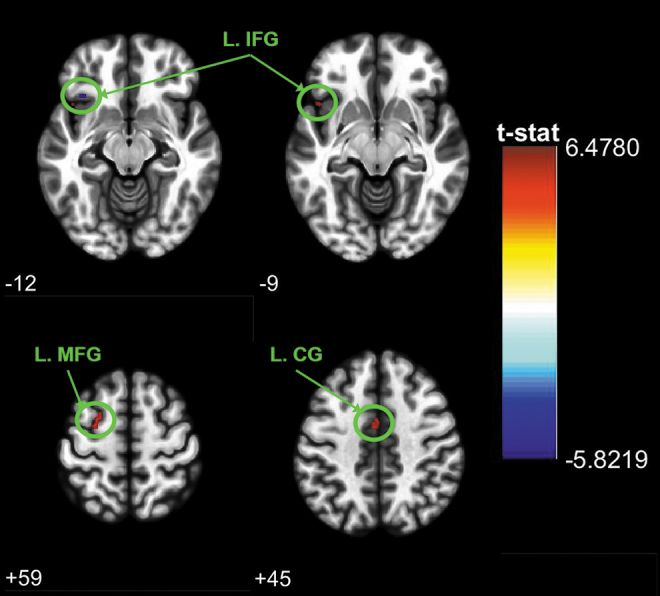
Regions of altered gray matter volume for mild traumatic brain injury (TBI) subjects over 1 year including 9 months of growth hormone (GH) therapy. Significant change in gray matter volume was determined with flexible factorial analysis using peak-level familywise error (FWE) corrected p < 0.05. Clusters ≥8 voxels are reported with blue clusters depicting decreased volume and red clusters depicting increased volume. Images are depicted at Montreal Neurological Institute (MNI) oriented axial slice number. Clusters with significantly altered gray matter include decreased volume in one portion of the left inferior frontal gyrus (L. IFG) (upper left image) along with a significant increase in another portion of the same L. IFG region (upper right image). Significant increases in gray matter volume were also seen in the left mid-frontal gyrus (lower left image) and the left cingulate gyrus (lower right image).
Correlations with altered GM volume
In our exploratory analysis, the change in 50% walk distance was the only MOI correlated to changes in GM volume. Subjects with the greatest increase in 50% walk distance had increased GM volume in the left superior parietal cortex (Fig. 6G) and decreased volume in the left cerebellar lobule VI (Fig. 6H).
Altered cortical thickness
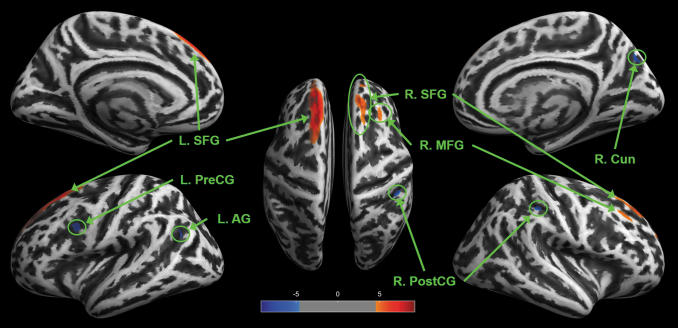
Using flexible factorial longitudinal analysis, GH treatment was associated with significant changes in cortical thickness with increased cortical thickness in regions within both the left and right superior frontal gyrus and the right mid frontal gyrus (Fig. 8; Table S5). Other regions experienced decreased cortical thickness including the left angular gyrus, left pre-central gyrus, right cuneus, and right post-central gyrus. Changes in cortical thickness were not correlated with pre/post treatment changes in any of the measures of interest.
FIG. 8.
Regions of altered cortical thickness for mild traumatic brain injury (TBI) subjects over 1 year including 9 months of growth hormone (GH) therapy. Significant change in cortical thickness was determined with flexible factorial analysis using peak-level familywise error (FWE) corrected p < 0.05. Clusters ≥8 voxels are reported with blue clusters depicting decreased cortical thickness and red clusters depicting increased cortical thickness. Significantly altered regions include the left superior frontal gyrus (L. SFG), left pre-central gyrus (L. PreCG), left angular gyrus (L. AG), right superior frontal gyrus (R. SFG), right mid -frontal gyrus (R. MFG), right cuneus (R. Cun), and right post-central gyrus (R. PostCG).
