Abstract
Respiratory infections and subsequent complications are one of the leading causes of high mortality in immunocompromised patients. Although chest radiograph and computed tomography are the commonly used diagnostic tools for the early diagnosis of lung manifestations of infections, they lack the specificity for the wide range of chest infections which can occur in immunocompromised patients. Systematic analysis of the imaging findings in correlation with the clinical settings along with comparison with the old images can expedite early and accurate diagnosis for subsequent appropriate management. Computer tomography findings in immunocompromised patients with respiratory infections, with regards to various clinical settings, will be discussed here.
Keywords: Chest infection, Pneumonia, Immunocompromised patient, AIDS, Cancer drugs, Computed tomography
Learning objectives.
After reading this article the reader will be able to:
-
(a)
Understand the classification, patterns and imaging features of the respiratory infections
-
(b)
Understand the role of clinical settings while interpreting computer tomography findings
-
(c)
Understand some of the mimics of chest infections
1. Introduction
Infections are very common in an immunocompromised host (ICH), which includes patients on chemotherapy (cancer), on immunosuppressive therapy (post transplantation patients, rheumatologic disorders) or acquired immunodeficiency diseases (AIDS, post-splenectomy), and their population is expanding globally.
Pneumonitis implies inflammation of the lung and in an immunocompromised patient (ICP) may occur due to disease progression, infections or secondary to non-infectious causes like drug induced toxicities [1], [2], [3], [4], [5], [21]. Lung inflammations due to infections are known as pneumonia. Infection in ICH may be primary or secondary due to the underlying noninfectious inflammatory condition of the lung, which further compromises the immune system of the body.
Respiratory infections, along with drug toxicity, are major concerns in more than two thirds of the ICPs. These are leading causes of therapy failure of the primary diseases, are often life threatening and are associated with high mortality and morbidity. Therefore, an accurate and quick diagnosis is a major cornerstone for the management team.
2. Classifications of pneumonia
Pneumonia can be classified, on the basis of different modes of acquirement of the infections, into community-acquired pneumonia (CAP), hospital acquired pneumonia (also referred as nosocomial pneumonia, HAP) or ventilator associated pneumonia (VAP) (Table 1 ). Pneumonia can be further stratified as mild, moderate or severe on the basis of clinical parameters and scoring systems like Pneumonia Severity Index (PSI) or CURB-65 [6]. These validated scoring systems assist healthcare professionals in determination of the clinical outcomes.
Table 1.
Classification of pneumonia.
| Mode of infection |
|
| Type of organism |
|
| Imaging pattern |
|
Another method of classification divides patients into typical or atypical groups, based on the clinical manifestations and the expected pathogens differ accordingly. Patient who present with classical symptoms like fever, rigors, chills, cough with expectoration, chest pain, dyspnea and whose chest radiographic findings are suggestive of common bacterial infections is considered to have typical pneumonia. On the other hand, atypical pneumonia usually presents with persistent low grade fever without the typical pneumonia symptoms and signs. The etiologic pathogen could be either a bacterial, viral, fungal or any other opportunistic organism.
The most common pathogens of CAP are Streptococcus pneumoniae, Mycoplasma pneumoniae, Chlamydia pneumoniae, Haemophilus influenzae, Legionella pneumoniae and respiratory viruses. VAP are likely due to gram negative infections such as Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae and Acinetobacter as well as gram positive pathogens like Staphylococcus aureus.
In the early stages of immunosuppression, such as in the induction phase of chemotherapy, infections are most likely bacterial in origin. Common isolated bacterial pathogens in ICH are Legionella, Mycoplasma and Chlamydia (Table 2 ). The predominant viral agents are rhinoviruses, coronaviruses, influenza virus, respiratory syncytial virus (RSV), adenovirus and parainfluenza virus. ICPs as a result of organ transplantation are vulnerable to infections with cytomegalovirus (CMV) and rarely Herpes virus. Coronavirus and Hantavirus infections are commonly observed during seasonal and non-seasonal outbreaks. Pneumonia due to multidrug-resistant organisms such as S. pneumoniae (DRSP) or S. aureus (MRSA) are often seen in ICPs. Viral infections can be also be complicated by secondary bacterial super-infections. Fungal infections are very common in ICH with leukemia, cancer chemotherapy or organ transplant, mainly seen in later stages of immunosuppression either as a primary infection or as a result of a super-infection. Pneumocystis jiroveci Pneumonia (PJP) constitutes the most frequent opportunistic fungal infection. Other common fungal infections reported are Aspergillus fumigatus, Candida albicans, and Cryptococcus neoformans. Histoplasmosis, Blastomycosis and Mucormycosis are not so common globally but are isolated in endemic areas (Table 2).
Table 2.
Common atypical infections in immunocompromised patients.
| Viral Infections | Herpes simplex viruses, Varicella-zoster virus, CMV, Measles virus, Adenoviruses |
| Opportunistic organisms | Legionella, Mycoplasma, Chlamydia, Nocardia |
| Fungal | Aspergillosis, Candida, Mucormycosis |
| Endemic: Blastomycosis, Histoplasmosis, Cryptococcus |
Non-infectious etiologies like drug toxicities, cardiac causes and organizing pneumonias should always be considered in the differential diagnosis of atypical and non-resolving pneumonias.
3. Radiological patterns of pneumonia
On the basis of imaging patterns, pneumonia is classified as lobar pneumonia, bronchopneumonia and interstitial or atypical pneumonia. Lobar and bronchopneumonia are usually bacterial in origin, whereas, viruses, parasites and fungi are likely pathogens in interstitial pneumonia. However, one should always keep in mind overlapping imaging features.
3.1. Lobar pneumonia
Radiographically, lobar pneumonia is manifested by homogeneous consolidation with air bronchogram involving one, or less commonly, multiple lobes. Segmental pneumonia shows consolidation involving one or more segments of a lobe and may occasionally manifest as a round opacity simulating a pulmonary mass, called round pneumonia. Frank consolidation and air bronchogram have been associated with a higher incidence of bacteremia.
