Abstract
The objective of this study was to evaluate under field conditions, whether daily administration of oregano essential oil is effective in preventing and/or diminishing the severity of neonatal diarrhea syndrome in calves aged less than 15 days. Ninety-one newborn calves from three dairy farms were assigned into two groups; “Eco” group (n = 46) calves were drenched with Greek oregano (Origanum vulgare ssp. Hirtum) essential oil (ECODIAR® liquid 5%) at the dose of 12.5 mg/kg body weight once per day until the age of 10 days. “Conts” group (n = 45) calves were left untreated and served as controls. All animals were monitored daily for the incidence of diarrhea until the age of 15 days and their fecal score was recorded. Fecal samples were collected on days 3, 6 and 10 for microbiological and parasitological evaluation. Average fecal score throughout the experiment, incidence of diarrhea, duration and severity of diarrhea episodes were significantly lower in Eco group compared to the controls. Daily administration of oregano essential oil in calves for the first 10 days of their life effectively diminishes the severity of naturally acquired diarrhea under field conditions and, under certain hygiene practices, possess a preventive effect against neonatal diarrhea syndrome.
Keywords: Oregano essential oil, Calves, Diarrhea, Treatment, Prevention
Highlights
-
•
We evaluated the efficacy of oregano essential oil against calf neonatal diarrhea syndrome.
-
•
Ninety-one calves from 3 farms were assigned in to two groups.
-
•
Oregano essential oil was orally drenched (12.5 mg/kg) once per day from birth until day 10.
-
•
Diarrhea incidence was significantly lower in calves receiving oregano oil than the controls.
-
•
The severity of diarrhea was significantly lower in calves receiving oregano oil than the controls.
1. Introduction
Neonatal calf diarrhea is the most common cause of illness and mortality in calves and a major cause of economic loss to cattle herds (Barragry, 1997, De la Fuente et al., 1999). Economic losses, on top to mortality, is associated with treatment, veterinary and labor costs as well as with reduced growth rates of the affected calves (Anderson et al., 2003, Ok et al., 2009). The most important infectious agents associated with the disease are enterotoxic Escherichia coli K99/F5 (ETEC), Rotavirus A (RVA), Bovine coronavirus (BCoV), and Cryptosporidium spp. with RVA and Cryptosporidium spp. being most frequently identified in fecal samples from young calves (Gulliksen et al., 2009, Bartels et al., 2010, Silverlås et al., 2010). Co-infections with more than one of these pathogens are frequently detected in diarrheic calves in clinical practice and are associated with increased morbidity and mortality rates (Blanchard, 2012).
It is well documented that diarrheic calves, regardless of the causative agent or agents of diarrhea, often have small intestinal overgrowth of E. coli bacteria. This overgrowth increases the severity of diarrhea, retards the recovery and increases the risk of bacteremia and death (Constable, 2004). In respect to this, oral administration of antibacterial agents that inhibit coliform bacterial overgrowth in calves with diarrhea might have beneficial effects on the disease outcome by decreasing the duration and the severity of the disease and by preventing the development of bacteremia.
Oregano essential oil could be used as such an antibacterial agent providing an antibiotic alternative for this purpose. It has been proven in many experiments in vitro that oregano essential oil and its main constituents, carvacrol and thymol, have strong antibacterial activity against gram negative bacteria and especially E. coli (Elgayyar et al., 2001, Si et al., 2008, Nazzaro et al., 2013). Apart from its antibacterial properties, oregano essential oil was also found to have antiviral (Pilau et al., 2011) and anticryptosporidial (Gaur et al., 2016) effects in vitro. Based on these properties it could be hypothesized that the administration of oregano essential oil in newborn calves might have beneficial effects not only in treating but also in preventing diarrhea syndrome. In the available literature there is not any relevant controlled research evaluating potential prophylactic or treatment effects of oregano essential oil on diarrhea in calves aged < 15 days. In the only available study it was observed that the administration of oregano leaves had comparable effects with neomycin on the duration of diarrhea in diarrheic calves aged 7 to 20 days without however using control group (Bampidis et al., 2006).
The objective of the present study was to evaluate under field conditions whether daily administration of oregano essential oil is effective in preventing and/or diminishing the severity of neonatal diarrhea syndrome in calves aged < 15 days.
2. Materials and methods
2.1. Animals and experimental design
Prior to the onset of the experiment the minimum required total sample size was calculated using General Linear Multivariate Model with Wilks Likelihood Ratioprocedure at the GLIMMPSE software (http://glimmpse.samplesizeshop.org/). The desired power was set at 0.8, the type I error rate at 0.05, the desired detectable difference of days with diarrhea at 2 days with standard deviation 1; the means scale factor was set at 0.5 and the variability scale factor at 2. The results of the analysis revealed that a minimum sample size of 66 calves (33 per group) was required (Power = 0.808).
Ninety-one newborn Holstein calves (48 females and 43 males) from three dairy farms were finally used in the study. They were all born to dams that were vaccinated against Rotavirus, Coronavirus and E. coli F5 (K99)/F41 antigens (Lactovac C; Zoetis, Hellas) one month prior to the expected day of calving. The calves were randomly alternately assigned into one of two groups according to their birth date in each farm until the total number of 30 calves per farm was reached. In Farm 1 however 31 calves were finally used due to a twin birth at the last calving. The experiment was run in all 3 farms simultaneously from September 10th 2016 to November 12th 2016. The animals of Eco group (n = 46) were orally drenched with Greek oregano (Origanum vulgare ssp. hirtum) essential oil (ECODIAR® liquid 5%; Ecopharm Hellas S.A., Kilkis, Greece) at the dose of 12.5 mg/kg body weight once per day for the first 10 days of their life whereas those of Conts group (n = 45) were left untreated and served as controls.
