Abstract
Background
Viral upper respiratory tract infections occur frequently among conscripts. Probiotics have reduced viral infections in children attending day care. Limited data are available on the effects of probiotics on the nasopharyngeal presence of respiratory viruses.
Objectives
To assess, whether probiotics could decrease nasopharyngeal occurrence of respiratory viruses in Finnish conscripts.
Study design
In a randomized, double-blind, placebo-controlled 90- and 150-day intervention study, 239 nasopharyngeal swab samples were collected from 192 symptomatic conscripts receiving daily chewable probiotic tablet containing Lactobacillus rhamnosus GG and Bifidobacterium animalis ssp. lactis BB-12 (46.9%) or control tablet (53.1%) on visits to a garrison's health care center due to symptoms of infection. The presence of respiratory viruses was tested by PCR-methods, and viral findings were compared between the intervention groups.
Results
184 (76.9%) nasopharyngeal samples were positive for at least one respiratory virus. Picornaviruses were the most common viruses and were detected in 155 (84.2%) of samples. Of these, 143 (92.3%) were rhinovirus-positive and 20 (12.9%) were enterovirus-positive. The control group had 83 (64%) and the probiotic group 72 (66%) picornavirus infections (p = 0.79). Monthly distribution of picornaviruses showed that there were less picornavirus findings after 3 months in the probiotic group than in the control group (p = 0.0069). However, probiotics did not reduce picornavirus occurrence in other months.
Conclusions
Overall, probiotics did not reduce viral occurrence in symptomatic conscripts. However, probiotics decreased the presence of picornaviruses after 3 months, which may imply that probiotics play a role against viruses causing common cold. Further investigations are necessary to clarify the mechanisms involved in order to target specific probiotics on specific respiratory viruses.
Abbreviations: RTI, respiratory tract infection; RV, human rhinovirus; EV, human enterovirus; L., lactobacillus; B., bifidobacterium; RSV, respiratory syncytial virus; PIV, parainfluenza virus; AdV, adenovirus; hMPV, human metapneumovirus; HBoV, human bocavirus
Keywords: Probiotic, Respiratory virus, Rhinovirus, Conscript
1. Background
The most common illnesses in humans are acute respiratory tract infections (RTIs) and the majority of them are common colds. Acute RTIs are also the leading cause of missed days of service among military conscripts. Annually approximately 27 000 conscripts are trained in Finland. In 2007 in the garrisons around Finland, over 25 000 upper RTI cases, 4300 maxillary sinus infections, and 1300 middle ear infections were diagnosed [1]. Moreover, approximately 1500 cases of bronchitis and 800 pneumonias were reported [1]. Military trainees are especially at high risk for epidemics of respiratory infection due to the crowded living conditions in barracks, environmental factors, and physical stress related to military training [2].
Over 200 virus types are known to cause acute RTIs. The most frequent causative agent of common cold and RTIs of varying severity are human rhinoviruses (RVs). Currently, the only effective antivirals and vaccines for the prevention and treatment of respiratory virus infections are available against influenza viruses. In addition, adenovirus vaccines against serotypes 4 and 7 are available in the U.S. army. However, for the common cold and against RVs there are virtually no effective treatment or medication. The only current approach is the prevention of virus transmission by avoiding close contact and hand hygiene [3].
Probiotics are defined as live microorganisms that confer a health benefit on the host [4]. Increasing evidence shows that probiotics may provide protection against RTIs [5], [6], which in most cases are viral origin. The use of specific probiotics such as Lactobacillus (L.) rhamnosus GG and Bifidobacterium (B.) animalis ssp. lactis BB-12 have shown promising results in reducing respiratory symptoms and illnesses especially in children [5], [7], [8], [9], [10], [11]. Moreover, combination of L. rhamnosus GG and B. lactis BB-12 have reduced the risk or the duration of RTIs in children and in young adults [12], [13]. The most convincing results of the effectiveness of probiotics in virus infections are studies of specific probiotics in alleviating the duration and severity of acute rotavirus gastroenteritis [14]. In respiratory virus infections, however, there are only few implications that probiotics may reduce viral occurrence in the nasopharynx [11], [15], [16].
2. Objectives
The aim of this study was to investigate the effects of regular consumption of probiotic combination L. rhamnosus GG and B. lactis BB-12 on the nasopharyngeal occurrence of respiratory viruses of two conscript groups suffering from respiratory infection attending military service in Finland from July 2012 to January 2013.
