Summary
Background
Middle East respiratory syndrome (MERS) is a new human disease caused by a novel coronavirus (CoV). Clinical data on MERS-CoV infections are scarce. We report epidemiological, demographic, clinical, and laboratory characteristics of 47 cases of MERS-CoV infections, identify knowledge gaps, and define research priorities.
Methods
We abstracted and analysed epidemiological, demographic, clinical, and laboratory data from confirmed cases of sporadic, household, community, and health-care-associated MERS-CoV infections reported from Saudi Arabia between Sept 1, 2012, and June 15, 2013. Cases were confirmed as having MERS-CoV by real-time RT-PCR.
Findings
47 individuals (46 adults, one child) with laboratory-confirmed MERS-CoV disease were identified; 36 (77%) were male (male:female ratio 3·3:1). 28 patients died, a 60% case-fatality rate. The case-fatality rate rose with increasing age. Only two of the 47 cases were previously healthy; most patients (45 [96%]) had underlying comorbid medical disorders, including diabetes (32 [68%]), hypertension (16 [34%]), chronic cardiac disease (13 [28%]), and chronic renal disease (23 [49%]). Common symptoms at presentation were fever (46 [98%]), fever with chills or rigors (41 [87%]), cough (39 [83%]), shortness of breath (34 [72%]), and myalgia (15 [32%]). Gastrointestinal symptoms were also frequent, including diarrhoea (12 [26%]), vomiting (ten [21%]), and abdominal pain (eight [17%]). All patients had abnormal findings on chest radiography, ranging from subtle to extensive unilateral and bilateral abnormalities. Laboratory analyses showed raised concentrations of lactate dehydrogenase (23 [49%]) and aspartate aminotransferase (seven [15%]) and thrombocytopenia (17 [36%]) and lymphopenia (16 [34%]).
Interpretation
Disease caused by MERS-CoV presents with a wide range of clinical manifestations and is associated with substantial mortality in admitted patients who have medical comorbidities. Major gaps in our knowledge of the epidemiology, community prevalence, and clinical spectrum of infection and disease need urgent definition.
Funding
None.
Introduction
Middle East respiratory syndrome (MERS) is a new human disease1 first reported from Saudi Arabia in September, 2012, after identification of a novel coronavirus (CoV) from a male Saudi Arabian patient who died from severe respiratory illness in June, 2012.1, 2 The virus was initially designated HCoV-EMC,2 but after global consensus it was renamed MERS-CoV.3 Since the first reported Saudi case of MERS-CoV, the Saudi Ministry of Health mandated that all patients with respiratory illnesses needing admission to intensive care should be tested for the virus, using any available clinical specimen. All laboratory-confirmed cases of MERS-CoV to date from Saudi Arabia have been reported to WHO.4, 5
Data for clinical characteristics of MERS are scant because of the small number of cases detected since appearance of the virus 15 months ago. Clinical information from individual or cluster case reports is recorded by the Program for Monitoring Emerging Diseases (ProMED) and is summarised through updates6, 7, 8, 9, 10 from WHO, the US Centers for Disease Control and Prevention (CDC), and the European Centre for Disease Prevention and Control (ECDC). Data suggest that MERS presents primarily with respiratory symptoms and that most patients with serious disease have either other medical comorbid disorders or immunosuppression. Case reports of family clusters and hospital outbreaks in Saudi Arabia,11, 12, 13 and other countries in the Middle East and Europe,14, 15, 16, 17, 18, 19, 20 also indicate that individuals with MERS-CoV can present with a range of respiratory and non-respiratory symptoms, with many manifestations of clinical disease. Furthermore, recent case reports from Saudi Arabia11, 12, 13, the UK,15, 16 France,17 Germany,18, 19, 20 Italy,21 and Tunisia22 suggest that mild respiratory illness might be part of a wider clinical spectrum of MERS-CoV infection. Two patients from Tunisia9, 22 and one from the UK16 had mild respiratory illnesses and they did not need to be admitted. Furthermore, initial presentations of MERS-CoV disease might not include respiratory symptoms initially.12, 13 The first patient reported from France17 of MERS-CoV infection presented initially with diarrhoea and abdominal pain and subsequently developed respiratory symptoms.
We report demographic, clinical, and laboratory characteristics of MERS in patients diagnosed in Saudi Arabia up to June 15, 2013. Additional information is included for 27 Saudi cases reported previously by us.11, 12, 13
Methods
Data collection
We reviewed clinical records, nursing charts, laboratory results, and imaging findings for all patients with laboratory-confirmed MERS-CoV infection who were reported by the Saudi Ministry of Health to WHO from Sept 1, 2012, to June 15, 2013. We obtained epidemiological, demographic, clinical, laboratory, and management outcome data and entered this information into standardised data collection forms (prepared by ourselves for this study). If data were missing from the records or clarification was needed, we gathered data by direct communication with attending doctors and other health-care providers. We cross-checked the number of cases we identified with samples reported as positive from the Saudi Ministry of Health regional reference virology laboratory in Jeddah. We obtained data for global MERS-CoV cases from ProMED, WHO, and CDC reports.
Procedures
Attending doctors took Dacron-flocked nasopharyngeal swabs from patients routinely and submitted these to the Ministry of Health laboratory for initial screening of MERS-CoV infection. Attending doctors also obtained deep respiratory samples (tracheal aspirates and bronchoalveolar lavage) from patients admitted to intensive-care units. Clinical samples were screened at the Ministry of Health laboratory with real-time RT-PCR.23, 24 Amplification targeted both the upstream E protein (upE gene) and ORF1a for confirmation, and these are standard assays used in Saudi Arabia for MERS-CoV testing. A case was confirmed as having infection if both assays were positive. In case of discordance, or if the result was judged a weak positive, another clinical sample was requested and analysed by the Ministry of Health laboratory. All patients had chest radiography. Chest CT was done in selected patients at the discretion of attending doctors when clinically indicated.