The experiment started at the day of calving (day 1) and lasted until day 15. Each calf was separated from its dam after calving, weighed, its navel was disinfected using an antibiotic spray (TERRAMYCIN™ AEROSOL SPRAY; Zoetis, Hellas) and was offered its first colostrum meal. The calves of Eco group were orally drenched immediately afterwards with the respective amount of oregano essential oil that was diluted with normal saline up to the volume of 60 ml with the aid of a feeding syringe of equal volume. The following days and until day 10, oregano essential oil was administered in the calves of this group at the same way after the morning feeding. The same procedures were followed in all farms.
Feces were scored every day throughout the experiment after the morning feeding by the same person who was blinded to group allocation using a three point scale with 1 = normal, 2 = intermediate and 3 = watery and the score was recorded. Calves with fecal scores ≥ 2 were considered diarrheic. Based on these records, number of days with diarrhea (fecal score ≥ 2) was determined and diarrhea index (DI) was calculated: DI = number of days with diarrhea × average fecal score on these days.
Fecal samples were collected on days 3, 6 and 10 of the experiment for microbiological and parasitological evaluation. The samples were obtained directly from the rectum using sterile latex gloves, were separated into 3 aliquots in sterile containers and were transferred refrigerated to the laboratories for analysis. On the same days, the calves of Eco group were weighed and the daily dosage of oregano essential oil was modified accordingly. On day 3 a blood sample was also collected via jugular vein-puncture (21 G) into evacuated glass tubes from all calves and transferred refrigerated in the laboratory for the evaluation of passive immunity transfer.
All calves were routinely clinically examined by the same person who was blinded to group allocation at the fecal sampling days (days 3, 6 and 10) and at the end of the experiment (day 15). In cases of diarrhea the animals were clinically evaluated daily until recovery. Diarrheic calves in all farms continued milk feeding and were receiving an extra meal per day with oral electrolytes (Diaproof K®; Virbac, Hellas). In cases of dehydration or inappetence the calves were receiving Lactated Ringers solution intravenously and, if blood serum glucose concentration was lower than 3 mmol/l (as determined on farm with a handheld glucose meter; FreeStyle Precision, Abbott, UK), a dextrose 10% solution was also administered intravenously. Calves with inappetence, hypoglycemia, depression and fever or hypothermia were also treated with antibiotics, based on antimicrobial susceptibility tests the last six months (Farm 2: enrofloxacin 5 mg/kg SC, Baytril®, Bayer Animal Health GmbH, Germany; Farms 1 and 3: ceftiofour 2.2 mg/kg SC, Excenel® RTU, Zoetis, Hellas) and non-steroid anti-inflammatory drugs (carprofen 1.4 mg/kg SC, Rimadyl® Cattle, Zoetis, Hellas). In calves of Eco group that were still diarrheic after day 10, administration of oregano essential oil continued until recovery.
2.2. Calf housing and feeding management
Newborn calves in Farm 1, were housed in individual pens in a separate building. Windows (1 m by 0,5 m) served as ventilation inlets, situated 1 m above the pens and oil heaters were used for heat supplementation when ambient temperature was below 4 °C. Each individual pen had a concrete floor area of 2 m2, with visual access to neighboring pens. Long stem wheat straw bedding was layed at a density of 70 kg/m3. Wet bedding was removed and replaced twice a week. All rails, gates, partitions, walls, feeders and floors were cleaned on a weekly basis and after each calf was removed from the building. A pressure water system and a broad spectrum disinfectant were used and adequate time for drying was provided. Farm 2 housed newborn calves outdoors, in individual polyethylene hutches with an outside run for the calves to move around. Hutches were situated on free draining concrete and were placed 0,3 m apart, enabling visual contact between calves. Each individual hatch had a concrete floor area of 2.4 m2. Newborn calves in Farm 3 were open housed in group pens partially enclosed under a metallic shed. Each group pen had a concrete floor area of 12 m2 for approximately 15 calves.
Clean, dry straw bedding which was disposed of after each batch of calves, was used in all Farms. Concrete feed troughs on the external side of the pens and water bowls mounted inside the pens were used in Farm 1. Feed and water buckets were secured outside individual pens and hutches in Farms 2 and 3, respectively.
Newborn calves were bottle-fed fresh colostrum from their respective mothers in all Farms. Each calf was fed 2 l of colostrum within 2 h after birth and another 2 l after 6 h. At days two and three of their lives, all calves were fed colostrum/milk from their respective dams, at 10% of their bodyweight. At four days old and afterwards, calves in all Farms consumed milk replacer meal. The nutrient analysis of the milk replacer used is presented in Table 1 . Milk replacer was offered at 39 °C, the optimal drinking temperature, twice a day. Calves were fed 10% of their bodyweight in milk replacer. Along with that, all Farms offered fresh water, which was available at all times and was replaced daily. The same calf starter (Table 2 ) was also offered starting at day five in all Farms. Approximately 250 g of calf starter per day were offered in a shallow bucket. The amount increased gradually, as calves started consuming all of the feed. All buckets were emptied and refreshed every 12 h with clean feed.
