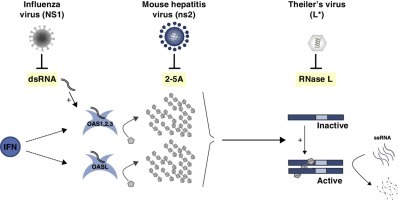
Graphical abstract
Highlights
-
•
The OAS/RNase L pathway was one of the first characterized IFN effector pathways.
-
•
2–5A molecules link ankyrin domains of two RNase L protomers to activate the enzyme.
-
•
Viruses evolved a variety of strategies to escape the OAS/RNase L host response.
-
•
Antagonism by viruses highlights the importance of RNase L as an antiviral defense.
-
•
Why do some viruses act upstream and others downstream of the pathway?
Abstract
The OAS/RNase L system was one of the first characterized interferon effector pathways. It relies on the synthesis, by oligoadenylate synthetases (OAS), of short oligonucleotides that act as second messengers to activate the latent cellular RNase L. Viruses have developed diverse strategies to escape its antiviral effects. This underscores the importance of the OAS/RNase L pathway in antiviral defenses. Viral proteins such as the NS1 protein of Influenza virus A act upstream of the pathway while other viral proteins such as Theiler's virus L* protein act downstream. The diversity of escape strategies used by viruses likely stems from their relative susceptibility to OAS/RNase L and other antiviral pathways, which may depend on their host and cellular tropism.
Current Opinion in Virology 2015, 15:19–26
This review comes from a themed issue on Viral immunology
Edited by Philip Stevenson
For a complete overview see the Issue and the Editorial
Available online 29th July 2015
http://dx.doi.org/10.1016/j.coviro.2015.07.002
1879-6257/© 2015 Elsevier B.V. All rights reserved.
Introduction
The OAS/RNase L system, discovered in the 1970s, was one of the first characterized interferon (IFN) effector pathways. The pioneering work of P. Lengyel's and I. Kerr's groups revealed a cellular RNase whose activity depended on IFN and correlated with the synthesis of 2′–5′ oligoadenylates (2–5A) in infected cells. This led to the model presented in Figure 1 , where IFN induces the expression of oligoadenylate synthetases (OAS) that synthesize 2–5A in response to viral infection. 2–5A act as second messengers to trigger dimerization and activation of latent RNase L. Activated RNase L cleaves viral and cellular single-stranded RNA (ssRNA) and thereby limits virus replication and triggers the apoptosis of infected cells.
Figure 1.
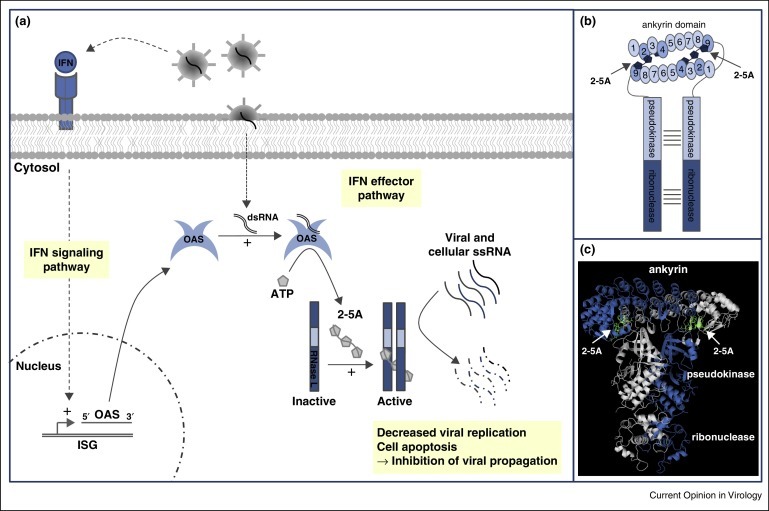
Activation of the OAS/RNase L pathway. (a) IFN secreted upon viral infection activates the transcription of hundreds of genes including oligoadenylate synthetase (OAS) genes. dsRNA resulting from viral replication switches on OAS, which convert ATP into 2′–5′ oligoadenylates (2–5A). 2–5A then bind to RNase L and trigger its dimerization and activation. RNase L degrades single-stranded viral and cellular RNA, decreasing viral replication and inducing apoptosis. (b,c) Schematic (b) and crystallographic structure (PDB ref 4OAV) (c) of active dimeric human RNase L, showing 2–5A bound to two RNase L protomers. 2–5A bind ankyrin repeats 2 and 4 of the first protomer and ankyrin repeat 9 of the other protomer (as well as the N-terminal lobe of the pseudokinase domain).