Correlations with altered cortical thickness
Our exploratory analysis did not reveal any significant correlations between regions of altered cortical thickness and the MOI explored in this study.
Discussion
We studied the effect of GH replacement on subjects with a history of mild TBI, insufficient GH secretion, and fatigue. Along with significant improvements in body composition, fatigue, and sleep, we identified associated changes in brain connectivity and morphology. In both the current study of patients affected by mild TBI and our previous study of community-dwelling patients following moderate to severe TBI,11 we selected subjects with fatigue and insufficient GH response. Taken together, these studies suggest that across the spectrum of TBI severity, GH secretion abnormalities and fatigue-related symptoms can occur and may be relieved with GH replacement.
From the outset of our studies, we have included subjects that responded abnormally to the glucagon stimulation test for GH secretion with a maximal response of ≤8 ng/dL of GH rather than a strict definition of GH deficiency based on a stimulation response to ≤3 ng/dL. Although GH therapy is established to affect cognition and behavior, these findings are from studies conducted primarily on patients with established GH deficiency.37 Other studies have also used a broader range of GH stimulation response levels as criteria for GH replacement.38 Although not controlled studies, clinical evidence suggests a neuroprotective and neurorehabilitative role for GH treatment even in the absence of GH deficiency.39–41 In light of these effects, it is unclear if the beneficial effects of GH replacement seen in this study are exclusively a result of correcting GH abnormality, or if supplemental GH administration would have similar effects with greater levels of endogenous GH secretion.
Others have proposed potential autoimmune and genetic components for susceptibility to hypopituitarism following TBI,42 and we likewise speculate that there may be a genetic, environmental, or morphological predisposition for some individuals to develop chronic inflammation in the brain following a severe/moderate, mild, or repetitive TBI. In this study we focused on patients with chronic fatigue symptoms, and propose that these persistent symptoms are associated with post-TBI hypopituitarism. Here, we demonstrate that GH replacement in these subjects is associated with significant changes in brain morphology and connectivity and improved fatigue symptoms.
Quality of life is impaired in patients with GH insufficiency following TBI,5 and long-term GH treatment improves quality of life for adult-onset hypopituitary patients.43 In the current study, GH replacement was broadly associated with improvements in many measures affecting quality of life. Previous studies by us and others have shown that patients with GH abnormalities and cognitive disorders following TBI showed significantly greater improvements in cognition with GH replacement than controls.6,38 In the current study, GH treatment was not associated with widespread cognitive improvements (Table 1). However, subjects were not selected for this study based on cognitive dysfunction, and the study was not powered to assess altered cognition. Subjects were, however, selected based on reported fatigue, and all measures used to assess subject-reported fatigue in this study were improved with GH treatment with all but one reaching statistical significance (Table 2).
Although questionnaire responses regarding mood were universally improved, there was no significant change in overall mood disturbance as assessed with the Profile of Mood States. However, subtests examining tension/anxiety and fatigue were significantly improved, and neuropsychological examination suggested a significant decrease in depressive symptoms (Table 1). Subjects also reported decreased sleep disturbance with GH treatment. Although many factors assessing quality of life were improved with GH treatment, it is not possible to determine the sequelae and mechanisms of symptom relief for interdependent measures of fatigue, sleep quality, and mood.
Consistent with other studies,44 GH replacement did not alter total body mass during the course of treatment, although shifts in body composition resulted from significant increases in lean mass and a trend toward decreased fat mass (Fig. 4; Table S2). The significant increase in REE in this study (Fig. 4; Table S2) is consistent with other studies that demonstrated that GH replacement in deficient subjects resulted in increased REE as a result of both increased lean mass and increased specific cellular metabolism through regulation of thyroid hormones.45 The slight but statistically significant decrease in bone mineral content seen following 9 months of GH treatment in this study (Table S2) is consistent with previous studies. As a regulator of bone remodeling, GH balances anabolic and catabolic processes of bone mineral resorption and generation. In adults with GH deficiency, GH treatment of ≤1 year is associated with short-term decreased bone mineral density, which is typically reversed with continued treatment46–48 and does not indicate a barrier to GH replacement.
