Abstract
Euterpe oleracea (EO) includes a large number of polyphenolic compounds such as phenolics, flavonoids, and anthocyanins that have antioxidant activities. E. oleracea was suggested to ease the oxidative stress and inflammation in brain cells. Our aim was to analyze the effects of E. oleracea on learning and memory. Seventy-two (250 ± 25 g) male Wistar albino rats were used for this study. The groups consisted of control, EO100 mg/kg, EO300 mg/kg, scopolamine 1.5 mg/kg, mecamylamine 7.5 mg/kg, combinations of scopolamine with EO100 mg/kg, EO300 mg/kg, and rivastigmine 1.5 mg/kg; and mecamylamine combined with EO100 mg/kg. Before the start of the study, E. oleracea doses were provided once a day for a period of 15 days and for a 6-day experimental period. Thirty minutes after intraperitoneal scopolamine and mecamylamine injections, gastrogavage was applied to each group. Ninety minutes after the drug treatments, locomotor activity and Morris water maze tests were performed. Rats were killed and each hippocampus was used for the quantification of acetylcholine (Ach). Statistical analyses were calculated using one-way and two-way analyses of variance (ANOVA), and a value of P < .05 was considered significant. In groups EO100 mg/kg and EO300 mg/kg the results did not show any significant changes on learning and memory compared with the control group. Mecamylamine and scopolamine enhanced the latency for the escape platform, and decreased the time spent in escape platform quadrant when the memory tests were applied in reference to the control value of P < .05. Scopolamine and mecamylamine combinations of EO100 mg/kg, EO300 mg/kg, and rivastigmine were proven to improve the memory. There was significant difference between the first and fifth days of the learning tests in all the groups, but no significant difference occurred between the groups. Ach levels in hippocampi supported all memory tests. We suggest that E. oleracea made no alterations on learning and memory, but still improved nicotinic and muscarinic receptor-mediated and impaired memory just as rivastigmine.
Keywords: Euterpe oleracea, learning, Morris water maze, rat, spatial memory
Introduction
Learning and memory regulate essential needed basic mental processes. Humans show mental functions unconsciously or involuntarily in each operation. Experiences cause and shape changes in behaviors, thus, different behavioral cases occur in learning.1 Memory provides retrievals and shows how behaviors are shaped in case of need. Interaction with the known information and remembering it when it is needed are basic cycles that come up with learning and memory processes.2 Cerebral cortex and limbic systems are efficient in this cycle and play important roles such as reading, recognition of the objects, cognition of a speech, and so on.2 It also serves as a solution region for spatial coordinates, found in the cerebral hemisphere; limbic association region plays an important role for motivation, emotions, and behaviors.2
Euterpe oleracea (EO) extract has been studied extensively as it has nutritional and phytochemical compositions.3 Chemically ∼90 substances have been described, which approximately consists of flavonoids (31%), phenolic compounds4 (23%), lignoids (11%), and anthocyanins (9%).5 It has been proved that these compounds have potent anti-inflammatory,6,7 cardioprotective, antitumoral,8–13 and antioxidant effects.14–16 E. oleracea extract has managed to decrease inflammatory and oxidative damages in brain cells.17 Studies about the compounds in E. oleracea extract have revealed that a group of polyphenolic flavonoids could enter the cytosol and reduce oxidative damage that is associated with inflammation within the cell.18,4 Antioxidants have different kinds of properties such as being a free radical scavenger, preventing many diseases.19 The in vitro evidence for the potent antioxidant and anti-inflammatory properties of E. oleracea extract are supported by studies conducted on humans.20 Two studies including a randomized, double-blind, placebo-controlled crossover study reported significant reductions in lipid peroxidation in healthy participants in ages between 19 and 52 years.4 Alzheimer's is the main (three-fourth of patients) and vascular disease is the second reason for dementia.21 Dementia is characterized by changes in behavior and decrease in daily actions and initial learning memory and cognitive functions.22 According to studies, change in the number of muscarinic acetylcholine (Ach) receptors and decay in their activity are main reasons for neurological diseases such as Alzheimer's,23 Down's syndrome, and parkinsonism.24,25 The aim of our experiment was to detect learning and memory in rats using E. oleracea extract, which is known for its antioxidant15 and anti-inflammatory6 effects. In addition, contribution of Ach muscarinic and nicotinic receptor was researched in these effects. Scopolamine as a muscarinic receptor antagonist and nicotinic receptor antagonist mecamylamine were used in this study.