Since the discovery of this pathway, the antiviral role of RNase L has been widely documented in vitro and in vivo, notably via RNase L-knockout mice [1]. Viruses have developed various strategies to escape the OAS/RNase L pathway, underlining its importance in the antiviral defense. Numerous reviews have covered the cellular and antiviral activities of RNase L [2, 3]. This review focuses on the many strategies whereby viruses escape this defense pathway and discusses their implication for the biology of RNase L.
OAS/RNase L pathway
The OAS family consists of homologous enzymes encoded by interferon-stimulated genes (ISGs). The three OAS (OAS1, OAS2 and OAS3) differ in their number of OAS domains, oligomerization level and type of synthesized 2–5A. In addition to OAS, human and mouse genomes encode ‘OAS-like’ (OASL) proteins. The unique human OASL is catalytically inactive. In mice, Oasl1 is inactive while Oasl2 can synthesize 2–5A (reviewed in [4, 5]). Binding of dsRNA triggers the catalytic activity of OAS, which convert ATP into 2–5A. 2–5A are short oligoadenylates linked by 2′,5′-phosphodiester bonds, whose general formula is [pxA(2′p5′A)n; x = 1–3; n ≥ 2] [6, 7]. The only known function of 2–5A is RNase L activation [8].
RNase L is a 741 amino acid latent endoribonuclease ubiquitously expressed in mammalian tissues [8]. It is described as a cytosolic enzyme, but is also detected in other subcellular compartments such as mitochondria where it regulates mitochondrial mRNA abundance [3, 9]. RNase L comprises three domains: (i) a N-terminal ankyrin domain composed of 9 ankyrin repeats (R1–R9) involved in 2–5A recognition; (ii) a central catalytically inactive pseudokinase domain which contributes to RNase L dimerization; and (iii) a C-terminal enzymatic domain that cleaves target RNA. Recent structural studies show that 2–5A bind R2 and R4 of one RNase L protomer, and R9 and the pseudokinase N-terminal lobe of the other, thereby triggering RNase L dimerization [10••, 11, 12••]. RNase L cleaves viral and cellular ssRNA with little specificity (UN^N sequence), leaving a 5′-OH and 3′-monophosphate [10••].
2–5A are degraded within minutes of their synthesis, by 2′-phosphodiesterases and phosphatases. This allows a tight regulation of RNase L activity, which mirrors OAS activity. RNase L activity can also be restrained by association with a cellular factor known as inhibitor/ATP-binding cassette, sub-family E member 1 (RLI/ABCE).
Mechanisms of antiviral activity
RNase L restricts viral propagation through both direct and indirect mechanisms that include:
-
(i)
Viral genome degradation: This is reported for EMCV [13] and is predicted for all ssRNA viruses.
-
(ii)
Viral mRNA degradation: This potentially affects both DNA and RNA viruses. It has been suggested that activation of OAS and consequent 2–5A production preferentially occur at sites of dsRNA production (i.e. close to RNA virus replication complexes), which may impart some specificity toward viral mRNA [13, 14].
-
(iii)
Cellular mRNA and rRNA degradation: rRNA damage should limit translation, including that of viral mRNA. Sustained degradation of cellular RNA, including mitochondrial RNA, leads to apoptosis, which reduces viral propagation.
-
(iv)
Amplification of IFN signaling: The release, by RNase L, of short RNA fragments into the cytoplasm can activate cytoplasmic helicases that, in turn, activate type I IFN synthesis, creating a positive feed-back in antiviral defense [15].
Antiviral activity
A major step forward in the analysis of RNase L was the development of RNase L-KO mice [1], which contributed to uncovering the role of RNase L in vivo, against encephalomyocarditis virus, Coxsackie virus B4 and West Nile virus [16, 17, 18].
Activity of the OAS/RNase L pathway was also demonstrated in vitro, against many viruses, in particular RNA viruses (Table 1 ). However, some RNA viruses, such as influenza A virus, Theiler's virus and murine hepatitis virus are hardly affected, because they express antagonist proteins (see following section and Figure 2 ).
Table 1.