Although lean mass was increased with GH treatment, this increase was not associated with significant increases in maximal physical performance in our current or previous study of TBI patients11 (Table 3). This contradicts some previous studies, which demonstrated improved exercise capacity in low GH adults with GH replacement,45 and suggests that the fatigue expressed following TBI in this study was central, without an obvious measured peripheral component. Although maximal physical performance was not increased with GH replacement, there was a consistent trend of altered performance at submaximal effort, including increased performance at submaximal effort for the 6 min walk distance and isokinetic leg extension, as well as decreased submaximal effort performance for measures of grip force in both dominant and non-dominant hands (Table 3).
Altered physical performance at subject-perceived submaximal effort suggests altered perception of effort or motor control. During physical exercise testing, both conscious and unconscious factors influence subject effort and force production.49 Scaling of perceived effort in generating forces may be largely regulated by central control, with afferent somatosensory feedback being important for central calibration of efferent output.50 In this way, changes to connectivity in brain networks responsible for processing somatosensory input may affect effort, particularly at subjective assessment of submaximal effort.
Studies examining altered functional connectivity in TBI patients demonstrate diverse changes associated with acute and chronic behavioral and cognitive deficits following injury. When interpreting TBI-related changes in functional connectivity, consideration must be given to timing related to injury and context of recognized networks.9 TBI-related disruption of canonical networks may result in increased compensatory non-canonical connectivity,51 and reversal of this altered connectivity may reflect recovery.
Altered functional connectivity in somatosensory-related networks in this study (Fig. 5; Table S3) may be related to altered perception of effort, fatigue, or motor control. Previous studies have demonstrated altered thalamic network connectivity in mild TBI subjects compared with healthy controls, and these changes in thalamic connectivity were correlated with reports of fatigue and symptom severity.14,15 In our study, growth hormone treatment was associated with significant increase in connectivity between the left thalamus and left post-central gyrus. In addition, the right frontal eye field (dorsal attention network), a region not typically associated with somatosensory networks, exhibited decreased connectivity with the left post-central gyrus potentially representing normalization (decreased compensatory connectivity) within this circuit.
The posterior cingulate cortex, a critical node in the default mode network, exhibited significantly decreased connectivity with lobule VIIb of the left cerebellum (Fig. 5; Table S3). The default mode network is tonically activated at rest, requiring deactivation to initiate subsequent tasks. Decreased connectivity between these two regions may represent normalizing of the default mode network with GH treatment, as these regions are not typically networked. These findings are consistent with a previous study of sub-acute post-concussive athletes which found increased whole-brain cerebellar connectivity associated with greater symptom severity, which they attributed to potential compensatory effects.52 Altered cerebellar connectivity seen in this study may be related to altered measures of quality of life, as lobule VIIb is associated with executive function and emotional processing.53
The right frontal eye field representing the dorsal attention network also demonstrated decreased connectivity with a cluster within the left post-central gyrus (somatosensory) (Fig. 5; Table S3). Because the post-central gyrus is not canonically associated with the frontal eye field, decreased connectivity in this region may also reflect decreased compensatory connectivity within the dorsal attention network.
Changes in functional connectivity with GH replacement in our study all involved the left post-central gyrus or left cerebellar lobule VIIb. These regions are strikingly similar to a recently published study comparing resting state functional connectivity in children with GH deficiency in children with idiopathic short stature.54 This study found that children with GH deficiency had altered connectivity in cerebellar, and sensorimotor networks including decreased whole-brain connectivity in the left post-central gyrus and left cerebellar lobule VIIb. This previous study proposed that post-central gyrus function may be affected by reduced GH levels, and proposed longitudinal studies examining changes in post-central gyrus function following hormone replacement. The high degree of parity between brain regions with altered connectivity in GH deficient children and those altered with GH replacement in our study provide strong evidence linking GH with somatosensory and cerebellar networks.
TBI is associated with altered brain morphology, including decreased GM prominently in frontal brain regions.55,56 Recently, former National Football League players exhibiting neuropsychiatric and cognitive symptoms were found to have elevated tau protein deposition bilaterally in superior frontal gyrus regions.57 In the current study, changes in brain morphology primarily included increased GM in left inferior and mid frontal gyrus regions (Fig. 7; Table S4). Additionally, cortical thickness increased bilaterally in superior fontal regions as well as in the right mid-frontal gyrus (Fig. 8; Table S5). Increases in GM volume and cortical thickness in frontal regions following GH replacement may be related to the improved quality of life and reduced cognitive fatigue. Although the indicators of increased cognition in this study were not significant, these behavioral assessments are often designed for severe impairments and, therefore, may not be sensitive to the changes observed in this study.