Table 1.
Nutrient and ingredient analysis of the milk replacer used in all three farms of the experiment.
| Milk replacer nutrient analysis | |
|---|---|
| Crude protein | 22.00% |
| Crude fat | 16.00% |
| Crude fibre | 1.10% |
| Calcium | 0.65% |
| Phosphorus | 0.45% |
| Sodium | 0.25% |
| Ash | 6.50% |
| Vitamin A (3a672a- retinyl acetate) | 25,000 IU/kg |
| Vitamin D3 (E671-cholecalciferol) | 5000 IU/kg |
| Cupric sulphate pentahydrate (E4- copper) | 7.00 mg/kg |
| Ferrous sulphate monohydrate (E1-iron) | 50.00 mg/kg |
| Calcium Iodate anhydrous (E2-iodine) | 0.15 mg/kg |
| Sodium selenite (E8-selenium) | 0.10 mg/kg |
| Manganous sulphate monohydrate (E5-manganese) | 35.00 mg/kg |
| Cobalt sulphate (E3-cobalt) | 0.60 mg/kg |
| Zinc sulphate monohydrate (E6-zinc) | 60.00 mg/kg |
| Butylated hydroxytoluene (E 321) | 30.00 mg/kg |
Ingredients: whey, wheat gluten, palm oil, lactose free whey, coconut oil, wheat flour, dextrose, calcium carbonate, antioxidant, vitamin and mineral premix.
Table 2.
Nutrient and ingredient analysis of the calf starter used in all three farms of the experiment.
| Calf starter nutrient analysis | |
|---|---|
| Crude protein | 20.00% |
| Oils & fats | 12.00% |
| Crude fibre | 2.00% |
| Sodium | 0.40% |
| Ash | 8.00% |
| Vitamin A (3a672a- retinyl acetate) | 10,000 IU/kg |
| Vitamin D3 (E671-cholecalciferol) | 3000 IU/kg |
| Vitamin E (3a700 – all-rac-alpha tocopherol acetate) | 100 IU/kg |
| Vitamin B12 | 100.00 μg/kg |
| Cupric sulphate pentahydrate (E4- copper) | 51.00 mg/kg |
| Ferrous sulphate monohydrate (E1-iron) | 333.30 mg/kg |
| Calcium iodate anhydrous (E2-iodine) | 4.70 mg/kg |
| Manganous sulphate monohydrate (E5-manganese) | 312.50 mg/kg |
| Zinc sulphate monohydrate (E6-zinc) | 277.80 mg/kg |
| Zinc chelate of amino acid hydrate (E6-zinc) | 200.00 mg/kg |
| Sodium selenite (E8-selenium) | 0.44 mg/kg |
| Ethoxyquin (E324) | 0.90 mg/kg |
| Propyl gallate (E310) | 50.00 mg/kg |
| Enterococcus faecium NCIMB 11181 (4b1708) | 1.25 × 109 CFU/kg |
Ingredients: toasted soya beans (produced from gm soya), whey powder, whey permeate powder, vegetable (palm) oil, oat flakes – heat treated, calcium carbonate, antioxidants, vitamin and mineral premix.
2.3. Parasitological analysis
The fecal samples were diluted (1:1) with tap water and passed through a sieve (no. 150) in a centrifuge tube, centrifuged at 500g for 3 min and smears of 20 μl of the sediment were dried, stained using the acid-fast Ziehl-Neelsen technique (Henriksen and Pohlenz, 1981), and examined at 1000 × magnification for Cryptosporidium spp. oocysts. Oocyst shedding was estimated semiquantitatively according to the average number of oocysts in 10 random fields. Calves found positive at at least one sampling were considered as positive for Cryptosporidium spp.
2.4. Virological analysis
Ten percent suspensions of each fecal sample were prepared in phosphate-buffered saline (PBS), and RNA was extracted according to RNA extraction method C described by Pappi et al. (2015). RNA extracts were stored at − 80 °C until testing. Two different real-time quantitative reverse transcription-PCR (qRT-PCR) assays were applied for the detection of RVA (Freeman et al., 2008) and BCoV (Decaro et al., 2008). Calves found positive even at one sapling were considered as positive for the respective virus.
2.5. Bacteriological analysis
To enumerate the population of E. coli in fecal samples viable plate count method was used. Initially 1 g of the sample was diluted in 9 subsequent ten-fold dilutions in phosphate buffer saline. One hundred microliters of each diluent was plated on TBX agar medium (Merck KGaA; Darmstadt, Germany) and incubated aerobically at 37 °C. Plates with 30 to 300 typical X-glucuronide positive colonies (blue in color) were calculated. Each colony was represented as colony forming unit (CFU) and measured with the formula shown below in CFUs/ml:
Furthermore 5 colonies per plate were selected and subcultured on Nutrient Agar plates (105540 Merck KGaA; Darmstadt, Germany). Total DNA of the E. coli isolates was further analyzed using a multiplex PCR system for the detection of enterotoxigenic, attaching, effacing, and shiga toxin producing genes (Franck et al., 1998).