Statistical analyses
We tabulated demographic, clinical, and laboratory descriptive data. We did not do detailed statistical analyses because of the self-selected nature of the cases. We did univariate analyses of the association of sex or increasing age with mortality, using binary logistic regression analysis. We judged p<0·05 significant.
Role of the funding source
No funding was received for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
47 cases of laboratory-confirmed MERS-CoV disease reported from Saudi Arabia between Sept 1, 2012, and June 15, 2013, were identified. All individuals except two were Saudi Arabian citizens. Identified cases of MERS-CoV included two clusters, one of three people in one family living in one villa,12 which were reported in October and November, 2012, and a hospital outbreak in Al-Hasa13 of 23 cases in April and May, 2013. Transfer of patients and readmissions resulted in cases and transmission in two health-care facilities. In the outbreak at Al-Hasa,13 the median incubation period was 5·2 days, with 95% of patients estimated to have symptom onset within 12·4 days. The estimated serial interval was 7·6 days.13 Table 1 shows the distribution over time of MERS-CoV infections and deaths, from Saudi Arabia and other countries.
Table 1.
Distribution of Middle East respiratory syndrome-coronavirus infections and deaths, by country (March 1, 2012, to July 21, 2013)
|
2012 |
2013 |
Total | Deaths (%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| March | April | May | June | July | Aug | Sept | Oct | Nov | Dec | Jan | Feb | March | April | May | June | July | |||
| Saudi Arabia | .. | .. | .. | 1 | .. | .. | .. | 3 | 1 | .. | 1 | 2 | 1 | 16 | 16 | 22 | 7 | 70 | 38 (54%) |
| Jordan | .. | 2* | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 2 | 2 (100%) |
| Qatar | .. | .. | .. | .. | .. | .. | 1† | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 1 | 1 (100%) |
| UK | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 3‡ | .. | .. | .. | .. | .. | .. | 3 | 2 (67%) |
| Germany§ | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 1 | .. | .. | 1 | .. | .. | 2 | 1 (50%) |
| France | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 2¶ | .. | .. | .. | 2 | 1 (50%) |
| Italy | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 1‖ | 2** | .. | 3 | 0 |
| Tunisia | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 2 | .. | .. | 2 | 0 |
| United Arab Emirates | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 5 | 5 | 0 |
| Global total | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 90 | 45 (50%) |
Patients who died in April, 2012, were reported retrospectively in September, 2012; further serological testing of this cluster in Jordan detected eight additional cases from contacts (six from the outbreak members, one household, and one health-care worker), but these have not been confirmed by real-time RT-PCR.
Transferred to UK for treatment.
Index case was linked to travel to Pakistan and Saudi Arabia; second and third cases had no travel history but were family members of the index case.
February case linked to travel from Abu Dhabi, United Arab Emirates, and was transferred to Germany for hospital treatment; case from May came from Qatar for treatment in Germany.
First case linked to travel to Dubai; the other was acquired nosocomially in a French hospital from the first patient.
First case had a travel link with Jordan.
Family member (2-year-old girl) and a coworker of the first case, who was presumed to be the index case.
Table 2 shows the distribution of cases by sex and age, and case-fatality rates. The male:female case ratio was 3·3:1. Of 47 patients, 28 (60%) died, and the case-fatality rate was similar between female and male patients (55% vs 61%; odds ratio 1·31, 95% CI 0·34–5·12; p=0·698). Case-fatality rates rose with increasing age, from 39% (seven of 18) in those younger than 50 years, to 48% (13 of 27) in the group aged under 60 years, and to 75% (15 of 20) in cases aged 60 years or older. Univariate analysis (by binary logistic regression) of age 60 years or older as a risk factor for mortality was not significant (odds ratio 3·23, 95% CI 0·91–11·42; p=0·069).
Table 2.
Mortality in 47 Saudi cases of Middle East respiratory syndrome
|
Female |
Male |
All |
|||||
|---|---|---|---|---|---|---|---|
| Total | Dead | Total | Dead | Total | Dead | ||
| Age range (years) | |||||||
| 10–19 | 1 | 0 | 1 | 0 | 2 | 0 | |
| 20–29 | 0 | 0 | 1 | 1 | 1 | 1 | |
| 30–39 | 0 | 0 | 5 | 2 | 5 | 2 | |
| 40–49 | 1 | 0 | 9 | 4 | 10 | 4 | |
| 50–59 | 2 | 1 | 7 | 5 | 9 | 6 | |
| 60–69 | 5 | 4 | 6 | 4 | 11 | 8 | |
| 70–79 | 1 | 0 | 4 | 4 | 5 | 4 | |
| 80–89 | 1 | 1 | 2 | 1 | 3 | 2 | |
| 90–99 | 0 | 0 | 1 | 1 | 1 | 1 | |
| Total | 11* | 6 (55%) | 36* | 22 (61%) | 47 | 28 (60%) | |
Male:female ratio 3·3:1.
The most common symptoms at presentation (table 3 ) were fever, fever with chills or rigors, cough, shortness of breath, and myalgia. Gastrointestinal symptoms were also frequent, including diarrhoea, vomiting, and abdominal pain.
Table 3.