Previous studies have demonstrated reduced connectivity within the default mode network following TBI.30,58 In the current study, reduced fatigue was correlated with increased connectivity between the posterior cingulate, a central hub of the default mode network, and the left pre-central gyrus, a primary motor region (Fig.6). Increased connectivity between the motor network and default mode network are counter to what might be expected with improvements in motivation and activation of movement with reduced fatigue symptoms.
Decreased fatigue symptoms were also correlated with greater connectivity between the right thalamus and the right temporo-parieto-occipital junction (Fig. 6). Previous studies have found altered thalamocortical connectivity following TBI,15 with alterations associated with chronic fatigue following injury;14 however, these studies did not identify altered thalamocortical connectivity specific to this region. Given the role of the thalamus as a central relay of somatosensory signaling and the somatosensory role within the temporo-parieto-occipital region, increased connectivity with these regions may represent a normalization of somatosensory processing of fatigue symptoms.
Hillary and coworkers59 found increased insular connectivity during 3–6 month recovery following TBI. In the current study, reduced fatigue symptoms were correlated with lower connectivity between the left supplemental motor area and the right insula (Fig. 6), which may represent a normalization of this network. The role of the insula in motivation for movement60 is consistent with insula/SMA connectivity changes relevant to altered fatigue.
Increased lean mass was correlated with lower whole-brain connectivity with the right superior occipital cortex (Fig. 6). Although body mass index has been correlated to altered resting state functional connectivity in networks associated with food intake and reward,61 the link between body composition and a primarily visual processing network is not clear.
In the current study, decreased sleep disturbance was correlated with greater connectivity between the right cerebellar lobules IV-V and the right superior frontal gyrus, specifically in the right apical pre-frontal region (Fig. 6). Although not examining connectivity between distant brain regions, a previous study found that patients with chronic insomnia had altered regional homogeneity of localized connectivity in regions including the right anterior cerebellum and frontal gyrus.62 The correlation of improved sleep quality and greater connectivity between known sleep-associated brain regions suggests a potential route in which GH replacement may affect sleep and potential sleep-associated aspects of fatigue.
Correlations between increased submaximal 50% effort walk distance and changes in connectivity and GM volume (Fig. 6) suggest a link between changes in motor control or perceived effort and sensorimotor brain regions. Increased 50% walk distance was correlated to greater connectivity between the right cerebellar lobule VIII and the left post-central gyrus. Although previous studies do not appear to highlight changes in connectivity between these two specific regions, their common sensorimotor link is consistent with altered motor control.
Increased 50% effort walk distance was also correlated with changes in GM volume in brain regions associated with movement, including increased GM in the left superior parietal region and decreased GM in left cerebellar lobule VI (Fig. 6). The left superior parietal region is associated with motor attention63 as well as self-perception and movement of limbs,64 and increased GM in this region may be related to changes in coordination and initiation of movement. The cerebellar lobule VI is primarily associated with the motor cortex,65 and regional volume decreases may reflect normalization after long-term compensatory inflation.
This manuscript is published in this journal issue along with two other manuscripts highlighting the etiology of persistent symptoms following TBI. One of these is an original research article that discovered the fecal microbiome profile of moderate to severe TBI patients remained altered years after the initial trauma.66 The other manuscript is a letter to the editor that describes a unified complex of symptoms that persists long-term in a subset of TBI patients including profound fatigue and altered cognition; we have dubbed this symptom complex brain injury associated fatigue and altered cognition (BIAFAC).67 Together, these three manuscripts characterize chronic symptoms that plague many patients following TBI and provide evidence to further explore therapeutic treatments to relieve those symptoms.
Conclusion
In this study we demonstrate that GH replacement in individuals with fatigue-related symptoms following mild TBI was associated with altered perception of submaximal effort, relief of fatigue, change in body composition, and decreased self-reported tension/anxiety, depressive symptoms, and sleep disturbance. As a novel aspect of this study, these changes were also associated with changes in brain morphology and regional connectivity. Changes in functional connectivity primarily occurred in sensorimotor-related networks that have been previously linked to GH deficiency.54 These changes may be related to the altered fatigue and perception of effort with GH treatment seen in this study. Additionally, changes in brain morphology included increased GM volume and cortical thickness in frontal brain regions, which may be related to improved quality of life measures and decreased cognitive fatigue. Although GH treatment demonstrated improvements in many measures of quality of life for subjects with fatigue following mild TBI, additional studies are required to elucidate the mechanisms involved, understand the sequelae of symptom relief, and better identify patient populations most likely to benefit from this treatment.