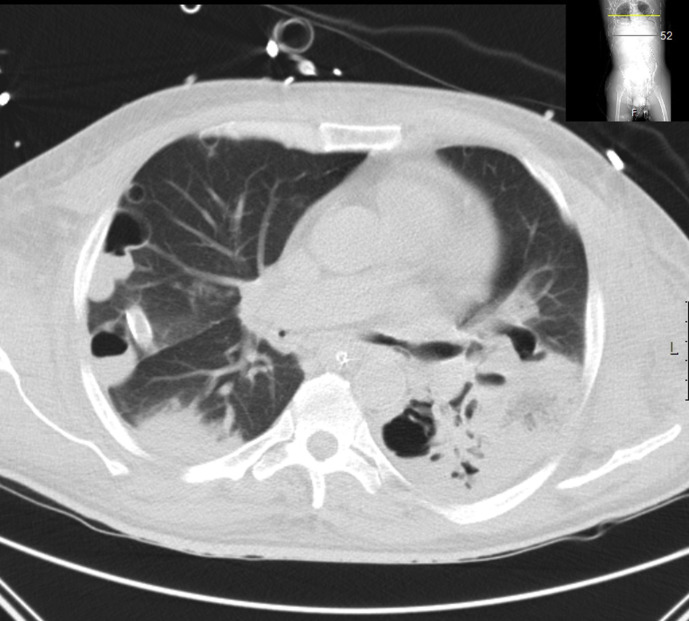
Klebsiella infection may show radiographic evidence of lobar expansion with bulging of interlobular fissures due to voluminous inflammatory exudates. Cavitation may also be present and there is a tendency to occur in the upper lobes. Legionella has a predilection for the lower lung fields. Radiologic resolution tends to lag far behind the clinical improvement by six to eight weeks (Fig. 1 ).
Fig. 1.
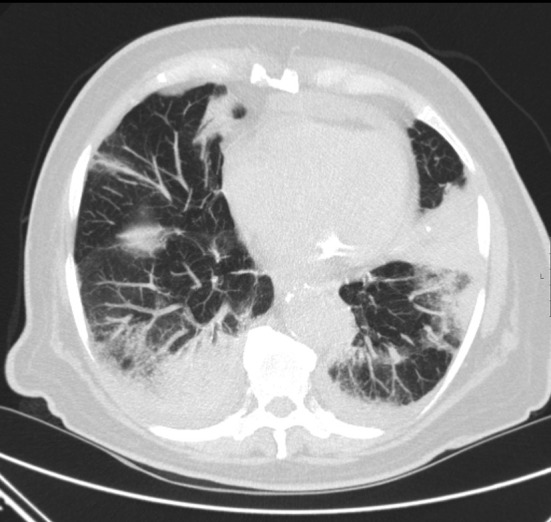
Patient with AML, CT showing Legionella pneumonia.
3.2. Bronchopneumonia
Bronchopneumonia, also known as multifocal or lobular pneumonia, is radiographically identified by its patchy appearance with peri-bronchial thickening and poorly defined air-space opacities. As the illness becomes more severe, consolidation involving respiratory bronchioles and alveoli results in the development of centrilobular nodular opacities or air-space nodules. The consolidation can progress further and coalesce to give a lobular or lobar pattern of involvement. Typically, air bronchogram are absent. The pathogens known to cause this pattern of pneumonia are particularly destructive. Thus, abscesses, pneumatoceles, and pulmonary gangrene may develop. Pathologically, bronchopneumonia stems from inflammation of large airways (bronchitis) with patchy (lobular) involvement.
In severe Staphylococcus infection, lobar enlargement with bulging of interlobular fissures can be seen. Abscess, cavitation (with air-fluid levels), and pneumatocele are not uncommon and 30–50% of patients develop pleural effusions, half of which are empyema. It may be noted that cavitation and associated pleural effusions are also observed in cases of anaerobic infections, gram-negative infections and tuberculosis.
In Pseudomonas infection, the radiographic findings tend to be nonspecific and are difficult to differentiate from the underlying lung disease. All the lobes are involved, with a predilection for the lower lobes. Necrosis and cavitation may occur. Invasive aspergillosis as well as pulmonary vasculitis with infarction can resemble bronchopneumonic pattern.
3.3. Interstitial pneumonia
Interstitial pneumonia is classified into focal and diffuse types. The radiological appearance results from that the edema and inflammatory cellular infiltrate into the interstitial tissue of the lung. The pathological development of interstitial pneumonia generally takes 1 of 2 forms: (1) an insidious infectious course that results in lymphatic infiltration of alveolar septa without parenchymal abnormality; (2) acute or rapidly progressive disease that results in diffuse alveolar damage affecting the interstitial and air spaces. Radiographically, this disease manifest with a reticular or reticulo-nodular pattern (Table 4 ).
Table 4.
CT changes in atypical Pneumonia.
| Pneumonia | GGO with lobular distribution | GGO diffuse pattern | Centrilobular nodules | Segmental consolidation | Interlobular septal thickening | Pleural effusion |
|---|---|---|---|---|---|---|
| Viral | ++ | +++ | ++ | + | ++ | – |
| Legionella | +++ | +++ | ++ | |||
| Mycoplasma | ++ | ++ | ++ | + | ||
| Chlamydia | ++ | ++ | ++ | + | ||
| Aspergillosis | ++ | + | ++ (+/− halo) | ++ | ||
| Candida | + | ++ | + |
+Sign indicate the relative frequency of the findings from lowest to highest.