Symptoms of Middle East respiratory syndrome in 47 Saudi cases at presentation
| Patients (n=47) | ||
|---|---|---|
| Fever | 46 (98%) | |
| Fever with chills or rigors | 41 (87%) | |
| Cough | 39 (83%) | |
| Dry | 22 (47%) | |
| Productive (sputum) | 17 (36%) | |
| Haemoptysis | 8 (17%) | |
| Shortness of breath | 34 (72%) | |
| Chest pain | 7 (15%) | |
| Sore throat | 10 (21%) | |
| Runny nose | 2 (4%) | |
| Abdominal pain | 8 (17%) | |
| Nausea | 10 (21%) | |
| Vomiting | 10 (21%) | |
| Diarrhoea | 12 (26%) | |
| Myalgia | 15 (32%) | |
| Headache | 6 (13%) | |
Of 47 patients, 42 (89%) needed intensive care and 34 (72%) had mechanical ventilation. The median time to mechanical ventilation was 7 days (range 3–11) and median time to death was 14 days (range 5–36). Most patients received oseltamivir and broad-spectrum antibiotics, which covered community-acquired and atypical pneumonia. Five individuals were also empirically started on fluconazole, two received steroids, five were treated with ribavirin, one was given interferon alfa, and five were infused with intravenous immunoglobulin.
45 (96%) of 47 patients had underlying comorbid medical disorders and only two people were previously healthy (table 4 ). Diabetes, chronic renal disease, chronic cardiac disease, and hypertension were the most frequent comorbid disorders. One patient was on long-term immunosuppressive treatment with steroids. The number of comorbidities in relation to mortality is shown in table 5 .
Table 4.
Comorbidities in 47 Saudi cases of Middle East respiratory syndrome
| Patients (n=47) | Deaths (%)* | |
|---|---|---|
| Any comorbidity | 45 (96%) | 28 (60%) |
| Diabetes | 32 (68%) | 21 (66%) |
| Chronic kidney disease | 23 (49%) | 17 (74%) |
| Chronic heart disease | 13 (28%) | 10 (77%) |
| Hypertension | 16 (34%) | 13 (81%) |
| Chronic lung disease | 12 (26%) | 10 (83%) |
| Obesity | 8 (17%) | 5 (63%) |
| Smoking | 11 (23%) | 7 (64%) |
| Malignant disease | 1 (2%) | 1 (100%) |
| Steroid use | 3 (6%) | 3 (100%) |
Proportion of patients who died according to comorbidity.
Table 5.
Number of comorbidities in relation to mortality
| Total | Dead | |
|---|---|---|
| None | 2 | 0 |
| 1 | 11 | 3 |
| 2 | 11 | 6 |
| 3 | 10 | 8 |
| 4 | 10 | 8 |
| 5 | 2 | 2 |
| 6 | 1 | 1 |
| Total | 47 | 28 (60%) |
All 47 patients had MERS-CoV infection confirmed by real-time RT-PCR testing, which was done by measuring cycle threshold (Ct) values for viral load. Ct data were available from 37 samples, and these ranged from 19·85 to more than 40, the limit of detection (appendix). The time between onset of symptoms and obtaining clinical samples varied. During the health-care-associated outbreak at Al-Hasa,13 most patients were sampled within 72 h of onset. For sporadic cases and family case clusters, intervals were longer (average 6 days).
Haematological abnormalities seen on admission were thrombocytopenia (17 [36%]), lymphopenia (16 [34%]), and lymphocytosis (five [11%]). 43 (91%) patients had normal neutrophil counts on admission, and monocyte counts were also normal. Concentrations of lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase were raised in 23 (49%), five (11%), and seven (15%) patients, respectively. Other liver-function test values were within the normal range.
None of the 47 blood cultures or respiratory-tract samples screened for bacterial, viral, or fungal pathogens on admission was positive. No cases of co-infection with MERS-CoV were recorded. Microbiological investigations excluded bacterial pathogens associated with community-acquired pneumonia.
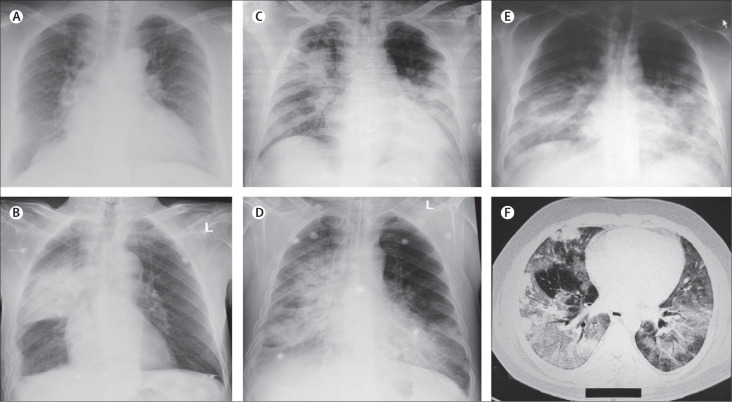
Abnormalities on chest radiography were noted in all 47 cases. Imaging findings ranged from minor to extensive unilateral and bilateral abnormalities and included increased bronchovascular markings, airspace opacities, patchy infiltrates, interstitial changes, patchy to confluent air-space consolidation, nodular opacities, reticular opacities, reticulonodular shadowing, pleural effusions, and total opacification of lung segments and lobes (figure 1A–E ). Chest CT was done in some cases if clinically indicated and at the request of the attending doctor (figure 1F).
Figure 1.
Imaging findings at presentation in Saudi patients with Middle East respiratory syndrome
(A) Chest radiograph of a 61-year-old man, showing bilateral fine reticulonodular air-space opacities, increased vascular markings, and cardiomegaly. (B) Chest radiograph of an 83-year-old man, showing right lung consolidation, right basal pleural thickening, and reticulonodular air-space opacities; rib fractures on the right are old. (C) Chest radiograph of a 56-year-old man, showing extensive bilateral extensive diffuse and focal alveolar space opacities, with opacification of the left lower lobe. (D) Chest radiograph of a 67-year-old man, showing extensive bilateral disease, with diffuse alveolar space densities, opacification, reticulonodular opacities, and bronchial wall thickening. (E) Chest radiograph of a 49-year-old man, showing extensive bilateral mid and lower zone disease, with diffuse reticulonodular alveolar space opacities. A thoracic CT scan in the same patient (F) shows extensive bilateral opacities and ground-glass reticulonodular shadowing and bronchiolar wall thickening.
