Abstract
Multiple sclerosis (MS) is a T cell driven autoimmune disease of the central nervous system (CNS). Despite its association with Epstein-Barr Virus (EBV), how viral infections promote MS remains unclear. However, there is increasing evidence that the CNS is continuously surveyed by virus-specific T cells, which protect against reactivating neurotropic viruses. Here, we discuss how viral infections could lead to the breakdown of self-tolerance in genetically predisposed individuals, and how the reactivations of viruses in the CNS could induce the recruitment of both autoaggressive and virus-specific T cell subsets, causing relapses and progressive disability. A disturbed immune surveillance in MS would explain several experimental findings, and has important implications for prognosis and therapy.
Trends
A huge body of evidence suggests that viral infections promote MS; however, no single causal virus has been identified. Multiple viruses could promote MS via bystander effects.
Molecular mimicry is an established pathogenic mechanism in selected autoimmune diseases. It is also well documented in MS, but its contribution to MS pathogenesis is still unclear.
Bystander activation upon viral infection could be involved in the generation of the autoreactive and potentially encephalitogenic T helper (Th)-1/17 central memory (Th1/17CM) cells found in the circulation of patients with MS.
Autoreactive Th1/17CM cells could expand at the cost of antiviral Th1CM cells in patients with MS, in particular in those undergoing natalizumab therapy, because these cells are expected to compete for the same homeostatic niche.
Autoreactive Th1/17 cells and antiviral Th1 cells are recruited to the CSF of patients with MS following attacks, suggesting that viral reactivations in the CNS induce the recruitment of pathogenic Th1/17 cells. Autoreactive Th1/17 cells in the CNS might also induce de novo viral reactivations in a circuit of self-induced inflammation.
Genetic and Environmental Factors Contribute to the Risk of MS
MS is the most common inflammatory autoimmune disorder of the CNS 1, 2. It is characterized by the destruction of the protective myelin sheath of neurons, resulting in macroscopic lesions in the brain and causing progressive disability. MS can be subdivided into relapsing–remitting (RR), primary progressive (PP) or secondary progressive (SP; i.e., the RR subtype worsening over time to SP-MS) forms. RR-MS is the dominant form at disease onset, and is characterized by acute clinical attacks followed by apparent disease stability. Symptoms can be alleviated with several therapies, but, in some patients, there is no beneficial effect and the disease may evolve to a SP form. PP-MS and SP-MS remain difficult to treat and are also mechanistically poorly understood [3].
The etiology of MS is still unknown, but both genetic and environmental factors contribute to the risk of developing MS 1, 2. The major genetic risk factor maps to the human leukocyte antigen (HLA) gene cluster, and the strongest risk is conferred by HLA-DRB1*15:01 in the class II region 4, 5. The principal function of MHC class II proteins is to present peptide ligands to CD4+ lymphocytes and these T cells are consequently believed to have a key pathogenic role in MS. However, the MHC class I cluster, which regulates cytotoxic lymphocyte responses, contains polymorphic regions that are associated with protection against MS [4]. Several other gene polymorphisms associated with MS are involved in immune responses, in particular in the activation and homeostasis of T cells [6], consistent with the concept that MS is a T cell-driven autoimmune disease.
The importance of the environment in determining whether a genetically susceptible individual develops MS has been underlined by studies of monozygotic twins and of genetically susceptible individuals migrating from low- to high-risk areas. The strongest environmental risk factors are Vitamin D deficiency, smoking, and viral infections [7]. Interestingly, infections with helminths have been shown to have a protective effect 7, 8. Among viral infections, EBV shows the strongest association, and it was estimated that EBV-induced infectious mononucleosis increases the risk of MS to a similar degree as the strongest genetic risk factor (HLA-DRB1*15:01) 4, 9, 10, 11. In addition to EBV, several other viruses have been implicated in MS [12], in particular neurotropic viruses, including human herpes virus-6 (HHV-6) [13], herpes zoster virus [14] and John Cunningham virus (JCV) [15], but also endogenous retroviruses [16]. Based on this evidence, a possible viral etiology of MS has been proposed 9, 13, 15, 17 and continues to stimulate intense research in the field (see Outstanding Questions).
The risk of life-threatening JCV-induced progressive multifocal leukoencephalopathy (PML) in patients with MS undergoing therapy with natalizumab [18], a therapeutic antibody that binds to the α4-integrin adhesion receptor and blocks lymphocyte migration to the CNS, has highlighted the importance of antiviral immune surveillance of the CNS. Indeed, the presence of a lymphatic system in the CNS has challenged the view of the CNS being an immune-privileged site 19, 20, and it is now widely accepted that the CNS is surveyed and protected by antiviral T cells [21] (Box 1 ).
Box 1. CNS Immune Privilege.
The notion that the CNS is a tolerogenic, ‘immune-privileged’ site, where immune reactions that occur in peripheral tissues are inefficient and slow, stems from seminal studies with transplanted allogenic tissues that were not or were only slowly rejected in the brain, unless animals had been immunized previously [150]. In addition, it is well known that entry of macromolecules and immune cells into the CNS from the blood is restricted by the BBB and, until recently, the CNS was also believed to lack lymphatic drainage. However, the presence of a lymphatic system of the meninges in the brain and of occasionally reactivating neurotropic viruses suggest that the CNS is constantly surveyed by the immune system, although in a manner that limits the type of collateral tissue damage that occurs in MS.
Alt-text: Box 1
Given this updated view of immune responses in the CNS, here we discuss different models of how viral infections could promote MS, and illustrate how a defective antiviral immune surveillance could be a driving force in its pathogenesis.
The Most Widely Studied Animal Models of MS Induce CNS Inflammation in the Absence of Viral Infections
Although the epidemiological data clearly indicate that viral infections are a critical risk factor for MS, the underlying mechanisms are poorly understood [12]. Animal models that induce experimental autoimmune encephalomyelitis (EAE) in the absence of viral infections by priming pathogenic CD4+ T cells with myelin antigens are widely used to study neuroinflammation and MS [22]. Self-tolerance has to be broken in these models by adjuvants such as CFA, which contain killed mycobacteria, intracellular pathogens that potently activate the innate immune system. Alternative models of MS, in which demyelination is induced by neurotropic viruses, such as mouse hepatitis virus or Theiler’s murine encephalomyelitis virus (TMEV), are less studied, but enable researchers to address how viral infections could promote MS [23]. TMEV induces chronic inflammation and demyelination in the brain and, importantly, both virus-specific and myelin-reactive effector T cells are generated in this MS model [23]. Thus, antiviral immune responses in the CNS can result in the breakdown of self-tolerance to myelin antigens, which is normally prevented by regulatory T cells (Tregs) [24]. In addition, viruses and antiviral T cells can also lead directly to damage and demyelination in the CNS 23, 25, 26, 27, 28. Thus, viral MS models are highly relevant, but less studied than are those of EAE, and this is one reason why the role of viruses in MS is still poorly understood.
The Antigen Specificity of Encephalitogenic T Cells Is Incompletely Defined
The use of myelin antigens to induce EAE is consistent with demyelination in the brain of patients with MS and with the presence of autoreactive CD4+ T cells recognizing myelin-derived antigens that are restricted by the major MS risk allele, HLA-DRB1*15:01 29, 30. However, myelin-reactive CD4+ T cells are rare and were found at similar frequencies in the peripheral blood of healthy individuals and of patients with MS [30]. However, myelin-reactive T cells have more proinflammatory properties in patients with MS than in healthy donors 31, 32, and are enriched in the CSF of patients with MS shortly after an attack 32, 33. Nevertheless, myelin-derived antigens are probably not the only relevant self-antigens in MS. In particular, pathogenic T cells in MS may have a degenerate T cell receptor (TCR) that cross-reacts with several structurally related self-peptides, or potentially even directly with the backbone of some MHC molecules 34, 35, 36, 37. These autoreactive T cells have a high pathogenic potential because they could be activated by any antigen-presenting cell (APC) that expresses MHC class II and co-stimulatory molecules. Finally, virus-specific CD4+ and CD8+ T cells could also cause collateral damage in the CNS following an antiviral immune response, in particular those that cross-react with relevant self-antigens due to a phenomenon known as ‘molecular mimicry’ 38, 39.