3. Study design
3.1. Subjects and intervention
This study is a part of larger randomized, double-blind, and placebo-controlled parallel group intervention study investigating whether L. rhamnosus GG and B. lactis BB-12 can decrease infections in conscripts in Gulf of Finland Naval Command in Upinniemi and Hamina (http://clinicaltrials.gov; identifier NCT01651195) (Kalima et al., manuscript). This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Ethics Committee of Helsinki and Uusimaa Hospital District. Written informed consent was obtained from all participants. Originally, a total of 982 healthy adults aged 18–28 years (mean 19.3 years), who fulfilled the inclusion criteria (Kalima et al., manuscript), were enrolled in the study from two intakes of conscripts in July 2012 (recruits) and in the end of the October 2012 (reserve officer candidates). Conscripts were randomized to receive either one chewing tablet (Orion Pharma Ltd., Espoo, Finland) containing 37.3% xylitol, 37.3% sorbitol, 7.5% microfibrous cellulose, 3.0% magnesium stearate, and 1.9% citrus flavor (n = 50.2%; control group), or the same tablet with 5 × 109 CFU of L. rhamnosus GG (ATCC 53103) and 2 × 109 CFU of B. animalis ssp. lactis (DSM 15954; Chr. Hansen A/S, Hoersholm, Denmark) (n = 49.8%; probiotic group) twice daily either from July to December 2012 (recruits, 150 days) or from October 2012 to January 2013 (reserve officer candidates, 90 days). During the intervention, whenever the conscripts suffered from upper RTI symptoms and sought treatment from the garrisons healthcare center, a nasopharyngeal swab sample was collected by a flocked-tip nylon swab (Copan, Diagnostics Murrieta, CA, USA) inserted into the nasopharynx through the nose. The swabs were placed immediately into a vial containing 3 mL universal transport medium (UTM-RT, Copan, Diagnostics, Murrieta, CA, USA), and then stored at −70 °C.
3.2. Viral nucleic acid extraction
For xTAG respiratory virus panel assay viral nucleic acids were purified from collected swab samples by MagNA Pure robot (Roche Diagnostics Ltd., United Kingdom) according to the manufacturer's instructions. For human rhino (RV) and enterovirus (EV) PCR assays, viral nucleic acids were purified as described [16].
3.3. PCR assays for respiratory viruses
3.3.1. The xTAG respiratory virus panel (RVP Fast) assay
The RVP Fast assay detects following respiratory viruses: influenza A and B viruses, respiratory syncytial viruses (RSV) A and B, parainfluenzaviruses 1–4 (PIV1-4), adenovirus (AdV), human metapneumovirus (hMPV), coronaviruses 229E, OC43, NL63 and HKU1, picornaviruses (EV/RV), and human bocavirus (HBoV). Additionally, the assay allows subtyping of influenza A virus to seasonal H1 or H3 viruses or to an unsubtypable influenza A virus. The assay was performed as described earlier [17]. Briefly, total nucleic acid from samples stored at −70 °C was isolated by MagNA Pure robot (Roche Diagnostics Ltd.) and the extracts were instantly subjected to the RVP Fast assay (Luminex Molecular Diagnostics Inc., Toronto, Canada).
3.3.2. EV and RV PCR assays
Real-time PCR for picornaviruses RV and EV was performed at the National Institute for Health and Welfare, as described previously [18], [19]. Curves rising above the threshold were considered positive. All real-time assays were executed with the Mx3005P analyzer (Stratagene, La Jolla, CA, USA).
3.4. Statistical analysis
We evaluated the difference of the number of subjects suffering from a specific virus between the two study groups. Each nasopharyngeal sample represented one virus infection. The conscripts were first analyzed separately, and then they were merged and analyzed together. For each virus, the analyses were performed on the monthly symptoms and the symptoms throughout the whole period independently. Because of the relatively small sample size, we applied Fisher's exact test to the contingency tables, where the frequency distribution of getting a virus over these two groups is displayed in a 2 × 2 matrix format. Results with p-value <0.05 were considered statistically significant. All analyses were conducted using R version 2.15.2 (The R Foundation for Statistical Computing).