Discussion
As of July 21, 2013, 90 laboratory-confirmed cases of MERS have been reported worldwide to WHO, including 45 deaths (table 1).25 The first two known patients with MERS-CoV infection (diagnosed retrospectively) died in April, 2012, at a public hospital in Zarqa, Jordan.14 All individuals with MERS have been linked directly or indirectly to one of four countries in the Middle East—namely Saudi Arabia, Jordan, Qatar, and the United Arab Emirates. The largest number of MERS-CoV cases has been reported from Saudi Arabia, where 70 cases are known to date. Countries outside Saudi Arabia—reporting a total of 20 patients with MERS—are Jordan,14 the UK,15, 16 Italy,21 Germany,19 France,17, 18 Tunisia,22 Qatar, and United Arab Emirates (table 1). Some cases of MERS-CoV infection have been reported in travellers returning from the Middle East and in their close contacts (some without a travel history),16, 17 suggesting person-to-person transmission. This type of transmission was confirmed by genome sequencing of MERS-CoV isolates from the Al-Hasa health-care-associated outbreak.13
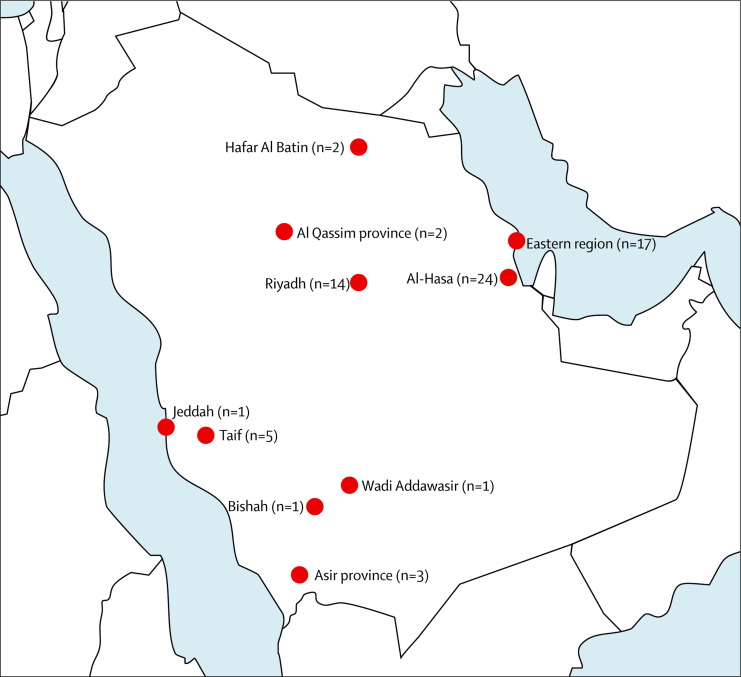
In Saudi Arabia, the first case of MERS-CoV infection occurred in June, 2012 (reported in September, 2012). Two main reporting periods subsequently became apparent. The first was the early period from June, 2012, until April 1, 2013, during which time nine cases were recorded in residents of central and western Saudi Arabia (Bishah, Al Qassim, and Riyadh; figure 2 ). These cases were predominantly male (90%), occurred sporadically, and included two small family clusters in Riyadh. In one family cluster,12 the index case resided in an extended household of 28 males and females, including 18 children. Secondary cases arose in two sons, and a grandson also became infected. During this first reporting period, no ill health was identified or reported among health-care workers; thus, transmission potential of MERS-CoV in the community seems low. In the second period, from April 1, 2013, to July 14, 2013, almost all cases arose in the eastern province of Saudi Arabia (Al-Hasa) and were associated mainly with transmission in hospitals, including three cases among family contacts and two cases in health-care workers.13 The rise in the number of MERS-CoV cases detected in Saudi Arabia during the second reporting period might not reflect increased transmission but rather could be attributable to augmented case detection as a result of enhanced screening and surveillance activities. Dissemination of information on MERS-CoV by the Saudi Ministry of Health has raised awareness among health-care providers and the public, leading to an increase in reports of suspected MERS-CoV cases, from 168 in April, 2013, to 524 in May, 2013 (currently about 70 reports daily, on average).
Figure 2.
Map of Saudi Arabia showing distribution of patients with Middle East respiratory syndrome by city or region
Transfer of patients and readmissions, in addition to sporadic community introductions, led to transmission of MERS-CoV between patients at four health-care facilities.13 The outbreak at Al-Hasa was brought under control quickly with infection-control measures.13 In the UK, MERS-CoV was transmitted to a family member who visited a patient with confirmed infection,16 and a recent report from France described patient-to-patient nosocomial transmission of MERS-CoV.17 In that case, a 64-year-old French man with a renal transplant fell ill with diarrhoea and fever on April 22, 2013, a week after returning from Dubai. Pneumonia was an incidental finding on chest radiography and he subsequently developed severe respiratory disease, from which he died. A second case of MERS-CoV infection was diagnosed subsequently at the same hospital in a patient on long-term steroid treatment. The respiratory presentation of the index case suggests transmission in the hospital room shared by both patients. However, infectiousness of faeces (because the index case presented with diarrhoea) at an early stage due to cross-contamination cannot be ruled out.