Molecular Mimicry Is unlikely to be the only Virus-Related Pathogenic Mechanism in MS
Molecular mimicry is the most frequently discussed mechanism for how viruses could induce autoimmunity and MS (Box 2 ), and excellent reviews have been published on this topic 34, 39, 40. Some TCRs recognize several similar peptides, and this cross-reactivity is relevant for autoimmunity [41] and might be exploited by pathogens, such as viruses, to avoid recognition by the adaptive immune system [42]. Indeed, autoreactive T cells are either deleted in the thymus, rendered unresponsive in the periphery, or even redirected to the Treg lineage that induces dominant immune suppression 43, 44. Some viral proteins contain peptide sequences that are similar to the self-proteins of their hosts [39]. In the case of MS, molecular mimicry between myelin basic protein (MBP) and the EBV latency antigen EBNA-1 is well documented, since CD8+ T cell clones isolated from patients with MS could be activated by both MBP- and EBNA-1-derived peptides [38]. In addition, CD4+ T cells that cross-reacted with both EBNA-1 and MBP were identified [45], and molecular mimicry might also be exploited by HHV-6 [13]. Interestingly, commensal bacteria are known to be essential for autoimmune demyelination [46], and T cells expressing a TCR that cross-reacted with MBP and a common bacterial peptide was able to induce MS-like disease in humanized mice [37]. However, while molecular mimicry is largely accepted as the driving force in some autoimmune diseases, such as Streptococcus-driven rheumatic fever, its relevance in MS is still debated 39, 40. Moreover, it was found T cell responses to antigens derived from EBV, JCV, and myelin were largely confined to T helper type 1 (Th1) and Th1/17 cell subsets in patients with MS [32]. Therefore, CD4+ T cell responses in MS against these viruses and myelin antigens are predominantly mediated by distinct Th cell subsets rather than by virus-specific T cell clones that cross-react with self-antigens. Nevertheless, Th1/17 cells isolated from the CSF reacted with autologous APC in the absence of exogenous antigens, and they might cross-react with peptides from other viruses or bacteria. Thus, although molecular mimicry is likely to contribute to the MS risk conferred by infections, other mechanisms are also likely to be important.
Box 2. Molecular Mimicry.
Mimicry is an evolutionary process whereby one organism acquires a similarity to another organism to obtain a survival advantage. Molecular mimicry is a phenomenon whereby molecules of a pathogen, in particular peptides, are similar to peptides of its host. T cells mount an immune response when they are activated by foreign peptides, but do not normally react against self-peptides. The latter is ensured by a combination of central and peripheral tolerance mechanisms, which lead to the deletion of highly autoreactive T cells in the thymus and ensures that T cells with an intermediate autoreactivity are not aberrantly activated in the periphery. The molecular mimicry hypothesis of autoimmunity proposes that T cells cause autoimmune disease following an antipathogen immune response when they cross-react with self-peptides from healthy, uninfected tissues. Molecular mimicry is thought to be a driving force in, for example, Streptococcus-driven rheumatic fever and has also been documented for the EBNA-1 protein of EBV and myelin basic protein in MS.
Alt-text: Box 2
The Complex Relationship between MS and Antiviral Immune Responses
In addition to CD4+ T cells, B cells and CD8+ T cells are also involved in human MS, as evidenced by their presence in the cerebrospinal fluid (CSF) and in demyelinated brain lesions in patients [47]. In particular, the production of oligoclonal antibodies in the CSF is highly characteristic for MS and, therefore, has been used as a supportive criterion for diagnosis 48, 49. Moreover, B cell depletion with rituximab reduces relapses in patients with RR-MS 50, 51. This therapeutic effect could reflect the capacity of B cells to present antigens to pathogenic T cells [52], since rituximab does not deplete plasma cells and antibody levels are poorly affected [51]. However, rituximab could also inhibit viral delivery to the brain, because B cells represent a cellular reservoir of both EBV and JCV [53] and, therefore, are a likely vehicle for these viruses to pass across the blood–brain barrier (BBB). Interestingly, secondary progressive MS is characterized by tertiary meningeal lymphoid structures, which might contain infected B cells as a constant local source of EBV [54]. Oligoclonal antibodies in the CSF were first thought to represent non-sense IgG [55], and their physiological relevance is a matter of debate. However, several groups found that they could react with neurotropic viruses 56, 57. Conflicting results have been published on EBV-specific antibodies in the CSF 9, 58, but several groups reported that antibodies against measles, Rubella, and herpes zoster viruses, known as the ‘MRZ reaction’, are present in 80–100% of patients with MS [57], and might predict whether patients with a clinically isolated syndrome (CIS) will go on to develop MS [49]. The presence of virus-specific antibodies in the CSF suggests that demyelination in MS is accompanied by antiviral immune responses. Consistent with this notion, EBV-specific CD8+ T cells are specifically expanded in the CSF of patients with MS [59], and CD8+ T cells interacting with lytically infected B cells have been identified in MS brain lesions 60, 61. However, whether the presence of EBV in brain lesions is characteristic for MS is debated [62]. Of note, several viruses persist in a latent stage, are neurotropic and, thus, might be present in the brain. Moreover, herpes simplex virus (HSV) was shown to trigger the generation of autoantibodies in the brain [63], and the neurotropic viruses HHV-6, JCV, and herpes zoster were proposed to have a role in MS 13, 15, 18. Direct detection of viral nucleic acids is limited to a minority of patients [64], but localized viral reactivation in the parenchyma might not be necessarily detectable in the CSF by standard techniques [65]. Of note, many viruses, including EBV and JCV, are efficiently controlled in healthy individuals, but can cause life-threatening infections in immuno-compromised individuals 66, 67. These findings raise the question whether patients with MS mount a somehow altered immune response against viruses, in particular in the tolerogenic environment of the CNS. The association of MHC class I polymorphisms with protection is consistent with an inefficient antiviral cytotoxic immune response in patients with MS. However, so far, antiviral immune responses in patients with MS were found to be either normal or even increased, in the case of EBV [60], and patients with MS treated with strong immune suppressants, with the notable exception of natalizumab, do not experience increased viral reactivations. Nevertheless, more qualitative approaches might be required to monitor antiviral immune responses in patients with MS 54, 68. For example, antiviral T cell responses are normally measured by IFN-γ production; however, central memory T cells (TCM), which could perform antiviral immune surveillance of the CNS (see below), produce only limited amounts of IFN-γ, but can be efficiently expanded with viral antigens [69]. In summary, the role of different viral infections in MS is debated, and more research is needed to understand the regulation of antiviral immune responses in patients with MS and how they might impact pathogenesis and disease progression.
T Cell Migration from Lymph Nodes to the CNS Is Required for Antiviral Immune Surveillance and Relapse
A required feature of T cells to not only induce relapse, but also perform antiviral immune surveillance is their capacity to migrate from lymph nodes to the CNS. Access to the CNS by immune cells is tightly controlled, and the brain is separated from the blood by the BBB [70]. Nevertheless, autoreactive effector T cells induced in the EAE model can spontaneously home to the CNS upon adoptive transfer and cause disease.