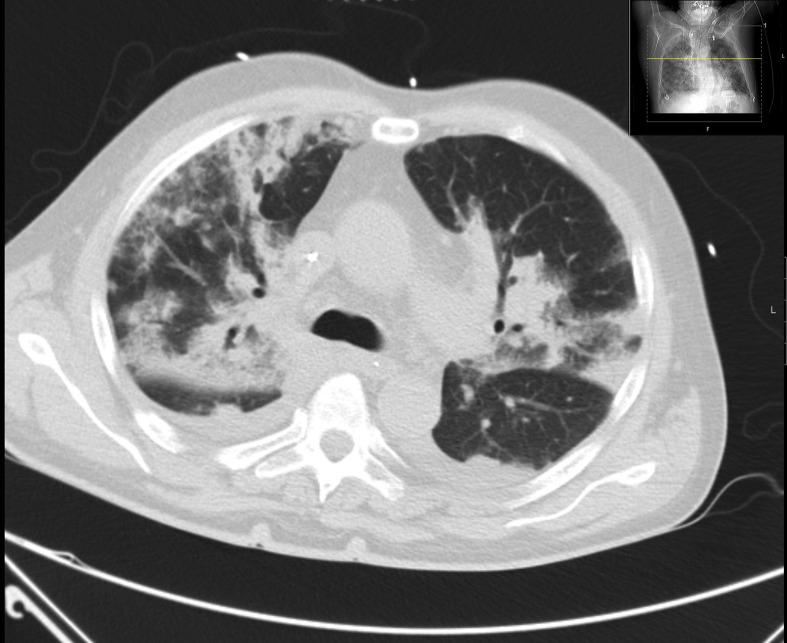
Legionella, Mycoplasma and chlamydial infections cause this pattern of pneumonia commonly. Legionella species usually cause a patchy, localized infiltrate in the lower lobes. Associated hilar adenopathy may be present. Pleural effusion is seen in up to 30% of cases. In rare instances, it may be associated with cavitation and a mass-like appearance (Fig. 2 ). The infiltrates in Mycoplasma pneumoniae can be unilateral, multi-lobular, or bilateral. In about 20% of patients, pleural effusion or hilar adenopathy may be present. Chlamydia pneumonia may be sub-segmental or more extensive in elderly patients; pleural effusions are rarely seen. Chest radiograph (CXR) reveals 50% resolution in four weeks. In 20% of cases, resolution takes longer than 9 weeks.
Fig. 2.
Patient with bronchial carcinoma under chemotherapy, CT showing septic lung embolism with cavitating lesions.
Aspergillosis is a mycotic disease caused by Aspergillus species, usually Aspergillus fumigatus. Pulmonary aspergillosis can be subdivided into five categories: (a) saprophytic aspergillosis (aspergilloma), (b) hypersensitivity reaction (allergic broncho-pulmonary aspergillosis), (c) semi-invasive (chronic necrotizing) aspergillosis, (d) airway-invasive aspergillosis (acute trachea-bronchitis, bronchiolitis, bronchopneumonia, obstructing bronchopulmonary aspergillosis), and (e) invasive pulmonary aspergillosis (IPA). IPA usually affects ICH and is reported as a vessel associated nodular lesion with “Halo sign”. It may present as peripheral consolidation.
Viral infection occurs very frequently in ICH [17], [18], [19]. Clinically, viral pneumonia in adults can be divided into two groups: atypical pneumonia in otherwise normal hosts and viral pneumonia in ICH. Influenza virus types A and B account for the majority of viral pneumonia in immune-competent adults. ICHs are susceptible to pneumonia caused by CMV and HSV, as well as measles and adenovirus (Table 2). The radiologic findings of viral pneumonia are variable and overlapping (Table 4). Specific etiological diagnosis of a viral pneumonia cannot be made on the basis of imaging features alone (Fig. 3 ). Clinical features such as patient age, immune status, time of year, illness in other family members, community outbreaks, different stages of the underlying disease at onset, severity and duration of symptoms, and presence of a rash remain important in diagnosing viral causes of atypical pneumonia in immune-competent as well as ICPs.
Fig. 3.
54 years old male with AML with fever, CT showing Influenza pneumonia.
4. Importance of clinical information in radiological interpretation
CXR is an essential tool for rapid diagnosis of lung changes and may also be help in follow up of the treatment response. However, it lacks specificity and often fails to show early and subtle findings of lung infections. Computed tomography (CT) of chest has become a mainstay tool for detecting early lung changes. There is better delineation and characterization of the patho-mechanism of the findings, enabling proper differential diagnosis including infection mimics [7]. The accuracy of radiological diagnosis can be improved significantly if it is correlated with the clinical background of the patient. Hence detailed information about the particular clinical setting including clinical presentation, past medical history such as exposure to tuberculosis et al., treatment history, and overall clinical condition is essential for better evaluation and proper diagnosis [8]. Pneumonia in a vulnerable ICH is influenced by several environmental and epidemiological factors like mode of exposure, i.e., CAP or HAP, history of previous infections and nature of ongoing drug therapy (cytotoxic, immunosuppressive) and stage of the disease.
Cytotoxic drugs causes neutropenia which is further complicated by increased risk of gram negative infections whereas immunosuppressive or immune-modulating drugs lead to increased risk of infections by causing defects in cell mediated immunity and phagocytosis.
Infections caused by Mycobacterium tuberculosis (Fig. 4 ), Pneumocystis jiroveci (PCP), Toxoplasma gondii and Varicella zoster virus are often on account of reactivation of past infections. Therefore, inquiring about past medical history suggestive of these infections is important.
Fig. 4.
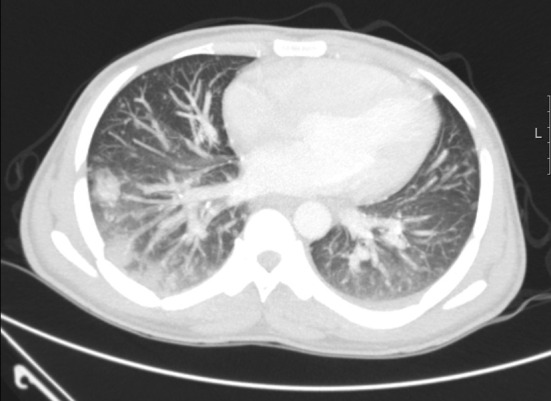
Patient with AIDS, CT showing tubercular bronchopneumonia.