2.6. Passive immunity transfer assessment
Blood samples were allowed to clot and serum was separated by low speed centrifugation (1600g for 15 min) and transferred into plastic vials. Serum total protein concentration was determined refractometrically with a temperature compensated refractometer (Reichert TS Meter refractometer, Model 1310400A, Reichert Scientific Instruments Buffalo, NY) according to the instructions of the manufacturer. Passive immunity transfer was considered as adequate when serum total protein values were ≥ 52 g/l (Tyler et al., 1996, Poulsen et al., 2010).
2.7. Ethics
All procedures were done according to the ethical standards in the Helsinki Declaration of 1975, as revised in 2000, as well as the national law and the guidelines of our Institutional Animal Care and Use Committee. According to the regulations of our Institution, a formal approval from the Ethical Committee was not required as long as samples were acquired for diagnostic or monitoring purposes under informed farmer consent.
2.8. Statistical analysis
Data were analyzed using the statistical program SPSS® 21. Normality of data distribution was assessed with Kolmogorov-Smirnov test and homogeneity of variances was evaluated with Levene test. Chi-square test was used to determine whether the percentage of calves with adequate passive transfer, the incidence of diarrhea, the percentage of calves that were positive to the evaluated infectious agents in total and in diarrheic animals as well as the percentage of animals that needed treatment were significantly different among groups and between farms. The effects of the fixed factors group, Farm, infectious agents detected (pathogens), as well as of their interactions on the fecal score, the number of days with diarrhea, the fecal score at the days with diarrhea and the diarrhea index were tested with Univariate Analysis of Variances. Repeated measures ANOVA was run to evaluate the effect of sampling day, of oregano essential oil administration (group) and their interactions (group × day) on the fecal output of E. coli and on the Ct values obtained from the RVA real-time RT-PCR. In cases of negative results a Ct value of 44 was set in order to facilitate the quantitative analysis. In both aforementioned models, the main effects of the fixed factors and of their interactions were tested with Bonferroni test. Friedman test was also run in order to determine the significance of the differences of Cryptosporidium spp. oocyst shedding at the three sampling days in total and within each group of calves. Kaplan-Meier survival analysis (MedCalc Software 9.2.1; MedCalc, Ostend, Belgium) was performed for plotting the time needed for the diarrheic calves either to recover after the onset of clinical signs at each group and for the comparison of the obtained curves. A value of P ≤ 0.05 was considered significant in all comparisons.
3. Results
The percentage of calves with adequate passive immunity transfer exceeded 90% (Table 3 ) and was not significantly different either between farms (P > 0.05) or among groups (P > 0.05). On average, the serum total protein concentration was not significantly different between groups (mean ± SE: 65.4 ± 1.7 and 61.6 ± 1.7 g/l for groups Eco and Conts, respectively; P > 0.05).
Table 3.
Incidence of diarrhea, percentages of calves with adequate passive immunity transfer and percentages of positive calves to Cryptosporidium spp., Rotavirus A (RVA), Bovine coronavirus (BCoV) and enterotoxic Escherichia coli K99/F5 (ETEC) in the three farms of the experiment (Farm 1, 2 and 3) and in calves drenched with oregano essential oil (Eco) or left untreated as controls (Conts).
| Eco | Conts | |||
|---|---|---|---|---|
| Diarrhea incidence (%) | Overall | 80.2 | 69.6a | 91.1b |
| Farm 1 | 71.0 | 50.0a | 93.3b | |
| Farm 2 | 80.0 | 80.0a | 80.0a | |
| Farm 3 | 90.0 | 80.0a | 100.0a | |
| Adequate passive transfer (%) | Overall | 92.3 | 93.5a | 91.1a |
| Farm 1 | 90.3 | 93.8a | 86.7a | |
| Farm 2 | 93.3 | 93.3a | 93.3a | |
| Farm 3 | 93.3 | 93.3a | 93.3a | |
| Cryptosporidium spp. positive (%) | Overall | 68.1 | 60.9a | 75.6a |
| Farm 1 | 74.2 | 68.8a | 80.0a | |
| Farm 2 | 70.0 | 60.0a | 80.0a | |
| Farm 3 | 60.0 | 53.3a | 67.7a | |
| RVA positive (%) | Overall | 91.2 | 89.1a | 93.3a |
| Farm 1 | 87.1 | 81.3a | 93.3a | |
| Farm 2 | 100.0 | 100.0a | 100.0a | |
| Farm 3 | 86.7 | 86.7a | 86.7a | |
| BCoV positive (%) | Overall | 7.7 | 8.7a | 6.7a |
| Farm 1 | 22.6 | 25.0a | 20.0a | |
| Farm 2 | 0 | 0a | 0a | |
| Farm 3 | 0 | 0a | 0a | |
| ETEC positive (%) | Overall | 0.0 | 0.0a | 0.0a |
| Farm 1 | 0 | 0a | 0a | |
| Farm 2 | 0 | 0a | 0a | |
| Farm 3 | 0 | 0a | 0a |
a,bDifferent superscripts at the same row denote significant difference (P < 0.05).