The migration of leukocytes into different tissues is controlled by specific adhesion molecules and chemokine receptors. The α4/β1-integrin is known to be a key adhesion molecule for CNS entry [70], although Th17 cells can home to the CNS independently of the α4/β1-integrin 71, 72. Nevertheless, natalizumab inhibits relapses and promotes JCV reactivation and PML in patients with MS, indicating that the α4/β1-integrin has a critical role for CNS homing of both pathogenic and protective, antiviral T cells. Integrins have to be activated by inside-out signaling and, in human Th1 cells, different chemokine receptors can induce α4/β1-driven adhesion [73]. However, the relevant chemokine receptors in CNS homing and MS are debated. One candidate is CCR6, which is induced by TGF-β and proinflammatory cytokines [74] and is stably expressed on human IL-17-producing Th17 cells 75, 76, 77. CCR6 was proposed to allow access of T cells to the CNS via the lumbar spinal cord upon EAE induction [78] or at steady state via the choroid plexus [79], paving the way for the consecutive recruitment of additional T cells upon ensuing inflammation. Inflammatory chemokine receptors implicated in CNS homing and MS are CXCR3 and CCR5 [80]. These two chemokine receptors are selectively expressed on IFN-γ-producing Th1 and Th1/17 cells, which fight viruses [81]; the CXCR3 ligand, CXCL10/IP-10, is induced upon viral infections in the canonical response to interferons [82]. Finally, CCR7 is also implicated in T cell migration in the CNS and MS [83]. This is somewhat surprising, since a key function of CCR7 on T cells is to mediate homing of naïve and CCR7-expressing TCM to lymph nodes, while homing of CCR7− effector memory T cells (TEM) to nonlymphoid tissues is predominantly mediated by inflammatory chemokine receptors, such as CCR5 [84]. However, relevant fractions of TCM express the α4/β1-integrin [84], CXCR3 [69], and CCR6 [77], suggesting that some TCM shuttle between lymph nodes and the CNS. Indeed, CCR7+ T cells are highly abundant in the CSF of patients with MS [85] and CCR7 ligands are expressed in the CNS, including in MS lesions [86]. The expression of CCR7 and of other chemokine receptors is dynamic in antigen-activated T cells [87] and, therefore, it is uncertain whether CCR7 expression reliably identifies TCM and TEM in the CNS of patients with active MS [85]. Nevertheless, an encephalitogenic role for TCM in MS is suggested by the therapeutic efficiency of fingolimod (FTY20), which sequesters naïve and TCM in lymph nodes, but spares TEM [88]. Furthermore, in the CSF of patients with natalizumab-treated MS, CCR7+VLA-4+ T cells were depleted, while CCR7−VLA-4− T cells were strongly enriched [72]. Since these patients had stable disease, these findings are consistent with the view that TCM-derived cells drive pathogenic CNS inflammation in MS. CCR7 ligands are also crucial for protective antiviral T cell responses in the CNS in mice [27], further suggesting that the CNS migrations of antiviral and pathogenic T cells rely on similar mechanisms. In summary, different migratory routes for T cells to reach the CNS have been described, but the α4/β1-integrin appears to be critical for both antiviral immune surveillance and relapse, while the identities of the relevant chemokine receptors are uncertain.
Different Cytokine Requirements of Pathogenic T Cells in MS and its Animal Models
The EAE model was instrumental for the identification of proinflammatory cytokines that can drive pathogenic CNS inflammation. A seminal finding in autoimmunity was that IL-23, but not the closely related cytokine IL-12, has a nonredundant pathogenic role 89, 90, 91. IL-12 potently induces Th1 cells, whereas IL-23 induces the maturation of Th17 cells, suggesting that Th17 but not Th1 cells are the key pathogenic cells. This concept was rapidly expanded to human organ-specific immune-mediated diseases, because single nucleotide polymorphisms (SNPs) in IL-23R, which reduces IL-23-mediated signaling [92], were shown to have a strong protective effect in psoriasis [93] and Crohn’s disease [94]. However, a similar protective effect of SNPs in IL-23R was not found for MS [95]; instead, polymorphisms in the gene locus encoding the IL-12-specific subunit p35 showed a strong association 4, 5, 6, 95. Interestingly, in viral encephalitis induced by neurotropic coronavirus in mice, IL-12, but not IL-23, enhanced morbidity, and this was associated with enhanced T cell IFN-γ production [25]. Thus, while IL-23 has a nonredundant pathogenic role in EAE, IL-12 appears to be relevant in viral MS mouse models and possibly also in human MS [95].
In the EAE model, different T cell subsets, including both Th1 and Th17 cells, could induce pathogenic neuroinflammation, although with different characteristics [89]. Th1/17 cells that co-produce IFN-γ and IL-17 have high pathogenic potential and are also enriched in brain lesions of patients with MS [96]. In humans, they can be induced from not only naïve T cells with IL-1β and IL-23 97, 98, but also from conventional Th17 cells with IL-1β and/or IL-12 32, 76, 99. IL-17 can enhance BBB permeability [100] and has neurotoxic potential [101]; in addition, promising results were obtained in a clinical trial with a neutralizing anti-IL-17 antibody in RR-MS [102]. However, deficiency for neither IL-17 103, 104 nor IFN-γ [105] completely prevents EAE induction, while GM-CSF is absolutely required 106, 107. The proposed pathogenic mechanism in EAE is that dendritic cell (DC)-derived IL-23 induces Th17 cells to produce GM-CSF, which in turn leads to the recruitment and activation of additional myeloid cells [89]. GM-CSF-producing T cells are also abundant in the CSF of patients with MS, but GM-CSF appears to be regulated differently in humans compared with mice 98, 108, and is produced not only by Th17 cells, but also by Th1 cells 32, 108. In summary, relevant differences exist in the regulation and pathogenicity of key proinflammatory cytokines, including IL-12, IL-23, and GM-CSF, in EAE, viral MS models, and patients with MS.
MS Could Be Initiated by Virus-Induced Bystander Activation of Autoreactive CCR6+ T Cells: The Original Sin?
An alternative mechanism that could explain a pathogenic role of viral infections in MS is bystander activation (Box 3 ). Viruses potently induce the maturation of DCs that consequently upregulate MHC and co-stimulatory molecules [109], thus favoring the activation of not only virus-specific, but also potentially autoreactive T cells. In addition, lytic viruses, such as JCV, can induce the death of myelin-producing oligodendrocytes [28], inducing the release and presentation of myelin-derived self-antigens in deep cervical lymph nodes by DCs [19] (Figure 1 ). The inhibition of bystander activation of autoreactive T cells is the task of Tregs [24], but several Treg subsets appear to be defective in patients with MS [110]. Moreover, the MS-associated polymorphisms in genes regulating T cell activation suggest that Th cells are more resistant to suppression [6]. However, naïve T cells have a high activation threshold and, therefore, it appears more likely that autoreactive memory T cells are aberrantly activated. Importantly, healthy individuals harbor a population of autoreactive memory T cells that secrete IL-10 in response to low-level TCR stimulation, such as self-MHC, to inhibit their own proliferation [74]. These autoreactive T cells express CCR6, possibly as a consequence of exposure to TGF-β at steady state and, thus, appear to be closely related to Th17 cells 74, 77. They are distinct from autoreactive CD25+ Tregs because they do not express Foxp3, and can also be distinguished from Foxp3− IL-10-producing regulatory ‘Tr1’ cells 111, 112, 113. Interestingly, some of these autoreactive CCR6+ memory T cells are specific for recall antigens, such as tetanus toxoid [74], suggesting that they have a degenerate TCR specificity. In response to optimal TCR stimulation or to recall antigens, they behave in a similar way to conventional memory T cells, suggesting that they contribute to protective recall responses in healthy individuals [74]. Intriguingly, patients with RR-MS have an expanded population of autoreactive CCR6+ T cells, which express CXCR3 and co-produce IL-17 and IFN-γ [32]. IL-10 production is reduced in CXCR3+CCR6+ T cells [32], suggesting that these autoreactive Th1/17 cells are more pathogenic [114] and have a reduced capacity to inhibit their own activation in response to low-level TCR stimulation [74]. Notably, Th1/17 cells can be induced from CCR6+ T cell precursors in response to IL-1β and/or IL-12 32, 76, 99, 115, proinflammatory cytokines that are produced by DCs in response to viruses 109, 116. A conversion of Th17 cells to IFN-γ-producing Th1/17 cells also occurs in inflamed tissues in mice in vivo 117, 118, 119. In addition, the expanded Th1/17 cells in patients with MS proliferated with myelin antigens, produced high levels of GM-CSF and expressed the α4/β1-integrin and CCR7 [32], indicating that these cells are potentially encephalitogenic. Consistent with this hypothesis, these Th1/17CM cells are selectively expanded in patients with RR-MS with a high disease severity score, and might represent a cellular ‘Sword of Damokles’. Following their generation, which could be a key pathogenic event in MS, these Th1/17CM cells probably no longer require IL-12, consistent with the unexpected failure of anti-IL-12/23p40 antibodies to inhibit relapse in established RR-MS [120]. In summary, virus-induced DC maturation and cytokine production could lead to the generation of potentially pathogenic Th1/17CM cells from autoreactive, IL-10-producing CXCR3−CCR6+ memory T cells, which are constitutively present in healthy individuals 32, 74 (Figure 1).
Box 3. Bystander Activation.