Similarly, HAP or nosocomial infections are caused by a specific group of organisms. The risk is increased by intubation, use of broad spectrum antibiotic, use of strong immunosuppressive drugs combined with Histamine 2 receptor blockers which reduce gastric acidity which in turn leads to increased colonization by gram negative bacilli, such as Pseudomonas, Klebsiella (Fig. 2), E. coli and Acinetobacter. Ventilators and central air-conditioning system are associated with Legionella and gram negative infections. Catheter and drainages increase the risk of S. aureus, P. aeruginosa and Candida in vulnerable hosts.
The type of immune defect also determines the specific pathogen to a larger extent. Complement system defects lead to infection by extracellular or encapsulated bacteria. Phagocytosis failure is mainly associated with bacterial and fungal infections. ICH with humoral immunodeficiency is at risk of getting infected with encapsulated bacteria like S. aureus, S. pneumoniae, H. influenza and PJP. Whereas, cell mediated immunodeficiency or T-cell defect, due to underlying primary disease or secondarily due to viral infections like CMV or Epstein–Barr virus, pose a high risk of opportunistic infections. In patients with AIDS, pathogenic agents are determined by the CD4 cell count-whether below 100 (viral and fungal) or above 100 (PCP and Mycobacterium). Immune Defects due to Splenectomy or hypo-splenismp redisposes to infection with encapsulated microorganisms such as S. pneumoniae, H. influenza and S. aureus [13], [14], [15].
Duration of immunosuppression also determines the specific pattern of chest infection. Gram-negative infections and S. aureus are often seen in the early phase of neutropenia, whereas opportunistic organisms like fungus are found in the later phases of neutropenia. Organ transplanted ICH are susceptible to infections with likely causative pathogen depends on the post-transplant phase. In the early phase, common bacterial infections are observed, whereas viral or fungal infections predominate in later phases (usually 6 months post-transplant). The likelihood of a particular pathogen depends on the type of immune defect, which is governed by the type of immunosuppressive therapy.
5. Evaluation of the radiological findings in respiratory infections
The radiological diagnosis should be corroborated by special laboratory tests like Polymerase chain reactions (PCR), serology, immunoassays (ELISA) and galactomannan test, whenever necessary. Bronchoscopy with broncho-alveolar lavage aids in detection and confirmation of PCP, Candida and other infections. Correct interpretation of abnormal radiological findings is limited by several factors, such as insufficient clinical information and failure in obtaining old radiological imaging for comparison. Furthermore, overlapping imaging features of different organisms, lack of experience of the radiologist, subtle findings which are difficult to correlate and co-morbidities (such as congestive heart failure, cor pulmonale, radiation induced changes etc.) pose further difficulty in correct assessment of radiological changes. Hence, it is suggested to adopt a comprehensive and holistic approach to the radiological diagnosis.
CXR and chest CT are the frontline diagnostic tools in early detection of respiratory infections. Chest ultrasonography is useful in evaluating para-pneumonic effusions and can be used to evaluate complications like pneumothorax or as an assist tool for thoracocentesis. Non-enhanced CT chest is the modality of choice when plain CXR is inconclusive or inadequate for interpretation. There are certain radiological features suggestive of pneumonia and when accompanied with presence of certain “special imaging signs” can clinch the diagnosis [9]. These characteristic findings with probable differential diagnosis will be discussed below (Table 3).
Table 3.
Classical imaging signs/pattern indicative of lung infections and probable differential diagnosis.
| Signs | Diagnosis | Differential diagnosis |
|---|---|---|
| Consolidation & air bronchogram | Pneumonia | Atelectasis, neoplasia, aspiration |
| Silhouette sign | Segmental or bronchopneumonia | Atelectasis, neoplasia |
| Bulging fissure sign | Lobar pneumonia, abscess | Neoplasia |
| Feeding vessel sign | Septic emboli | Metastasis |
| Air fluid level sign | Empyema, abscess | Neoplasia, Wegener'sgranulomatosis |
| Inhomogeneous enhancement | Abscess, empyema | Neoplasia |
| Split pleura sign | Empyema, bronchopulmonary fistula | Postoperative and pleurodesis associated changes |
| Ground glass opacities (GGO) | Atypical pneumonia | Neoplasia, drug toxicities, cardiac failure, vasculitis |
| Halo sign | Aspergillosis | Pseudomonas, HSV, CMV, Wegener granulomatosis, |
| Air crescent sign | Aspergillosis | Hydatid cyst of lung |
| Monad sign | Mycetoma | Wegener's granulomatosis, neoplasia |
| Reverse Halo | Aspergillosis, cryptogenic organizing pneumonia | Tuberculosis, bacterial infections, Sarcoidosis, Wegener's granulomatosis |
| Crazy paving | PCP, viral like influenza | Alveolar proteinosis, pulmonary edema and hemorrhages |
| Miliary pattern | Tuberculosis | Metastasis |
5.1. Consolidation
Consolidation is the most common and easily interpretable findings on CXR and CT. It occurs mainly due to opacification of the air spaces by exudates and is usually bacterial in origin. Air tracks (bronchioles) are visualized when the walls are effaced by surrounding consolidation. This is known as air bronchogram sign and a reliable sign of lung infection. Focal consolidation can also be seen in other conditions like non-obstructive atelectasis, neoplasia, aspiration and organizing pneumonia. Hence, these should be closely analyzed to differentiate from each other. For instance, lung volume loss with subsequent elevation of diaphragm, vascular crowding and mediastinal shift are normally due to atelectasis, whereas bulging fissure (bulging fissure sign) along with air bronchogram suggests pneumonia. Bulging fissure sign is most often seen in upper lobe pneumonia caused by Klebsiella and pneumococcal infections. Neoplasia, large abscesses, infected bullae and other lesions can also present with this sign and a comparison with old films is utmostly helpful.
5.2. Silhouette sign
Silhouette sign is another very useful sign in detecting subtle changes of chest infection. This sign is characterized by obscuring of normal air interfaces of the thorax. Thoracic aperture, thoracic wall, para-mediastinal spaces and peri-cardiac spaces are the areas where this sign is well illustrated. It can also be seen in space occupying lesions, atelectasis and localized effusions.