Our study design has several limitations that do not allow for more detailed statistical analyses. First, patients were self-selected because of referral to hospital and being screened actively for MERS-CoV by nature of their serious clinical condition. Second, we did a retrospective study of clinical case records. Third, case record entry was not done uniformly. Fourth, comorbidity data were unavailable for admissions without MERS-CoV infection and from the community population. Finally, follow-up data after discharge were missing. Despite these limitations, we have been able to delineate further the epidemiological, demographic, and clinical characteristics of 47 Saudi patients with MERS-CoV infection. The main findings of our study are that most patients present with serious respiratory disease, need to be admitted to hospital, and 60% of them die. Adults of both sexes are affected, at a mean age of 56 years (range 14–94). A quarter of patients had accompanying gastrointestinal symptoms, including diarrhoea and vomiting, and many cases occurred in individuals with chronic underlying comorbid medical disorders. Half of all patients had two medical comorbidities: diabetes and chronic renal disease. A study from Saudi Arabia showed that more than 50% of Saudi people older than 50 years have diabetes.26 However, the large number of people with MERS-CoV infection and chronic renal disease might have been biased by the Al-Hasa hospital outbreak, which happened in the renal unit. Further case-control studies are needed to define the effect of comorbidities on susceptibility to, and associated mortality from, MERS-CoV infection.
WHO's case definition for MERS-CoV, which was used by the Saudi Ministry of Health for screening,5, 6 includes fever of higher than 38°C, thus self-selecting patients who are clinically ill. This classification precludes identification of the full range of clinical presentations and misses asymptomatic, subclinical, and mild cases. Furthermore, since the first case report from Saudi Arabia, in September, 2012, the Ministry of Health has recommended mandatory testing for MERS-CoV of all cases of respiratory illness requiring admission to intensive care. This directive has biased selection and detection of more severe cases of MERS-CoV infection and might have skewed the observed high case-fatality rates. Clinical symptoms, laboratory investigations, and imaging findings of MERS-CoV are similar to those noted in other community-acquired respiratory-tract infections. For example, imaging features of MERS generally resemble those seen in patients with community-acquired pneumonia. Progression from unilateral focal air-space opacities to multifocal or bilateral involvement was frequent in patients admitted to the intensive-care unit with MERS pneumonia. The radiographic appearance of MERS lung disease shares common features with pneumonia of other causes.
Clusters of MERS-CoV infection within families indicate that a range of clinical illness occurs. Reports from Tunisia22 and the United Arab Emirates of infections in siblings whose father's illness was a probable case of MERS-CoV infection, and the case report from the UK,16 show that siblings who are not immunocompromised manifest only mild respiratory illness and do not need admission. In the UK family cluster,16 of 33 close contacts (20 household and 13 non-household), only two cases of MERS-CoV infection were confirmed (6% attack rate); one individual had mild illness and the other had severe illness. Importantly, no cases of MERS-CoV infection were reported in 59 health-care workers who were in contact with the index case without wearing full personal protective equipment.
Recent discussions have focused on similarities of clinical and laboratory features between MERS-CoV and severe acute respiratory syndrome-coronavirus (SARS-CoV) infections.27, 28, 29, 30, 31, 32, 33, 34 SARS originated in southern China in November, 2002, spread to Hong Kong, and was transmitted rapidly worldwide, resulting in 8422 cases and 916 deaths (case-fatality rate 11%). On the basis of our findings, the clinical features of MERS-CoV disease bear some resemblance to those seen in patients with disease caused by SARS-CoV. In patients with MERS, fever, cough, and dyspnoea are the major symptoms in those admitted to hospital. Other common presenting symptoms include chills, rigor, headache, myalgia, and malaise. Although respiratory failure is a major clinical feature, mild disease and atypical presentation with diarrhoea have been reported in cases of MERS and SARS. Common laboratory features of SARS32, 33, 34 recorded on admission include infiltrates on chest radiographs and lymphopenia, whereas thrombocytopenia and raised amounts of lactate dehydrogenase and alanine aminotransferase have been noted in some cases. Although our data show similarities in clinical presentation between cases of MERS and SARS, some important differences are also present (table 6 ).
Table 6.
Comparison of features of MERS-CoV infection and SARS-CoV outbreaks
| MERS-CoV | SARS, global27, 28, 29, 30, 31, 32, 33, 34 | ||
|---|---|---|---|
| Demographic factors | |||
| Date of first case report (place) | April, 2012 (Jordan);June, 2012 (first Saudi case) | November, 2002 (China) | |
| Mean (95% CI) incubation period (days) | 5·2 (1·9–14·7); range 2–13 | 4·6 (3·8–5·8); range 2–14 | |
| Serial interval (days) | 7·6 | 8·4 | |
| Age distribution | 98% adults, 2% children | 93% adults, 5–7% children | |
| Mean (range) age (years) | 56 (14–94) | 39·9 (1–91) | |
| Sex distribution | 77% male, 23% female | 43% male, 57% female | |
| Sex ratio (male:female) | 3·3:1 | 1:1·3 | |
| Clinical features | |||
| Mortality | 55% | 0–40% | |
| Case-fatality rate (overall) | Undefined | 9·6% | |
| In patients with comorbidities | 60% | 1–2% | |
| Mean time from onset to death (days) | 16·5 | 23·7 | |
| Presenting symptoms | .. | .. | |
| Fever >38°C | 98% | 99–100% | |
| Chills or rigors | 87% | 15–73% | |
| Cough | 83% | 62–100% | |
| Dry cough | 47% | 29–75% | |
| Productive cough | 36% | 4–29% | |
| Haemoptysis | 17% | 0–1% | |
| Headache | 13% | 20–56% | |
| Myalgia | 32% | 45–61% | |
| Malaise | 38% | 31–45% | |
| Shortness of breath | 72% | 40–42% | |
| Nausea | 21% | 20–35% | |
| Vomiting | 21% | 20–35% | |
| Diarrhoea | 26% | 20–25% | |
| Sore throat | 21% | 13–25% | |
| Rhinorrhoea | 4% | 2–24% | |
| Comorbidities | 96% | 10–30% | |
| Diabetes | 68% | 24% | |
| Chronic renal disease | 49% | 2–6% | |
