Abstract
Background
Because of the rapid change in economic development and lifestyle in China, and the ageing population, concerns have grown that chronic obstructive pulmonary disease (COPD) could become epidemic. An up-to-date nationwide estimation of COPD prevalence in China is needed.
Methods
We did a cross-sectional survey of a nationally representative sample of individuals from mainland China aged 40 years or older. The primary outcome was COPD, defined according to the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) lung function criteria.
Findings
Between Dec 29, 2014, and Dec 31, 2015, 66 752 adults were recruited to the study population. The estimated standardised prevalence of COPD was 13·6% (95% CI 12·0–15·2). The prevalence of COPD differed significantly between men and women (19·0%, 95% CI 16·9–21·2 vs 8·1%, 6·8–9·3; p<0·0001), mainly because of a significant difference in smoking status between men and women (current smokers 58·2% vs 4·0%). The prevalence of COPD differed by geographic region, with the highest prevalence in southwest China (20·2%, 95% CI 14·7–25·8) and the lowest in central China (10·2%, 8·2–12·2). Among adults with COPD, 56·4% (95% CI 53·7–59·2) had mild disease (GOLD stage I), 36·3% (34·3–38·3) had moderate disease (GOLD stage II), 6·5% (5·5–7·4) had severe disease (GOLD stage III), and 0·9% (0·6–1·1) had very severe disease (GOLD stage IV).
Interpretation
In a large, nationally representative sample of adults aged 40 years or older, the estimated overall prevalence of COPD in China in 2014–15 was 13·6%, indicating that this disease has become a major public-health problem. Strategies aimed at prevention and treatment of COPD are needed urgently.
Funding
Chinese Central Government, the Ministry of Science and Technology of The People's Republic of China, and the National Natural Science Foundation of China.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide, inducing substantial economic, social, and health-care burden.1 Nearly three million people died of the disease in 2016, which corresponds to around 5·4% of all deaths globally.2 Although the burden of COPD is large in high-income countries,3 around 90% of deaths from this disease still occur in low-income and middle-income countries.2, 4 China—the largest developing country—has undergone rapid economic growth and the population has seen an increase in life expectancy and substantial changes in lifestyle. In 2016, COPD was the fifth leading cause of death in China.5 The total number of deaths from the disease is projected to keep increasing unless urgent action is taken to reduce underlying risk factors, particularly tobacco use.6
In previous studies of COPD in China,2, 7, 8, 9, 10, 11, 12 prevalence has ranged from 2% to 21%, but these studies have included small sample sizes, incorporated no post-bronchodilator test, used outdated data, or been restricted to local occupational or urban cohorts. Only one nationwide study has included a post-bronchodilator spirometry examination, but it was undertaken in only seven provinces and cities in China between 2002 and 2004.7 In that study, the overall prevalence of COPD in China was 8·2%.
As a common and preventable disease, reliable estimation of the prevalence of COPD is needed to plan effective national prevention strategy and treatment programmes for COPD management. We aimed to provide an up-to-date estimate of the prevalence of COPD in China using data from a survey done in 2014–15 of a nationally representative sample of the Chinese population.
Methods
Study design and participants
We did a nationwide cross-sectional study, using the integrated national disease surveillance point (DSP) system from the Chinese Center for Disease Control and Prevention (China CDC; total 605 DSPs covering 24% of the Chinese population) to obtain a nationally representative sample of the general population in China.13, 14 We used a complex, multistage, probability sampling strategy, which is described in the appendix (p 2). The overall response rate, using the standard definition by the American Association for Public Opinion Research, was 96·3% (appendix pp 13, 14).15 After three attempts on three different days, we could not access and obtain data for 3·8% of sampled families. We replaced these families with another household of similar structure in the same village or residential area (appendix p 15).
Research in context.
Evidence before this study
We did a systematic review of PubMed, EBSCO, SinoMed (China BioMedical Literature Service System), CNKI (China National Knowledge Infrastructure), and Wanfang Database up to February, 2018, with search terms related to COPD, prevalence, and China (appendix p 6). We restricted our search to the English and Chinese languages. Our search identified 45 relevant studies. Previous studies of the prevalence of chronic obstructive pulmonary disease (COPD) in China (range 2–21%) had small sample sizes, did not include a post-bronchodilator test, used outdated data, or were restricted to local, occupational, or urban cohorts. Only one published study in which a post-bronchodilator spirometry examination was done was nationwide, but it was undertaken only in seven provinces or cities of China from 2002 to 2004, and the overall prevalence of COPD was estimated at 8·2%.
Added value of this study
We did a large nationally representative survey of 66 752 Chinese adults in 2014–15. Our study provides up-to-date estimation of the prevalence of COPD in China. We estimated that 13·6% of Chinese adults aged 40 years or older had COPD. A significant difference in the prevalence of COPD was noted between men and women. To our knowledge, our study provides the first direct comparison of COPD prevalence among seven major geographic regions in China within one survey. The prevalence of COPD varied substantially between geographic areas in China—it was highest in southwest China and lowest in central China. According to Global Initiative for Chronic Obstructive Lung Disease (GOLD) lung function criteria, 56·4% of individuals with COPD had mild disease (GOLD stage I), 36·3% had moderate disease (GOLD stage II), 6·5% had severe disease (GOLD stage III), and 0·9% had very severe disease (GOLD stage IV). This pattern changed strikingly from the previous nationwide study in China, in which only 24% of patients had mild disease, because we identified more patients diagnosed with mild COPD.
Implications of all the available evidence
COPD has become a major public-health problem in China, and strategies aimed at disease prevention and treatment are needed urgently. The high prevalence of smoking in China suggests that screening for COPD, at least in high-risk populations, could be essential in primary-care units. Avoidance of direct and indirect exposure to tobacco smoke is of primary importance, not only for prevention of COPD but also for reducing the risk of cardiovascular disease, cancer, and diabetes. Intervention strategies could have a pronounced effect on the future trajectory of COPD in China.
We included Chinese citizens (aged 40 years or older) who had been living in their current residence for at least 6 months within the year before the survey, to exclude new immigrants. We excluded people who lived in a communal residence (eg, a university dormitory, military unit, or nursing home), individuals with cognitive, language, or mental disorders who could not participate in the interview, people with cancer (newly diagnosed or under treatment), individuals with paraplegia, women who were pregnant or breastfeeding, and individuals who did not provide written informed consent.
The study protocol was approved by the ethics review committee of the National Center for Chronic and Non-Communicable Disease Control and Prevention, China CDC. All participants provided written informed consent.