5.3. Feeding vessel
Feeding vessel sign is a very useful sign of septic emboli in CT scan when cavitating or non-cavitating nodules are associated with a pulmonary vessel [10].
5.4. Air fluid level sign
Air fluid level sign is suggestive of abscess and empyema and mainly caused by S. aureus and Klebsiella. The wall of this cavitation may enhance in homogeneously on CT scan with contrast. A chest wall or fissure based focal opacity, also called split pleural sign, and is normally suggestive of empyema. It may also be illustrated in case of hemothorax, pleurodesis and post lobectomy.
5.5. Ground glass opacities (GGO)
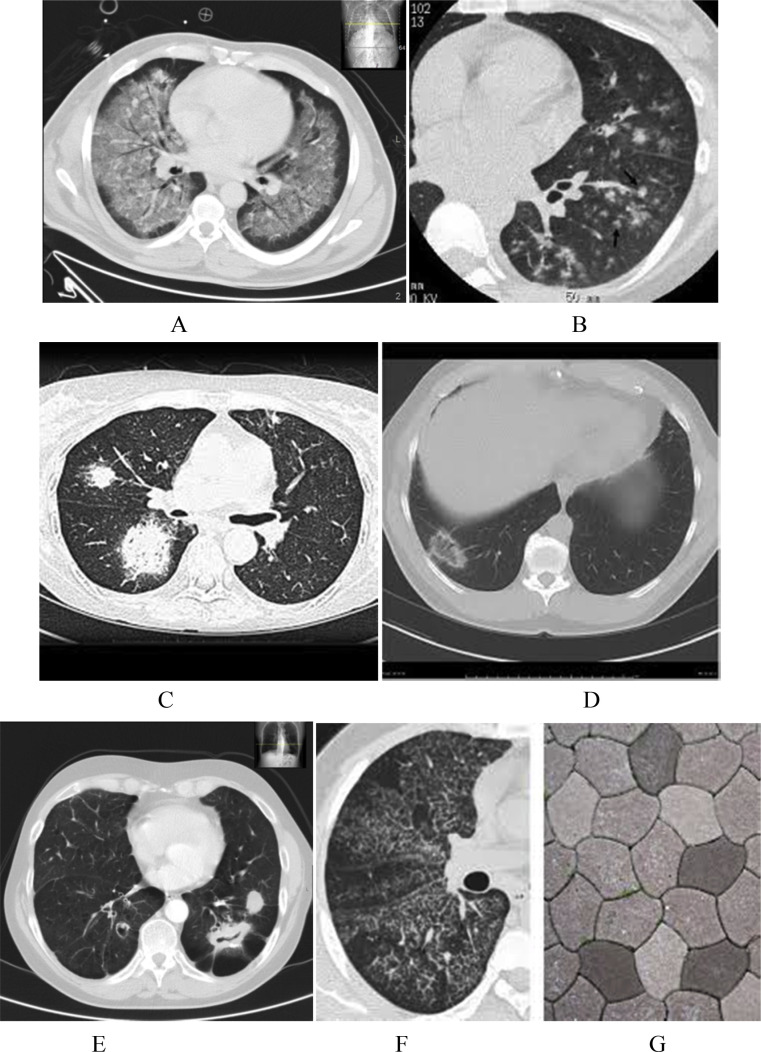
Ground glass opacities (GGO) are defined as nonspecific high attenuation of the lung parenchyma in CT scan due to decrease in air content or partial effacement resulting from exudates in the alveolar space (Fig. 5 a). Hence normal lung markings are not obscured in contrast to frank alveolar consolidations. This is not a very specific sign for infection and can be seen in other conditions like pulmonary congestion, interstitial lung disease, vasculitis, etc. The pattern of distribution of GGO with other accompanying signs may suggest a particular type of pneumonia. For example GGO confined to central and upper lobes with sparing of the subpleural spaces is very specific for PCP [16]. Tree-in-Bud sign (Fig. 5b) is a well-appreciated sign seen in various conditions such as infectious bronchiolitis, aspiration and cystic fibrosis. Terminal bronchioles are normally not perceived on CT scan because of their very thin walls and caliber (less than 1 mm). When peripheral areas of lung are opacified or plugged by exudates, they cause the appearance of a V or Y shaped branching tree pattern accompanied by bud-like nodules. Normally this sign spares the subpleural and peri-fissure areas (Fig. 5b).
Fig. 5.
CT scans showing different radiological signs: (a) ground glass opacities, (b)Tree and bud sign (c) Halo sign, (d) reverse Halo sign, (e) air crescent sign, (f) crazy pavement sign,.
5.6. Halo sign
Halo sign is a well-appreciated CT scan sign in a clinical setting of fever with neutropenia and is always suggestive of invasive aspergillosis (Fig. 5c) [12]. The focal infarction with surrounding hemorrhage is perceived as a Halo. Halo sign is a good prognostic predictor for response to therapy. Reverse halo sign is seen seldom in aspergillus or mucormycosis infection where the lesion shows consolidation around a central area of ground glass like changes with interception lines (Fig. 5d).
5.7. Air crescent and monad sign
Air crescent and monad sign are other signs suggestive of fungal infections (Fig. 5e). Separation of the necrotic infective mass by a crescent air space in response to therapy is known as Air crescent sign. It implicates good response to treatment. This is to be differentiated from an air crescent space resulting from secondary fungal infection with mycetoma (fungal ball) in a preexisting cavity, known as Monad sign. This is a bad sign and usually causes hemoptysis and needs surgical or interventional management.
5.8. Crazy paving sign
Crazy paving sign is due to alveolar opacity resulting from exudates accompanied by septal thickening leading to a characteristic picture of pedestrian path pattern, normally seen in alveolar proteinosis (Fig. 5f). PCP and viral infections may seldom manifest this sign.
5.9. Miliary pattern
Miliary pattern consists of widespread distribution of tiny nodules of less than 3 mm in size. Random type of military pattern is centrilobular and is characteristic of hematogenous spread of either mycobacterium infection or lung metastases. On the other hand, non-random type is peri-lymphatic in distribution and is a common feature of sarcoidosis [13], [14].