4. Results
4.1. Nasopharyngeal samples
A total of 239 nasopharyngeal swab samples were collected from 192 symptomatic conscripts (102 (53.1%) in the control group, and 90 (46.9%) in the probiotic group) from visits to garrison's healthcare centers during the intervention period. At least one nasopharyngeal swab sample was collected from each conscript (mean 1, range 1–3). The monthly distribution of collected nasopharyngeal samples of conscript groups between the intervention groups is shown in Table 1 . The collected samples were almost equally distributed between the intervention groups, except in reserve officer candidates, as there were fewer collected samples in December and January in the probiotic group.
Table 1.
Monthly distribution of collected nasopharyngeal swab samples of conscripts between the intervention groups and conscript groups from July 2012 to January 2013 in Finland.
| Month | No. (%) of collected nasopharyngeal swab samples |
||||
|---|---|---|---|---|---|
| Recruits |
Reserve officer candidates |
Total | |||
| Control | Probiotic | Control | Probiotic | ||
| July | 51 (53) | 45 (47) | – | – | 96 |
| August | 20 (43) | 27 (57) | – | – | 47 |
| September | 17 (57) | 13 (43) | – | – | 30 |
| October | 8 (57) | 6 (43) | 0 (0) | 1 (100) | 15 |
| November | 1 (33) | 2 (67) | 4 (57) | 3 (43) | 10 |
| December | 1 (50) | 1 (50) | 16 (70) | 7 (30) | 25 |
| January | – | – | 11 (73) | 4 (27) | 15 |
| Total | 98 (51) | 94 (49) | 31 (67) | 15 (33) | 238a |
a Month information was not available from 1 sample
4.2. Virological findings and probiotic intervention
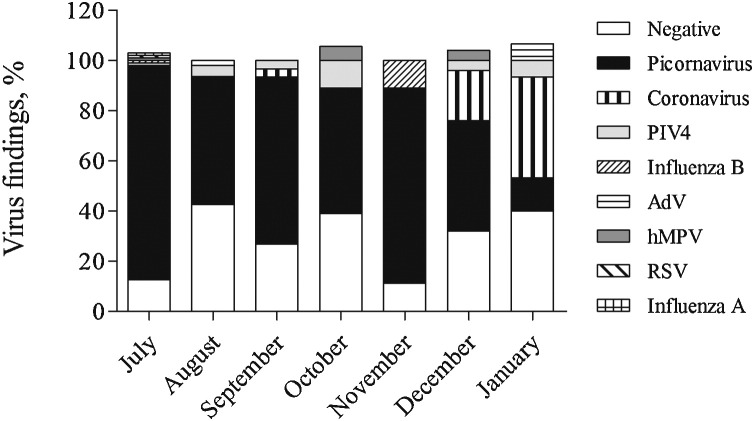
Of the 239 collected nasopharyngeal samples, 184 (76.9%) were positive for at least one respiratory virus. Co-infections with two, three, or four viruses were found in 2.9% of the samples studied. The majority of the virus containing samples were picornavirus-positive (n = 155; 84.2%), followed by human coronaviruses (NL63, OC43) (n = 8; 6.5%), PIV 4 (n = 8; 4.3%), hMPV (n = 3; 1.6%), AdV (n = 2; 1.1%), influenza B virus (n = 2; 1.1%), influenza A virus (n = 1; 0.5%), and RSV (n = 1; 0.5%). PIV 1–3 and HBoV were not detected in any of the specimens. RV and EV PCR- screening of picornavirus-positive samples revealed that out of 155 picornavirus-positive samples, 143 (92.3%) were RV-positive and 20 (12.9%) were EV-positive. All EV-positive specimens were also positive with RV PCR-assay. Viruses were detected in all months during the study. Picornaviruses were the most prevalent viruses in July 2012 to December 2012, whereas human coronaviruses peaked in January 2013 (Fig. 1 ).
Fig. 1.
Monthly distribution (%) of virus positive and negative findings in all conscripts who participated in the intervention study from July 2012 to January 2013 in Finland. The numbers of positive and negative findings were compared with the total number of collected samples in each month. Abbreviations: PIV, parainfluenza virus; AdV, adenovirus, hMPV, human metapneumovirus; RSV, respiratory syncytial virus.