Materials and Methods
Animals
Seventy-two (250 ± 25 g) male (n = 8) Wistar rats were obtained from Institutional Animal Care Facility (TICAM) in Eskişehir Osmangazi University (ESOGU). Animals were kept under ambient conditions at 22–24°C and 12 h light–12 h dark cycle for 1 week before the experiment. Pellet diets (Rodent Diet CE-2, Turkey, Inc.) and tap water were given to the experimental animals ad libitum. The experiments were approved by the Animal Care Committees of ESOGU (18-2-2016; 503-1).
Drugs and chemicals
E. oleracea was purchased from Mountain Fresh Herbal Capsules, Inc., (United Kingdom). Rivastigmine was obtained from Exelon, Novartis. Mecamylamine hydrochloride was purchased from Santa Cruz Biotechnology (USA). Scopolamine hydrobromide trihydrate was purchased from Acros Organics, ketamine and xylazine were obtained from TICAM. Enzyme-linked immunosorbent assay (ELISA) kit was purchased from Elabscience.
Assessment of locomotor activity
Locomotor activity was assessed using automated apparatus (AMS 02 Animal Activity Monitoring system). Animals were placed individually in clear Perspex cages (30 × 30 × 30 cm) and the number of compliance transit cages was recorded for the subsequent 5-min period.26,27 Activity of locomotor was calculated on the sixth day, after 85 min the drug was injected for all the experimental groups.
Morris water maze
Morris water maze (MWM) was used as a model for spatial learning and memory experiments.
The MWM is a circular pool (diameter 150 cm; height 60 cm) filled with water (25°C ± 1°C) that included small blue balls.28 MWM was divided into four quadrants B, H, G, and D, and three equally spaced points served as starting positions around the edge of the pool. The escape platform is located in the pool, 15 cm away from the border of the pool, and 1 cm above the water surface during the learning familiarization session, and 1 cm below the water surface during memory sessions. Video tracking was conducted with a video camera focused on the full diameter of the pool. Navigation parameters were analyzed by the Ethovision 3.1 video analysis system (version 9; Noldus Ethovision XT, Wageningen, the Netherlands). Three important data were evaluated with MWM: escape latency (sec) for the time required to find the hidden platform during acquisition sessions, time spent (sec) in the quadrant with platform, and the mean distance to the platform (cm). Rats were trained in the MWM for 5 days (familiarization session, S1, S2, S3, and S4). As soon as a rat climbed onto the platform, trial was stopped and escape latency was recorded,29 and did not climb onto the platform in 60 sec, trial was stopped during acquisition.30 Twenty-four hours after acquisition session, trial was used to assess the spatial memory retention of the location of the hidden platform. Rats were injected rivastigmine and E. oleracea. After 30 min, they were injected scopolamine and mecamylamine that were used to damage the memory. The percent of mean time spent for each quadrant was recorded. Rats were monitored for the time spent in escape platform quadrant, for the distance to the platform zone, and speed and distance that the rats covered by video tracking system.31
Evaluation of Ach in hippocampi
Rats were anesthetized with xylazine (15 mg/kg) and ketamine (60 mg/kg). Rats were decapitated for isolated brain tissue. Then, one cerebral hemisphere was homogenized in saline phosphate tampon. Ach levels were quantified using spectrophotometric method and were read at 450 nm with an ELISA reader.32
Statistical analysis
SPSS software 10.0 pocket program was used for statistical analysis (SPSS, Inc., Chicago, IL, USA). Learning parameters for groups and days were assessed using one-way and two-way ANOVA. Ach levels and memory were evaluated using one-way ANOVA. Student's t test was used for first and fifth days. Data are given as mean and standard error of the mean.
The results at the P < .05 level were accepted as statistically significant.