6. Practical approach
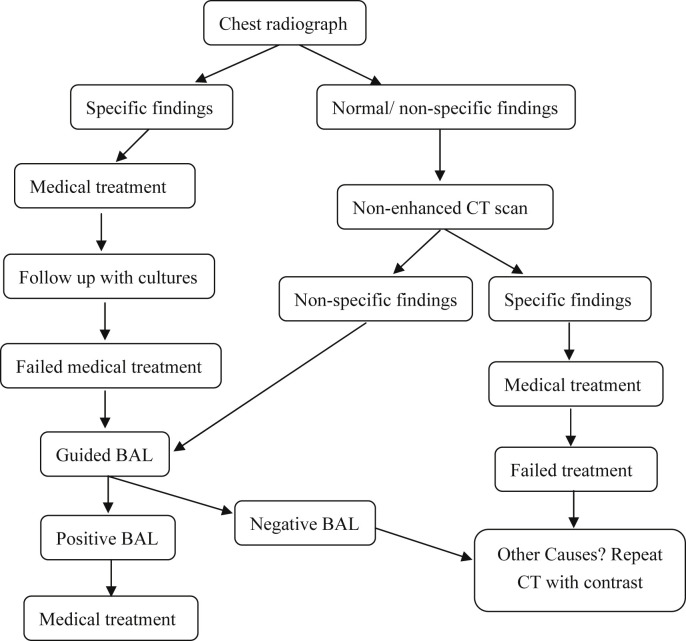
As mentioned above, it is not always easy to pin point a specific pathogen for the cause of the pneumonia on the basis of imaging findings alone. Furthermore, old pulmonary lung changes, cardiac insufficiency, atelectasis and pleural effusions can mask the new changes resulting from the current infection. Hence, we recommend an algorithm (Fig. 6 ) to be followed, for proper evaluation of the various lung changes in order to have a conclusive diagnosis and follow up.
Fig. 6.
Algorithm for patient suspected of having respiratory infections.
Due to regular follow up regime of our ICPs (many western countries), pneumonia with frank lobar consolidations are hardly observed these days. Patchy, segmental or non-segmental consolidations are the usual radiological presentation of typical bacterial pneumonia in case of ICH with acute onset of fever. However, in hospitalized patients with similar radiological features without any relevant clinical and laboratory findings consistent with lung infection, a possible diagnosis of atelectasis, old changes and organizing pneumonias following a course of antibiotics should be considered. Other accompanying features like loss of lung volume and vascular crowding may assist in the interpretation. Basal infiltrates and atelectasis are difficult to differentiate from each other. In addition, a likely pneumonic infiltrate overlying a basal lung collapse should always be considered, if clinically suspected.
In our experience, tree-in-bud phenomenon is often seen either as a footprint of old infection which can be verified by comparing with old films or as a sign of a fresh infective bronchiolitis usually caused by bacterial or fungal infections (Fig. 7 ).
Fig. 7.
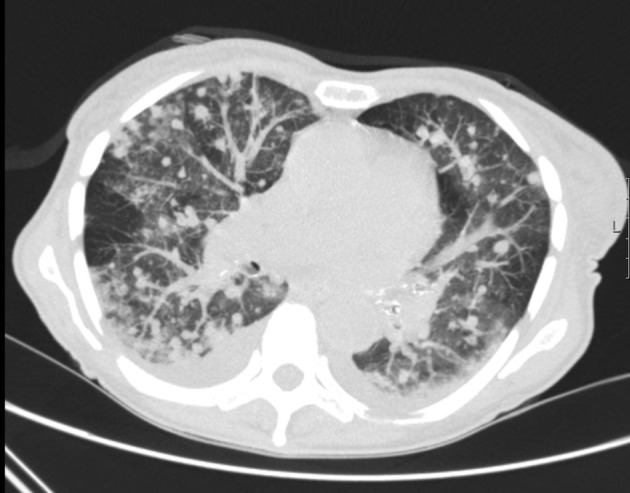
CT scan of 49 years old male with bone marrow transplantation with viral infection followed by Aspergillosis.
In our clinical setting, tuberculosis is not very common in ICH. It is mainly seen in patients with AIDS or as reactivation of an old tuberculosis infection in the migrant subgroups from the endemic areas. In AIDS patients, tuberculosis may cause minimal lung changes (with or without effusion), and necrotic mediastinal lymphadenopathy is often seen. Cavitating and military lung lesions are observed in the advanced disease (Fig. 4).
Nodular opacities (with or without cavitation) are commonly a manifestation of septic emboli from infected catheter, endocarditis, etc. and patients should be screened for such sources of infection (Fig. 2). Rarely, Wegener's granulomatosis can present in a similar fashion. In cases with nodular lung changes with mediastinal lymphadenopathy, one should always consider sarcoidosis as an alternative coincidental diagnosis, particularly in a younger age group. Nodular opacities may be seen in fungal infections, occurring secondary to virus infections.
Typical pneumonia, due to bacterial infections, is common in early stages of immunosuppression and is easy to diagnose based on clinical and laboratory parameters and their response to therapy. Atypical pneumonia is not uncommon as well and can lead to serious complications with high morbidity and mortality, if not timely diagnosed and treated.
Generally, viral pneumonia manifests as ground glass opacities in early stages, mainly in upper and middle lung fields and a tendency of consolidation in later stages (Fig. 3, Fig. 7). Reactive small mediastinal lymph nodes can be seen on CT scan. Influenza virus shows midfield changes more than upper lobe changes as well as early consolidations. Pneumatoceles and pneumothorax are not observed in viral infections, although these are frequent features of PCP (Fig. 8 ). A complicated viral infection may rapidly proceed to acute respiratory distress syndrome (ARDS). PJP seems more frequent compared to Legionella, mycoplasma and chlamydial lung infections in our ICHs.
Fig. 8.