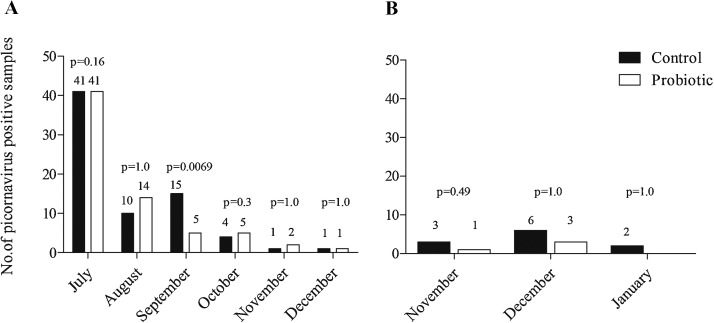
Table 2 shows the distribution of detected respiratory viruses in the nasopharyngeal samples of conscripts groups between the intervention groups. Overall, fewer viruses were detected in the probiotic group than in the control group, although the differences were not statistically significant. Probiotics did not decrease the total number of picornaviruses, RV, or EV in recruits, in reserve officer candidates, or in conscript groups combined. Fig. 2 shows the monthly distribution of picornavirus findings between the intervention groups in military recruits and reserve officer candidates. The monthly examination of picornavirus positive samples revealed that in September there were three times fewer picornaviruses (mainly RV) in the probiotic group (p = 0.0069) (Fig. 2). In other months studied, there were no significant differences in picornavirus positive samples between the intervention groups. Moreover, there were not any differences between the probiotic and the control group in the monthly distribution of other respiratory virus findings.
Table 2.
Distribution of respiratory viruses (%) between the intervention groups in 238 nasopharyngeal samples collected from symptomatic conscripts between July 2012 and January 2013 in Finland. There were no statistical differences in viral findings between the intervention groups. Abbreviations: RSV, respiratory syncytial virus; AdV, adenovirus; PIV, parainfluenza virus; RV, human rhinovirus; EV, human enterovirus; hMPV, human metapneumovirus.
| Virus | Recruits1 |
Reserve officer candidates2 |
Total3 |
P1 | P2 | P3 | |||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 98) | Probiotic (n = 94) | Control (n = 31) | Probiotic (n = 15) | Control (n = 129) | Probiotic (n = 109) | ||||
| RSV | 0 | 1 | 0 | 0 | 0 | 1 | 1.0 | 1.0 | 0.46 |
| Influenza A | 0 | 1 | 0 | 0 | 0 | 1 | 1.0 | 1.0 | 0.46 |
| Influenza B | 0 | 1 | 0 | 1 | 0 | 2 | 1.0 | 0.33 | 0.21 |
| AdV | 1 | 0 | 1 | 0 | 2 | 0 | 1.0 | 1.0 | 0.50 |
| PIV4 | 3 | 3 | 0 | 2 | 3 | 5 | 1.0 | 0.10 | 0.48 |
| Picornavirus | 72 | 68 | 11 | 4 | 83 | 72 | 0.87 | 0.74 | 0.79 |
| RV | 65 | 65 | 10 | 3 | 75 | 68 | 0.76 | 0.49 | 0.51 |
| EV | 7 | 9 | 4 | 0 | 11 | 9 | 0.6 | 0.29 | 1.0 |
| hMPV | 2 | 0 | 1 | 0 | 3 | 0 | 0.49 | 1.0 | 0.25 |
| Coronavirus | 1 | 0 | 8 | 3 | 9 | 3 | 1.0 | 1.0 | 0.23 |
| Total | 79 | 74 | 21 | 10 | 100 | 84 | 0.86 | 1.0 | 1.0 |
recruits.
reserve officer candidates.
total.
Fig. 2.
Monthly distribution of picornavirus findings in military recruits (A) and reserve officer candidates (B) between the intervention groups from July 2012 to January 2013 in Finland. Differences between the groups were analyzed using the Fisher exact-test.
5. Discussion
This study investigated whether regular consumption of probiotic combination of Lactobacillus rhamnosus GG and Bifidobacterium lactis BB-12 would decrease the nasopharyngeal occurrence of respiratory viruses in conscripts attending military service. Picornaviruses, in particular rhinoviruses, were the most common agents found in symptomatic conscripts. Probiotics did not decrease the total number of picornaviruses either in military recruits or in reserve officer candidates. However, the results showed that military recruits receiving probiotics had significantly fewer picornaviruses, after 3 months of intervention in September. The reducing effect was not observed in other months studied or in other viruses either in military recruits or in reserve officer candidates. The findings are possibly explained by the fact that conscripts were enrolled in the study in different months (July vs. end of October) and therefore different respiratory viruses were circulating in the population due to seasonal variation. In addition, probiotic's picornavirus reducing effect after 3 months of intervention imply that in order for probiotic to be effective, sufficient “priming time” is required. In otitis-prone children, probiotic combination was also able to reduce human bocavirus load after 3 months [15]. The reason why reducing effect was not observed in other viruses may be that different probiotic strains may have diverse antiviral mechanisms.