Effect of the OAS/RNase L pathway on viral infection
| Virus | Effect of the OAS/RNase L pathway on viral infection in vitro (cell culture) or in vivoa |
|---|---|
| RNA viruses | |
| Picornaviridae | Induction of 2–5A production by dsRNA in replication complexes |
| Encephalomyocarditis virus | Effect of dominant negative RNase L or overexpression of OAS1 In vivo: increased infection and mortality of RNase L-KO mice |
| Coxsackievirus B4 | In vivo: increased infection and mortality of RNase L-KO mice |
| Theiler's virusb | In vitro: increased replication in RNase L-KO macrophages |
| Poliovirus | Minor effect of RNase L overexpression or dominant negative RNase L |
| Flaviviridae | Induction of 2–5A production by dsRNA in replication complexes and by 5′ and 3′UTR structures |
| Hepatitis C virus | Degradation of viral genome by RNase L |
| West Nile virus | Degradation of viral genome by RNase L Dominant negative RNase L renders cells more permissive In vivo: enhanced susceptibility of RNase L-KO mice (footpad inoculation) |
| Togaviridae | |
| Sindbis virus |
In vitro: increased replication in RNase L-KO fibroblasts Minor effect in vivo (TDc mice versus IFNAR-KO mice) |
| Coronaviridae | |
| Murine hepatitis virusb | In vitro and in vivo: increased replication and mortality of the ns2 mutant in RNase L-KO macrophages and mice |
| Ortho/Para-myxoviridae | |
| Syncytial respiratory virus | Minor effect of OAS inhibition or RLI expression In vitro and in vivo: inhibition of infection by a 2–5A/oligonucleotide complex |
| Influenza A virusb | Increased replication of the NS1 mutant in RNase L-KO or RNase L-KD fibroblasts |
| Reoviridae | |
| Reovirus | Minor or deleterious effects in RNase L-KO fibroblasts |
| DNA viruses | |
| Poxviridae | |
| Vaccinia virus | In vivo: minor effect (TDc versus IFNAR mice) |
| Herpesviridae | |
| Herpes simplex virus 1 |
In vitro: effects of the McKrae strain In vivo: contradictory effects, depending on the viral strain and the inoculation route |
| Herpes simplex virus 2 | Deleterious proinflammatory effect of RNase L |
| Polyomaviridae | |
| Simian virus 40 | No cleavage is observed in vitro |
| Retrovirus and HBV | |
| Retroviridae | HIV: TAR sequence can activate the OAS but is inhibited by Tat |
| Human immunodeficiency virus |
In vitro: RNase L overexpression inhibits HIV replication RNase L inhibition or RLI overexpression activates HIV replication |
| Hepadnaviridae | |
| Hepatitis B virus | Identical HBV replication in RNase L-KO HBV transgenic mice |
Detailed references can be found in the review [42], from which this table was adapted.
In absence of the inhibitory viral protein.
TD, triply deficient mice: RNase L, PKR and Mx.
Figure 2.
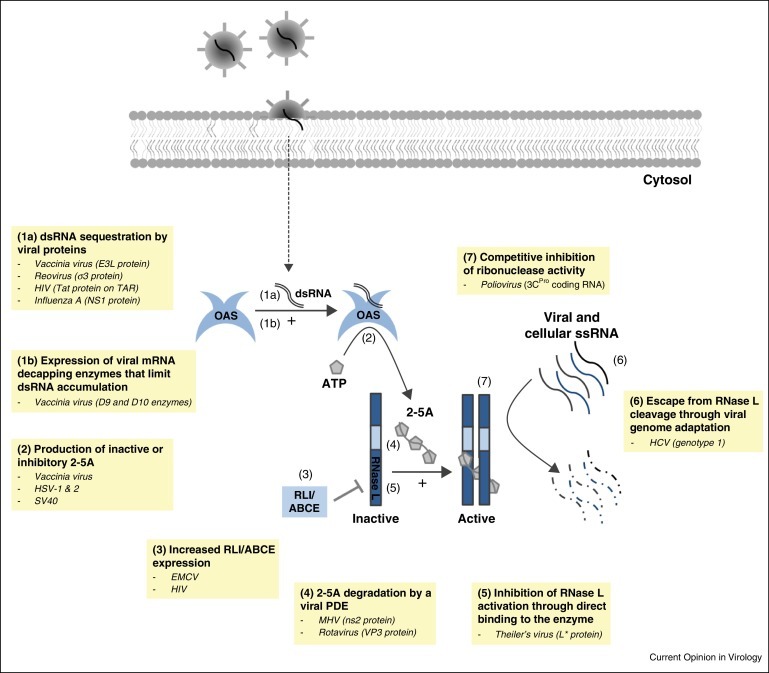
Strategies developed by viruses to escape the OAS/RNase L pathway.
Inhibition of OAS/RNase L system by viruses
Many viruses counteract the antiviral activity of OAS/RNase L (Figure 2). Some viruses act upstream of the pathway by masking dsRNA or by acting on OAS enzymes. Others act downstream, through 2–5A degradation or RNase L inhibition.
dsRNA sequestration by a viral protein
Some viruses sequester dsRNA and thereby prevent OAS activation. Examples of proteins with this action include Influenza A virus NS1 [19], vaccinia virus (VV) E3L [20] and the σ3 outer capsid protein of reoviruses [21], which remarkably both plays a structural role in the capsid and counteracts antiviral responses.