| Chronic heart disease | 28% | 10% | |
| Malignant disease | 2% | 3% | |
| Hypertension | 34% | 19% | |
| Obesity | 17% | .. | |
| Smoking | 23% | 17% | |
| Viral hepatitis | Not known | 27% | |
| Ventilatory support needed | 80% | 14–20% | |
| Laboratory results | |||
| Chest radiography abnormalities | 100% | 94–100% | |
| Leucopenia (<4·0 × 109 cells per L) | 14% | 25–35% | |
| Lymphopenia (<1·5 × 109 cells per L) | 34% | 68–85% | |
| Thrombocytopenia (<140 × 109 cells per L) | 36% | 40–45% | |
| Increased amount of lactate dehydrogenase | 49% | 50–71% | |
| Increased amount of alanine aminotransferase | 11% | 20–30% | |
| Increased amount of aspartate aminotransferase | 15% | 20–30% | |
After the Al-Hasa health-care-associated outbreak,13 global concern grew about the pandemic potential of MERS-CoV.35 Mathematical models developed recently36, 37 allow conjecture on the pandemic potential of MERS-CoV. Breban and colleagues37 defined the rate of spread by analysis of 55 of the first 64 laboratory-confirmed cases of MERS-CoV infection. The basic reproduction number (R0; ie, the number of secondary cases every index patient is expected to infect in a fully susceptible population) of MERS-CoV is 0·69 in the worst-case scenario and 0·60 in a more optimistic scenario. These researchers concluded that MERS-CoV does not yet have pandemic potential, which would occur if R0 reached 1. Breban and colleagues' analysis shows that the chance of MERS-CoV having an R0 greater than 1 is very small. Data to assess any changes in incidence of admissions or mortality due to pneumonia are not available in the WHO Eastern Mediterranean region. Overall, as yet, evidence is scant throughout this WHO region to support the possibility of either transmission of MERS-CoV from asymptomatic infected individuals or ongoing, low prevalence, mildly symptomatic illness in the community.
Reducing the rate of introduction of MERS-CoV into human beings is unpredictable because the source of the virus is not yet known. We are searching vigorously for the source of MERS-CoV in animal hosts and other potential reservoirs and for transmission routes to individuals. Active surveillance, achieving early detection, rapid diagnosis, and isolation of MERS cases has been in place in Saudi Arabia to achieve early control of the virus. The likelihood that MERS will follow a path similar to that of SARS remains unlikely. The virus has been circulating for 15 months without reaching a form capable of causing a pandemic. Proactive surveillance for severe acute respiratory illness caused by MERS-CoV is ongoing across Saudi Arabia. As of July 14, 2013, more than 3000 respiratory-tract samples had been tested for the presence of MERS-CoV at the Saudi Ministry of Health reference laboratory in Jeddah. WHO's definition of a probable case of MERS-CoV infection5 includes individuals with severe acute respiratory illness with no known cause and with an epidemiological link to a confirmed case. Until the transmission characteristics of MERS-CoV are better understood,34 patients under investigation, and probable and confirmed cases of infection, should be managed in health-care facilities with standard contact and droplets precautions. As information becomes available, these recommendations will be re-evaluated and updated as needed.
Long-term sequelae of patients who recover from acute MERS are not yet known and need to be defined. Clinical follow-up of patients who recovered from SARS shows radiological, functional, and psychological abnormalities of varying degrees.38 In the early rehabilitation phase, many patients complained of limitations in physical function from general weakness or shortness of breath. Lung function testing 6–8 weeks after hospital discharge showed a mild or moderate restrictive pattern consistent with muscle weakness in 6–20% of individuals. Psychobehavioural problems such as anxiety and depression were not uncommon in the early recovery phase and improved over time for most patients.
Our study was not designed to ascertain prognostic predictive values. The ability of clinical, laboratory, and other features to distinguish MERS-CoV from other respiratory-tract infections will need appropriately controlled studies with large numbers of patients. In the SARS epidemic, all laboratory variables (apart from absolute neutrophil counts) distinguished SARS poorly from other causes of community-acquired pneumonia. The comparatively few cases of MERS-CoV disease identified to date restricts the certainty of any comparison of epidemiological, demographic, and clinical features. Our study brings to light major knowledge gaps and a range of unanswered questions on the epidemiology, natural history, clinical presentation, course of disease, optimum management, and eventual control of MERS-CoV infection (panel 1 ). The source of infection, predisposing factors for susceptibility, and predictive factors for poor outcome remain unknown and need further investigation. Case-control and community studies are also needed to define accurately the clinical range of disease caused by MERS-CoV. Early recognition and rapid initiation of infection-control precautions are currently the most important strategies for controlling nosocomial MERS-CoV infections. At the moment, no tests are available to rule out MERS among patients with febrile respiratory illnesses, and development of a range of rapid and accurate diagnostic tests is needed urgently.39 With availability of improved laboratory methods,40 important questions about transmission, risk factors, clinical course, and natural history of MERS-CoV infection need to be addressed at once (panel 2 ).
Panel 1. Key knowledge gaps and research priorities.
Epidemiology
-
1)
What is the origin and the natural reservoir for MERS-CoV?*
-
2)
What is the source of MERS-CoV exposure and infection acquired outside health-care facilities: in the household or the community (animals, foodstuff, water, sewage, other)?*
-
3)
What is the range of genetic diversity among MERS-CoV isolates, their evolution, and importance?
-
4)
What is the full range of clinical expression of MERS-CoV disease in the community (asymptomatic carriage to mild, moderate, and severe disease)? What proportion of cases are severe, mild, or asymptomatic? What are the community infection and transmission rates?*
-
5)
Are healthy asymptomatic MERS-CoV carriers important, and what is their role in disease transmission?
-
6)
What is the age and sex distribution of community cases of MERS-CoV infection?