Results
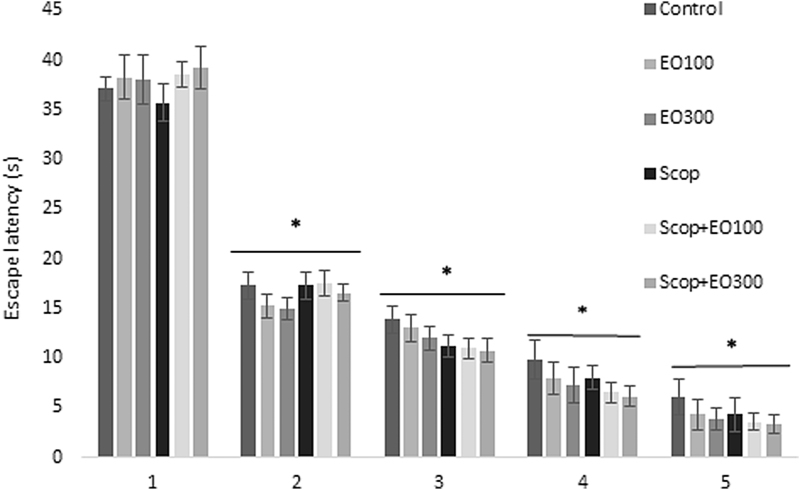
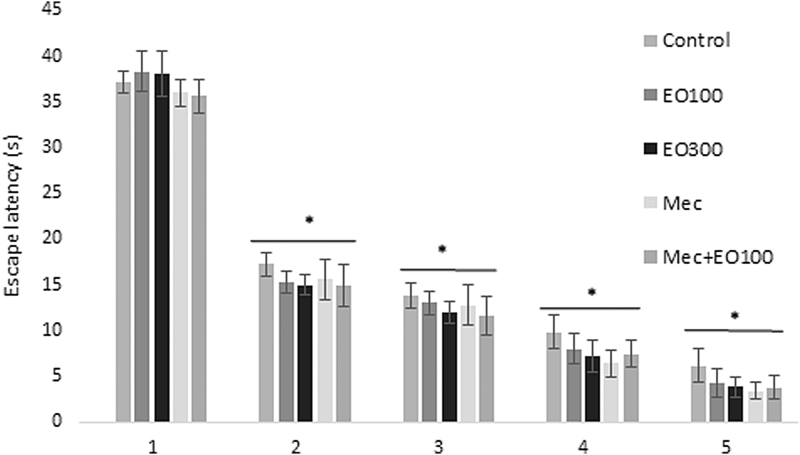
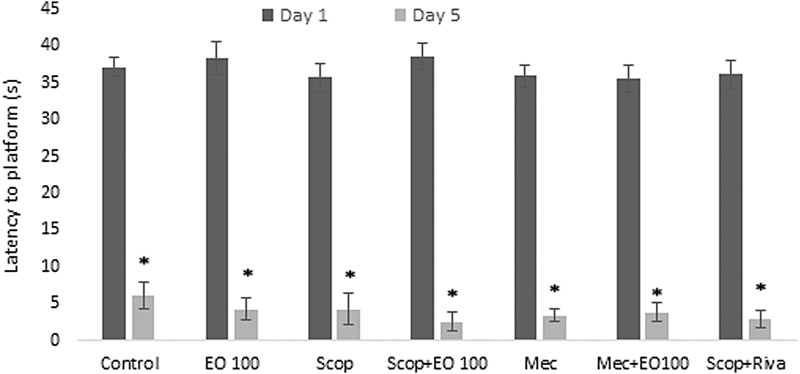
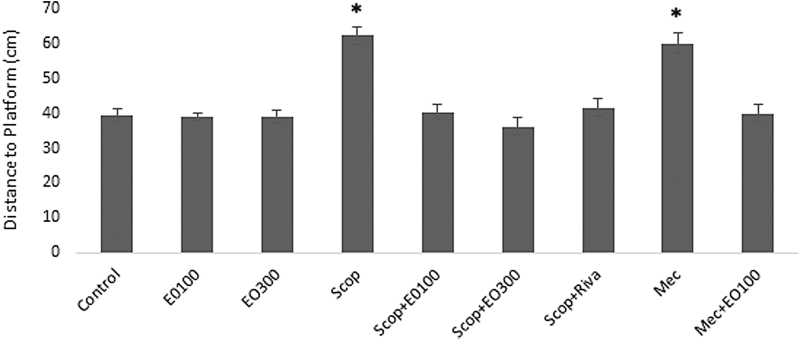
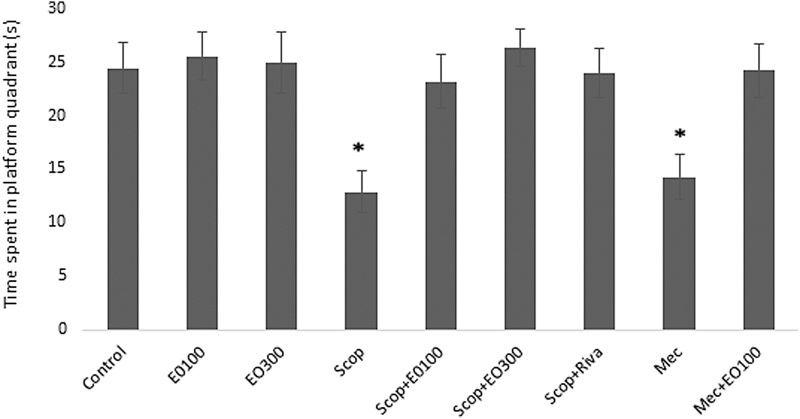
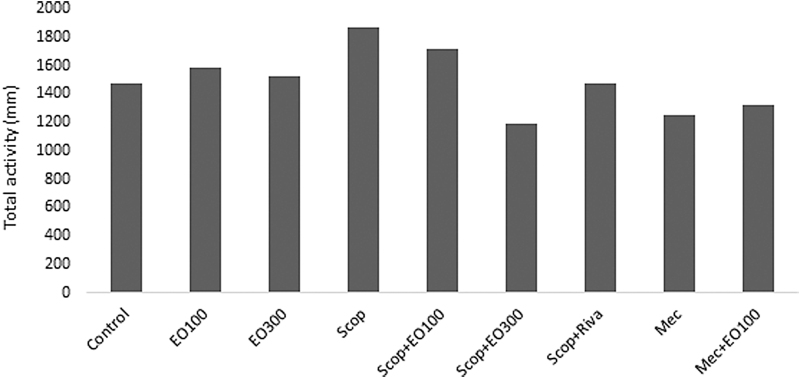
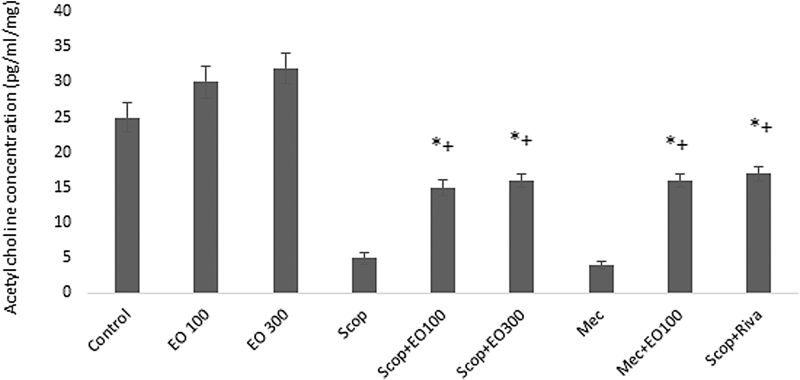
Evaluations for learning: escape latency (sec), which was defined as the time required to find the hidden platform, significantly decreased on the first day in all groups and as a result of that we observed enhanced learning rate related to that day (P < .05). E. oleracea did not contribute to learning very effectively in any doses (P > .05) (Figs. 1 and 2). When we evaluated learning for scopolamine and mecamylamine on the first and fifth days, the time spent for the accession to the platform was substantively lengthened out and on-platform stage shortened the time just as other groups. Results at P < .05 were considered statistically significant (Figs. 3–5). There were no statistically significant differences between control group, EO100, and 300, between scopolamine+EO100, scopolamine+EO300, scopolamine+rivastigmine, and mecamylamine+EO100 for mean distance to platform, and time spent in the quadrant that included the escape platform (P > .05) (Figs. 3–5). The assessments of scopolamine+EO100 and scopolamine+EO300 and mecamylamine+EO100 were similar in the control group. Thus, the memory that was