The human immunodeficiency virus (HIV) Tat protein binds to tar, a dsRNA structure in the HIV mRNA, to prevent OAS activation by tar [22].
Expression of viral mRNA decapping enzymes
In cells infected by DNA viruses, dsRNA can arise by convergent transcription from opposite DNA strands. With vaccinia virus, up to 15% of polyA RNA synthesized late in replication is predicted to form duplexes. However, VV encodes two decapping enzymes, D9 and D10, that degrade methylated mRNA cap structures and render them susceptible to cellular 5′ exonuclease Xrn1. Accordingly, infection with a D9 and D10 catalytic mutant virus triggers a drastic increase in dsRNA-mediated activation of OAS and PKR, as does the depletion of Xrn1 [23••, 24••].
2–5A degradation by a viral phosphodiesterase
A recently discovered mechanism to prevent RNase L activation is the expression of 2′,5′-phosphodiesterases (PDE) that degrade 2–5A into ATP and AMP. PDE activity was first shown for the ns2 protein of mouse hepatitis virus (MHV), a coronavirus. A catalytically inactive ns2-H126R mutant virus was strongly attenuated for liver infection of wild type but not RNase L-KO mice [25].
The C-terminal domain of rotavirus protein VP3 was identified, by sequence data mining, as another potential 2′,5′-PDE. In recombinant MHV viruses, rotavirus VP3 can substitute for ns2 and rescue replication in bone marrow-derived macrophages and in mouse liver. Sequence alignments suggest that a similar PDE occurs in all group A and likely group B and C rotavirus strains [26, 27].
Production of inactive or inhibitory 2–5A
It was proposed that some DNA viruses hijack OAS to promote the synthesis of inactive or inhibitory 2–5A. For instance, upon herpes simplex virus (HSV-1 and HSV-2) infection, 2–5A synthesis is induced but 2–5A accumulation does not contribute to ribosomal RNA degradation [28]. Likewise, in simian virus 40 (SV40) and vaccinia virus infection of IFN-primed cells, 2–5A concentration can reach 2–5 μM without inducing a clear RNase L activation [29, 30]. These 2–5A molecules, which remain to be analyzed, could include phosphorylated and unphosphorylated 2–5A as well as related compounds inactive on RNase L.
Increased RLI/ABCE expression
EMCV infection induces RLI/ABCE [31], which correlates with RNase L inhibition. Accordingly, RLI/ABCE overexpression partially suppresses the action of IFN against EMCV [32]. Similar observations were made for HIV-1 [33]. Interpretation of these data is, however, complicated by the contribution of RLI/ABCE to HIV-1 capsid assembly.
Inhibition of RNase L activation through direct binding to the enzyme
Theiler's murine encephalomyelitis virus (TMEV) encodes an L* accessory protein which enhances macrophage infection in vitro and is required to persist in the mouse central nervous system [34]. To date, L* is the only viral protein shown to inhibit RNase L through a direct protein–protein interaction. This activity of L* is species-specific [35].
Competitive inhibition of ribonuclease activity
Poliovirus antagonizes RNase L through a highly structured hairpin in its genomic RNA. This hairpin in the 3C protein coding region acts as a cleavage-resistant substrate of RNase L. This renders poliovirus RNA resistant to RNase L cleavage despite hundreds of UU and UA dinucleotides [36].
Escape from RNase L cleavage through genome adaptation
Hepatitis C virus (HCV) genotype 1 has evolved to decrease the number of cleavage sites recognized by RNase L. As a result, HCV1 is more resistant to IFN than HCV2 or HCV3. Viral strains resistant to RNase L have fewer UU and UA dinucleotides (the main RNase L targets). Moreover, silent mutations in these cleavage sites accumulate during IFN therapy [37].
Discussion
Amplification and bottleneck in the RNase L pathway
It is surprising that the OAS/RNase L pathway starts with a two-step amplification, which activates a single enzyme (Figure 3 ). Indeed, IFN strongly induces the expression of several OAS, which, upon viral dsRNA binding, synthesize large amounts of 2–5A. Then, 2–5A activate a single target, RNase L, which is present in low amounts in the cell and therefore limits the cellular response. One may wonder why evolution did not select a more direct way to activate RNase L.
Figure 3.