We are not aware of similar clinical studies conducted with young adults investigating the effects of probiotics on the nasal occurrence of respiratory viruses. Only few clinical studies in children imply that specific probiotics, such as L. rhamnosus GG, may have an impact in reducing the occurrence of respiratory viruses. In healthy children attending day care, children receiving L. rhamnosus GG in milk seemed to have fewer influenza virus A in the upper respiratory tract, although the difference was not statistically significant between the probiotic and the control group [16]. In otitis-prone children, combination of probiotics, including L. rhamnosus GG, reduced significantly the nasopharyngeal presence of human bocavirus DNA [15]. In preterm infants, L. rhamnosus GG reduced significantly the incidence of rhinovirus-induced episodes [11]. However, properties between probiotic species vary and are highly strain specific and the combination of probiotics may have different effects than single strains [20].
Probiotics may mediate their antiviral effects against rhinoviruses possibly by enhancing cellular immunity in the airways and increasing the activity of natural killer cells and macrophages, such as production of interferons. Probiotics may also hinder the absorption of rhinovirus to a host cell via binding to the intercellular adhesion molecule-1. For instance, in macrophage cell culture L. rhamnosus LC705 prevented influenza A virus replication and production of viral proteins by inducing type I interferon-dependent gene activation [21]. In mice, L. rhamnosus GG protected from influenza A virus infection by enhancing respiratory cell-mediated immune responses (interleukin-1β, tumor necrosis factor, and monocyte chemotactic protein-1), which up-regulated lung natural killer cells [22]. In rats with endotoxin-induced intestinal damage, Bifidobacterium infantis decreased the expression of intercellular adhesion molecule-1 [23]. However, mechanisms behind the antiviral effects of L. rhamnosus GG and B. lactis BB-12 against rhinoviruses should be further examined.
The most prevalent viruses in this study were picornaviruses. We found divergences between PCR-detection of picornaviruses by xTAG respiratory virus panel assay and RVs and EVs by separate screening assays. This could be partly explained by the fact that utilized PCR assays are targeted for different gene regions. In addition, it is possible that natural isolates of EV cross-react with the non-coding region-targeting primers and probe used in RV-specific real-time RT-PCR assay, and vice versa, possibly affecting the results [18], [24]. The final identification between RV and EV isolates should be executed via molecular typing of the capsid coding genes VP4-VP2 and VP1, respectively. However, at present the confined sensitivity is limiting the use of the typing assays in case of the positive findings with high C t-values in the real-time PCRs.
The abundance of picornavirus (of which most were RVs) findings was expected as RV is the most prevalent cause of common cold, particularly during early fall when up to 90% of the detected viruses among adolescents are RVs [25]. In the present study, RVs were detected in each month from July to January, with peak occurrence in July. This result was interesting in light of previous reports, where the peak prevalence of RV infections in the Northern hemisphere occurred in autumn and in spring [26]. However, RV infections have also been detected in July in the Finnish military in another study, although the detection rate was much lower than in our study [18]. The finding in the present study may reflect the fact that as large cohort of military recruits enter simultaneously to the semi-closed environment of a garrison, the infections spread rapidly due to high infection pressure. However, typing data to proof that there was an outbreak of RV infection caused by a small number of RV types during the recruitment period in July would be necessary. In contrast to picornaviruses, adenoviruses and influenza viruses, which are found commonly among military conscripts [27], [28], [29], occurred in the present study much lower prevalence. It should be noticed, however, that the results of the viral findings are slightly biased as the number of collected nasopharyngeal samples gradually decreased toward the end of the study due to the discontinuation of conscripts in the intervention trial [Kalima et al., manuscript]. On the influenza season 2012–2013 in Finland, the season peaked between January 2013 and March 2013 and therefore, the majority of these infections were missed in the present study.