damaged by scopolamine and mecamylamine was recovered by EO100 and EO300 doses (Fig. 3). The combination of scopolamine+rivastigmine showed similar effects with the combinations of scopolamine+EO100 and EO300. So, both the E. oleracea doses recovered the damaged memory like rivastigmine (Figs. 3–5). Statistically, both doses of E. oleracea improved damaged memory that was affected by scopolamine (muscarinic receptor antagonist) and mecamylamine (nicotinic receptor antagonist) (P < .05) (Figs. 4 and 5). These effects were the same with the effects of rivastigmine (P > .05) (Figs. 4 and 5). Locomotor activity: There was no statistically significant difference in all groups (P > .05) (Fig. 6). Ach in hippocampus: As per control group, the levels of Ach (pg/[mL·mg protein]) decreased significantly in both mecamylamine and scopolamine groups (P < .05). But these levels were obtained for the control group by the combinations of both EO100, EO300 and rivastigmine that was used for the treatment of cognitive deficits, and there were no significant differences compared with the control group (P > .05) (Fig. 7).
FIG. 1.
Escape latency of control, EO100 mg/kg, EO300 mg/kg, scopolamine, and scopolamine combined with EO100 mg/kg and EO300 mg/kg. Learning speed accelerated in all groups in parallel with number of days. *P < .05 comparison of all groups to day 1. EO, Euterpe oleracea; Scop, scopolamine.