Procedures
All people who fulfilled the inclusion criteria were invited to an interview. Trained staff from local health stations or community clinics administered a comprehensive questionnaire to obtain information on demographic characteristics, medical history, COPD-specific risk factors, and respiratory symptoms. COPD-specific risk factors used in our analyses were indoor exposure to biomass, indoor exposure to coal, and exposure to dust or chemicals in the workplace. We defined indoor exposure to biomass as household use of biomass fuels (including wood, grass, crop residues, and animal dung) for cooking or heating. We defined indoor exposure to coal as household use of coal fuels (including coal, lignite, and kerosene). Measurement of indoor air pollution is complex because of its temporal and spatial distribution within the household and characteristics of the residence's ventilation. Therefore, we used qualitative levels (yes or no) for indoor exposure to biomass or coal in our analyses. We defined exposure to dust or chemicals in the workplace as cumulative exposure to airborne dust or hazardous chemical gases in the workplace (including agricultural operations) for at least 12 months. Airborne dust refers to dirt, sand, powder, mineral dust, flue dust, and smog in the work environment; hazardous chemical gases refer to gases and vapours that are harmful to human beings, including gasoline, pesticides, lampblack, ammonia, sulphur dioxide, carbon monoxide, mercury, benzene, and hydrogen sulphide. We also used qualitative levels (yes or no) for exposure to dust or chemicals in the workplace in our analyses.
Trained staff did spirometry on all participants who were eligible for the procedure, using the same brand of spirometer (MasterScreen Pneumo, Jaeger, Germany). Spirometry was performed following recommendations by the American Thoracic Society.1, 16 Full details of the method used and eligibility criteria for spirometry are provided in the appendix (p 3). After initial prebronchodilator spirometry, participants who were allergic to salbutamol or had a resting heart rate of 100 bpm or higher were excluded from post-bronchodilator testing. We administered 400 μg salbutamol (Ventolin; GlaxoSmithKline, Middlesex, UK) to all eligible participants, then post-bronchodilator spirometry was done after 15 min. We measured both prebronchodilator and post-bronchodilator forced vital capacity (FVC), FEV1, forced expiratory volume in 6 s (FEV6), and peak expiratory flow. We did further testing of individuals with a post-bronchodilator FEV1:FVC less than 70%. These tests consisted of the modified Medical Research Council (mMRC) dyspnoea score and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 ABCD assessment tool,1 and we also offered additional chest radiography.
All interviewers and spirometry operators were from local health stations or community clinics in the participants' residential area; we trained all staff before the study, according to a standard protocol. For spirometry results, we used a quality grade (A, B, C, D, F) based on acceptable manoeuvres and repeatability of FEV1 and FVC, as applied elsewhere7 and described in the appendix (p 3). We judged grades A, B, and C acceptable for analysis. Quality assessment for every participant was done within 24 h of the test, and we offered to repeat examinations with grades D and F. In our survey, 96·5% and 96·8% of prebronchodilator and post-bronchodilator examinations were grade C or higher, respectively (79·6% and 82·5% were grade A, and 94·8% and 95·5% were grade A or B).
Outcomes
The primary outcome of our study was prevalence of COPD. We used GOLD lung function criteria to define individuals with COPD—ie, those with a post-bronchodilator FEV1:FVC less than 70%.1 Although GOLD 2017 criteria state that COPD should be considered in people with appropriate symptoms and substantial exposure to noxious stimuli, we used the simple criterion for COPD definition, for comparison with other studies.7, 17, 18 However, we also calculated the proportion of individuals with respiratory symptoms. We categorised COPD in accordance with GOLD staging criteria: GOLD stage I (mild disease, corresponding to FEV1 ≥80% predicted), GOLD stage II (moderate disease, FEV1 ≥50% to <80% predicted), GOLD stage III (severe disease, FEV1 ≥30% to <50% predicted), and GOLD stage IV (very severe disease, FEV1 <30% predicted). We obtained predicted values of FEV1 for normal lung function and the lower limited normal (LLN) of FEV1 from a nationwide study of reference values for spirometry in the Chinese population.19 Other outcomes of our study were the awareness rate (defined as the proportion of individuals who knew their diagnosis of COPD among all patients with COPD), suspected COPD by spirometry (defined as the proportion of individuals who had undergone spirometry before the survey among all patients with COPD), and treatment rate (defined as the proportion of individuals taking treatments for COPD among all patients with COPD).
Statistical analysis
We estimated standardised prevalence (with 95% CIs) in the overall population, in seven major regions of China (northwest, north, northeast, central, southwest, south, and east), in subgroups of sex, age, location (urban or rural), stages of economic development (underdeveloped, intermediately developed, or developed), and smoking status (current, former, and never) and exposure (pack-years), and by COPD-specific risk factors. We selected subgroup variables before analyses were done. We calculated standardised prevalence using weighted coefficients to represent the overall Chinese adult population aged 40 years or older. Weighted coefficients accommodated the sampling scheme for unequal probabilities of sample selection and the post-stratification weights, which harmonised the sample structure of the survey with that of the 2010 census of the Chinese population. We categorised every surveillance point into under-developed, intermediately developed, or developed according to the region's gross domestic product per person in 2015. For analyses of COPD severity, we present unweighted estimations because only patients with COPD were included. We used the χ2 test to compare prevalence. We calculated p values for trend using the Cochran-Armitage trend test for proportions. We used multivariable logistic regression to assess the association of risk factors with the odds of COPD. We applied four models with progressively increased adjustment of risk factors to assess region-specific odds ratios (ORs) and 95% CIs. We calculated projections using the overall Chinese population aged 40 years and older in 2010 multiplied by the estimated prevalence based on the sampling weight. For sex-specific projections we used sex-specific estimations of the prevalence. All p values were two-tailed and were not adjusted for multiple testing. We judged p values less than 0·05 significant. However, Benjamin and colleagues20 suggested that statistical significance should be redefined (eg, use the threshold of 0·005 for claims of new discoveries), particularly when the sample size is large. We, therefore, provided exact p values when 0·0001 or larger in all conditions, to help interpretation. We did all statistical analysis with SAS version 9.4 and Stata version 14.1. This Article follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cross-sectional studies (appendix pp 4, 5).21
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Between Dec 29, 2014, and Dec 31, 2015, 77 974 people were contacted to take part in the survey, of whom 75 107 (96·3%) were interviewed. 2282 individuals were not eligible for spirometry (appendix p 11). Prebronchodilator and post-bronchodilator examinations were completed by 68 984 people. In total, 66 752 participants took part in the interview and had acceptable post-bronchodilator spirometry examinations (grade A, B, or C) and were included in analyses. The general characteristics of participants included and excluded in the analyses are presented in the appendix (p 16). Sex and smoking status were broadly similar between groups; however, compared with those excluded from analyses, people included were younger, were more likely to live in urban areas, had a higher educational level, and were less likely to have a history of tuberculosis.