Bystander activation is a process whereby an adaptive immune response against a specific pathogen leads to the activation not only of pathogen-specific T cells, but also of ‘bystander’ T cells that are not specific for the pathogen. Two different mechanisms have been described: bystander T cell activation can occur in a TCR-independent fashion via homeostatic cytokines, such as IL-7 or IL-15. The latter allows established CD8 memory T cells and antiviral CD4+ T cells to survive despite the high number of new effector and memory T cells that are generated to protect against a new invading pathogen. This has been well documented in the case of viral infections in mice, and TCR-independent proliferation induced by cytokines has been documented in humans. Second, bystander T cells can be activated via the TCR, because pathogens induce DC maturation and, thus, upregulate MHC and co-stimulatory molecules. This TCR-driven bystander activation is particularly important for autoreactive T cell responses, and can be further modulated by cytokines.
Alt-text: Box 3
Figure 1.
Viral Infections Could Induce Bystander Generation of Pathogenic T Helper (Th)-1/17Cells. Local reactivations of neurotropic viruses in the central nervous system (CNS) parenchyma (A) leads to tissue damage and the uptake of both viral and myelin-derived antigens by dendritic cells (DCs). Virus-activated DCs then migrate via the cerebrospinal fluid (CSF) to draining deep cervical lymph nodes (B), present both viral and myelin-derived antigens to naïve and central memory T cells, and produce antiviral and proinflammatory cytokines, such as IL-12 and IL-1β. This not only leads to the priming of virus-specific Th1 cells, but could also result in the inappropriate stimulation of autoreactive CCR6+ T cells that are present in healthy individuals. Under the influence of IL-1β and/or IL-12, T cell receptor (TCR)-activated autoreactive CCR6+ central memory T cells (TCM) could acquire CXCR3 expression and IFN-γ-producing capacities, and downregulate IL-10 production, thus differentiating into potentially encephalitogenic Th1/17CM cells that are specifically expanded in patients with relapsing–remitting multiple sclerosis (RR-MS).
Competition between Autoreactive and Antiviral T Cells Could Favor Relapses and PML
Genome-wide association studies have identified SNPs in the loci of IL-2Rα and IL-7Rα as MS-associated risk factors 6, 121. These cytokine receptors not only control the homeostasis of CD4+ regulatory and Th cells, respectively 81, 122, but are also essential for protective CD8+ T cell responses 123, 124. Notably, the risk-conferring SNP of the IL-7R reduces IL-7R expression 6, 121, suggesting that IL-15, which controls memory T cell homeostasis together with IL-7 69, 125, 126, 127, 128, is particularly important in patients with MS. Based on T cell repopulation studies following therapeutic lymphocyte depletion with anti-CD52 antibodies, it was previously proposed that IL-15-dependent T cell homeostasis might be disturbed in MS [129]. It was found that, while Th1/17CM cells were expanded in patients with MS with a high disease score, conventional Th1 cells were selectively decreased, in particular Th1CM cells in patients treated with natalizumab [32]. The latter was unexpected, since natalizumab leads to the accumulation of proinflammatory T cells in the circulation [130]. Notably, in healthy individuals, both Th1 and Th1/17 cells respond to viruses 77, 99, including JCV [32]. Conversely, in patients with RR-MS, Th1/17CM cells failed to respond to JCV, but instead proliferated spontaneously with autologous DCs [32]. Consequently, the natalizumab-associated decrease in Th1CM cells might explain the risk of patients with MS developing PML following prolonged natalizumab treatment. Indeed, patients with PML and MS treated with natalizumab have an impaired JCV-specific Th1 response [131], but additional studies are needed to establish whether a decrease in Th1CM cells is associated with the risk of PML.
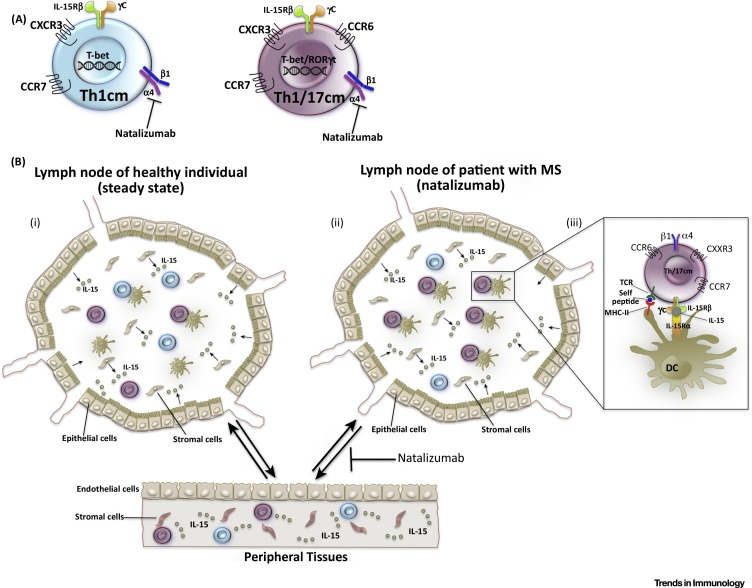
What could be the mechanism of the selective shift from Th1 to Th1/17 cells in the analyzed patients with MS? Among CD4+ T cells, only the Th1 and Th1/17 subsets express high levels of T-bet [32], which induces not only IFN-γ and CXCR3 [81], but also the IL-2/15Rβ chain (CD122), consequently rendering T cell homeostasis sensitive to IL-15 (Figure 2 A) 69, 126. Importantly, persistence of antiviral, but not of conventional, CD4 memory T cells requires IL-15, and they compete with other CD122+ lymphocytes for IL-15 in vivo [126]. In humans, TCM proliferate slowly in the steady state [132], and CXCR3+ ‘pre-Th1’ cells, which contain both Th1CM and Th1/17CM cells, express the highest levels of CD122 among CD4+ TCM, and proliferate most extensively with IL-15 in the absence of TCR stimulation 32, 69, 84. Thus, Th1CM and Th1/17CM cells are expected to compete for IL-15 in secondary lymphoid organs [133], and this competition could be intensified when natalizumab limits their access to IL-15-rich peripheral tissues, such as bone marrow or the lungs [134]. Of note, the lung is a niche for resting, myelin-reactive memory T cells in Lewis rats with EAE [135]. Moreover, the bone marrow is a key site for memory T cell maintenance 136, 137, and human antiviral CD4+ T cells are enriched at this site [136]. Interestingly, natalizumab inhibits bone marrow homing of stem cells [138], although it is unclear whether it also interferes with T cell homing to bone marrow or the lung 130, 139. In lymph nodes, DCs are expected to expand preferentially autoreactive Th1/17CM cells in the absence of viral reactivation [32] (Figure 2B). Indeed, an important function of DCs is to present self-MHC to CD4+ T cells at steady state to induce naïve T cell survival [140] and allow secondary expansions of CD4+ memory T cells [141]. Moreover, DCs also trans-present IL-15 to lymphocytes (Figure 2B), including CD4+ T cells 142, 143, 144, and could induce the survival and IL-15-dependent proliferation of antiviral Th1 cells [126]. Thus, competition for self-MHC and IL-15 presented by DCs in lymph nodes is a possible mechanism whereby autoreactive, pathogenic Th1/17CM cells could expand selectively at the cost of virus-specific, protective Th1CM cells in patients with natalizumab-treated MS (Figure 2). Whatever the mechanism, such disequilibrium would render patients with MS more vulnerable not only to relapses, but also to JCV 67, 131. In summary, CD4+ T cell homeostasis appears to be disturbed in patients with MS, but more research is needed to understand the maintenance of antiviral and autoreactive helper T cells in humans, and the equilibrium between Th1CM and Th1/17CM cells.
Figure 2.
Hypothetical Mechanism of Competition between Protective Central Memory T Helper Type 1 (Th1CM) and Pathogenic Th1/17CM Cells. (A) Th1CM and Th1/17CM cells express increased levels of the transcription factor T-bet, which induces the expression of the IL-2/15Rβ-chain that renders T cells responsive to the homeostatic cytokine IL-15, which is required to maintain antiviral CD4 memory in the absence of antigen. (B) In healthy individuals (i) Th1CM and Th1/17CM cells are in equilibrium, and both compete successfully for IL-15, which is most abundant in peripheral tissues, but is also produced by stromal and epithelial cells in lymph nodes. In addition, they occasionally interact with dendritic cells (DCs) in lymph nodes and sense self-major histocompatibility complexes (MHCs). In patients with multiple sclerosis (MS) (ii) autoreactive Th1/17CM cells could expand at the cost of virus-specific Th1CM cells, because they proliferate with self-MHC-presenting DCs in lymph nodes during the remission phase. In addition, natalizumab could limit the access to IL-15 in peripheral tissues and, thus, intensify competition. However, while Th1/17CM cells might have preferential access to IL-15 that is trans-presented on IL-15Rα by DCs (iii), Th1CM cells might be less fit under these conditions and die by neglect.