Supplementary Material
Acknowledgments
For assistance in functional MRI (fMRI) analysis, we thank Kathleen Hupfeld, Vincent Koppelmans, and Kaitlin Cassady. We also thank Dr. Satya Kolar for helpful comments in the drafting of the manuscript and Dr. Tiana Shiver for assistance in patient recruitment.
Funding Information
This research was funded by the TIRR Foundation by grant from the Transitional Learning Center of Galveston (014-106). The Institute for Translational Sciences at the University of Texas Medical Branch provided clinical support funded in part by a Clinical and Translational Science Award (UL1TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health. Additional support was provided by a Collaborative Research Travel Grant from the Burroughs Wellcome Fund (1016348). Pfizer generously supplied the growth hormone and placebo for this study (WI1906888).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Lieberman S.A., Oberoi A.L., Gilkison C.R., Masel B.E., and Urban R.J. (2001). Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J. Clin. Endocrinol. Metab. 86, 2752–2756 [DOI] [PubMed] [Google Scholar]
- 2. Agha A., Rogers B., Sherlock M., O'kelly P., Tormey W., Phillips J., and Thompson C.J. (2004). Anterior pituitary dysfunction in survivors of traumatic brain injury. J. Clin. Endocrinol. Metab. 89, 4929–4936 [DOI] [PubMed] [Google Scholar]
- 3. Tanriverdi F., Senyurek H., Unluhizarci K., Selcuklu A., Casanueva F.F., and Kelestimur F. (2006). High risk of hypopituitarism after traumatic brain injury: a prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J. Clin. Endocrinol. Metab. 91, 2105–2111 [DOI] [PubMed] [Google Scholar]
- 4. Schneider H.J., Kreitschmann-Andermahr I., Ghigo E., Stalla G.K. and Agha A. (2007). Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA 298, 1429–1438 [DOI] [PubMed] [Google Scholar]
- 5. Kelly D.F., McArthur D.L., Levin H., Swimmer S., Dusick J.R., Cohan P., Wang C., and Swerdloff R. (2006). Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J. Neurotrauma 23, 928–942 [DOI] [PubMed] [Google Scholar]
- 6. High W.M. Jr., Briones-Galang M., Clark J.A., Gilkison C., Mossberg K.A., Zgaljardic D.J., Masel B.E., and Urban R.J. (2010). Effect of growth hormone replacement therapy on cognition after traumatic brain injury. J. Neurotrauma 27, 1565–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giuliano S., Talarico S., Bruno L., Nicoletti F.B., Ceccotti C., and Belfiore A. (2017). Growth hormone deficiency and hypopituitarism in adults after complicated mild traumatic brain injury. Endocrine 58, 115–123 [DOI] [PubMed] [Google Scholar]
- 8. Zgaljardic D.J., Durham W.J., Mossberg K.A., Foreman J., Joshipura K., Masel B.E., Urban R., and Sheffield-Moore M. (2014). Neuropsychological and physiological correlates of fatigue following traumatic brain injury. Brain Inj. 28, 389–397 [DOI] [PubMed] [Google Scholar]
- 9. McDonald B.C., Saykin A.J., and McAllister T.W. (2012). Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav. 6, 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryan L.M., and Warden D.L. (2003). Post concussion syndrome. Int. Rev. Psychiatry 15, 310–316 [DOI] [PubMed] [Google Scholar]
- 11. Mossberg K.A., Durham W.J., Zgaljardic D.J., Gilkison C.R., Danesi C.P., Sheffield-Moore M., Masel B.E., and Urban R.J. (2017). Functional changes after recombinant human growth hormone replacement in patients with chronic traumatic brain injury and abnormal growth hormone secretion. J. Neurotrauma 34, 845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stevens M.C., Lovejoy D., Kim J., Oakes H., Kureshi I., and Witt S.T. (2012). Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav. 6, 293–318 [DOI] [PubMed] [Google Scholar]