FIG. 2.
Escape latency of control, EO100 mg/kg, EO300 mg/kg, mecamylamine, and mecamylamine combined with EO100 mg/kg dose. Learning speed accelerated in all groups in parallel with the number of days. *P < .05 comparison of all groups to day 1. Mec, mecamylamine.
FIG. 3.
Latency to platform by comparison between days 1 and 5 among the groups of control, EO100 mg/kg, scopolamine, mecamylamine, and their combinations with EO100 mg/kg (E. oleracea) and rivastigmine. *P < .05 comparison of all groups to day 1. Riva, rivastigmine.
FIG. 5.
Distance (cm) of rats to platform area on sixth day for memory evaluation among the groups of control, EO100 mg/kg, EO300 mg/kg, scopolamine, mecamylamine, and scopolamine combinations with EO100 mg/kg, EO300 mg/kg, and rivastigmine. *P < .05 compared with control group.
FIG. 4.
Time spent (sec) in platform quadrant on sixth day for memory evaluation among the groups of control, EO100 mg/kg, EO300 mg/kg, scopolamine, mecamylamine, and scopolamine combination with EO100 mg/kg and EO300 mg/kg. *P < .05 compared with control group.
FIG. 6.
Total movements (mm) of rats for locomotor activities in all groups. P > .05 compared with the control group. EO100, EO100 mg/kg; EO300, EO300 mg/kg.
FIG. 7.
Comparison of Ach levels (pg/[mL·mg protein]) according to control in all groups. *P < .05 EO100 and 300 mg/kg; +P < .05 comparison of Ach levels according to scopolamine, mecamylamine, and their combinations with EO100 and 300 mg/kg and rivastigmine. Ach, acetylcholine; EO100, EO100 mg/kg; EO300, EO300 mg/kg.
Discussion
It has been shown that age-related cognitive impairment is accompanied by cognitive declines and associated with an increased risk of dementia and neurodegenerative diseases that deeply affects a person's ability to function physically, socially, and emotionally.23 Recent bioactivity studies of the compounds in E. oleracea extract have revealed that a group of polyphenolic flavonoids could enter the cytosol and reduce oxidative damage that is associated with inflammation within the cell.17,4 Being a free radical scavenger prevents many diseases.18
Flavonoids have strong antioxidant activities and anti-inflammatory properties.33 Thus, the enhancing effects on learning and memory of E. oleracea in naive rats and impaired rats were evaluated in this study. Both the E. oleracea doses recovered the damaged memory like rivastigmine. E. oleracea did not contribute to learning very effectively.
Using MWM and radial arm maze tests for learning and memory in cognitive functions were implemented in terms of the relationship between MWM or radial arm maze and human learning memory.29,34 We preferred MWM for the evaluation of cholinergic system that has both muscarinic and nicotinic effects. The blockage of muscarinic receptors with scopolamine especially decreased the spatial learning in MWM test.31,35,36 Data from all groups showed that escape latency (sec) decreased during acquisition sessions for the evaluation of learning, so there are not much differences between all groups. Time spent (sec) in the quadrant with platform increased in control, EO100, EO300, and rivastigmine groups and decreased in scopolamine and mecamylamine groups. Distance to the platform (cm) increased in both scopolamine and mecamylamine groups. Many studies showed that both mecamylamine37 and scopolamine38 damaged cognitive functions. In our study, the memory that was damaged by scopolamine was improved by EO100 and EO300 doses. E. oleracea had a positive effect on muscarinic cholinergic receptors. When used 21 days, E. oleracea doses did not cause any significant difference in learning and memory compared with the control group (P > .05). There was significant difference on learning tests between the first and the fifth days in all groups (P < .001). But, there were no significant differences between groups (P > .05). E. oleracea had an effect on memory that was damaged by mecamylamine as a nicotinic receptor antagonist.39 EO100 improved the impaired memory caused by mecamylamine. E. oleracea's memory improving effect is through nicotinic and cholinergic pathways. Mecamylamine and scopolamine enhanced the latency of finding the escape platform and decreased the time spent in escape platform quadrant on memory tests (P < .05). Hippocampus (CA1–CA3) and amygdala are located in the limbic system. They store the information and have a role in information, retrieval, solving memory, and 3D spatial problems.40