Relapses Could Be Triggered by Viral Reactivations in the CNS that Induces the Recruitment of Autoreactive Bystander Th1/17Cells
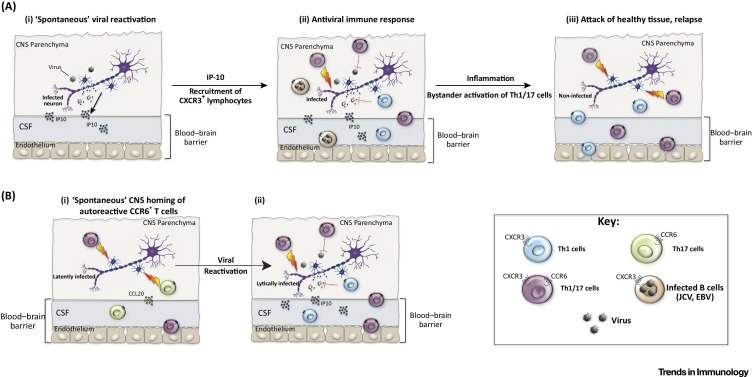
As discussed above, at least two different pathways could lead to the recruitment of pathogenic T cells to the CNS, and consequently to relapses. On the one hand, autoreactive T cells could enter the CNS in the absence of infections via CCR6 78, 79 and, on the other hand, viral reactivations in the CNS could induce CXCL10/IP10 and consequently attract autoreactive and/or virus-specific CXCR3+ T cells [82]. Notably, since Th1/17 cells co-express CXCR3 and CCR6, they could use either pathway to reach the CNS. However, antiviral Th1 cells that lack CCR6 expression are strongly enriched in the CSF of patients with active MS [32], suggesting that the IP10/CXCR3 axis is relevant for relapses. Consistent with this notion, in the circulation of patients with MS where CNS homing is blocked by natalizumab, there is a selective increase in CXCR3+ B cells [145] and CXCR3-expressing Th1/17 cells 32, 130. The requirements for CXCL10/IP10 in different animal models of MS is variable [82], but a role for CXCL10/IP10 in MS is further suggested by the fact that it is expressed in brain lesions of patients with MS [80], and that SNPs in its gene locus are associated with a worse prognosis [146]. In healthy individuals, CXCR3+ Th1 and CXCR3+CCR6+ Th1/17 cells respond to viruses and express the α4/β1-integrin and, thus, both could contribute to the physiological antiviral immune surveillance in the CNS [147] (Figure 3 A, Key Figure). Conversely, in patients with MS, Th1 and Th1/17 cells responded selectively to viral and myelin-derived antigens [32], respectively, suggesting that, while Th1 cells also mediate antiviral immune responses in patients with MS, Th1/17 cells could attack uninfected tissue and promote relapses (Figure 3A). In this scenario, relapses could be triggered by viral reactivation that lead to bystander recruitment of autoreactive Th1/17 cell to the CNS via IP10/CXCR3. EBV is one obvious candidate virus to induce bystander Th1/17 cell recruitment, but the high frequency of JCV-specific Th1 cells in the CSF of patients with active MS [32] also supports a role for JCV reactivation in relapses [15]. This ‘bystander recruitment model’ has important implications for MS therapy, because it predicts that selective targeting of autoreactive Th1/17 cells could be as efficient as natalizumab therapy, but would not induce PML if antiviral Th1 cells were spared. Surprisingly little is known about the signals that induce neurotropic viruses to switch from latency to a lytic stage and, therefore, stress is often suggested as a common explanation 15, 148. In the case of JCV, TNF-α, which is produced by effector T cells upon antigenic activation, can deliver critical reactivation signals [149]. Therefore, it is possible that autoreactive Th1/17 cells induce de novo viral reactivation in the CNS, thus fueling a vicious feed-forward loop that leads to the recruitment of new waves of pathogenic T cells and possibly also of virus-infected B cells [53]. Alternatively, it is possible that autoreactive Th1/17 cells home first to the CNS during the remission phase of MS via CCR6, and induce then viral reactivation and the recruitment of antiviral Th1 cells via IP-10 (Figure 3 B). However, the latter model fails to fully explain why relapses are characteristic for patients with MS, since healthy individuals also harbor autoreactive CCR6+ T cells [74] that produce substantial amounts of GM-CSF and also some IL-17 [32]. In any case, the concomitant enrichment of virus-specific Th1 and myelin-reactive Th1/17 cells in the CSF of patients with active MS further underlines the close relationship of antiviral immune responses and MS, and warrants further investigation.
Figure 3.
Key Figure: Viral Reactivation Could Lead to Bystander Recruitment of Autoreactive Central Memory T Helper Type 1/17 (Th1/17CM) Cells
(A) In healthy individuals, the central nervous system (CNS) is surveyed by antiviral T cells. Upon viral reactivation (i), IP-10 is induced via IFNs and leads to the recruitment of antiviral CXCR3+ T cells, likely both Th1/17- and Th1 cells, to the CNS parenchyma from the blood stream (ii). Antiviral T cells rapidly control the virus, and tissue repair mechanisms ensure that damage to the CNS is limited. Relapses could be triggered when the same immune surveillance mechanism leads to the erroneous bystander recruitment of autoreactive Th1/17CM cells to the CNS in patients with multiple sclerosis (MS). In addition, infected CXCR3+ B cells could be recruited that transport viruses, such as Epstein-Barr virus (EBV) and John Cunningham virus (JCV) to the CNS. Th1/17CM cells in patients with MS, but not in healthy individuals, react with myelin-derived self-antigens and, thus, could attack healthy, uninfected tissues, inducing extensive tissue damage and relapses (iii). (B) Alternatively, autoreactive Th1/17CM cells could home spontaneously to the CNS parenchyma via CCR6 during the remission phase of patients with MS (i), and induce de novo viral reactivations and, consequently, the recruitment of CXCR3+ lymphocytes (ii). Reactivation of viruses in the CNS induced by autoreactive Th1/17CM cells could either trigger relapses or represent an amplification loop of virus-induced relapses. Notably, autoreactive Th17 cells in healthy individuals could also induce viral reactivation in the CNS by this mechanism, but they are less pathogenic.
Concluding Remarks and Future Perspectives
How infections promote autoimmune diseases is a fascinating and clinically relevant topic. In MS, the field has been dominated by the search for a single causative infectious agent and, due to strong epidemiological evidence, EBV has attracted the most attention. The intriguing molecular mimicry hypothesis has further underlined a possible role of EBV. However, accumulating evidence indicates that several neurotropic viruses, including JCV, could have a role in MS, and it is also possible that different viruses could be important in individual patients with MS. In this review, we discussed some poorly understood, intensively debated, and understudied aspects in the field. We have proposed possible mechanisms for how viral infections could generate pathogenic Th1/17CM cells from autoreactive memory cells upon bystander activation, how these Th1/17CM cells could progressively expand at the cost of protective, antiviral Th1 cells in the remission phase, and how they could finally be recruited to the CNS upon viral reactivation to promote relapses. Notably, the molecular mimicry concept and this bystander generation/recruitment model are not mutually exclusive. However, the finding that JCV-specific and autoreactive T cells in patients with MS are largely segregated into two different subsets of Th1 and Th1/17 cells with different properties suggests that selective targeting of Th1/17 cells could inhibit relapses without inducing PML. More research on antiviral immune responses in animal models of MS and in patients will be necessary to unravel the complex relationships between viruses and MS, to predict relapses, and, ultimately, to develop more efficient and/or selective therapies.
Outstanding Questions.
Is there a single virus that causes MS, or could several different viruses promote MS in a partially redundant manner by bystander mechanisms?
Do viral reactivations in the CNS lead to the recruitment of proinflammatory, myelin-reactive T cells? Do relapses triggered by autoreactive T cells induce viral reactivations?
How are pathogenic immune responses in relapses regulated and terminated? What is the role of different Treg subsets in MS?