Table 1 presents characteristics of the 66 752 individuals included in analyses. Mean age was 54·9 years (SD 11·1), body-mass index (BMI) was 24·7 kg/m2 (SD 3·5), and 33 615 (50%) participants were women. With respect to smoking status, 20 059 (31·4% [95% CI 30·0–32·7]) were current smokers, of whom 18 793 (58·2% [56·1–60·3]) were men and 1266 (4·0% [2·7–5·3]) were women (p<0·0001). Overall, 1656 (2·5% [95% CI 2·3–2·8]) of the population had been admitted to hospital for severe pulmonary disease in childhood, 28 914 (40·7% [34·0–47·4]) had indoor exposure to biomass fuel, 22 881 (34·3% [27·1–41·6]) had indoor exposure to coal, 29 808 (46·6% [40·0–49·1]) had exposure to dust or chemicals in the workplace, and 1247 (1·6% [1·4–1·9]) had a history of tuberculosis.
Table 1.
Demographic characteristics and exposures of study participants from the general Chinese population aged 40 years or older
| Total (n=66 752) | Men (n=33 137) | Women (n=33 615) | ||
|---|---|---|---|---|
| Age (years) | 54·9 (11·1) | 54·7 (10·9) | 55·0 (11·3) | |
| Body-mass index (kg/m2) | 24·7 (3·5) | 24·6 (3·5) | 24·8 (3·6) | |
| Residence | ||||
| Urban | 32 009/66 752 (48·2% [42·3–54·2]) | 14 624/33 137 (48·4% [42·4–54·4]) | 17 385/33 615 (48·0% [42·0–54·1]) | |
| Rural | 34 743/66 752 (51·8% [45·8–57·7]) | 18 513/33 137 (51·6% [45·6–57·6]) | 16 230/33 615 (52·0% [45·9–58·0]) | |
| Economic development | ||||
| Underdeveloped | 23 637/66 752 (34·4% [25·0–43·8]) | 12 106/33 137 (34·5% [25·3–43·7]) | 11 531/33 615 (34·3% [24·6–44·1]) | |
| Intermediately developed | 21 020/66 752 (24·2% [16·1–32·4]) | 10 137/33 137 (24·1% [15·9–32·3]) | 10 883/33 615 (24·4% [16·1–32·7]) | |
| Developed | 22 095/66 752 (41·4% [33·2–49·5]) | 10 894/33 137 (41·4% [33·5–49·3]) | 11 201/33 615 (41·3% [32·6–50·0]) | |
| Educational level | ||||
| Primary school and lower | 33 693/66 733 (47·1% [42·3–52·0]) | 14 454/33 130 (39·1% [34·8–43·4]) | 19 239/33 603 (55·3% [49·6–61·0]) | |
| Secondary school | 30 213/66 733 (48·4% [44·6–52·2]) | 17 068/33 130 (55·5% [52·1–58·8]) | 13 145/33 603 (41·2% [36·5–45·9]) | |
| Higher and further education | 2827/66 733 (4·5% [3·1–5·9]) | 1608/33 130 (5·4% [3·9–6·9]) | 1219/33 603 (3·5% [2·1–4·9]) | |
| Smoking status | ||||
| Never smoker | 40 070/66 567 (59·8% [58·3–61·4]) | 8296/33 077 (25·6% [23·3–27·8]) | 31 774/33 490 (94·8% [93·3–96·4]) | |
| Former smoker | 6438/66 567 (8·8% [8·2–9·4]) | 5988/33 077 (16·2% [15·2–17·3]) | 450/33 490 (1·2% [0·8–1·5]) | |
| Current smoker | 20 059/66 567 (31·4% [30·0–32·7]) | 18 793/33 077 (58·2% [56·1–60·3]) | 1266/33 490 (4·0% [2·7–5·3]) | |
| Smoking exposure* (pack-years) | ||||
| None to less than ten | 5801/24 775 (23·4% [22·1–24·7]) | 5029/23 201 (21·7% [20·4–23·0]) | 772/1574 (49·0% [45·1–53·0]) | |
| Ten to less than 25 | 6593/24 775 (26·6% [25·5–27·7]) | 6162/23 201 (26·5% [25·5–27·6]) | 431/1574 (27·4% [23·9–30·9]) | |
| 25 to less than 50 | 8610/24 775 (34·8% [33·7–35·8]) | 8321/23 201 (35·9% [34·8–36·9]) | 289/1574 (18·4% [15·6–21·1]) | |
| 50 or more | 3771/24 775 (15·2% [14·0–16·4]) | 3689/23 201 (15·9% [14·7–17·1]) | 82/1574 (5·2% [3·9–6·5]) | |
| Risk factors for COPD | ||||
| Hospital admission due to severe pulmonary disease in childhood | 1656/66 730 (2·5% [2·3–2·8]) | 819/33 129 (2·7% [2·3–3·1]) | 837/33 601 (2·4% [2·0–2·7]) | |
| Indoor exposure to biomass for cooking or heating | 28 914/66 686 (40·7% [34·0–47·4]) | 14 922/33 106 (40·7% [34·2–47·2]) | 13 992/33 580 (40·6% [33·5–47·8]) | |
| Indoor exposure to coal for cooking or heating | 22 881/66 676 (34·3% [27·1–41·6]) | 11 665/33 102 (34·5% [27·5–41·5]) | 11 216/33 574 (34·1% [26·4–41·8]) | |
| Exposure to dust or chemicals in the workplace | 29 808/66 722 (46·6% [40·0–49·1]) | 16 561/33 121 (49·7% [45·4–54·0]) | 13 247/33 601 (39·4% [34·3–44·4]) | |
| History of tuberculosis | 1247/66 730 (1·6% [1·4–1·9]) | 779/33 129 (2·1% [1·8–2·4]) | 468/33 601 (1·2% [1·0–1·4]) | |
| Spirometry | ||||
| Pre-bronchodilator FEV1 | 2·63 (0·70) | 2·99 (0·69) | 2·26 (0·49) | |
| Post-bronchodilator FEV1 | 2·68 (0·70) | 3·05 (0·69) | 2·31 (0·49) | |
| Pre-bronchodilator FVC | 3·45 (0·88) | 3·99 (0·78) | 2·89 (0·59) | |
| Post-bronchodilator FVC | 3·45 (0·87) | 3·99 (0·77) | 2·89 (0·58) | |
| Pre-bronchodilator FEV1:FVC | 76% (9) | 75% (9) | 78% (8) | |
| Post-bronchodilator FEV1:FVC | 78% (9) | 76% (9) | 80% (7) | |
Data are number exposed/total participants (% [95% CI]) or mean (SD). Weighted estimations were used. COPD=chronic obstructive pulmonary disease. FVC=forced vital capacity.
Only assessed in participants who were former or current smokers; data missing for 1722 participants.