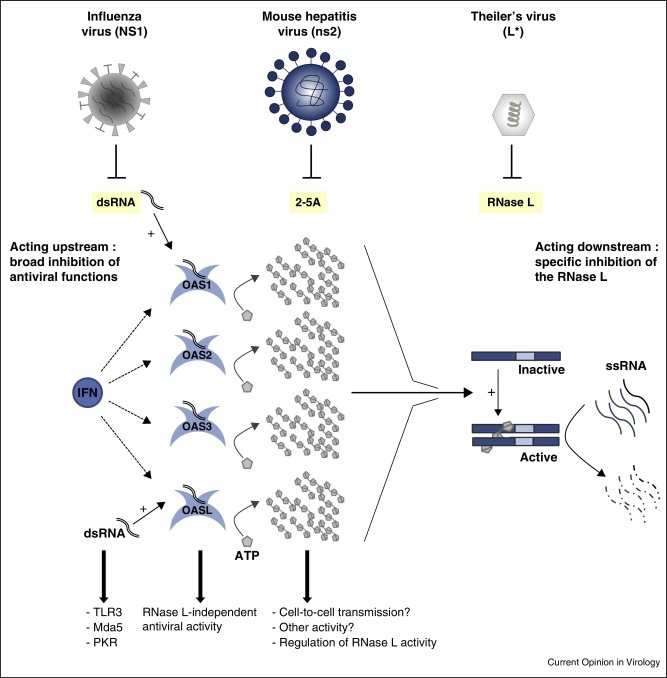
Inhibition of upstream or downstream steps of the OAS/RNase L pathway. Influenza virus NS1, mouse hepatitis virus ns2 and Theiler's virus L* proteins are examples that act on different steps of the pathway. NS1 is a broad spectrum inhibitor that acts upstream, by sequestering dsRNA. It therefore concomitantly inhibits other antiviral pathways that depend on dsRNA such as PKR, Mda5 and TLR3. ns2 degrades 2–5A and thus acts downstream of OAS. It is unclear whether 2–5A have functions other than RNase L activation. L* directly interacts with RNase L, which ensures a specific effect.
One reason may be that factors upstream of the cascade have additional functions. This is indeed the case for some OAS, which have RNase L-independent antiviral activity [38•, 39]. For example, upon dsRNA binding, the catalytically inactive human OASL can activate RIG-I. Even more surprising is the intensity of 2–5A production since these compounds have a unique known function, the activation of RNase L [8]. First, it is possible that 2–5A have another function. Second, the use of 2–5A as second messengers may allow tight regulation of RNase L, as 2–5A are quickly degraded by phosphodiesterases. Third, some analogy exists between the OAS/RNase L pathway and the cGAS-STING pathway, which leads to IFN expression. Both involve the synthesis of non-canonical nucleotides acting as second messengers: 2–5A for OAS/RNase L and cyclic AMP-GMP for the cGAS/STING pathway. Cyclic AMP–GMP can be transferred between cells, in a gap junction-dependent manner, to activate IFN synthesis in neighboring cells [40••]. 2–5A could similarly signal to neighboring cells to prime the RNase L-mediated positive feed-back into the IFN response.
Acting upstream or downstream of the RNase L pathway?
Almost every step of the OAS/RNase L pathway is targeted by a viral protein. Inhibiting the downstream effector enzyme (RNase L), as does TMEV L* [35], would seem to be the most efficient mechanism. Indeed, RNase L is much less abundant than OAS that are induced by IFN or than 2–5A that are synthesized by activated OAS. Nonetheless, some viruses target molecules upstream of the pathway, such as dsRNA or 2–5A, despite their abundance. Targeting dsRNA may less potently counteract RNase L activity, but also inhibits other antiviral pathways such as PKR, Mda5 and TLR3. Targeting 2–5A, as coronaviruses and rotaviruses do, is a more difficult-to-understand strategy, unless 2–5A have an alternative function that would also be inhibited by these viruses (Figure 3).
Is there a host/tissue specificity for RNase L activity?
It is noteworthy that RNA viruses, like TMEV or MHV, devote a protein to inhibiting RNase L, despite their limited coding capacity [25, 35]. This indicates that the OAS/RNase L pathway exerts strong selective pressure. The fact that both viruses are murine may suggest that the OAS/RNase L pathway is particularly active in this species. However, human enteric (HEC4408 strain) and respiratory (OC43 strain) coronaviruses produce a protein, homologous to MHV ns2, likely sharing phosphodiesterase activity. Moreover, human rotaviruses encode a PDE [27].