How could pathogenic T cells be targeted without interfering with antiviral immune surveillance? Could their generation be prevented in high-risk individuals?
Are humoral and cellular antiviral immune responses altered in patients with MS? What is the role of CTL, B cells, and antibodies in MS?
What is the role of viruses in progressive MS? Is the same immune response compartmentalized in the CNS, or is it a completely different response?
Glossary
- Blood–brain barrier (BBB)
comprises two physical barriers of endothelial cells and the parenchymal base membrane that strongly limit the access of macromolecules and cells to the CNS parenchyma. The endothelial and parenchymal basement membranes define the inner and outer limits of the perivascular space, which is filled by cerebrospinal fluid (CSF), which drains macromolecules and immune cells into deep cervical lymph nodes.
- Central memory (TCM) and effector memory T (TEM) cells
distinguished by CCR7 expression in humans and by CD62L expression in mice; these cells home preferentially to lymphoid and nonlymphoid tissues, respectively. TEM produce higher levels of proinflammatory cytokines than do TCM, but both TCM and TEM contain Th1, Th17, and Th1/17 cells. Moreover, TCM express adhesion and chemokine receptors, enabling them to home to the CNS.
- Clinically isolated syndrome (CIS)
a first attack is classified as CIS unless MS is diagnosed following the demonstration of lesion dissemination in space over time at MRI or the occurrence of a second clinical attack.
- Experimental autoimmune encephalopathy (EAE)
the most-studied animal model of MS. CNS inflammation and disability is induced by immunization with myelin antigens and adjuvants, or by the adoptive transfer of activated, myelin-specific Th cells.
- Progressive multifocal leukoencephalopathy (PML)
an often fatal demyelinating CNS disease caused by uncontrolled JCV replication. It is largely limited to individuals where CD4+ T cell responses are impaired, such as patients with AIDS or MS, where CNS immune surveillance is inhibited by natalizumab.
- T helper 1 (Th1) and T helper 17 (Th17) cells
uncommitted naïve CD4+ T cells can differentiate upon antigenic activation under the influence of different cytokines to IFN-γ-producing Th1- or IL-17-producing Th17 cells. Th1 cells are induced by IL-12 and mediate protection against intracellular pathogens, such as viruses, while Th17 cells are induced by TGF-β, IL-1, IL-6, and Il-23, and are vital for dealing with extracellular bacteria and fungi. Th1/17 cells co-produce IFN-γ and IL-17 and can respond to both intra- and extracellular pathogens.
References
- 1.Dendrou C.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 2.Nylander A., Hafler D.A. Multiple sclerosis. J. Clin. Invest. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ontaneda D. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol. 2015;14:208–223. doi: 10.1016/S1474-4422(14)70264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollenbach J.A., Oksenberg J.R. The immunogenetics of multiple sclerosis: a comprehensive review. J. Autoimmun. 2015;64:13–25. doi: 10.1016/j.jaut.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuvich R.L. Genetics and pathogenesis of multiple sclerosis. Semin. Immunol. 2009;21:328–333. doi: 10.1016/j.smim.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussman J.P. GWAS analysis implicates NF-kappaB-mediated induction of inflammatory T cells in multiple sclerosis. Genes Immun. 2016;17:305–312. doi: 10.1038/gene.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correale J., Gaitan M.I. Multiple sclerosis and environmental factors: the role of vitamin D, parasites, and Epstein-Barr virus infection. Acta Neurol. Scand. 2015;132:46–55. doi: 10.1111/ane.12431. [DOI] [PubMed] [Google Scholar]
- 8.Walsh K.P. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J. Immunol. 2009;183:1577–1586. doi: 10.4049/jimmunol.0803803. [DOI] [PubMed] [Google Scholar]
- 9.Tselis A. Evidence for viral etiology of multiple sclerosis. Semin. Neurol. 2011;31:307–316. doi: 10.1055/s-0031-1287656. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen T.R. Multiple sclerosis after infectious mononucleosis. Arch. Neurol. 2007;64:72–75. doi: 10.1001/archneur.64.1.72. [DOI] [PubMed] [Google Scholar]
- 11.International Multiple Sclerosis Genetics Consortium Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakalacheva K. Viral triggers of multiple sclerosis. Biochim. Biophys. Acta. 2011;1812:132–140. doi: 10.1016/j.bbadis.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broccolo F. Possible role of human herpesvirus 6 as a trigger of autoimmune disease. Sci. World J. 2013;2013:867389. doi: 10.1155/2013/867389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotelo J. On the viral hypothesis of multiple sclerosis: participation of varicella-zoster virus. J. Neurol. Sci. 2007;262:113–116. doi: 10.1016/j.jns.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Stoner G.L. Implications of progressive multifocal leukoencephalopathy and JC virus for the etiology of MS. Acta Neurol. Scand. 1991;83:20–33. [PubMed] [Google Scholar]
- 16.Christensen T. Association of human endogenous retroviruses with multiple sclerosis and possible interactions with herpes viruses. Rev. Med. Virol. 2005;15:179–211. doi: 10.1002/rmv.465. [DOI] [PubMed] [Google Scholar]
- 17.Christensen T. Human herpesviruses in MS. Int. MS J. 2007;14:41–47. [PubMed] [Google Scholar]
- 18.Berger J.R., Houff S. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol. Res. 2006;28:299–305. doi: 10.1179/016164106X98198. [DOI] [PubMed] [Google Scholar]
- 19.Louveau A. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prineas J.W. Multiple sclerosis: presence of lymphatic capillaries and lymphoid tissue in the brain and spinal cord. Science. 1979;203:1123–1125. doi: 10.1126/science.424741. [DOI] [PubMed] [Google Scholar]
- 21.Russo M.V., McGavern D.B. Immune Surveillance of the CNS following Infection and Injury. Trends Immunol. 2015;36:637–650. doi: 10.1016/j.it.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher J.M. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsunoda I., Fujinami R.S. Neuropathogenesis of Theiler’s murine encephalomyelitis virus infection, an animal model for multiple sclerosis. J. Neuroimmune Pharmacol. 2010;5:355–369. doi: 10.1007/s11481-009-9179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervantes-Barragan L. Regulatory T cells selectively preserve immune privilege of self-antigens during viral central nervous system infection. J. Immunol. 2012;188:3678–3685. doi: 10.4049/jimmunol.1102422. [DOI] [PubMed] [Google Scholar]
- 25.Kapil P. Interleukin-12 (IL-12), but not IL-23, deficiency ameliorates viral encephalitis without affecting viral control. J. Virol. 2009;83:5978–5986. doi: 10.1128/JVI.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aly L. Central role of JC virus-specific CD4+ lymphocytes in progressive multi-focal leucoencephalopathy-immune reconstitution inflammatory syndrome. Brain. 2011;134:2687–2702. doi: 10.1093/brain/awr206. [DOI] [PubMed] [Google Scholar]
- 27.Cupovic J. Central nervous system stromal cells control local CD8(+) T cell responses during virus-induced neuroinflammation. Immunity. 2016;44:622–633. doi: 10.1016/j.immuni.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollebo H.S. Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann. Neurol. 2015;77:560–570. doi: 10.1002/ana.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellmerich S. Disease-related epitope spread in a humanized T cell receptor transgenic model of multiple sclerosis. Eur. J. Immunol. 2004;34:1839–1848. doi: 10.1002/eji.200324044. [DOI] [PubMed] [Google Scholar]
- 30.Elong Ngono A. Frequency of circulating autoreactive T cells committed to myelin determinants in relapsing-remitting multiple sclerosis patients. Clin. Immunol. 2012;144:117–126. doi: 10.1016/j.clim.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 2015;7:287ra74. doi: 10.1126/scitranslmed.aaa8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paroni M. Recognition of viral and self-antigens by TH1 and TH1/TH17 central memory cells in patients with multiple sclerosis reveals distinct roles in immune surveillance and relapses. J. Allergy Clin. Immunol. 2017 doi: 10.1016/j.jaci.2016.11.045. Published online February 1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J. Exp. Med. 1994;179:973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markovic-Plese S. Degenerate T-cell receptor recognition, autoreactive cells, and the autoimmune response in multiple sclerosis. Neuroscientist. 2009;15:225–231. doi: 10.1177/1073858409332404. [DOI] [PubMed] [Google Scholar]
- 35.Stinissen P., Hellings N. Activation of myelin reactive T cells in multiple sclerosis, a possible role for T cell degeneracy? Eur. J. Immunol. 2008;38:1190–1193. doi: 10.1002/eji.200838371. [DOI] [PubMed] [Google Scholar]
- 36.Bhardwaj V. Degenerate recognition of a dissimilar antigenic peptide by myelin basic protein-reactive T cells: Implications for thymic education and autoimmunity. J. Immunol. 1993;151:5000–5010. [PubMed] [Google Scholar]