Acute upper respiratory tract infections, also known as the common cold, are mainly caused by rhinoviruses. Due to its many different serotypes, developing a vaccine for rhinoviruses is unlikely. Other preventive and clinically useful measures, which should be simple, low cost, and without adverse reactions are needed in order to effectively control these infections. Along with proper hand hygiene, probiotic therapy could be an attractive and safe alternative in the prevention or alleviation of rhinovirus infections. In the present study, we observed fewer picornaviruses, predominantly rhinoviruses, in nasopharyngeal samples of military conscripts after 3 months use of probiotics L. rhamnosus GG and B. lactis BB-12. However, this reducing effect was not seen in the total number of picornavirus findings or in other months studied. Further studies revealing specific antiviral mechanism of probiotics L. rhamnosus GG and B. lactis BB-12 against rhinoviruses are warranted.
Funding
This study was supported by Scientific Advisory Board for Defense [grant number MAT819] in Finland and a grant from a special governmental subsidy for health sciences research in Finland.
Competing interests
None declared.
Ethical approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Ethics Committee of Helsinki and Uusimaa Hospital District. Written informed consent was obtained from all participants.
Acknowledgments
The authors are grateful to the personnel of the garrison's healthcare centers for collecting the nasopharyngeal swab samples. Eija Nenye-Lehtonen and Leena Juvonen are thanked for helping with the viral nucleic acid extraction. Svetlana Kaijalainen is acknowledged for helping with the picornavirus PCR-analyses. We are also grateful for Orion Ltd. for providing the study products.
Contributor Information
Liisa Lehtoranta, Email: liisa.lehtoranta@helsinki.fi.
Anne Pitkäranta, Email: anne.pitkaranta@helsinki.fi.
References
- 1.Vartti A.-M., Mäkitie I., Aro A., Henriksson M., Jormanainen V., Nikkari S. The perceptions and knowledge of conscripts concerning influenza in the beginning of military service. Suomen Lääkärilehti. 2009;64:3303–3310. Summary http://www.laakarilehti.fi/e/summary.html?type=4/news_id=7845/Influenza (in Finnish) [Google Scholar]
- 2.Juvonen R., Bloigu A., Peitso A., Silvennoinen-Kassinen S., Saikku P., Leinonen M. Risk factors for acute respiratory tract illness in military conscripts. Respirology. 2008;13:575–580. doi: 10.1111/j.1440-1843.2008.01299.x. [DOI] [PubMed] [Google Scholar]
- 3.Savolainen-Kopra C., Korpela T., Simonen-Tikka M.L., Amiryousefi A., Ziegler T., Roivainen M. Single treatment with ethanol hand rub is ineffective against human rhinovirus-hand washing with soap and water removes the virus efficiently. J Med Virol. 2012;84:543–547. doi: 10.1002/jmv.23222. [DOI] [PubMed] [Google Scholar]
- 4.FAO/WHO . World Health Oraganization; 2002. Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food.ftp://ftp.fao.org/es/esn/food/wgreport2.pdf [Google Scholar]
- 5.Hao Q., Lu Z., Dong B.R., Huang C.Q., Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2011;9:CD006895. doi: 10.1002/14651858.CD006895.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Popova M., Molimard P., Courau S., Crociani J., Dufour C., Le Vacon F. Beneficial effects of probiotics in upper respiratory tract infections and their mechanical actions to antagonize pathogens. J Appl Microbiol. 2012;113:1305–1318. doi: 10.1111/j.1365-2672.2012.05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatakka K., Savilahti E., Pönkä A., Meurman J.H., Poussa T., Näse L. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322:1–5. doi: 10.1136/bmj.322.7298.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumpu M., Kekkonen R.A., Kautiainen H., Järvenpää S., Kristo A., Huovinen P. Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2012;66:1020–1023. doi: 10.1038/ejcn.2012.62. [DOI] [PubMed] [Google Scholar]
- 9.Hojsak I., Snovak N., Abdovic S., Szajewska H., Misak Z., Kolacek S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2010;29:312–316. doi: 10.1016/j.clnu.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Taipale T., Pienihäkkinen K., Isolauri E., Larsen C., Brockmann E., Alanen P. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br J Nutr. 2011;105:409–416. doi: 10.1017/S0007114510003685. [DOI] [PubMed] [Google Scholar]