Another trait common to MHV and TMEV is macrophage tropism. In macrophages, OAS and, to a lesser extent, RNase L basal expression is higher than in other cell types [35, 41•]. Incidentally, the L* protein was first described as a protein that facilitates the infection of macrophages. Similar observations were made that ns2 promotes MHV replication specifically in macrophages [41•]. The PDE activity of the rotavirus VP3 is not required to infect enterocytes of small intestinal villi but might contribute to infecting a subpopulation of plasmacytoid dendritic cells thought to play an important role in virus dissemination [27].
In conclusion, viruses have developed various strategies to escape the OAS/RNase L pathway, underlining its physiological importance. The multiplicity of evasion strategies may stem from the diversity of viral replication cycles and from the variety of antiviral defenses exerted by different cell types and organisms. Some viruses rather act upstream, on triggers of the OAS/RNase L pathway, thereby antagonizing other antiviral pathways that depend on the same triggers. Others act downstream for more selective RNase L inhibition. This latter option may reflect a tropism for macrophages, in which the OAS/RNase L system is particularly active [35, 41•].
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank Daniel Gonzalez-Dunia for critical reading of the manuscript.
MD is the recipient of a PhD fellowship from the Belgian FRIA. This work was supported by IAP-P7/45 BELVIR (BELSPO) and by the Belgian fund for medical research (FRSM, PDR #T.0185.14)
References
- 1.Zhou A., Paranjape J., Brown T.L., Nie H., Naik S., Dong B., Chang A., Trapp B., Fairchild R., Colmenares C. Interferon action and apoptosis are defective in mice devoid of 2’,5’-oligoadenylate-dependent RNase L. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisbal C., Silverman R.H. Diverse functions of RNase L and implications in pathology. Biochimie. 2007;89:789–798. doi: 10.1016/j.biochi.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan-Laun S.E., Ezelle H.J., Li X.L., Hassel B.A. RNase-L control of cellular mRNAs: roles in biologic functions and mechanisms of substrate targeting. J Interferon Cytokine Res. 2014;34:275–288. doi: 10.1089/jir.2013.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovanessian A.G., Justesen J. The human 2′–5′oligoadenylate synthetase family: unique interferon-inducible enzymes catalyzing 2′–5′ instead of 3′–5′ phosphodiester bond formation. Biochimie. 2007;89:779–788. doi: 10.1016/j.biochi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Justesen J., Hartmann R., Kjeldgaard N.O. Gene structure and function of the 2′–5′-oligoadenylate synthetase family. Cell Mol Life Sci. 2000;57:1593–1612. doi: 10.1007/PL00000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerr I.M., Brown R.E. pppA2′p5′A2′p5′A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978;75:256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerr I.M., Brown R.E., Hovanessian A.G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977;268:540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- 8.Zhou A., Hassel B.A., Silverman R.H. Expression cloning of 2–5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993;72:753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- 9.Le Roy F., Bisbal C., Silhol M., Martinand C., Lebleu B., Salehzada T. The 2–5A/RNase L/RNase L inhibitor (RLI) [correction of (RNI)] pathway regulates mitochondrial mRNAs stability in interferon alpha-treated H9 cells. J Biol Chem. 2001;276:48473–48482. doi: 10.1074/jbc.M107482200. [DOI] [PubMed] [Google Scholar]
- 10••.Han Y., Donovan J., Rath S., Whitney G., Chitrakar A., Korennykh A. Structure of human RNase L reveals the basis for regulated RNA decay in the IFN response. Science. 2014;343:1244–1248. doi: 10.1126/science.1249845. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the structure of dimeric human RNase L. This paper and that of Huang et al. [12••] show how 2–5A interact with ankyrin repeats of RNase L to trigger dimerization.
- 11.Han Y., Whitney G., Donovan J., Korennykh A. Innate immune messenger 2–5A tethers human RNase L into active high-order complexes. Cell Rep. 2012;2:902–913. doi: 10.1016/j.celrep.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 12••.Huang H., Zeqiraj E., Dong B., Jha B.K., Duffy N.M., Orlicky S., Thevakumaran N., Talukdar M., Pillon M.C., Ceccarelli D.F. Dimeric structure of pseudokinase RNase L bound to 2–5A reveals a basis for interferon-induced antiviral activity. Mol Cell. 2014;53:221–234. doi: 10.1016/j.molcel.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; Structure of dimeric RNase L from sus scrofa. This article, along with that of Han et al. [10••] describes the structure of dimeric RNase L from sus scrofa. They reveal how 2–5A interact with ankyrin repeats of RNase L to trigger dimerization of the enzyme.