Hippocampi region in the brain plays a role in spatial learning,41 associating different true-life experiences.42 Cholinergic neurons show connection network that is the main factor for maintaining normal cognitive relationship with learning and memory, emotional, and behavioral functions.43 We used anticholinergic scopolamine and mecamylamine for their memory damaging effect. Ach levels in hippocampi homogenates (pg/[mL·mg protein]) of scopolamine and mecamylamine groups significantly decreased compared with the control. There are no statistically significant differences in doses of EO100 and EO300. All combinations of E. oleracea approached to control group in Ach levels (P > .05). E. oleracea actually acts like rivastigmine especially in terms of memory and hippocampus Ach levels. Some treatment follows to increase Ach levels via using precursors, acetylcholinesterase inhibitors just as physostigmine, metrifonate, and galantamine that emerge nonspecific inhibition and butyryl-cholinesterase, donepezil and rivastigmine that are specific44 muscarinic and nicotinic Ach agonists.45 Rivastigmine stimulates the release of Ach from synapses in hippocampus and cortex,46 and is widely used for Alzheimer's disease.47 So, we used rivastigmine as a reference drug in our study. E. oleracea is effective at least as much as rivastigmine in cognitive functions deficits. We did not comment whether rivastigmine has nicotinic contribution because we did not combine it with mecamylamine. Many studies show that there is a positive correlation between hippocampal Ach release and locomotor activity.48 There were no statistically significant differences between all groups about the total number of movements in locomotor activity test (P > .05). Scopolamine and mecamylamine combinations with E. oleracea groups did not show an alteration in motor and motivational functions just as rivastigmine did.
In conclusion, E. oleracea that we studied has dose-independent effects related to learning and memory and it corrects corrupted memory without making a significant difference in learning. It is concluded that this correction is as effective as rivastigmine. E. oleracea's memory corrective effect is through nicotinic and muscarinic cholinergic pathways.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by Eskişehir Osmangazi University Commission of Scientific Researches under the project number of 2016-1168.
References
- 1. Pittenger C, Kandel ER: In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos Trans R Soc Lond B Biol Sci 2003;358:757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gasparini L, Racchi M, Binetti G, Trabucchi M, Solerte SB, Alkon D, Etcheberrigaray R, Gibson G, Blass J, Paoletti R, Govoni S: Peripheral markers in testing pathophysiological hypotheses and diagnosing Alzheimer's disease. FASEB J 1998;12:17–34 [DOI] [PubMed] [Google Scholar]
- 3. Schauss AG, Wu X, Prior RL, et al. :. Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (Acai). J Agricul Food Chem 2006;54:8598–8603 [DOI] [PubMed] [Google Scholar]
- 4. Da Costa CA, Ognibene DT, Cordeiro VSC, de Bem GF, Santos IB, Soares RA, Resende AC: Effect of Euterpe oleracea Mart. seeds extract on chronic ischemic renal injury in renovascular hypertensive rats. J Med Food 2017;20:1002–1010 [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi KK, Pereira LF, Lamarão CV, Lima ES, da Veiga-Junior VF: Amazon acai: Chemistry and biological activities: A review. Food Chem 2015;179:137–151 [DOI] [PubMed] [Google Scholar]
- 6. Jensen GS, Wu X, Patterson KM, et al. : In vitro and in vivo antioxidant and anti inflammatory capacities of an antioxidantrich fruit and berry juice blend. Results of a pilot and randomized, double-blind, placebo-controlled, crossover study. J Agric Food Chem 2008;56:8326–8333 [DOI] [PubMed] [Google Scholar]
- 7. Xie C, Kang J, Li Z, Schauss AG, Badger TM, Nagarajan S, Wu T, Wu X: The açaí flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κ;B activation and MAPK pathway. J Nutr Biochem 2012;23:1184–1191 [DOI] [PubMed] [Google Scholar]
- 8. Dias MM, Noratto G, Martino HS, Arbizu S, Peluzio Mdo C, Talcott S, Ramos AM, Mertens-Talcott SU: Pro-apoptotic activities of polyphenolics from açai (Euterpe oleracea Martius) in human SW-480 colon cancercells. Nutr Cancer 2014;66:1394–1405 [DOI] [PubMed] [Google Scholar]