- 13. Messé A., Caplain S., Pélégrini-Issac M., Blancho S., Lévy R., Aghakhani N., Montreuil M., Benali H., and Lehéricy S. (2013). Specific and evolving resting-state network alterations in post-concussion syndrome following mild traumatic brain injury. PloS One 8, e65470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nordin L.E., Möller M.C., Julin P., Bartfai A., Hashim F., and Li T.-Q. (2016). Post mTBI fatigue is associated with abnormal brain functional connectivity. Sci. Rep. 6, 21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang L., Ge Y., Sodickson D.K., Miles L., Zhou Y., Reaume J., and Grossman R.I. (2011). Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology 260, 831–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arce V.M., Devesa P., and Devesa J. (2013). Role of growth hormone (GH) in the treatment on neural diseases: from neuroprotection to neural repair. Neurosci. Res. 76, 179–186 [DOI] [PubMed] [Google Scholar]
- 17. Mendoza T.R., Wang X.S., Cleeland C.S., Morrissey M., Johnson B.A., Wendt J.K., and Huber S.L. (1999). The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85, 1186–1196 [DOI] [PubMed] [Google Scholar]
- 18. Benjamini Y., and Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc, 57, 289–300 [Google Scholar]
- 19. Brandt J., and Benedict R.H. (2001). Hopkins Verbal Learning Test––Revised: Professional Manual Psychological Assessment Resources, Lutz, FL
- 20. Benedict R.H. (1997). Brief Visuospatial Memory Test––Revised: Professional Manual Psychological Assessment Resources, Lutz, FL
- 21. Klove H. (1963). Grooved Pegboard Test. Med. Clin. North. Am. 46, 1647–1658 [PubMed] [Google Scholar]
- 22. Delis D.C., Kaplan E., and Kramer J.H. (2001). Delis-Kaplan Executive Function System (D-KEFS). Psychological Corporation, San Antonio, TX [Google Scholar]
- 23. Stern R.A., and White T. (2003). NAB, Neuropsychological Assessment Battery: Administration, Scoring, and Interpretation Manual Psychological Assessment Resources, Lutz, FL
- 24. Beck A.T., Steer R.A., and Brown G.K. (1996). Manual for the Beck Depression Inventory–II. Psychological Corporation, San Antonio, TX [Google Scholar]
- 25. Wechsler D. (2008). Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV). The Psychological Corporation: San Antonio [Google Scholar]
- 26. Stein K.D., Jacobsen P.B., Blanchard C.M., and Thors C. (2004). Further validation of the multidimensional fatigue symptom inventory–short form. J. Pain Symptom Manage. 27, 14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buysse D., Reynolds C. 3rd, Monk T., Berman S., and Kupfer D. (1989). The Pittsburg Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 [DOI] [PubMed] [Google Scholar]
- 28. Whitfield-Gabrieli S., and Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141 [DOI] [PubMed] [Google Scholar]
- 29. Johnson B., Zhang K., Gay M., Horovitz S., Hallett M., Sebastianelli W., and Slobounov S. (2012). Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage 59, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou Y., Milham M.P., Lui Y.W., Miles L., Reaume J., Sodickson D.K., Grossman R.I., and Ge Y. (2012). Default-mode network disruption in mild traumatic brain injury. Radiology 265, 882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Churchill N., Hutchison M.G., Leung G., Graham S., and Schweizer T.A. (2017). Changes in functional connectivity of the brain associated with a history of sport concussion: a preliminary investigation. Brain Inj. 31, 39–48 [DOI] [PubMed] [Google Scholar]
- 32. Salamone J.D., Correa M., Farrar A., and Mingote S.M. (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191, 461–482 [DOI] [PubMed] [Google Scholar]
- 33. Dobryakova E., DeLuca J., Genova H.M., and Wylie G.R. (2013). Neural correlates of cognitive fatigue: cortico-striatal circuitry and effort–reward imbalance. J. Int. Neuropsychol. Soc. 19, 849–853 [DOI] [PubMed] [Google Scholar]
- 34. Bonnelle V., Manohar S., Behrens T., and Husain M. (2015). Individual differences in premotor brain systems underlie behavioral apathy. Cereb. Cortex 26, 807–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaser C., and Dahnke R. (2016). CAT–a computational anatomy toolbox for the analysis of structural MRI data. HBM 2016, 336–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dahnke R., Yotter R.A., and Gaser C. (2013). Cortical thickness and central surface estimation. Neuroimage 65, 336–348 [DOI] [PubMed] [Google Scholar]