The infectious agents related with neonatal diarrhea syndrome that were detected at repeated fecal samplings are presented in Table 3. The percentage of animals found positive in RVA and Cryptosporidium spp. was not significantly different between groups (P > 0.05) or farms (P > 0.05). BCoV was detected only in Farm 1 and the percentage of positive animals was similar among groups (P > 0.05). Fecal shedding of Cryptosporidium spp. oocysts was not significantly different between groups at any sampling day (P > 0.05). Friedman repeated measures analysis revealed that fecal shedding of oocyst was significantly reduced on days 6 and 10 compared to day 3 in Eco group (mean ranks: 2.60, 1.88 and 1.52 for days 3, 6 and 10, respectively; x 2 = 19.79, P < 0.001), whereas the reduction was not significant in the control group (mean ranks: 2.16, 2.00 and 1.84 for days 3, 6 and 10, respectively; x 2 = 1.29, P > 0.05). Average Ct values obtained from Rotavirus real-time RT-PCR were not significantly affected either by oregano oil administration (mean ± SE: 37.43 ± 1.64 and 34.53 ± 1.52 for groups Eco and Conts, respectively; P > 0.05), or by sampling day (P > 0.05), or their interactions (P > 0.05). Fecal shedding of E. coli was also not significantly affected by group (mean ± SE: 7.84 ± 0.09 and 7.92 ± 0.08 log cfu/ml for groups Eco and Conts, respectively; P > 0.05) or day of sampling (P > 0.05), or their interactions (P > 0.05).
Diarrhea was recorded in 73 out of 91 calves (80.2%) that were used in the experiment (Table 3). The incidence of diarrhea was significantly lower (P < 0.05) in calves receiving oregano oil (Eco group) compared to the controls (P < 0.05; Table 3). Within farms, the incidence of diarrhea in Farm 1 was also significantly lower in Eco group than the Conts one (P < 0.05: Table 3), whereas in Farms 2 and 3 no significant difference was recorded among groups (P < 0.05; Table 3). Thirteen out of 73 (17.8%) diarrheic calves had two episodes of diarrhea during the experimental period, without, however, the percentages being significantly different between groups (25% and 12% for Eco and Conts groups, respectively; P > 0.05).
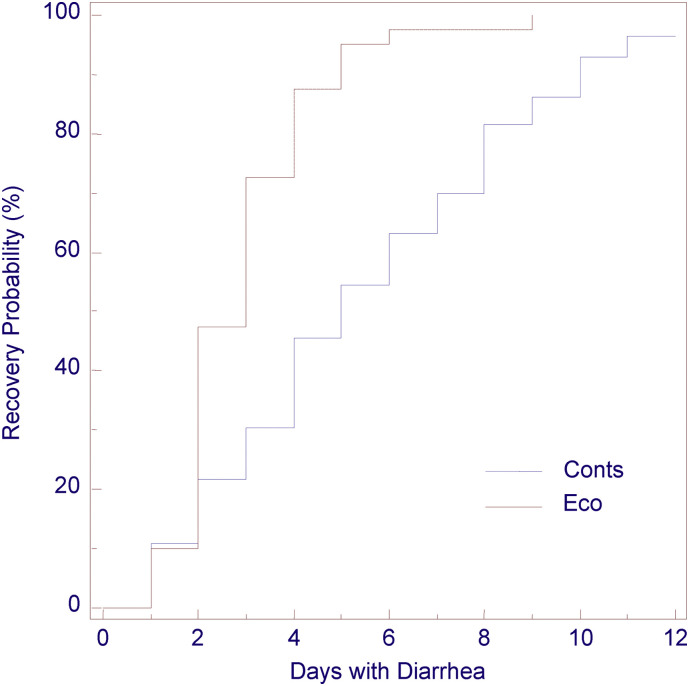
The average age of diarrhea onset was not significantly different between groups (mean ± SE: 5.89 ± 0.43 and 6.07 ± 0.45 days for groups Eco and Conts, respectively; P > 0.05). In Eco group all diarrhea cases started before day 10 and no new case was recorded between days 10 and 15 of the study. The combinations of infectious agents detected in diarrheic calves are presented in Table 4 ; the percentages of positive animals at each agent were not significantly different among groups (P > 0.05; Table 2). As it is shown in Table 5 , average fecal score recorded in calves throughout the experiment, average number of days that calves had diarrhea and average diarrhea index were significantly affected only by the daily administration of oregano oil and were significantly lower in Eco group compared to the controls (P < 0.05). However, fecal score on days with diarrhea was not significantly affected by the group of animals. The percentage of calves that needed treatment was significantly lower (P < 0.05) in Eco group than the controls (Table 6 ). Significantly lower was also the percentage of animals that needed IV fluids and antibiotic treatment in Eco group than the Conts (P < 0.05; Table 6). The survival curves depicting the time until recovery at diarrhea episodes in both groups are presented in Fig. 1 . The analysis revealed that the median time until recovery was significantly lower (P < 0.05) in Eco group (3 days) compared to the controls (5 days). No death was recorded in any experimental group.
Table 4.
Percentage (%) of diarrheic calves positive to the infectious agent or agents (RVA: Rotavirus A; BCoV: Bovine coronavirus; ETEC: enterotoxic Escherichia coli K99/F5) detected at the repeated fecal samplings in the group of animals that received oregano essential oil (Eco) and those left untreated (Conts).
| Pathogens | Eco (%) |
Conts (%) |
|---|---|---|
| RVA | 34.4a | 24.4a |
| Cryptosporidium spp. | 6.3a | 7.3a |
| RVA + Cryptosporidium spp. | 53.1a | 63.4a |
| RVA + BCoV | 6.3a | 0.0a |
| RVA + Cryptosporidium spp. + BCoV | 0.0a | 4.9a |
| ETEC | 0.0a | 0.0a |
Different superscripts at the same row denote significant difference (P < 0.05).