-
7)
What is the seasonal pattern of MERS-CoV infection?
-
8)
What is the potential for person-to-person transmission of MERS-CoV in the community?*
-
9)
What is the incubation period for community cases of MERS-CoV?
-
10)
Do super-spreaders of MERS-CoV exist in the community?*
-
11)
How long after recovery do individuals shed the virus, and from which sites?
Transmission, natural history, pathogenesis, and clinical course
-
1)
What is the mode of acquisition of MERS-CoV infection?*
-
2)
What is the natural history of infection? How many people develop severe progressive disease?*
-
3)
What is the excretion pattern and viral kinetics during infection, clinical course, and recovery or death?*
-
4)
Does susceptibility to infection and progression to development of disease differ by age?
-
5)
What host factors (HLA, genetic, other) predispose to infection and severe disease?
-
6)
What are the protective immune mechanisms against MERS-CoV?
-
7)
What are the pathogenic mechanisms underlying disease severity?
-
8)
Can limited autopsy or biopsy studies provide insights into pathological features of MERS-CoV infections?
-
9)
What are clinical and laboratory prognostic values for predicting cure or death?*
-
10)
What are the long-term sequelae of MERS-CoV infections?
Diagnostics
-
1)
What is the excretion pattern of the virus and when is the best time for sampling?
-
2)
Can data for viral load kinetics be generated?*
-
3)
What are the best clinical specimens for optimum RT-PCR yield and identification of cases?*
-
4)
What range of RT-PCR tests are available? What are their sensitivities and specificities?
-
5)
What serological tests are available?* What are their sensitivities and specificities?*
-
6)
What do negative tests in contacts mean?
-
7)
What are the diagnostic test outcomes by sample type and sampling date?
-
8)
Can reliable and implementable rapid point-of-care tests for epidemiological studies or for diagnosis be developed?*
Inpatient management and treatment
-
1)
What is the best clinical management for MERS-CoV cases?*
-
2)
What proportions of MERS-CoV patients need intensive care and ventilation?
-
3)
What are the roles of antiviral agents (eg, ribavirin), steroids, immunomodulating agents (eg, interferon alfa, acetylcysteine), intravenous immunoglobulin, and convalescent plasma?
-
4)
What are the prognostic predictive values of clinical and laboratory values in MERS-CoV infections?
Infection control
-
1)
What are optimum infection control measures?*
-
2)
When is MERS-CoV shedding highest during the course of the illness?*
-
3)
What is the concentration of MERS-CoV in the lungs and in samples of sputum, urine, and faeces?
-
4)
Does MERS-CoV viral load correlate with severity of illness or efficiency of transmission?
-
5)
Does MERS-CoV shedding occur after clinical recovery?* If so, for how long?
-
6)
Is MERS-CoV shedding and transmission related to a clinically more severe illness or to a higher degree of infectiousness (so-called super-spreaders)?
-
7)
How stable is MERS-CoV under different environmental conditions (dry surface, in suspension, in faecal matter, or in urine, sputum, or vomit)?*
-
8)
How can efficient disinfection of MERS-CoV be achieved?*
-
9)
Can passive immunisation prevent MERS-CoV infections?
-
10)
What are protective immune responses to MERS-CoV, and can vaccines be developed?*
Panel 2. Research in context.
Systematic review
We abstracted and analysed available demographic, clinical, and laboratory data from confirmed cases of MERS-CoV infection (available at the Ministry of Health, Riyadh, Saudi Arabia), which were reported by the Saudi Ministry of Health to WHO, between June 1, 2012, and June 15, 2013. We searched PubMed and ProMED websites on June 15, 2013, for all relevant English language publications with the terms “MERS-CoV”, “novel coronavirus”, “Middle East”, and “HCoV-EMC”. We included all reports from Saudi Arabia.2, 11, 12, 13 Data for MERS-CoV infections from other countries (reported on ProMED, in CDC reports, by the European Centre for Disease Prevention and Control and by the UK's Health Protection Agency [now Public Health England], and in PubMed) were also reviewed.
Interpretation
In our previous reports of 27 cases from Saudi Arabia we presented selected limited data on patients' demographics, symptoms before presentation, comorbidities, and laboratory data. We now show in a series of 47 cases, including the previous 27, more detailed analysis of patients' demographics, age range and sex-related prevalence and mortality, full range of symptoms and comorbidities at presentation, and the number of comorbidities related to mortality. Scant information is available about the epidemiological and clinical characteristics of MERS-CoV infections, and limited data come from individual, family, and hospital case reports. We present the largest case series to date of MERS-CoV infections and provide further information on demographic, clinical, epidemiological, and laboratory features of patients. In individuals admitted with medical comorbidities of MERS, a wide range of clinical manifestations can be seen, and they are associated with substantial mortality. We have identified major knowledge gaps in the source, community prevalence, clinical spectrum, laboratory features, and epidemiology of MERS-CoV infections. Further definition of the clinical and epidemiological range of MERS in the community is a priority and needs multidisciplinary research.
Although current data on MERS-CoV infections are biased by high case fatality in admitted patients with medical comorbidities, since June 15, 2013, the Saudi Ministry of Health has further reported MERS-CoV infections in 14 asymptomatic individuals (three children <15 years, seven health-care workers, and four family members) after screening contacts of confirmed MERS-CoV cases. Two further asymptomatic cases have been recorded among female health-care workers in the eastern region and Al-Hasa. These findings are important because the cases we present here might be just the tip of an iceberg of a range of clinical illness caused by MERS-CoV. Apart from enhanced and active surveillance for cases, we need to develop rapid, accurate, serological diagnostic tests for case-control studies.