- 37. Nyberg F. and Hallberg M. (2013). Growth hormone and cognitive function. Nat. Rev. Endocrinol. 9, 357. [DOI] [PubMed] [Google Scholar]
- 38. Reimunde P., Quintana A., Castañón B., Casteleiro N., Vilarnovo Z., Otero A., Devesa A., Otero-Cepeda X., and Devesa J. (2011). Effects of growth hormone (GH) replacement and cognitive rehabilitation in patients with cognitive disorders after traumatic brain injury. Brain Inj. 25, 65–73 [DOI] [PubMed] [Google Scholar]
- 39. Devesa J., Díaz-Getino G., Rey P., García-Cancela J., Loures I., Nogueiras S., Hurtado de Mendoza A., Salgado L., González M., and Pablos T. (2015). Brain recovery after a plane crash: treatment with growth hormone (GH) and neurorehabilitation: a case report. Int. J. Mol. Sci. 16, 30470–30482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Devesa J., Lema H., Zas E., Munín B., Taboada P., and Devesa P. (2016). Learning and memory recoveries in a young girl treated with growth hormone and neurorehabilitation. J. Clin. Med. 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Devesa J., Núñez I., Agra C., Bejarano A., and Devesa P. (2018). Treatment with growth hormone (GH) increased the metabolic activity of the brain in an elder patient, not gh-deficient, who suffered mild cognitive alterations and had an ApoE 4/3 genotype. Int. J. Mol. Sci. 19, 2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanriverdi F., Schneider H.J., Aimaretti G., Masel B.E., Casanueva F.F., and Kelestimur F. (2015). Pituitary dysfunction after traumatic brain injury: a clinical and pathophysiological approach. Endocr. Rev. 36, 305–342 [DOI] [PubMed] [Google Scholar]
- 43. Elbornsson M., Horvath A., Götherström G., Bengtsson B.-Å., Johannsson G., and Svensson J. (2017). Seven years of growth hormone (GH) replacement improves quality of life in hypopituitary patients with adult-onset GH deficiency. Eur. J. Endocrinol. 176, 99–109 [DOI] [PubMed] [Google Scholar]
- 44. Whitehead H.M., Boreham C., McIlrath E.M., Sheridan B., Kennedy L., Atkinson A.B., and Madden D.R. (1992). Growth hormone treatment of adults with growth hormone deficiency: results of a 13-month placebo controlled cross-over study. Clin. Endocrinol. 36, 45–52 [DOI] [PubMed] [Google Scholar]
- 45. Carroll P.V., Christ E.R., Bengtsson B.A., Carlsson L., Christiansen J.S., Clemmons D., Hintz R., Ho K., Laron Z., Sizonenko P., Sönksen P.H., Tanaka T., and Thorne M. (1998). Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J. Clin. Endocrinol. Metab. 83, 382–395 [DOI] [PubMed] [Google Scholar]
- 46. Molitch M.E., Clemmons D.R., Malozowski S., Merriam G.R., and Vance M.L. (2011). Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 1587–1609 [DOI] [PubMed] [Google Scholar]
- 47. Vandeweghe M., Taelman P., and Kaufman J.M. (1993). Short and long-term effects of growth hormone treatment on bone turnover and bone mineral content in adult growth hormone-deficient males. Clin. Endocrinol. 39, 409–415 [DOI] [PubMed] [Google Scholar]
- 48. Barake M., Klibanski A., and Tritos N.A. (2014). Effects of recombinant human growth hormone therapy on bone mineral density in adults with growth hormone deficiency: a meta-analysis. J. Clin. Endocrinol. Metab. 99, 852–860 [DOI] [PubMed] [Google Scholar]
- 49. Takarada Y., and Nozaki D. (2014). Maximal voluntary force strengthened by the enhancement of motor system state through barely visible priming words with reward. PloS One 9, e109422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lafargue G., Paillard J., Lamarre Y., and Sirigu A. (2003). Production and perception of grip force without proprioception: is there a sense of effort in deafferented subjects? Eur. J. Neurosci. 17, 2741–2749 [DOI] [PubMed] [Google Scholar]
- 51. Stevens M.C., Lovejoy D., Kim J., Oakes H., Kureshi I., and Witt S.T. (2012). Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav. 6, 293–318 [DOI] [PubMed] [Google Scholar]