- 11.Luoto R., Ruuskanen O., Waris M., Kalliomäki M., Salminen S., Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133:405–413. doi: 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rautava S., Salminen S., Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy – a randomised, double-blind, placebo-controlled study. Br J Nutr. 2009;101:1722–1726. doi: 10.1017/S0007114508116282. [DOI] [PubMed] [Google Scholar]
- 13.Smith T.J., Rigassio-Radler D., Denmark R., Haley T., Touger-Decker R. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2012;1:1–9. doi: 10.1017/S0007114512004138. [DOI] [PubMed] [Google Scholar]
- 14.Guarino A., Vecchio A.L., Canani R.B. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastroenterol. 2009;25:18–23. doi: 10.1097/MOG.0b013e32831b4455. [DOI] [PubMed] [Google Scholar]
- 15.Lehtoranta L., Söderlund-Venermo M., Nokso-Koivisto J., Toivola H., Blomgren K., Hatakka K. Human bocavirus in the nasopharynx of otitis-prone children. Int J Pediatr Otorhinolaryngol. 2012;76:206–211. doi: 10.1016/j.ijporl.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumpu M., Lehtoranta L., Roivainen M., Rönkkö E., Ziegler T., Söderlund-Venermo M. The use of the probiotic Lactobacillus rhamnosus GG and viral findings in the nasopharynx of children attending day care. J Med Virol. 2013;85:1632–1638. doi: 10.1002/jmv.23623. [DOI] [PubMed] [Google Scholar]
- 17.Jokela P., Piiparinen H., Mannonen L., Auvinen E., Lappalainen M. Performance of the Luminex xTAG respiratory viral panel fast in a clinical laboratory setting. J Virol Methods. 2012;182:82–86. doi: 10.1016/j.jviromet.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savolainen-Kopra C., Blomqvist S., Kaijalainen S., Auvinen E., Lappalainen M. All known human rhinovirus species are present in sputum specimens of military recruits during respiratory infection. Viruses. 2009;1:1178–1189. doi: 10.3390/v1031178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blomqvist S., Klemola P., Kaijalainen S., Paananen A., Simonen M.L., Vuorinen T. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49–54. doi: 10.1016/j.jcv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Bron P.A., Tomita S., Mercenier A., Kleerebezem M. Cell surface-associated compounds of probiotic lactobacilli sustain the strain-specificity dogma. Curr Opin Microbiol. 2013;16:262–269. doi: 10.1016/j.mib.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen M., Pietilä T.E., Kekkonen R.A., Kankainen M., Latvala S., Pirhonen J. Nonpathogenic Lactobacillus rhamnosus activates the inflammasome and antiviral responses in human macrophages. Gut Microbes. 2012;3:510–522. doi: 10.4161/gmic.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harata G., He F., Hiruta N., Kawase M., Kubota A., Hiramatsu M. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol. 2010;50:597–602. doi: 10.1111/j.1472-765X.2010.02844.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang W., Ye Q., Liu W. Effect of bifidobacteria on ileum intercellular adhesion molecule-1 expression in young rats with endotoxin-induced intestinal damage. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:375–378. [PubMed] [Google Scholar]
- 24.Simonen-Tikka M.L., Klemola P., Suomenrinne S., Kaijalainen S., Söderström D., Savolainen-Kopra C. Virus infections among young children-the first year of the INDIS study. J Med Virol. 2013;85:1678–1684. doi: 10.1002/jmv.23625. [DOI] [PubMed] [Google Scholar]
- 25.Mäkelä M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimäki M. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monto A.S. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin Ther. 2002;24:1987–1997. doi: 10.1016/S0149-2918(02)80093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray G.C., Callahan J.D., Hawksworth A.W., Fisher C.A., Gaydos J.C. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5:379–385. doi: 10.3201/eid0503.990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajon A.E., Moseley J.M., Metzgar D., Huong H.S., Wadleigh A., Ryan M.A. Molecular epidemiology of adenovirus type 4 infections in US military recruits in the postvaccination era (1997–2003) J Infect Dis. 2007;196:67–75. doi: 10.1086/518442. [DOI] [PubMed] [Google Scholar]
- 29.Aho M., Lyytikäinen O., Nyholm J.E., Kuitunen T., Rönkkö E., Santanen R. Outbreak of 2009 pandemic influenza A(H1N1) in a Finnish garrison – a serological survey. Euro Surveill. 2010;11:45. [PubMed] [Google Scholar]