- 13.Li X.L., Blackford J.A., Hassel B.A. RNase L mediates the antiviral effect of interferon through a selective reduction in viral RNA during encephalomyocarditis virus infection. J Virol. 1998;72:2752–2759. doi: 10.1128/jvi.72.4.2752-2759.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsen T.W., Baglioni C. Mechanism for discrimination between viral and host mRNA in interferon-treated cells. Proc Natl Acad Sci U S A. 1979;76:2600–2604. doi: 10.1073/pnas.76.6.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malathi K., Dong B., Gale M., Jr., Silverman R.H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flodstrom-Tullberg M., Hultcrantz M., Stotland A., Maday A., Tsai D., Fine C., Williams B., Silverman R., Sarvetnick N. RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J Immunol. 2005;174:1171–1177. doi: 10.4049/jimmunol.174.3.1171. [DOI] [PubMed] [Google Scholar]
- 17.Samuel M.A., Whitby K., Keller B.C., Marri A., Barchet W., Williams B.R., Silverman R.H., Gale M., Jr., Diamond M.S. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J Virol. 2006;80:7009–7019. doi: 10.1128/JVI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherbik S.V., Paranjape J.M., Stockman B.M., Silverman R.H., Brinton M.A. RNase L plays a role in the antiviral response to West Nile virus. J Virol. 2006;80:2987–2999. doi: 10.1128/JVI.80.6.2987-2999.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min J.Y., Krug R.M. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2′–5′ oligo (A) synthetase/RNase L pathway. Proc Natl Acad Sci U S A. 2006;103:7100–7105. doi: 10.1073/pnas.0602184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang H.W., Watson J.C., Jacobs B.L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1992;89:4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huismans H., Joklik W.K. Reovirus-coded polypeptides in infected cells: isolation of two native monomeric polypeptides with affinity for single-stranded and double-stranded RNA, respectively. Virology. 1976;70:411–424. doi: 10.1016/0042-6822(76)90282-8. [DOI] [PubMed] [Google Scholar]
- 22.Schroder H.C., Ugarkovic D., Wenger R., Reuter P., Okamoto T., Muller W.E. Binding of Tat protein to TAR region of human immunodeficiency virus type 1 blocks TAR-mediated activation of (2′–5′)oligoadenylate synthetase. AIDS Res Hum Retroviruses. 1990;6:659–672. doi: 10.1089/aid.1990.6.659. [DOI] [PubMed] [Google Scholar]
- 23••.Burgess H.M., Mohr I. Cellular 5′–3′ mRNA exonuclease Xrn1 controls double-stranded RNA accumulation and anti-viral responses. Cell Host Microbe. 2015;17:332–344. doi: 10.1016/j.chom.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; See annotation to Ref. [24••].
- 24••.Liu S.W., Katsafanas G.C., Liu R., Wyatt L.S., Moss B. Poxvirus decapping enzymes enhance virulence by preventing the accumulation of dsRNA and the induction of innate antiviral responses. Cell Host Microbe. 2015;17:320–331. doi: 10.1016/j.chom.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; These two articles, published back-to-back, uncovers a new mechanism used by vaccinia virus to limit the formation of RNA duplexes that might induce IFN production. Interestingly, the mechanism involves two vaccinia virus encoded decapping enzymes, which cooperate with the cellular exonuclease Xrn1, to trigger the degradation of viral mRNA.
- 25.Zhao L., Jha B.K., Wu A., Elliott R., Ziebuhr J., Gorbalenya A.E., Silverman R.H., Weiss S.R. Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell Host Microbe. 2012;11:607–616. doi: 10.1016/j.chom.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden K.M., Hu L., Jha B.K., Sankaran B., Weiss S.R., Silverman R.H., Patton J.T., Prasad B.V. Structural basis for 2′–5′-oligoadenylate binding and enzyme activity of a viral RNase L antagonist. J Virol. 2015 doi: 10.1128/JVI.00701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R., Jha B.K., Ogden K.M., Dong B., Zhao L., Elliott R., Patton J.T., Silverman R.H., Weiss S.R. Homologous 2′,5′-phosphodiesterases from disparate RNA viruses antagonize antiviral innate immunity. Proc Natl Acad Sci U S A. 2013;110:13114–13119. doi: 10.1073/pnas.1306917110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cayley P.J., Davies J.A., McCullagh K.G., Kerr I.M. Activation of the ppp(A2′p)nA system in interferon-treated, herpes simplex virus-infected cells and evidence for novel inhibitors of the ppp(A2′p)nA-dependent RNase. Eur J Biochem. 1984;143:165–174. doi: 10.1111/j.1432-1033.1984.tb08355.x. [DOI] [PubMed] [Google Scholar]