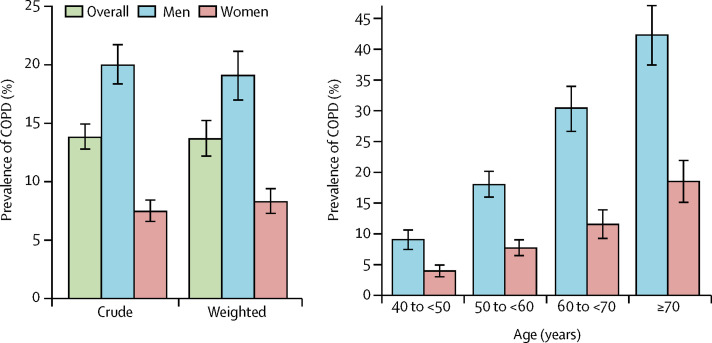
9134 participants in the survey had an FEV1:FVC ratio less than 70% and were diagnosed with COPD. The overall standardised prevalence of COPD in Chinese adults aged 40 years or older was, therefore, estimated as 13·6% (95% CI 12·0–15·2), which accorded with crude estimates (figure , appendix p 17). The age-specific prevalence of COPD rose significantly with increasing age (p<0·0001 for both men and women). The prevalence of COPD differed significantly between men (19·0% [95% CI 16·9–21·2]) and women (8·1% [6·8–9·3]; difference in prevalence 10·9%, 95% CI 9·5–12·4; p<0·0001). The sex difference is most probably attributable to the notable difference in smoking status between men and women (table 1). For current and former smokers, the prevalence of COPD was broadly similar between men and women when assessed by pack-years, and it increased significantly with increments of pack-year (p<0·0001; table 2 ). Overall, the prevalence of COPD was 8·7% (95% CI 7·3–10·1) in never smokers, 22·6% (19·6–25·5) in former smokers, and 20·4% (18·1–22·6) in current smokers.
Figure.
Prevalence of COPD in the general Chinese population aged 40 years or older
(A) Crude and weighted prevalence of COPD, overall and in men and women. (B) Age-specific prevalence of COPD in men and women. Bars represent the prevalance and error bars the 95% CI. Data are presented in the appendix (p 17). COPD=chronic obstructive pulmonary disease.
Table 2.
Prevalence of COPD in the general Chinese population aged 40 years or older, by demographic, social, and economic characteristics
|
Overall |
Men |
Women |
||||
|---|---|---|---|---|---|---|
| Cases/total (n/N) | Prevalence of COPD (95% CI) | Cases/total (n/N) | Prevalence of COPD (95% CI) | Cases/total (n/N) | Prevalence of COPD (95% CI) | |
| Residence | ||||||
| Urban | 3877/32 009 | 12·2% (11·0–13·4) | 2666/14 624 | 16·7% (15·0–18·3) | 1211/17 385 | 7·6% (6·4–8·8) |
| Rural | 5257/34 743 | 14·9% (12·7–17·2) | 3969/18 513 | 21·2% (18·2–24·3) | 1288/16 230 | 8·6% (6·9–10·2) |
| p value | .. | 0·0007 | .. | 0·0001 | .. | 0·15 |
| Economic development | ||||||
| Under-developed | 3167/23 637 | 14·2% (11·0–17·4) | 2398/12 106 | 20·6% (16·2–24·9) | 769/11 531 | 7·7% (5·5–9·9) |
| Intermediately developed | 2640/21 020 | 12·5% (10·6–14·5) | 1868/10 137 | 17·5% (14·4–20·5) | 772/10 883 | 7·6% (6·1–9·0) |
| Developed | 3327/22 095 | 13·8% (11·2–16·4) | 2369/10 894 | 18·7% (15·5–21·9) | 958/11 201 | 8·7% (6·4–11·0) |
| p value for trend | .. | 0·86 | .. | 0·51 | .. | 0·50 |
| Educational level* | ||||||
| Primary and lower | 5217/33 693 | 16·0% (14·1–18·0) | 3479/14 454 | 24·4% (21·4–27·4) | 1738/19 239 | 10·0% (8·5–11·5) |
| Secondary school | 3685/30 213 | 11·7% (10·1–13·3) | 2971/17 068 | 16·1% (14·2–18·0) | 714/13 145 | 5·7% (4·2–7·2) |
| Higher or further education | 229/2827 | 8·7% (6·6–10·9) | 182/1608 | 10·4% (7·4–13·4) | 47/1219 | 6·0% (3·4–8·7) |
| p value for trend | .. | <0·0001 | .. | <0·0001 | .. | 0·0002 |
| Smoking status* | ||||||
| Never smoker | 3326/40 070 | 8·7% (7·3–10·1) | 1107/8296 | 12·8% (10·5–15·1) | 2219/31 774 | 7·6% (6·3–8·8) |
| Former smoker | 1445/6438 | 22·6% (19·6–25·5) | 1380/5988 | 22·9% (19·9–26·0) | 65/450 | 17·4% (12·3–22·5) |
| Current smoker | 4346/20 059 | 20·4% (18·1–22·6) | 4140/18 793 | 20·7% (18·4–23·0) | 206/1266 | 15·8% (12·1–19·6) |
| p value | .. | <0·0001 | .. | <0·0001 | .. | <0·0001 |
| Smoking exposure†(pack-years) | ||||||
| None to less than ten | 877/5801 | 15·1% (12·1–18·1) | 802/5029 | 15·9% (12·7–19·2) | 75/772 | 9·6% (6·5–12·7) |
| Ten to less than 25 | 1208/6593 | 16·8% (14·6–18·9) | 1134/6162 | 16·8% (14·5–19·1) | 74/431 | 16·6% (12·3–20·9) |
| 25 to less than 50 | 2153/8610 | 24·6% (21·8–27·3) | 2078/8321 | 24·4% (21·7–27·2) | 75/289 | 28·8% (20·3–37·3) |
| 50 or more | 1184/3771 | 31·0% (27·6–34·3) | 1159/3689 | 31·0% (27·6–34·4) | 25/82 | 30·6% (17·1–44·2) |
| p value for trend | .. | <0·0001 | .. | <0·0001 | .. | <0·0001 |
| Hospital admission for severe pulmonary disease in childhood* | ||||||
| Yes | 350/1656 | 21·5% (18·9–24·1) | 222/819 | 25·8% (22·0–29·7) | 128/837 | 16·4% (12·5–20·2) |
| No | 8781/65 074 | 13·4% (11·8–15·0) | 6410/32 310 | 18·8% (16·7–21·0) | 2371/32 764 | 7·9% (6·6–9·1) |
| p value | .. | <0·0001 | .. | 0·0008 | .. | <0·0001 |
| Indoor exposure to biomass for cooking or heating* | ||||||
| Yes | 4602/28 914 | 16·3% (13·8–18·7) | 3399/14 922 | 22·9% (19·5–26·4) | 1203/13 992 | 9·4% (7·6–11·3) |
| No | 4519/37 772 | 11·8% (10·1–13·4) | 3225/18 184 | 16·3% (14·3–18·4) | 1294/19 588 | 7·1% (5·7–8·6) |
| p value | .. | 0·0002 | .. | <0·0001 | .. | 0·0259 |
| Indoor exposure to coal for cooking or heating* | ||||||
| Yes | 3237/22 881 | 12·8% (10·9–14·7) | 2361/11 665 | 17·8% (15·6–20·1) | 876/11 216 | 7·6% (5·9–9·3) |
| No | 5882/43 795 | 14·0% (12·1–15·9) | 4263/21 437 | 19·6% (17·0–22·3) | 1619/22 358 | 8·3% (6·9–9·7) |
| p value | .. | 0·27 | .. | 0·22 | .. | 0·84 |
| Exposure to dust or chemicals in the workplace* | ||||||
| Yes | 4550/29 808 | 15·4% (13·5–17·4) | 3517/16 561 | 20·7% (18·2–23·2) | 1033/13 247 | 8·6% (7·0–10·3) |