Table 5.
Average (mean ± SE) fecal score at the 15 days of the study, number of days with diarrhea, fecal score at the days with diarrhea and diarrhea index in calves receiving oregano oil (Eco) and those left untreated (Conts).
| Fecal score | Days with diarrhea | Diarrhea fecal score | Diarrhea index | ||
|---|---|---|---|---|---|
| Eco | 1.20 ± 0.05 | 3.86 ± 0.41 | 2.42 ± 0.06 | 9.52 ± 1.09 | |
| Conts | 1.52 ± 0.06 | 5.18 ± 0.42 | 2.44 ± 0.06 | 12.97 ± 1.21 | |
| P | Group | *** | * | NS | * |
| Farm | NS | NS | NS | NS | |
| Pathogen | NS | NS | NS | NS | |
| Group × farm | NS | NS | NS | NS | |
| Group × pathogen | NS | NS | NS | NS | |
| Farm × pathogen | NS | NS | NS | NS | |
| Group × farm × pathogen | NS | NS | NS | NS | |
*P < 0.05; ***P < 0.001; NS: no significant (P > 0.05).
Table 6.
Number and percentage (%) of the 73 diarrheic calves that needed treatment (IV fluids or IV fluids and antibiotics) in the group of animals that received oregano essential oil (Eco) and those left untreated (Conts).
| n |
Eco |
Conts |
||
|---|---|---|---|---|
| Calves needed treatment | 36 | 9 (28.1%)a | 27 (65.9%)b | |
| Treatment | IV fluids | 19 | 6 (31.6%)a | 13 (53.8%)a |
| IV fluids & antibiotics | 17 | 3 (17.6%)a | 14 (82.4%)b | |
a,bDifferent superscripts at the same row denote significant difference (P < 0.05).
Fig. 1.
Kaplan-Meier survival curve depicting the time needed for recovery after the onset of diarrhea at the 32 diarrheic calves in the group of animals receiving oregano essential oil (Eco) and at the 41 diarrheic calves in the control group (Conts).
4. Discussion
The objective of the present study was to investigate under field conditions whether daily administration of oregano essential oil could prevent the occurrence and diminish the severity of the diarrhea syndrome in newborn calves. In order to evaluate its possible preventive effect against diarrhea, oregano essential oil was administered to the calves of Eco group for the first 10 days of their life, given that the great majority of the diarrhea cases commence during this period, as occurred at the present study. It was selected to continue the administration of the oregano essential oil in calves of Eco group that remained diarrheic beyond the age of 10 days in order to be able to estimate its possible treatment effects as well. In the available literature there is not standardized dosage for oregano oil in calves. In a former experiment the dosage of 10 mg/kg was used (Bampidis et al., 2006). According to our experience in practice, diarrheic calves that weight about 40 kg are empirically orally drenched by the farmers with 10 ml of ECODIAR liquid 5%, which corresponds to the dosage rate of 12.5 mg/kg that was used at the present experiment. Oregano oil, even at the concentration of 5%, has very strong flavor; in order to avoid any milk consumption refusals it was selected not to mix it with milk but to administer it immediately after feeding as an oral drench, by diluting the drenched quantity with normal saline until 60 ml. The followed practice ensured complete milk consumption and was not associated with discomfort of the calves during administration.
The percentage of calves with adequate passive immunity transfer using 52 g/l as the cut-off value for serum total protein concentration exceeded the critical level of 90% (McGuirk, 2010) in all farms. These results indicate that the farms selected for the study had successful colostrum management practices (McGuirk, 2010) and that the distribution of calves with failure and adequate passive transfer is equal between groups. Additionally, as expected, the non-significantly different serum total proteins concentrations among groups imply that the administration of oregano oil does not impair the acquirement of passive immunity.
The most commonly detected infectious agent at the repeated fecal samplings was RVA. The detection of this virus in all three farms of the study and the high percentage of RVA positive animals is in accordance to our observations that this virus is prevalent to the great majority of the dairy herds and that, as occurs worldwide (Bartels et al., 2010, Cho et al., 2013, Al Mawly et al., 2015), is a major cause of diarrhea in neonatal calves in our country. The hardly identical percentage of positive animals and the insignificant differences on the virus shedding among groups indicate that oregano essential oil administration does not prevent the infection of calves with RVA. This is in accordance to in vitro observations that carvacrol, the main component of oregano oil, does not possess antiviral activity against rotavirus when added before but only when added after the inoculation of cell cultures with the virus (Pilau et al., 2011).
Cryptosporidium spp. was the second most commonly detected infectious factor at this study, with similar percentage of positive calves between farms. However, even though non statistically significant, the percentage of positive animals in Eco group was lower compared to the controls. This finding was constant in all 3 farms and implies that oregano oil might possibly be associated with reduced infection rates. Supporting towards to this point of view is the significant reduction of oocyst shedding from day 3 to day 10 of the experiment in Eco group whereas it remained constant in the control group. Such an effect warrants further evaluation and cannot be excluded, given that oregano oil is effective against other protozoa in vivo in other animal species (Giannenas et al., 2003, Toulah et al., 2012, Mohiti-Asli and Ghanaatparast-Rashti, 2015). Furthermore, it was recently proved in an in vitro study that at certain concentrations oregano oil as well as carvacrol alone effectively reduced (50%) the infectivity of Cryptosporidium parvum on cell cultures (Gaur et al., 2016).