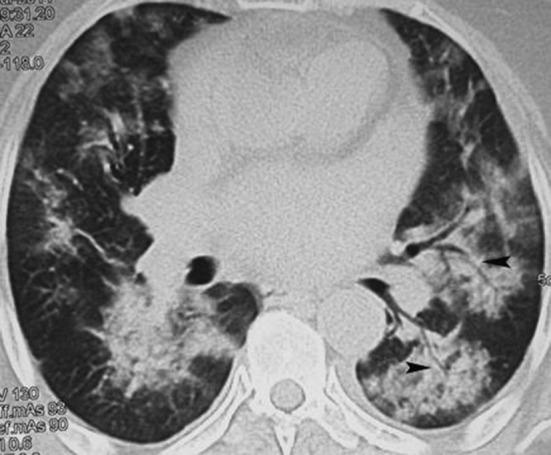
55 years old male with AML with fever, CT showing PCP.
Fungal infections comprise the third major group of pneumonias. IPA (with the characteristic Halo sign) is likely to be more frequent than Candida infections. Reverse Halo sign is not a commonly seen entity in our practice.
7. Infection mimics
It is very important and imperative that radiologists be aware of lung changes, which may mimic lung infection. These are caused by various conditions like cardiogenic conditions, cryptogenic organizing pneumonia (COP) (Fig. 9 ), progressive cancer disease with lymphangitis and chemotherapy associated lung changes. Hence recognizing the relevant findings, correlating with the clinical findings and course of management can provide a complete differential diagnosis and thus play an important role in patient care.
Fig. 9.
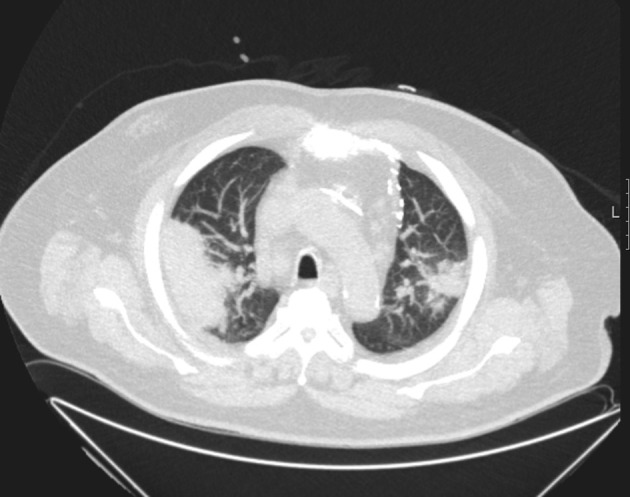
78 years old male with myelodysplastic syndrome, CT showing crytogenic organizing pneumonia.
GGO due to fluid retention in bedridden ICH is common. CHF in old patients may present with typical findings of central and lower lobe dominant GGO accompanying with small effusions (Fig. 10 ). Atelectasis is inadvertently found in such conditions. Progressive dyspnea without fever may be a feature of lymphangitis and normally shows CT findings of perihilar and segmental reticular or reticulo-nodular opacities. COP is not very uncommon, and should be considered when consolidation persists or increases during the course of the treatment whereby the laboratory findings are not suggestive of active infection [11]. In case of viral infections, increasing nodular opacities or consolidation, and superinfection (such as accompanied with bacterial, pneumocystis or fungal infections) should be considered.
Fig. 10.
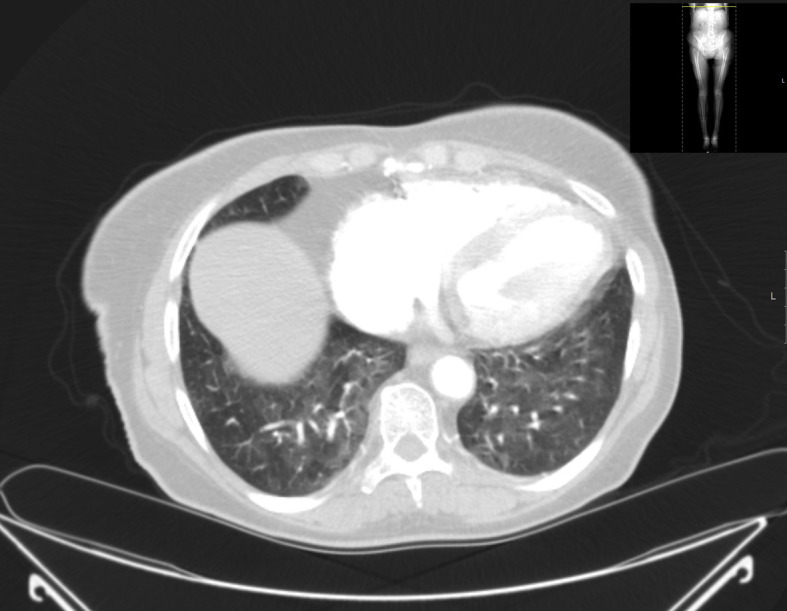
Cancer patient, with dyspnea and without fever showing GGO due to mild CHF.
The effect of drug toxicities on the lung can manifest as infiltrates (interstitial, alveolar or both), pulmonary edema and hypersensitivity pattern and changes secondary to cardiotoxic CHF. They may be asymptomatic coincidental findings or may present with progressive dyspnea. Some of the newer drugs may also be accompanied with neutropenia and fever [20]. Chemotherapy induced patchy pneumonitis is not uncommon and should be considered in differential diagnosis when approaching lung parenchymal changes [21]. These are anticipated changes in later stages of chemotherapy regime. However, Paclitaxel and anti EGFR agents may show similar changes in the earlier cycles of chemotherapy. Many cytotoxic agents and the new agents may show GGO and consolidation (mTOR antagonist like Everolimus, Temsirolimus, Bleomycin) or a COP like image. Some drugs (Gemcitabine) may cause capillary leakage syndrome and few agents may cause interstitial changes due to heart failure (Doxorubicin).
8. Conclusions
CT chest is, in particular, a very sensitive radiological tool for detection of the early signs of respiratory infections in sick ICPs. The specificity of the interpretation can be improved significantly through a comprehensive approach taking into consideration of the clinical setting, past infections and treatment history. Used appropriately, CT scan is a very valuable tool for early detection and differential diagnosis of lung infections in ICPs and therefore for the subsequent reduction of morbidity and mortality highly vulnerable patients.