- 52. Churchill N.W., Hutchison M.G., Graham S.J., and Schweizer T.A. (2018). Connectomic markers of symptom severity in sport-related concussion: Whole-brain analysis of resting-state fMRI. Neuroimage Clin. 18, 518–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stoodley C.J., and Schmahmann J.D. (2009). Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44, 489–501 [DOI] [PubMed] [Google Scholar]
- 54. Hu Y., Liu X., Ye P., Chen X., Jiang L., Chen T., Fu Y., Xie X., Shan X., and Yan Z. (2018). Differences in the functional connectivity density of the brain between individuals with growth hormone deficiency and idiopathic short stature. Psychoneuroendocrinology 103, 67–75 [DOI] [PubMed] [Google Scholar]
- 55. Gale S.D., Baxter L., Roundy N., and Johnson S. (2005). Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry 76, 984–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wilde E.A., Hunter J.V., Newsome M.R., Scheibel R.S., Bigler E.D., Johnson J.L., Fearing M.A., Cleavinger H.B., Li X., and Swank P.R. (2005). Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J. Neurotrauma 22, 333–344 [DOI] [PubMed] [Google Scholar]
- 57. Stern R.A., Adler C.H., Chen K., Navitsky M., Luo J., Dodick D.W., Alosco M.L., Tripodis Y., Goradia D.D., and Martin B. (2019). Tau positron-emission tomography in former National Football League players. N. Engl. J. Med. 380, 1716–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sharp D.J., Beckmann C.F., Greenwood R., Kinnunen K.M., Bonnelle V., De Boissezon X., Powell J.H., Counsell S.J., Patel M.C., and Leech R. (2011). Default mode network functional and structural connectivity after traumatic brain injury. Brain 134, 2233–2247 [DOI] [PubMed] [Google Scholar]
- 59. Hillary F.G., Slocomb J., Hills E.C., Fitzpatrick N.M., Medaglia J.D., Wang J., Good D.C., and Wylie G.R. (2011). Changes in resting connectivity during recovery from severe traumatic brain injury. Int. J. Psychophysiol. 82, 115–123 [DOI] [PubMed] [Google Scholar]
- 60. Tinaz S., Para K., Vives-Rodriguez A., Martinez-Kaigi V., Nalamada K., Sezgin M., Scheinost D., Hampson M., Louis E.D., and Constable R.T. (2018). Insula as the interface between body awareness and movement: a neurofeedback-guided kinesthetic motor imagery study in Parkinson's disease. Front. Hum. Neurosci. 12, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kullmann S., Heni M., Veit R., Ketterer C., Schick F., Haring H.U., Fritsche A., and Preissl H. (2012). The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum. Brain Mapp. 33, 1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dai X.J., Peng D.C., Gong H.H., Wan A.L., Nie X., Li H.J., and Wang Y.X. (2014). Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr. Dis. Treat. 10, 2163–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rushworth M.F., Johansen-Berg H., Gobel S.M., and Devlin J.T. (2003). The left parietal and premotor cortices: motor attention and selection. Neuroimage 20, Suppl. 1, S89–100 [DOI] [PubMed] [Google Scholar]
- 64. Felician O., Romaiguere P., Anton J.L., Nazarian B., Roth M., Poncet M., and Roll J.P. (2004). The role of human left superior parietal lobule in body part localization. Ann. Neurol. 55, 749–751 [DOI] [PubMed] [Google Scholar]
- 65. Bernard J.A., Seidler R.D., Hassevoort K.M., Benson B.L., Welsh R.C., Wiggins J.L., Jaeggi S.M., Buschkuehl M., Monk C.S., Jonides J., and Peltier S.J. (2012). Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front. Neuroanat. 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Urban R.J., Pyles R.B., Stewart C.J., Ajami N.J., Randolph K.M., Durham M.J., Danesi C.P., Dillon E.L., Summons J.R., Singh C.K., Morrison M., Kreber L.A., Masel B., Miller A.L., Wright T.J., and Sheffield-Moore M. (in press). Altered fecal microbiome years after traumatic brain injury. J. Neurotrauma [Epub ahead of print; DOI: 10.1089/neu.20XX.6688] [DOI] [PubMed] [Google Scholar]
- 67. Urban R.J. (in press). A treatable syndrome in patients with traumatic brain injury. J. Neurotrauma [Epub ahead of print; DOI: 10.1089/neu.20XX.6689] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.