- 29.Hersh C.L., Brown R.E., Roberts W.K., Swyryd E.A., Kerr I.M., Stark G.R. Simian virus 40-infected, interferon-treated cells contain 2′,5′-oligoadenylates which do not activate cleavage of RNA. J Biol Chem. 1984;259:1731–1737. [PubMed] [Google Scholar]
- 30.Rice A.P., Kerr S.M., Roberts W.K., Brown R.E., Kerr I.M. Novel 2′,5′-oligoadenylates synthesized in interferon-treated, vaccinia virus-infected cells. J Virol. 1985;56:1041–1044. doi: 10.1128/jvi.56.3.1041-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinand C., Salehzada T., Silhol M., Lebleu B., Bisbal C. RNase L inhibitor (RLI) antisense constructions block partially the down regulation of the 2–5A/RNase L pathway in encephalomyocarditis-virus-(EMCV)-infected cells. Eur J Biochem. 1998;254:248–255. doi: 10.1046/j.1432-1327.1998.2540248.x. [DOI] [PubMed] [Google Scholar]
- 32.Bisbal C., Martinand C., Silhol M., Lebleu B., Salehzada T. Cloning and characterization of a RNAse L inhibitor. A new component of the interferon-regulated 2–5A pathway. J Biol Chem. 1995;270:13308–13317. doi: 10.1074/jbc.270.22.13308. [DOI] [PubMed] [Google Scholar]
- 33.Martinand C., Montavon C., Salehzada T., Silhol M., Lebleu B., Bisbal C. RNase L inhibitor is induced during human immunodeficiency virus type 1 infection and down regulates the 2–5A/RNase L pathway in human T cells. J Virol. 1999;73:290–296. doi: 10.1128/jvi.73.1.290-296.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Eyll O., Michiels T. Influence of the Theiler's virus L* protein on macrophage infection, viral persistence, and neurovirulence. J Virol. 2000;74:9071–9077. doi: 10.1128/jvi.74.19.9071-9077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorgeloos F., Jha B.K., Silverman R.H., Michiels T. Evasion of antiviral innate immunity by Theiler's virus L* protein through direct inhibition of RNase L. PLoS Pathog. 2013;9:e1003474. doi: 10.1371/journal.ppat.1003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han J.Q., Townsend H.L., Jha B.K., Paranjape J.M., Silverman R.H., Barton D.J. A phylogenetically conserved RNA structure in the poliovirus open reading frame inhibits the antiviral endoribonuclease RNase L. J Virol. 2007;81:5561–5572. doi: 10.1128/JVI.01857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J.Q., Barton D.J. Activation and evasion of the antiviral 2′–5′-oligoadenylate synthetase/ribonuclease L pathway by hepatitis C virus mRNA. RNA. 2002;8:512–525. doi: 10.1017/s1355838202020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Ibsen M.S., Gad H.H., Andersen L.L., Hornung V., Julkunen I., Sarkar S.N., Hartmann R. Structural and functional analysis reveals that human OASL binds dsRNA to enhance RIG-I signaling. Nucleic Acids Res. 2015;43:5236–5248. doi: 10.1093/nar/gkv389. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article shows a long-sought RNase L-independent activity of human OASL. Upon dsRNA binding, OASL binds to RIG-I, thereby activating the IFN signaling pathway.
- 39.Zhu J., Zhang Y., Ghosh A., Cuevas R.A., Forero A., Dhar J., Ibsen M.S., Schmid-Burgk J.L., Schmidt T., Ganapathiraju M.K. Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor. Immunity. 2014;40:936–948. doi: 10.1016/j.immuni.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Ablasser A., Goldeck M., Cavlar T., Deimling T., Witte G., Rohl I., Hopfner K.P., Ludwig J., Hornung V. cGAS produces a 2′–5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article shows that cyclic AMP–GMP products synthesized by the recently characterized cGAS enzyme can spread from cell to cell in order to activate the STING/IFN pathway in neighboring cells.
- 41•.Zhao L., Birdwell L.D., Wu A., Elliott R., Rose K.M., Phillips J.M., Li Y., Grinspan J., Silverman R.H., Weiss S.R. Cell-type-specific activation of the oligoadenylate synthetase-RNase L pathway by a murine coronavirus. J Virol. 2013;87:8408–8418. doi: 10.1128/JVI.00769-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; These authors discovered a novel family of viral phosphodiesterases that degrade 2–5A and thereby inhibit RNase L. A similar enzyme was later detected in rotaviruses. This paper further shows the influence of this activity in vivo.
- 42.Silverman R.H. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J Virol. 2007;81:12720–12729. doi: 10.1128/JVI.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