- 37.Harkiolaki M. T cell-mediated autoimmune disease due to low-affinity crossreactivity to common microbial peptides. Immunity. 2009;30:348–357. doi: 10.1016/j.immuni.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Wucherpfennig K.W., Strominger J.L. Molecular mimicry in T cell-mediated autoimmunity, viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sospedra M., Martin R. Molecular mimicry in multiple sclerosis. Autoimmunity. 2006;39:3–8. doi: 10.1080/08916930500484922. [DOI] [PubMed] [Google Scholar]
- 40.Rose N.R. Molecular mimicry and clonal deletion, a fresh look. J. Theor. Biol. 2015;375:71–76. doi: 10.1016/j.jtbi.2014.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson R.W. T cell receptor cross-reactivity between similar foreign and self peptides influences naïve cell population size and autoimmunity. Immunity. 2015;42:95–107. doi: 10.1016/j.immuni.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger G. Escape of pathogens from the host immune response by mutations and mimicry. Possible means to improve vaccine performance. Med. Hypotheses. 2015;85:664–669. doi: 10.1016/j.mehy.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Kyewski B., Klein L. A central role for central tolerance. Annu. Rev. Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz R.H. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 45.Lunemann J.D. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-gamma and IL-2. J. Exp. Med. 2008;205:1763–1773. doi: 10.1084/jem.20072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berer K. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 47.Magliozzi R. B-cell enrichment and Epstein-Barr virus infection in inflammatory cortical lesions in secondary progressive multiple sclerosis. J. Neuropathol. Exp. Neurol. 2013;72:29–41. doi: 10.1097/NEN.0b013e31827bfc62. [DOI] [PubMed] [Google Scholar]
- 48.Housley W.J. Biomarkers in multiple sclerosis. Clin. Immunol. 2015;161:51–58. doi: 10.1016/j.clim.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Brettschneider J. IgG antibodies against measles, rubella, and varicella zoster virus predict conversion to multiple sclerosis in clinically isolated syndrome. PLoS One. 2009;4:e7638. doi: 10.1371/journal.pone.0007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salzer J. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology. 2016;87:2074–2081. doi: 10.1212/WNL.0000000000003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehmann-Horn K. Targeting B cells in the treatment of multiple sclerosis: recent advances and remaining challenges. Ther. Adv. Neurol. Disord. 2013;6:161–173. doi: 10.1177/1756285612474333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molnarfi N. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J. Exp. Med. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chapagain M.L., Nerurkar V.R. Human polyomavirus JC (JCV) infection of human B lymphocytes: a possible mechanism for JCV transmigration across the blood-brain barrier. J. Infect. Dis. 2010;202:184–191. doi: 10.1086/653823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serafini B. Epstein-Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: implications for viral persistence and intrathecal B-cell activation. J. Neuropathol. Exp. Neurol. 2010;69:677–693. doi: 10.1097/NEN.0b013e3181e332ec. [DOI] [PubMed] [Google Scholar]
- 55.Mattson D.H. Oligoclonal IgG in multiple sclerosis and subacute sclerosing panencephalitis brains. J. Neuroimmunol. 1982;2:261–276. doi: 10.1016/0165-5728(82)90059-5. [DOI] [PubMed] [Google Scholar]
- 56.Pohl D. Intrathecal antibody production against Epstein-Barr and other neurotropic viruses in pediatric and adult onset multiple sclerosis. J. Neurol. 2010;257:212–216. doi: 10.1007/s00415-009-5296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jarius S. The intrathecal, polyspecific antiviral immune response: Specific for MS or a general marker of CNS autoimmunity? J. Neurol. Sci. 2009;280:98–100. doi: 10.1016/j.jns.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Castellazzi M. Epstein-Barr virus-specific intrathecal oligoclonal IgG production in relapsing-remitting multiple sclerosis is limited to a subset of patients and is composed of low-affinity antibodies. J. Neuroinflammation. 2014;11:188. doi: 10.1186/s12974-014-0188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lossius A. High-throughput sequencing of TCR repertoires in multiple sclerosis reveals intrathecal enrichment of EBV-reactive CD8+ T cells. Eur. J. Immunol. 2014;44:3439–3452. doi: 10.1002/eji.201444662. [DOI] [PubMed] [Google Scholar]
- 60.Angelini D.F. Increased CD8+ T cell response to Epstein-Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 2013;9:e1003220. doi: 10.1371/journal.ppat.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serafini B. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willis S.N. Epstein-Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain. 2009;132:3318–3328. doi: 10.1093/brain/awp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Armangue T. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann. Neurol. 2014;75:317–323. doi: 10.1002/ana.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mancuso R. Detection of viral DNA sequences in the cerebrospinal fluid of patients with multiple sclerosis. J. Med. Virol. 2010;82:1051–1057. doi: 10.1002/jmv.21764. [DOI] [PubMed] [Google Scholar]
- 65.Marzocchetti A. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J. Clin. Microbiol. 2005;43:4175–4177. doi: 10.1128/JCM.43.8.4175-4177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pietersma F. Immune surveillance of EBV–infected B cells and the development of non-Hodgkin lymphomas in immunocompromised patients. Leuk. Lymphoma. 2008;49:1028–1041. doi: 10.1080/10428190801911662. [DOI] [PubMed] [Google Scholar]
- 67.Gasnault J. Critical role of JC virus-specific CD4 T-cell responses in preventing progressive multifocal leukoencephalopathy. AIDS. 2003;17:1443–1449. doi: 10.1097/00002030-200307040-00004. [DOI] [PubMed] [Google Scholar]
- 68.Delbue S. JC virus urinary excretion and seroprevalence in natalizumab-treated multiple sclerosis patients. J. Neurovirol. 2014;21:645–652. doi: 10.1007/s13365-014-0268-0. [DOI] [PubMed] [Google Scholar]
- 69.Rivino L. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engelhardt B., Ransohoff R.M. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Rothhammer V. Th17 lymphocytes traffic to the central nervous system independently of alpha4 integrin expression during EAE. J. Exp. Med. 2011;208:2465–2476. doi: 10.1084/jem.20110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schneider-Hohendorf T. VLA-4 blockade promotes differential routes into human CNS involving PSGL-1 rolling of T cells and MCAM-adhesion of TH17 cells. J. Exp. Med. 2014;211:1833–1846. doi: 10.1084/jem.20140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clissi B. Chemokines fail to up-regulate beta 1 integrin-dependent adhesion in human Th2 T lymphocytes. J. Immunol. 2000;164:3292–3300. doi: 10.4049/jimmunol.164.6.3292. [DOI] [PubMed] [Google Scholar]
- 74.Rivino L. CCR6 is expressed on an IL-10-producing, autoreactive memory T cell population with context-dependent regulatory function. J. Exp. Med. 2010;207:565–577. doi: 10.1084/jem.20091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinfelder S. Epigenetic modification of the human CCR6 gene is associated with stable CCR6 expression in T cells. Blood. 2011;117:2839–2846. doi: 10.1182/blood-2010-06-293027. [DOI] [PubMed] [Google Scholar]
- 76.Annunziato F. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acosta-Rodriguez E.V. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 78.Arima Y. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell. 2012;148:447–457. doi: 10.1016/j.cell.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 79.Reboldi A. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 80.Balashov K.E. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geginat J. The CD4-centered universe of human T cell subsets. Semin. Immunol. 2013;25:252–262. doi: 10.1016/j.smim.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Tsunoda I. Distinct roles for IP-10/CXCL10 in three animal models, Theiler’s virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Mult. Scler. 2004;10:26–34. doi: 10.1191/1352458504ms982oa. [DOI] [PubMed] [Google Scholar]
- 83.Noor S., Wilson E.H. Role of C-C chemokine receptor type 7 and its ligands during neuroinflammation. J. Neuroinflammation. 2012;9:77. doi: 10.1186/1742-2094-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sallusto F. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 85.Kivisakk P. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann. Neurol. 2004;55:627–638. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- 86.Krumbholz M. CCL19 is constitutively expressed in the CNS, up-regulated in neuroinflammation, active and also inactive multiple sclerosis lesions. J. Neuroimmunol. 2007;190:72–79. doi: 10.1016/j.jneuroim.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 87.Sallusto F. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur. J. Immunol. 1999;29:2037–2045. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 88.Mehling M. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71:1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- 89.Becher B., Segal B.M. T(H)17 cytokines in autoimmune neuro-inflammation. Curr. Opin. Immunol. 2011;23:707–712. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gran B. Role of the IL-12/IL-23 system in the regulation of T-cell responses in central nervous system inflammatory demyelination. Crit. Rev. Immunol. 2004;24:111–128. doi: 10.1615/critrevimmunol.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- 91.Cua D.J. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 92.Sarin R. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9560–9565. doi: 10.1073/pnas.1017854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nair R.P. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat. Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Limbergen J. The genetics of Crohn’s disease. Annu. Rev. Genomics Hum. Genet. 2009;10:89–116. doi: 10.1146/annurev-genom-082908-150013. [DOI] [PubMed] [Google Scholar]