- 9. Silva DF, Vidal FC, Santos D, Costa MC, Morgado-Díaz JA, do Desterro Soares Brandão Nascimento M, de Moura RS: Cytotoxic effects of Euterpe oleracea Mart. in malignant cell lines. BMC Complement Altern Med 2014;14:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fragoso MF, Romualdo GR, Ribeiro DA, Barbisan LF: Açai (Euterpe oleracea Mart.) feeding attenuates dimethylhydrazine-induced rat colon carcinogenesis. Food Chem Toxicol 2013;58:68–76 [DOI] [PubMed] [Google Scholar]
- 11. Fragoso MF, Prado MG, Barbosa L, Rocha NS, Barbisan LF: Inhibition of mouse urinary bladder carcinogenesis by açai fruit (Euterpe oleraceae Martius) intake. Plant Foods Hum Nutr 2012;67:235–241 [DOI] [PubMed] [Google Scholar]
- 12. Zapata-Sudo G, da Silva JS, Pereira SL, Souza PJ, de Moura RS, Sudo RT: Oral treatment with Euterpe oleracea Mart. (açaí) extract improves cardiac dysfunction and exercise intolerance in rats subjected to myocardial infarction. BMC Complement Altern Med 2014;14:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silva Santos V, Almeida Teixeira GH, Barbosa F Jr.: Açaí (Euterpe oleracea Mart.): A tropical fruit with high levels of essential minerals-especially manganese-andits contribution as a source of natural mineral supplementation. J Toxicol Environ Health A 2014;77:80–89 [DOI] [PubMed] [Google Scholar]
- 14. Honzel D, Carter SG, Redman KA, et al. : Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: Generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J Agric Food Chem 2008;56:8319–8325 [DOI] [PubMed] [Google Scholar]
- 15. Cesar LT, de Freitas Cabral M, Maia GA, de Figueiredo RW, de Miranda MR, et al. : Effects of clarification on physicochemical characteristics, antioxidant capacity and quality attributes of açai (Euterpe oleracea Mart.) juice. J Food Sci Technol 2014;51:3293–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chin YW, Chai HB, Keller WJ, Kinghorn AD: Lignans and other constituents of the fruits of Euterpe oleraceae (acai) with antioxidant and cytoprotective activities. J Agric Food Chem 2008;56:7759–7764 [DOI] [PubMed] [Google Scholar]
- 17. Wong DY, Musgrave IF, Harvey BS, Smid SD: Açaí (Euterpe oleraceae Mart.) berry extract exerts neuroprotective effects against β-amyloid exposure in vitro. Neurosci Lett 2013;556:221–226 [DOI] [PubMed] [Google Scholar]
- 18. Schauss AG, Wu X, Prior RL, Ou B, et al. : Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (ac¸ai). J Agric Food Chem 2006;54:8604–8610 [DOI] [PubMed] [Google Scholar]
- 19. Wani SA, Kumar P: Antioxidants and its properties as affected by extrusion process: A review. Recent Pat Food Nutr Agric 2015;7:108–114 [DOI] [PubMed] [Google Scholar]
- 20. Schauss AG: Ac¸ai (Euterpe oleracea Mart.): A macro and nutrient rich palm fruit from the Amazon rain forest with demonstrated bioactivities in vitro and in vivo. In: Bioactive Foods in Promoting Health: Fruits and Vegetables. (Watson RR, Preedy V, eds.). Academic Press, Oxford, 2010, pp. 479–490 [Google Scholar]
- 21. Meng X, D'Arcy C: Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One 2012;7:e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knopman DS: The initial recognition and diagnosis of dementia. Am J Med 1998;104:2S–12S; discussion 39S–42S. [DOI] [PubMed] [Google Scholar]
- 23. Pepeu G, Grazia Giovannini M: The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res 2017;1670:173–184 [DOI] [PubMed] [Google Scholar]
- 24. Bymaster FP, Carter PA, Yamada M, et al. : Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci 2003;17:1403–1410 [DOI] [PubMed] [Google Scholar]
- 25. Caulfield MP, Birdsall NJ: International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 1998;50:279–290 [PubMed] [Google Scholar]
- 26. Pirondi S, Kuteeva E, Giardino L, Ferraro L, Antonelli T, Bartfai T, Ogren SO, Hökfelt T, Calzà L: Behavioral and neurochemical studies on brain aging in galanin over expressing mice. Neuropeptides 2005;39:305–312 [DOI] [PubMed] [Google Scholar]
- 27. Sonkusare S, Srinivasan K, Kaul C, Ramarao P: Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci 2005;77:1–14 [DOI] [PubMed] [Google Scholar]