Concerning the other pathogens, BCoV was detected only in one farm and to a small number of animals and no ETEC or other enteropathogenic E. coli strain was identified in any calf. However, the possibility that some strains of E. coli were undetected cannot be excluded since fecal E. coli strains are not representative of small intestinal strains (Constable, 2004). As is commonly observed under field conditions with naturally acquired diarrhea cases (Van Metre et al., 2008), the majority of diarrheic calves at the present study had mixed infections. However, the analysis of the obtained data revealed that the presence of single or mixed infections in the diarrheic calves did not have different impact on the diarrhea course given that the factor “pathogens” did not affect either the duration or the severity of diarrhea.
The overall incidence of diarrhea at the present experiment was significantly lower in calves drenched with oregano essential oil compared to the controls. However, this trend was not observed in all farms but only in Farm 1. So, it cannot be supported with certainty that oregano oil administration has preventive effect against neonatal diarrhea syndrome and the hypothesis should be further investigated. However, taking into account that the hygiene management practices in Farm 1 were more intensive compared to the other farms, it can be supported that this effect might be possible if oregano oil is drenched to calves that are exposed to low amounts of infectious agents.
The significantly lower number of days with diarrhea, the lower diarrhea index, the shorter duration of the diarrhea episodes and the lower number of calves required treatment in Eco group compared to the controls, indicate that daily administration of oregano essential oil can effectively diminish the severity of neonatal diarrhea syndrome in calves. This effect could be attributed to the inhibition of coliform bacterial overgrowth in the small intestine of diarrheic calves by oregano essential oil due to its strong antibacterial activity against E. coli (Elgayyar et al., 2001, Si et al., 2008, Nazzaro et al., 2013). In support to this point of view, Bampidis et al. (2006) observed that oregano essential oil drenching had comparable results with neomycin administration per os on the treatment outcome of diarrhea due to E. coli in calves. Furthermore, the significantly lower number of calves with systemic illness that needed antimicrobial and supportive therapy in Eco group compared to the controls, which is suggestive of lower cases of bacteremia (Constable, 2004), could also possibly imply that oregano essential oil might inhibit intestinal coliform overgrowth. However, this hypothesis has to be further investigated. The detection of similar number of E. coli in the fecal samples of calves in Eco and control group is not contrary to the proposed mode of action of oregano essential oil, given that E. coli numbers in the large intestine do not reflect the numbers in the small intestine (Constable, 2004).
The main conclusion of this study is that daily administration of Greek oregano essential oil in calves for the first 10 days of their life effectively diminishes the severity of naturally acquired diarrhea under field conditions. Furthermore, it seems that under certain hygiene management practices oregano essential oil administration might possess a preventive effect against neonatal calf diarrhea syndrome. Although further research is necessary in order to determine the exact mechanisms of these effects, oregano essential oil appears to be a promising adjunct to antibiotics for the management of diarrhea in neonatal calves.
Acknowledgments
Conflict of interest
None of the authors has any personal or financial relationships with people or organisations that could inappropriately influence or bias the content of this paper.
Acknowledgments
Acknowledgements
This research has been funded by the Research Committee of Aristotle University of Thessaloniki (grant number 93297).
References
- Al Mawly J., Grinberg A., Prattley D., Moffat J., French N. Prevalence of endemic enteropathogens of calves in New Zealand dairy farms. N. Z. Vet. J. 2015;63:147–152. doi: 10.1080/00480169.2014.966168. [DOI] [PubMed] [Google Scholar]
- Anderson D.C., Kress P.D.D., Bernardini T.M.M., Davis K.C., Boss D.L., Doornbos D.E. The effect of scours on calf weaning weight. Prof. Anim. Sci. 2003;19:399–403. [Google Scholar]
- Bampidis V.A., Christodoulou V., Florou-Paneri P., Christaki E. Effect of dried oregano leaves versus neomycin in treating newborn calves with colibacillosis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2006;53:154–156. doi: 10.1111/j.1439-0442.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- Barragry T. Calf diarrhoea. Ir. Vet. J. 1997;50:49–58. [Google Scholar]