| No | 4576/36 914 | 12·1% (10·6–13·6) | 3110/16 560 | 17·3% (15·2–19·5) | 1466/20 354 | 7·7% (6·4–9·0) |
| p value | .. | <0·0001 | .. | 0·0003 | .. | 0·20 |
| Body-mass index (kg/m2) | ||||||
| <18·5 | 362/1515 | 22·3% (18·9–25·8) | 265/818 | 29·7% (24·3–35·2) | 97/697 | 14·5% (10·6–18·5) |
| 18·5–23·9 | 4521/28 588 | 16·0% (14·1–17·8) | 3496/15 001 | 23·2% (20·7–25·7) | 1025/13 587 | 8·2% (7·0–9·5) |
| 24·0–27·9 | 3119/25 830 | 11·7% (10·3–13·0) | 2190/12 554 | 15·9% (13·9–17·8) | 929/13 276 | 7·4% (6·3–8·5) |
| ≥28·0 | 1132/10 819 | 10·9% (8·2–13·5) | 684/4764 | 13·7% (10·9–16·6) | 448/6055 | 8·4% (5·6–11·1) |
| p value for trend | .. | 0·0004 | .. | <0·0001 | .. | <0·0001 |
COPD=chronic obstructive pulmonary disease.
Data missing for educational level (n=19), smoking status (n=185), hospital admission for severe pulmonary disease in childhood (n=22), indoor exposure to biomass for cooking or heating (n=66), indoor exposure to coal for cooking or heating (n=76), and exposure to dust or chemicals in the workplace (n=30).
Assessed in participants who were former or current smokers; 1722 current or former smokers had data missing for frequency (cigarettes per day or week) or duration of smoking.
Table 3 presents the estimated prevalence of COPD in the seven regions of China. Prevalence was highest in southwest China (20·2% [95% CI 14·7–25·8]) and lowest in central China (10·2% [8·2–12·2]). In the multivariable logistic models for COPD, compared with central China, ORs adjusted for age and sex were 2·33 (95% CI 1·55–3·50) for southwest China and 1·97 (1·30–2·99) for northeast China (appendix p 19). Adjusted ORs were similar to these after further adjustments for residential area, educational level, smoking status, hospital admission for severe pulmonary disease in childhood, indoor exposure to biomass for cooking or heating, indoor exposure to coal for cooking or heating, exposure to dust or chemicals in the workplace, family history of pulmonary disease, history of tuberculosis, economic development, season of the survey, climate, and outdoor air pollution risk factors (ie, yearly average temperature, humidity, particulate matter with a diameter less than 2·5 μm [PM2·5], and ozone levels in DSPs in 2015).
Table 3.
Prevalence of COPD by regions in China
| Cases/total (n/N) | Prevalence of COPD (95% CI) | Men (n/N) | Prevalence of COPD in men (95% CI) | Women (n/N) | Prevalence of COPD in women (95% CI) | p value | |
|---|---|---|---|---|---|---|---|
| Southwest China | 1798/10 887 | 20·2% (14·7–25·8) | 1291/5464 | 28·3% (20·7–36·0) | 507/5423 | 12·1% (8·5–15·7) | <0·0001 |
| Northeast China | 1004/6980 | 15·6% (10·4–20·7) | 602/3254 | 18·7% (12·9–24·5) | 402/3726 | 12·5% (7·5–17·6) | 0·0001 |
| North China | 1223/9103 | 13·7% (7·2–20·2) | 861/4249 | 17·8% (10·5–25·2) | 362/4854 | 9·6% (4·2–15·1) | <0·0001 |
| Northwest China | 1135/7895 | 13·6% (9·5–17·6) | 812/4078 | 18·0% (13·1–23·0) | 323/3817 | 8·4% (5·6–11·3) | <0·0001 |
| East China | 2324/17 716 | 12·1% (10·4–13·9) | 1757/8828 | 17·9% (15·1–20·7) | 567/8888 | 6·4% (5·3–7·4) | <0·0001 |
| South China | 757/6303 | 11·0% (9·8–12·2) | 595/3277 | 15·9% (13·7–18·1) | 162/3026 | 5·7% (3·4–7·9) | <0·0001 |
| Central China | 893/7868 | 10·2% (8·2–12·2) | 717/3987 | 15·7% (12·7–18·7) | 176/3881 | 4·5% (2·9–6·1) | <0·0001 |
Regions of China are shown in the appendix (p 2). COPD=chronic obstructive pulmonary disease.
Table 4 shows the severity of COPD, by GOLD stage. Among patients with COPD, 56·4% (95% CI 53·7–59·2) of patients were GOLD stage I, 36·3% (34·3–38·3) were GOLD stage II, 6·5% (5·5–7·4) were GOLD stage III, and 0·9% (0·6–1·1) were GOLD stage IV. The corresponding prevalence of COPD of GOLD stage II or higher in the whole population was 6·1% (95% CI 5·4–6·7), 8·3% (7·3–9·3) in men and 3·8% (3·2–4·3) in women (appendix p 17). The prevalence of respiratory symptoms (ie, cough, sputum, wheezing, and dyspnoea) in patients with COPD is shown in the appendix (p 20). Overall, around a third of individuals with COPD had at least one respiratory symptom (33·7% [95% CI 31·6–35·8]), with about two-thirds showing no symptoms. The frequency of respiratory symptoms increased with GOLD severity stage (p<0·0001). Comorbidities of individuals with COPD are shown in the appendix (p 21). 21·3% (95% CI 19·7–22·8) of people with COPD also had hypertension, 5·9% (5·2–6·7) had a history of exacerbation in the past year, and 3·0% (2·5–3·4) had a history of admission to hospital for COPD. Among the 9101 individuals diagnosed with COPD with complete information on mMRC score, 4·5% had an mMRC score of 2 or higher, and 90·9% were categorised as A with the GOLD ABCD assessment tool (appendix p 22). Only 0·9% (95% CI 0·6–1·1) of patients diagnosed with COPD were aware their condition was known as COPD (appendix p 23); 18·9% (17·2–20·5) were aware they had been diagnosed with chronic pulmonary disease (defined as COPD, chronic bronchitis, or emphysema). Of all patients with COPD, only 5·9% (95% CI 4·9–6·9) had ever been tested by spirometry before the survey and 11·7% (10·2–13·2) were taking treatments for COPD.