Financial disclosures
None.
Footnotes
Peer review under responsibility of Beijing You'an Hospital affiliated to Capital Medical University.
References
- 1.Rubin R.H., Ferraro M.J. Understanding and diagnosing infectious complications in the immunocompromised host. Hematol Oncol Clin North Am. 1993;7:795–812. [PubMed] [Google Scholar]
- 2.Chanock S. Evolving risk factors for infectious complications of cancer therapy. Hematol Oncol Clin North Am. 1993;7:771–793. [PubMed] [Google Scholar]
- 3.Ketai L., Jordan K., Marom E.M. Imaging infection. Clin Chest Med. 2008;29:77–105. doi: 10.1016/j.ccm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Heussel C.P., Kauczor H.U., Heussel G., Fischer B., Mildenberger P., Thelen M. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. AJR Am J Roentgenol. 1997;169:1347–1353. doi: 10.2214/ajr.169.5.9353456. [DOI] [PubMed] [Google Scholar]
- 5.Worthy S., Kang E.Y., Muller N.L. Acute lung disease in the immunocompromised host: differential diagnosis at high-resolution CT. Semin Ultrasound CT MR. 1995;16:353–360. doi: 10.1016/0887-2171(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers J.D., Singanayagam A., Akram A.R., Mandal P., Short P.M., Choudhury G. Severity assessment tools for predicting mortality in HAP. Thorax. 2010;65(10):878–883. doi: 10.1136/thx.2009.133280. [DOI] [PubMed] [Google Scholar]
- 7.Demirkazik F.B., Akin A., Uzun O., Akpinar M.G., Ariyuerek M.O. CT findings in immnunocompromised patients with pulmonary infections. Diagn Interv Radiol. 2008;14:75–82. [PubMed] [Google Scholar]
- 8.Oh Y.W., Effmann E.L., Godwin J.D. Pulmonary infections in immnunocompromised hosts: the importance of correlating the conventional radiological appearance with clinical setting. Radiology. 2000;217:647–657. doi: 10.1148/radiology.217.3.r00dc35647. [DOI] [PubMed] [Google Scholar]
- 9.Walker C.M., Abbott G.F., Greene R.E., Shepard J.O., Vummuidi D., Digumarthy S.R. Imaging pulmonary infection: classic signs & patterns. AJR. 2014;202:479–492. doi: 10.2214/AJR.13.11463. [DOI] [PubMed] [Google Scholar]
- 10.Kuhlman J.E., Fishman E.K., Teigen C. Pulmonary septic emboli: diagnosis with CT. Radiology. 1990;174:211–213. doi: 10.1148/radiology.174.1.2294550. [DOI] [PubMed] [Google Scholar]
- 11.Kim S.J., Lee K.S., Ryu Y.H., Yoon Y.C., Choe K.O., Kim T.S. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR. 2003;180:1251–1254. doi: 10.2214/ajr.180.5.1801251. [DOI] [PubMed] [Google Scholar]
- 12.Kuhlman J.E., Fishman E.K., Siegelman S.S. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985;157:611–614. doi: 10.1148/radiology.157.3.3864189. [DOI] [PubMed] [Google Scholar]
- 13.Sider L., Gabriel H., Curry D.R., Pham M.S. Pattern recognition of the pulmonary manifestations of AIDS on CT scans. Radio Graph. 1993;13:771–784. doi: 10.1148/radiographics.13.4.8356267. [DOI] [PubMed] [Google Scholar]
- 14.Burrill J., Williams C.J., Bain G., Conder G., Hine A.L., Misra R.R. Tuberculosis: a radiologic review. Radio Graph. 2007;27:1255–1273. doi: 10.1148/rg.275065176. [DOI] [PubMed] [Google Scholar]
- 15.Masur H., Ognibene F.P., Yarchoan R., Shelhamer J.H., Baird B.F., Travis W. CD4 counts as predictors of opportunistic pneumonias in HIV infection. Ann Intern Med. 1989;111:223–231. doi: 10.7326/0003-4819-111-3-223. [DOI] [PubMed] [Google Scholar]
- 16.Kanne J.P., Yandow D.R., Meyer C.A. Pneumocystis jiroveci pneumonia: high-resolution CT findings in patients with and without HIV infection. AJR. 2012;198:W555–W561. doi: 10.2214/AJR.11.7329. [web] [DOI] [PubMed] [Google Scholar]
- 17.Franquet T., Müller N.L., Giménez A., Guembe P., de La Torre J., Bagué S. Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radio Graph. 2001;21:825–837. doi: 10.1148/radiographics.21.4.g01jl03825. [DOI] [PubMed] [Google Scholar]
- 18.McAdams H.P., Rosado-de-Christenson M.L., Templeton P.A., Lesar M., Moran C.A. Thoracic mycoses from opportunistic fungi: radiologic-pathologic correlation. Radio Graph. 1995;15:271–286. doi: 10.1148/radiographics.15.2.7761633. [DOI] [PubMed] [Google Scholar]
- 19.Chong S., Lee K.S., Yi C.A., Chung M.J., Kim T.S., Han J. Pulmonary fungal infection: imaging findings in immunocompetent and immnunocompromised patients. Eur J Radiol. 2006;59:371–383. doi: 10.1016/j.ejrad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Rosewarne D., Reynolds J., Trotter S.E., Burge P.S. Viral pneumonias in adults: radiologic and pathologic findings. Radio Graph. 2002;22:S137–S149. doi: 10.1148/radiographics.22.suppl_1.g02oc15s137. [DOI] [PubMed] [Google Scholar]
- 21.Torrisi J.M., Schwartz L.H., Gollub M.J., Ginsberg M.S., Bosl G.J., Hricak H. CT findings of chemotherapy -induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology. 2011;258(1):41–56. doi: 10.1148/radiol.10092129. [DOI] [PubMed] [Google Scholar]