- Bartels C.J.M., Holzhauer M., Jorritsma R., Swart W.A.J.M., Lam T.J.G.M. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves. Prev. Vet. Med. 2010;93:162–169. doi: 10.1016/j.prevetmed.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard P.C. Diagnostics of dairy and beef cattle diarrhea. Vet. Clin. N. Am. Food Anim. Pract. 2012;28:443–464. doi: 10.1016/j.cvfa.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.I., Han J.I., Wang C., Cooper V., Schwartz K., Engelken T., Yoon K.Y. Case-control study of microbiological etiology associated with calf diarrhea. Vet. Microbiol. 2013;166:375–385. doi: 10.1016/j.vetmic.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable P.D. Antimicrobial use in the treatment of calf diarrhea. J. Vet. Intern. Med. 2004;18:8–17. doi: 10.1111/j.1939-1676.2004.tb00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Fuente R., Luzón M., Ruiz-Santa-Quiteria J.A., García A., Cid D., Orden J.A., García S., Sanz R., Gómez-Bautista M. Cryptosporidium and concurrent infections with other major enterophatogens in 1 to 30-day-old diarrheic dairy calves in central Spain. Vet. Parasitol. 1999;80:179–185. doi: 10.1016/S0304-4017(98)00218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Elia G., Campolo M., Desario C., Mari V., Radogna A., Colaianni M.L., Cirone F., Tempesta M., Buonavoglia C. Detection of bovine coronavirus using a Taq-Man-based real-time RT-PCR assay. J. Virol. Methods. 2008;151:167–171. doi: 10.1016/j.jviromet.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgayyar M., Draughon F.A., Golden D.A., Mount J.R. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J. Food Prot. 2001;64:1019–1024. doi: 10.4315/0362-028x-64.7.1019. [DOI] [PubMed] [Google Scholar]
- Franck S.M., Bosworth B.T., Moon H.W. Multiplex PCR for enterotoxigenic, attaching and effacing, and Shiga toxin-producing Escherichia coli strains from calves. J. Clin. Microbiol. 1998;36:1795–1797. doi: 10.1128/jcm.36.6.1795-1797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M.M., Kerin T., Hull J., McCaustland K., Gentsch J. Enhancement of detection and quantification of rotavirus in stool using a modified real-time RT-PCR assay. J. Med. Virol. 2008;80:1489–1496. doi: 10.1002/jmv.21228. [DOI] [PubMed] [Google Scholar]
- Gaur S., Kuhlenschmidt T.B., Kuhlenschmidt M.S., AndradeJ E. Oregano essential oil and carvacrol reduce Cryptosporidium parvum infectivity of HCT-8 cells. FASEB J. 2016;30(1 Suppl):668.12. doi: 10.1016/j.parint.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Giannenas I., Florou-Paneri P., Papazahariadou M., Christaki E., Botsoglou N.A., Spais A.B. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Tierernahr. 2003;57:99–106. doi: 10.1080/0003942031000107299. [DOI] [PubMed] [Google Scholar]
- Gulliksen S.M., Jor E., Lie K.I., Hamnes I.S., Løken T., Åkerstedt J., Østerås O. Enteropathogens and risk factors for diarrhea in Norwegian dairy calves. J. Dairy Sci. 2009;92:5057–5066. doi: 10.3168/jds.2009-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen S.A., Pohlenz J.F. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet. Scand. 1981;22:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirk S. School of Veterinary Medicine, University of Wisconsin-Madison; USA: 2010. Herd-based Problem Solving: Failure of Passive Transfer.https://www.vetmed.wisc.edu/dms/fapm/fapmtools/8calf/calf_herd_FPT_Troubleshooting.pdf (accessed 4/4/2017) [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prev. Vet. Med. 2015;120:195–202. doi: 10.1016/j.prevetmed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ok M., Güler L., Turgut K., Ok Ü., Sen I., Gündüz I.K., Birdane M.F., Güzelbektes H. The studies on the aetiology of diarrhoea in neonatal calves and determination of virulence gene markers of Escherichia coli strains by multiplex PCR. Zoonoses Public Health. 2009;56:94–101. doi: 10.1111/j.1863-2378.2008.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappi P.G., Chaintoutis S.C., Dovas C.I., Efthimiou K.E., Katis N.I. Development of one-tube real-time qRT-PCR and evaluation of RNA extraction methods for the detection of Eggplant mottled dwarf virus in different species. J. Virol. Methods. 2015;212:59–65. doi: 10.1016/j.jviromet.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Pilau M.R., Alves S.H., Weiblen R., Arenhart S., Cueto A.P., Lovato L.T. Antiviral activity of the Lippiagraveolens (Mexican oregano) essential oil and its main compound carvacrol against human and animal viruses. Braz. J. Microbiol. 2011;42:1616–1624. doi: 10.1590/S1517-838220110004000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen K.P., Foley A.L., Collins M.T., McGuirk S.M. Comparison of passive transfer of immunity in neonatal dairy calves fed colostrum or bovine serum-based colostrum replacement and colostrum supplement products. J. Am. Vet. Med. Assoc. 2010;237:949–954. doi: 10.2460/javma.237.8.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H., Hu J., Liu Z., Zeng Z.L. Antibacterial effect of oregano essential oil alone and in combination with antibiotics against extended-spectrum β-lactamase-producing Escherichia coli. FEMS Immunol. Med. Microbiol. 2008;53:190–194. doi: 10.1111/j.1574-695X.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- Silverlås C., de Verdier K., Emanuelson U., Mattsson J.G., Björkman C. Cryptosporidium infection in herds with and without calf diarrhoeal problems. Parasitol. Res. 2010;107:1435–1444. doi: 10.1007/s00436-010-2020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulah F.H., Esmail H.A., Khan S. The efficacy of Origanum vulgare on Eimeria tenella. J. Egypt. Soc. Parasitol. 2012;42:245–250. [PubMed] [Google Scholar]
- Tyler J.W., Hancock D.D., Parish S.M., Rea D.E., Besser T.E., Sanders S.G., Wilson L.K. Evaluation of 3 assays for failure of passive transfer in calves. J. Vet. Intern. Med. 1996;10:304–307. doi: 10.1111/j.1939-1676.1996.tb02067.x. [DOI] [PubMed] [Google Scholar]
- Van Metre D.C., Tennant B.C., Whitlock R.H. Infectious diseases of the gastrointestinal tract. In: Divers T.J., Peel S.F., editors. Rebhun's Diseases of Dairy Cattle. 2nd edition. Saunders, Elsevier; St. Louis: 2008. pp. 200–294. [Google Scholar]