Table 4.
Severity of COPD according to GOLD criteria
|
Overall (n=9134) |
Men (n=6635) |
Women (n=2499) | ||||
|---|---|---|---|---|---|---|
| Cases (n) | Proportion (95% CI) | Cases (n) | Proportion (95% CI) | Cases (n) | Proportion (95% CI) | |
| GOLD stage I (mild) | 5153 | 56·4% (53·7–59·2) | 3764 | 56·7% (53·9–59·5) | 1389 | 55·6% (51·7–59·4) |
| GOLD stage II (moderate) | 3313 | 36·3% (34·3–38·3) | 2344 | 35·3% (33·3–37·3) | 969 | 38·8% (35·5–42·0) |
| GOLD stage III (severe) | 590 | 6·5% (5·5–7·4) | 457 | 6·9% (5·8–8·0) | 133 | 5·3% (4·1–6·5) |
| GOLD stage IV (very severe) | 78 | 0·9% (0·6–1·1) | 70 | 1·1% (0·8–1·4) | 8 | 0·3% (0·1–0·5) |
COPD=chronic obstructive pulmonary disease. GOLD=Global Initiative for Chronic Obstructive Lung Disease.
The prevalence of COPD was broadly similar across categories of economic development but was higher in rural residents (p=0·0007), in individuals who had been admitted to hospital for severe pulmonary disease in childhood (p<0·0001), in those with indoor exposure to biomass for cooking or heating (p=0·0002), in people with exposure to dust or chemicals in the workplace (p<0·0001), and in those with lower BMI categories (p=0·0004; table 2). In the multivariable logistic model, older age, male sex, former or current cigarette smoking, hospital admission for severe pulmonary disease in childhood, exposure to dust or chemicals in the workplace, a family history of lung disease, and a history of tuberculosis were all associated significantly with increased risk of COPD (table 5 ). The population attributable fractions for smoking status and exposure to dust or chemicals in the workplace are shown in the appendix (p 24).
Table 5.
Multivariable-adjusted odds ratios for COPD in China
| Odds ratio (95% CI) | p value | ||
|---|---|---|---|
| Age at survey (years) | 1·06 (1·05–1·07) | <0·0001 | |
| Sex (men) | 1·94 (1·69–2·23) | <0·0001 | |
| Rural residence | 1·09 (0·90–1·32) | 0·38 | |
| Educational level | |||
| Primary school and lower | 1·31 (0·95–1·80) | 0·09 | |
| Secondary school | 1·18 (0·88–1·59) | 0·26 | |
| Higher or further education | 1·00 (ref) | .. | |
| Smoking status | |||
| Never smoker | 1·00 (ref) | .. | |
| Former smoker | 1·56 (1·30–1·88) | <0·0001 | |
| Current smoker | 1·87 (1·60–2·19) | <0·0001 | |
| Economic development | |||
| Developed | 1·00 (ref) | .. | |
| Intermediately developed | 0·83 (0·61–1·12) | 0·21 | |
| Under-developed | 0·94 (0·67–1·32) | 0·71 | |
| Hospital admission for severe pulmonary disease in childhood | 2·09 (1·70–2·56) | <0·0001 | |
| Indoor exposure to biomass for cooking or heating | 1·12 (0·90–1·39) | 0·29 | |
| Indoor exposure to coal for cooking or heating | 0·93 (0·76–1·14) | 0·47 | |
| Exposure to dust or chemicals in the workplace | 1·27 (1·13–1·42) | 0·0001 | |
| Family history of lung disease | 1·55 (1·39–1·73) | <0·0001 | |
| History of tuberculosis | 1·73 (1·33–2·23) | <0·0001 | |
| Body-mass index (kg/m2) | |||
| <18·5 | 1·12 (0·93–1·35) | 0·25 | |
| 18·5–23·9 | 1·00 (ref) | .. | |
| 24·0–27·9 | 0·81 (0·75–0·88) | <0·0001 | |
| ≥28·0 | 0·83 (0·68–1·02) | 0·07 | |
COPD=chronic obstructive pulmonary disease.
Discussion
From the findings of our large, nationally representative survey undertaken in China in 2014–15, we have estimated that the prevalence of COPD is 13·6% in Chinese adults aged 40 years and older. This finding is strikingly higher than the previous nationwide estimate for 2002–04 of 8·2%.7 With around 568·0 million adults aged 40 years or older living in mainland China in 2010, a projected 77·2 million Chinese adults (54·3 million men and 22·9 million women) might have COPD. The annual total costs of COPD range from US$1964 to $3449 per patient, representing 33–40% of average household income in China22 and corresponding with a total cost of $151·6 billion to $266·3 billion per year for the whole country. The percentage increase in prevalence of COPD from 2004 to 2015 is 65·9%, indicating a serious public-health burden in China.
The prevalence of COPD in China we report in our study (13·6% in 2014–15) is higher than the global estimate of the prevalence of COPD (11·7% in 2010).23 Compared with other studies using the same diagnostic criteria (GOLD) in the same age group (age 40 years or older), the latest estimate we report from China is similar to the prevalence of COPD in the USA (14·0% using post-bronchodilator test in 2010)24 and Japan (13·6% in 2005),25 higher than the estimate from South Korea (12·4% in 2011),26 and lower than estimates in three Latin American cities in the PLATINO study (São Paulo in Brazil, Santiago in Chile, and Montevideo in Uruguay [14·8–19·7%]).18 The GOLD criteria we used in our study apply a fixed ratio of FEV1:FVC of less than 70% for a diagnosis of COPD. Findings suggest use of a fixed ratio might lead to more frequent diagnosis of COPD in the elderly population and less frequent diagnosis in adults younger than 45 years, particularly for mild disease.1 Therefore, we did a sensitivity analysis using a cutoff based on the lower limit of normal (appendix p 12), which gave a prevalence of COPD of 15·0% (95% CI 13·2–16·8) in China.
Cigarette smoking is a well-known risk factor that contributes substantially to COPD.17, 27 Divergent smoking patterns between men and women accounted for much of the noted difference between sexes in prevalence of COPD in China. The prevalence of current and former smoking was increased slightly in our study (40·2%) from that reported in the previous nationwide survey of COPD (38·4% in 2004).7 Furthermore, within every category of smoking exposure, the prevalence of COPD also rose—eg, from 22·6% in 2004 to 30·5% in 2015 in the group with 45 or more pack-years—suggesting that factors other than smoking exposure might play a part in COPD. Except for the lowest BMI category (<18·5 kg/m2), the prevalence of COPD was nearly doubled in the other categories of BMI than in the previous survey. We noted that the BMI category of 24·0–27·9 kg/m2 had a lower OR of 0·81 (95% CI 0·75–0·88), but this result could be attributable to reverse causality in view of the cross-sectional study design and residual confounding by smoking status. Compared with the previous study of COPD prevalence in China,7 the population in our survey comprised a significantly higher proportion of individuals exposed to dust or chemicals in the workplace (including agricultural operations)—from 20·5% in 2004 to 46·6% in 2015—and this factor could account for the pronounced increase in COPD prevalence. Although in our study, compared with the 2004 survey,7 a lower proportion of participants had severe pulmonary disease in childhood, we noted that some degree of recall bias might have existed with the current study design.