- 28. Mutlu O, Ulak G, Kokturk S, Komsuoglu Celikyurt I, Tanyeri P, Akar F, Erden F: Effects ofa dragonfly (Anax i.) homeopathic remedy on learning, memory andcell morphology in mice. Homeopathy 2016;105:96–101 [DOI] [PubMed] [Google Scholar]
- 29. Yau JL, Noble J, Hibberd C, Rowe WB, Meaney MJ, Morris RG, Seckl JR: Chronic treatment with the antidepressant amitriptyline prevents impairments in water maze learning in aging rats. J Neurosci 2002;22:1436–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D'Hooge R, De Deyn PP: Applications of the Morris water maze in the study of learning and memory. Brain Res Rev 2001;36:60–90 [DOI] [PubMed] [Google Scholar]
- 31. Brandeis R, Brandys Y, Yehuda S: The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci 1989;48:29–69 [DOI] [PubMed] [Google Scholar]
- 32. Scarr E, Gibbons AS, Neo J, Udawela M, Dean B: Cholinergic connectivity: It's implications for psychiatric disorders. Front Cell Neurosci 2013;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kang J, Li Z, Wu T, et al. : Antioxidant capacities and antiinflammatory effects of flavonoid compounds from acai pulp (Euterpe oleracea Mart.). Food Chem 2010;122:610–617 [Google Scholar]
- 34. Aydin E, Hritcu L, Dogan G, Hayta S, Bagci E: The effects of inhaled Pimpinella peregrina essential oil on scopolamine-induced memory impairment, anxiety, and depression in laboratory rats. Mol Neurobiol 2016;53:6557–6567 [DOI] [PubMed] [Google Scholar]
- 35. Mutlu O, Ulak G, Belzung C: Effects of nitric oxide synthase inhibitors 1-(2-trifluoromethylphenyl)—Imidazole (TRIM) and 7-nitroindazole (7-NI) on learning and memory in mice. Fundam Clin Pharmacol 2011;25:368–377 [DOI] [PubMed] [Google Scholar]
- 36. Warburton DM, Rusted JM: Cholinergic control of cognitive resources. Neuropsychobiology 1993;28:43–46 [DOI] [PubMed] [Google Scholar]
- 37. Newman LA, Gold PE: Attenuation in rats of impairments of memory by scopolamine, a muscarinic receptor antagonist, by mecamylamine, a nicotinic receptor antagonist. Psychopharmacology (Berl) 2016;233:925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deb D, Bairy KL, Nayak V, Rao M: Comparative effect of lisinopril and fosinopril in mitigating learning and memory deficit in scopolamine-induced amnesic rats. Adv Pharmacol Sci 2015;2015:521718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Javadi P, Rezayof A, Sardari M, Ghasemzadeh Z: Brain nicotinic acetylcholine receptors are involved in stress induced potentiation of nicotinereward in rats. J Psychopharmacol 2017;31:945–955 [DOI] [PubMed] [Google Scholar]
- 40. Waxman A, Chugani H, Seibyl J: Medical imaging in neurological disorders. J Am Pharm Assoc (Wash) 2002;42(5 Suppl 1):S48–S49 [DOI] [PubMed] [Google Scholar]
- 41. Murty VP, LaBar KS, Hamilton DA, Adcock RA: Is all motivation good for learning? Dissociable influences of approach and avoidance motivation in declarative memory. Learn Mem 2011;18:712–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumaran D, Hassabis D, Spiers HJ, Vann SD, Vargha-Khadem F, Maguire EA: Impaired spatial and non-spatial configural learning in patients with hippocampal pathology. Neuropsychologia 2007;45:2699–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luchicchi A, Bloem B, Viaña JN, Mansvelder HD, Role LW: Illuminating the role of cholinergic signaling in circuits of attention and emotionally salient behaviors. Front Synaptic Neurosci 2014;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH: Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J 1978;2:1457–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilson C, Lee MD, McCarron JG: Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J Physiol 2016;594:7267–7307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamaguchi Y, Takeda K, Hino M: Combination effects of ZSET1446/ST101 with memantine on cognitive function and extracellular acetylcholine in the hippocampus. J Pharmacol Sci 2013;123:347–355 [DOI] [PubMed] [Google Scholar]
- 47. Elmegeed GA, Ahmed HH, Hashash MA, Abd-Elhalim MM, El-kady DS: Synthesis of novel steroidal curcumin derivatives as anti-Alzheimer's disease candidates: Evidences-based on in vivo study. Steroids 2015;101:78–89 [DOI] [PubMed] [Google Scholar]
- 48. Kikuchi T, Tan H, Mihara T, et al. : Effects of volatile anesthetics on the Circadian rhythms of rat hippocampal acetylcholine release and locomotor activity. Neuroscience 2013;237:151–160 [DOI] [PubMed] [Google Scholar]