- 95.Ramos P.S. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011;7:e1002406. doi: 10.1371/journal.pgen.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kebir H. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 97.Cosmi L. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kastirr I. IL-21 Is a Central Memory T Cell-Associated Cytokine That Inhibits the Generation of Pathogenic Th1/17 Effector Cells. J. Immunol. 2014;193:3322–3331. doi: 10.4049/jimmunol.1400775. [DOI] [PubMed] [Google Scholar]
- 99.Duhen T., Campbell D.J. IL-1beta promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J. Immunol. 2014;193:120–129. doi: 10.4049/jimmunol.1302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kebir H. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang Z. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat. Neurosci. 2013;16:1401–1408. doi: 10.1038/nn.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knier B. Novel monoclonal antibodies for therapy of multiple sclerosis. Expert Opin. Biol. Ther. 2014;14:503–513. doi: 10.1517/14712598.2014.887676. [DOI] [PubMed] [Google Scholar]
- 103.Kroenke M.A., Segal B.M. IL-23 modulated myelin-specific T cells induce EAE via an IFNgamma driven, IL-17 independent pathway. Brain Behav. Immun. 2011;25:932–937. doi: 10.1016/j.bbi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Komiyama Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 105.Willenborg D.O. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 106.Codarri L. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 107.McQualter J.L. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J. Exp. Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Noster R. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci. Transl. Med. 2014;6:241ra80. doi: 10.1126/scitranslmed.3008706. [DOI] [PubMed] [Google Scholar]
- 109.Geginat J. Immunity to Pathogens Taught by Specialized Human Dendritic Cell Subsets. Front. Immunol. 2015;6:527. doi: 10.3389/fimmu.2015.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kleinewietfeld M., Hafler D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013;25:305–312. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haringer B. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J. Exp. Med. 2009;206:1009–1017. doi: 10.1084/jem.20082238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Facciotti F. IL-10-producing forkhead box protein 3-negative regulatory T cells inhibit B-cell responses and are involved in systemic lupus erythematosus. J. Allergy Clin. Immunol. 2016;137:318–321. doi: 10.1016/j.jaci.2015.06.044. e5. [DOI] [PubMed] [Google Scholar]
- 113.Battaglia M. Tr1 cells: from discovery to their clinical application. Semin. Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 114.McGeachy M.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 115.Lexberg M.H. IFN-gamma and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur. J. Immunol. 2010;40:3017–3027. doi: 10.1002/eji.201040539. [DOI] [PubMed] [Google Scholar]
- 116.Nizzoli G. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T cell responses. Blood. 2013;122:932–942. doi: 10.1182/blood-2013-04-495424. [DOI] [PubMed] [Google Scholar]
- 117.Hirota K. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geginat J. Plasticity of human CD4 T cell subsets. Front. Immunol. 2014;5:630. doi: 10.3389/fimmu.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Geginat J. Reverse plasticity: TGF-beta and IL-6 induce Th1-to-Th17-cell transdifferentiation in the gut. Eur. J. Immunol. 2016;46:2306–2310. doi: 10.1002/eji.201646618. [DOI] [PubMed] [Google Scholar]
- 120.Haghikia A. Therapies for multiple sclerosis: translational achievements and outstanding needs. Trends Mol. Med. 2013;19:309–319. doi: 10.1016/j.molmed.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 121.Gregory S.G. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat. Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 122.Park J.H. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 123.Feau S. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat. Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaech S.M. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 125.Lenz D.C. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Purton J.F. Antiviral CD4+ memory T cells are IL-15 dependent. J. Exp. Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Geginat J. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 128.Tan J.T. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cox A.L. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur. J. Immunol. 2005;35:3332–3342. doi: 10.1002/eji.200535075. [DOI] [PubMed] [Google Scholar]
- 130.Kivisakk P. Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurology. 2009;72:1922–1930. doi: 10.1212/WNL.0b013e3181a8266f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perkins M.R. Changes in JC virus-specific T cell responses during natalizumab treatment and in natalizumab-associated progressive multifocal leukoencephalopathy. PLoS Pathog. 2012;8:e1003014. doi: 10.1371/journal.ppat.1003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Macallan D.C. Rapid turnover of effector-memory CD4(+) T cells in healthy humans. J. Exp. Med. 2004;200:255–260. doi: 10.1084/jem.20040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cui G. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1915–1920. doi: 10.1073/pnas.1318281111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jung Y.W. CCR7 expression alters memory CD8 T-cell homeostasis by regulating occupancy in IL-7- and IL-15-dependent niches. Proc. Natl. Acad. Sci. U. S. A. 2016;113:8278–8283. doi: 10.1073/pnas.1602899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Odoardi F. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 136.Okhrimenko A. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc. Natl. Acad. Sci. U. S. A. 2014;111:9229–9234. doi: 10.1073/pnas.1318731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Di Rosa F. Two Niches in the Bone Marrow: a Hypothesis on Life-long T Cell Memory. Trends Immunol. 2016;37:503–512. doi: 10.1016/j.it.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 138.Saure C. Natalizumab and impedance of the homing of CD34+ hematopoietic progenitors. Arch. Neurol. 2011;68:1428–1431. doi: 10.1001/archneurol.2011.238. [DOI] [PubMed] [Google Scholar]
- 139.Mattoscio M. Hematopoietic mobilization: potential biomarker of response to natalizumab in multiple sclerosis. Neurology. 2015;84:1473–1482. doi: 10.1212/WNL.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J. Exp. Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kassiotis G. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat. Immunol. 2002;3:244–2450. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 142.Nizzoli G. IL-10 promotes homeostatic proliferation of human CD8(+) memory T cells and, when produced by CD1c(+) DCs, shapes naïve CD8(+) T-cell priming. Eur. J. Immunol. 2016;46:1622–1632. doi: 10.1002/eji.201546136. [DOI] [PubMed] [Google Scholar]
- 143.McGill J. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J. Exp. Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen X.L. IL-15 trans-presentation regulates homeostasis of CD4(+) T lymphocytes. Cell Mol. Immunol. 2014;11:387–397. doi: 10.1038/cmi.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saraste M. Natalizumab treatment leads to an increase in circulating CXCR3-expressing B cells. Neurol. Neuroimmunol. Neuroinflamm. 2016;3:e292. doi: 10.1212/NXI.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Galimberti D. CXCL10 haplotypes and multiple sclerosis: association and correlation with clinical course. Eur. J. Neurol. 2007;14:162–167. doi: 10.1111/j.1468-1331.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 147.Kivisakk P. T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin. Exp. Immunol. 2002;129:510–518. doi: 10.1046/j.1365-2249.2002.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Preston C.M., Efstathiou S. Molecular basis of HSV latency and reactivation. In: Arvin A., editor. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; 2007. pp. 1–15. [Google Scholar]
- 149.Wollebo H.S. Cooperative roles of NF-kappaB and NFAT4 in polyomavirus JC regulation at the KB control element. Virology. 2012;432:146–154. doi: 10.1016/j.virol.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Medawar P.B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]