In our study, the prevalence of COPD varied substantially by geographic area of China. Differences in genetic background, culture, socioeconomic level, climate and geographic features of the residential area, and lifestyle patterns might all contribute to disparities across regions.14 The relatively large sample size of our survey enabled subgroup analyses to have enough participants in seven major regions in China. Specifically, participants in southwest China were reported to have the highest prevalence of COPD (20·2%). The prevalence of current and former smoking and exposure to indoor air pollution from biomass or coal were broadly similar across regions. A higher proportion of individuals exposed to dust or chemicals in the workplace was recorded in the southwest region (appendix p 18). However, the estimated OR for southwest China remained significant after adjustment for these traditional risk factors of COPD, as well as for outdoor air pollution, climate information, and season of the survey (appendix p 19). The southwest region consisted of four provinces and one municipality, including Tibetan participants (appendix p 25). COPD prevalence was highest in Chongqing and Sichuan but relatively low in Tibet, consistent with findings of previous reports in which an inverse correlation between altitude and COPD was noted.18 Thus, reasons for the geographic variation in COPD prevalence are difficult to ascertain from cross-sectional surveillance data. Further investigation is needed to investigate and find the causes.
In our survey, 56%, 36%, and 7% of patients with COPD had mild, moderate, and severe or very severe disease, respectively, whereas in the previous survey from 2004 these proportions were 24%, 46%, and 26%, respectively (4% were unknown stage).7 The current pattern in China is now close to that in the USA, with corresponding proportions of 56%, 34%, and 5%.24 However, the pattern in China has changed strikingly from 2004 to 2015 because more patients are now diagnosed with mild COPD. Furthermore, around two-thirds of patients with COPD were asymptomatic, which has risen from a third in 2004. The previous survey was undertaken in 2002–04 when severe acute respiratory syndrome (SARS) began spreading rapidly around the world, particularly in China. Moreover, fewer than 1% of people were aware they had COPD, fewer than 19% were aware they had chronic pulmonary disease, and fewer than 6% had ever been examined by spirometry, indicating the real challenge for COPD prevention in China.
The US Preventive Services Task Force (USPSTF) recommends against screening for COPD in asymptomatic adults because of scant evidence showing a benefit of early detection and treatment.28, 29 However, findings of a 2-year, multicentre, double-blind, randomised controlled trial in patients with COPD of GOLD stage I or II in China showed a significant and clinically relevant higher FEV1 in patients assigned tiotropium than in those allocated placebo.30 Moreover, for the population with at least one risk factor or respiratory symptom, evidence suggests that screening by spirometry has changed management and outcomes related to COPD.1, 29 Even when spirometry is not available, an alternative nine-item discriminant function model—ie, age, sex, BMI, smoking index, wheezing, cough, dyspnoea, living environment, and occupational exposure—for peak expiratory flow measurement as a screening method achieved a sensitivity of 89% and specificity of 82% in a Chinese general population.31 The high prevalence of smoking in China suggests that screening for COPD—at least for those in the high-risk population—could be essential in primary-care units.
Primary prevention of COPD requires reduction or avoidance of personal exposure to common risk factors. For example, legislation is needed to ban smoking in public places, to replace cooking with biomass or coal with biogas, to install exhaust fans in households, and to develop clean vehicle fuel and promote public transportation to reduce outdoor air pollution.32 Avoidance of direct and indirect exposure to tobacco smoke is of primary importance, not only for COPD but also for cardiovascular disease, cancer, and diabetes. Findings of a cluster-randomised controlled trial suggested that a community-based integrated intervention strategy, including systematic health education, intensive and individualised interventions (eg, personalised instructions for smoking cessation, advice for better stoves, and encouragement on adequate excercises), treatment, and rehabilitation, is well-suited for early prevention and management of COPD in China.33 All these intervention strategies could have a pronounced effect on the future trajectory of COPD in China.
Our study has several strengths. The survey was undertaken in a large, nationally representative sample of the general population in China, based on the integrated national DSP system and following a strict quality assurance and control programme to ensure data validity and reliability. All participants included in the analysis had spirometry with both prebronchodilator and post-bronchodilator examinations for diagnosis of COPD. To our knowledge, the large sample size in our study allows us to provide the first direct comparison of COPD prevalence among major geographic regions in China within one survey (appendix pp 6–10). We have not only applied the GOLD criteria with a fixed ratio of FEV1:FVC less than 70% but also used a cutoff based on LLN values to investigate the effect caused by different diagnostic criteria.
Our study also has several limitations. First, the predicted normative values of FEV1 were derived from a nationwide study in a Chinese population.19 That study had 24 centres throughout the country and included 7115 Chinese individuals aged 4–81 years. In our study, 112 (1·2%) of 9134 people with COPD were older than 81 years; the predicted value of these patients was, therefore, adopted for those aged 80 years. Second, non-permanent residents were not included in the study because of the sampling design. Moreover, 8355 participants (11% of the total sample) were excluded from the analysis for reasons including non-eligibility for spirometry and the post-bronchodilator test (appendix p 11). Strict rules for spirometry exclude patients with more severe disease for safety reasons, and their exclusion could have affected our estimates. Third, smoking status (and smoking exposure) was self-reported and not validated biochemically using exhaled carbon monoxide or amount of cotinine. Finally, we cannot eliminate the possibility that we misclassified people with asthma and other diseases as having COPD.34 Of 6810 individuals with a post-bronchodilator FEV1:FVC less than 70% who underwent additional chest radiography, 191 (2·8%) had bronchiectasis, 252 (3·7%) had non-active pulmonary tuberculosis, and 37 (0·5%) had lung lesions, indicating the possible overestimation from this perspective.
Among adults aged 40 years or older in China in 2014–15, the estimated overall prevalence of COPD is 13·6%, indicating that COPD has become a major public-health problem in China. Urgent action needs to be taken to reduce further increases in the disease burden.
Acknowledgments
Acknowledgments
We thank all research staff from local Centers for Disease Control and Prevention and local hospitals for collection of data. We also thank Chen Wang and Ting Yang (China–Japan Friendship Hospital), Kewu Huang and Yong Lu (Beijing Chao-Yang Hospital), Yahong Chen (Peking University Third Hospital), Yumin Zhou and Yi Gao (Guangzhou Institute of Respiratory Disease), Yanfei Guo (Beijing Hospital), Binmiao Liang (West China Hospital, Sichuan University), and Xuhua Zhang (General Hospital of Ningxia Medical University) for advice and support on study design, staff training, and quality control of the study. This study was supported by grants from the Chinese Central Government (key project of public health programme, grant no 2014814), the Ministry of Science and Technology of the People's Republic of China (national key R&D programme of China, 2016YFC1303905), and the National Natural Science Foundation of China (grant no 81473043, 81230066, and 91546120).
Contributors
LF and LW had full access to all data in the study. LW and YH take responsibility for the integrity of the data and the accuracy of the data analysis. LF, PG, LW, and YH contributed to study design and obtained funding for the study. LF, HB, BW, YF, SC, JF, NW, and LW contributed to data collection. All authors contributed to analysis and interpretation of data. LF, PG, HB, and XT contributed to writing of the report. LF, PG, HB, XT, JJ, KL, LW, and YH contributed to critical revision.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2017 report) http://goldcopd.org/download/326/wms-GOLD-2017-FINAL.pdf (accessed March 19, 2018).
- 2.Adeloye D, Chua S, Lee C. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5:020415. doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW. Trends and patterns of differences in chronic respiratory disease mortality among US counties, 1980–2014. JAMA. 2017;318:1136–1149. doi: 10.1001/jama.2017.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousquet J, Khaltaev NG, Cruz AA. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. 2007. http://www.who.int/gard/publications/GARD%20Book%202007.pdf?ua=1 (accessed March 5, 2018).
- 5.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Chronic respiratory diseases: burden of COPD. http://www.who.int/respiratory/copd/burden/en/index.html (accessed March 5, 2018).
- 7.Zhong N, Wang C, Yao W. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176:753–760. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 8.Bao H, Fang L, Wang L. Prevalence of chronic obstructive pulmonary disease among community population aged ≥40 in China: a meta-analysis on studies published between 1990 and 2014. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37:119–124. doi: 10.3760/cma.j.issn.0254-6450.2016.01.026. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 9.Qiu J, Zhang YN, Chen J. Prevalence of chronic obstructive pulmonary disease in Ningxia Hui Autonomous Region of China. Zhonghua Jie He He Hu Xi Za Zhi. 2013;36:265–268. (in Chinese). [PubMed] [Google Scholar]
- 10.Smith M, Li L, Augustyn M. Prevalence and correlates of airflow obstruction in approximately 317 000 never-smokers in China. Eur Respir J. 2014;44:66–77. doi: 10.1183/09031936.00152413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YC, Lin JM, Li CY, Lee LT, Guo YL, Sung FC. Prevalence and risks of chronic airway obstruction: a population cohort study in Taiwan. Chest. 2007;131:705–710. doi: 10.1378/chest.06-1829. [DOI] [PubMed] [Google Scholar]
- 12.Kurmi OP, Li L, Smith M. Regional variations in the prevalence and misdiagnosis of air flow obstruction in China: baseline results from a prospective cohort of the China Kadoorie Biobank (CKB) BMJ Open Respir Res. 2014;1:e000025. doi: 10.1136/bmjresp-2014-000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Wu X, Lopez AD. An integrated national mortality surveillance system for death registration and mortality surveillance, China. Bull World Health Organ. 2016;94:46–57. doi: 10.2471/BLT.15.153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Gao P, Zhang M. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Association for Public Opinion Research (AAPOR) Standard definitions: final dispositions of case codes and outcome rates for surveys. 2016. http://www.aapor.org/AAPOR_Main/media/publications/Standard-Definitions20169theditionfinal.pdf (accessed March 5, 2018).
- 16.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 17.Buist AS, McBurnie MA, Vollmer WM. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 18.Menezes AMB, Perez-Padilla R, Jardim JRB. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 19.Jian W, Gao Y, Hao C. Reference values for spirometry in Chinese aged 4–80 years. J Thorac Dis. 2017;9:4538–4549. doi: 10.21037/jtd.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin D, Berger J, Johannesson M. Redefine statistical significance. Nat Hum Behav. 2018;2:6–10. doi: 10.1038/s41562-017-0189-z. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava K, Thakur D, Sharma S, Punekar YS. Systematic review of economic burden in symptomatic chronic obstructive pulmonary disease (COPD) patients. Value Health. 2014;17:A173–A174. (abstr PRS26). [Google Scholar]
- 23.Ko FWS, Wong GWK. Drug treatment for early-stage COPD. N Engl J Med. 2017;377:988–989. doi: 10.1056/NEJMe1707929. [DOI] [PubMed] [Google Scholar]
- 24.Tilert T, Dillon C, Paulose-Ram R, Hnizdo E, Doney B. Estimating the US prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Respir Res. 2013;14:103. doi: 10.1186/1465-9921-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukahori S, Matsuse H, Takamura N. Prevalence of chronic obstructive pulmonary diseases in general clinics in terms of FEV1/FVC. Int J Clin Pract. 2009;63:269–274. doi: 10.1111/j.1742-1241.2008.01873.x. [DOI] [PubMed] [Google Scholar]
- 26.Hong JY, Jung JY, Lee MG. Changes in the prevalence of COPD in Korea between 2001 and 2011 in the KNHANES data. Respir Med. 2017;125:12–18. doi: 10.1016/j.rmed.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Yin P, Jiang CQ, Cheng KK. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet. 2007;370:751–757. doi: 10.1016/S0140-6736(07)61378-6. [DOI] [PubMed] [Google Scholar]
- 28.US Preventive Services Task Force (USPSTF) Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372–1377. doi: 10.1001/jama.2016.2638. [DOI] [PubMed] [Google Scholar]
- 29.Press VG, Cifu AS, White SR. Screening for chronic obstructive pulmonary disease. JAMA. 2017;318:1702–1703. doi: 10.1001/jama.2017.15782. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Zhong NS, Li X. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377:923–935. doi: 10.1056/NEJMoa1700228. [DOI] [PubMed] [Google Scholar]
- 31.Tian J, Zhou Y, Cui J. Peak expiratory flow as a screening tool to detect airflow obstruction in a primary health care setting. Int J Tuberc Lung Dis. 2012;16:674–680. doi: 10.5588/ijtld.11.0429. [DOI] [PubMed] [Google Scholar]
- 32.Guan W-J, Ran P-X, Zhong N-S. Prevention and management of COPD in China: successes and major challenges. Lancet Respir Med. 2016;4:428–430. doi: 10.1016/S2213-2600(16)30092-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Hu G, Wang D. Community based integrated intervention for prevention and management of chronic obstructive pulmonary disease (COPD) in Guangdong, China: cluster randomised controlled trial. BMJ. 2010;341:c6387. doi: 10.1136/bmj.c6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373:1241–1249. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.