Contributors
This study was initiated, designed, and undertaken as a major priority issued under the auspices of the Global Center for Mass Gatherings Medicine (GCMGM), Ministry of Health, Riyadh, Saudi Arabia. Board members of the GCMGM (AAA-R, ZAM, AIZ, and RFA-H) initiated a range of MERS-CoV studies, and ZAM, AIZ, and AAA-R oversaw all aspects of this research. AA, JAA-T, AAA-R, FAA-R, SA-H, AA-B, HF, WNA-N, HHB, RFA-H, HQM, and ZAM obtained and collated patients' data. AA and JAA-T compiled and finalised the database. ZAM, AIZ, and AA wrote the first draft of the manuscript, and AA and JAA-T contributed to several subsequent drafts. All authors contributed to the final report.
Conflicts of interest
We declare that we have no conflicts of interest.
Acknowledgments
Acknowledgments
We thank staff of the Ministry of Health, Riyadh, Saudi Arabia, and Adam Zumla (UCL School of Pharmacy, London, UK) and Matthew Bates (UNZA-UCLMS Project, Lusaka, Zambia) for technical and administrative support. AIZ acknowledges support from: the National Institute of Health Research, Biomedical Research Centre, UCL Hospital, London, UK; the European and Developing Countries Clinical Trials Partnership, The Hague, Netherlands; and EC-FW7 (European Commission's seventh framework programme for research), Brussels, Belgium.
Contributors
This study was initiated, designed, and undertaken as a major priority issued under the auspices of the Global Center for Mass Gatherings Medicine (GCMGM), Ministry of Health, Riyadh, Saudi Arabia. Board members of the GCMGM (AAA-R, ZAM, AIZ, and RFA-H) initiated a range of MERS-CoV studies, and ZAM, AIZ, and AAA-R oversaw all aspects of this research. AA, JAA-T, AAA-R, FAA-R, SA-H, AA-B, HF, WNA-N, HHB, RFA-H, HQM, and ZAM obtained and collated patients' data. AA and JAA-T compiled and finalised the database. ZAM, AIZ, and AA wrote the first draft of the manuscript, and AA and JAA-T contributed to several subsequent drafts. All authors contributed to the final report.
Conflicts of interest
We declare that we have no conflicts of interest.
Footnotes
Urgent key research priorities.
Supplementary Material
References
- 1.Centers for Disease Control and Prevention (CDC) Severe respiratory illness associated with a novel coronavirus: Saudi Arabia and Qatar, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:820. [PubMed] [Google Scholar]
- 2.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.de Groot RJ, Baker SC, Baric RS. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Global alert and response (GAR): Middle East respiratory syndrome coronavirus (MERS-CoV)—update. June 7, 2013. http://www.who.int/csr/don/2013_06_07/en/index.html (accessed June 11, 2013).
- 5.WHO Global alert and response (GAR): novel coronavirus summary and literature update. May 17, 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130517/en/index.html (accessed July 12, 2013).
- 6.WHO Global alert and response (GAR): novel coronavirus infection—update (Middle East respiratory syndrome coronavirus) May 23, 2013. http://www.who.int/csr/don/2013_05_23_ncov/en/index.html (accessed July 13, 2013).
- 7.Centers for Disease Control and Prevention (CDC) Update: severe respiratory illness associated with Middle East respiratory syndrome coronavirus (MERS-CoV)—worldwide, 2012–2013. MMWR Morb Mortal Wkly Rep. 2013;62:480–483. [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control (ECDC) Rapid risk assessment: update—severe respiratory disease associated with a novel coronavirus. Dec 7, 2012. http://www.ecdc.europa.eu/en/publications/Publications/20121207-Novel-coronavirus-rapid-risk-assessment.pdf (accessed June 10, 2013).
- 9.European Centre for Disease Prevention and Control (ECDC) Epidemiological update: additional confirmed cases of Middle East respiratory syndrome coronavirus (novel coronavirus) in France, Saudi Arabia, and Tunisia. May 23, 2013. http://www.ecdc.europa.eu/en/press/news/lists/news/ecdc_dispform.aspx?list=32e43ee8%2de230%2d4424%2da783%2d85742124029a&id=921&rootfolder=%2fen%2fpress%2fnews%2flists (accessed July 5, 2013).
- 10.European Centre for Disease Prevention and Control (ECDC) Table: confirmed cases of Middle East coronavirus infection reported in Europe (n=11 of 53 cases reported worldwide) June 3, 2013. http://www.ecdc.europa.eu/en/healthtopics/Documents/Novel-coronavirus-cases-table.pdf (accessed July 18, 2013).
- 11.AlBarrak AM, Stephens GM, Hewson R, Memish ZA. Recovery from severe novel coronavirus infection. Saudi Med J. 2012;33:1265–1269. [PubMed] [Google Scholar]
- 12.Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 13.Assiri A, McGeer A, Perl TM. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013 doi: 10.1056/NEJMoa1306742. published online June 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hijawi B, Abdallat M, Sayaydeh A. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19(suppl 1):S12–S18. [PubMed] [Google Scholar]
- 15.Bermingham A, Chand MA, Brown CS. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]
- 16.Health Protection Agency (HPA) UK Novel Coronavirus Investigation team Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. 2013;18:20427. doi: 10.2807/ese.18.11.20427-en. [DOI] [PubMed] [Google Scholar]
- 17.Mailles A, Blanckaert K, Chaud P. First cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill. 2013;18:20502. [PubMed] [Google Scholar]
- 18.Guery B, Poissy J, el Mansouf L, and the MERS-CoV study group Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–2272. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchholz U, Müller MA, Nitsche A. Contact investigation of a case of human novel coronavirus infection treated in a German hospital, October–November 2012. Euro Surveill. 2013;18:20406. [PubMed] [Google Scholar]
- 20.Drosten C, Seilmaier M, Corman VM. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013 doi: 10.1016/S1473-3099(13)70154-3. published online June 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ProMED-mail . MERS-CoV—Eastern Mediterranean (16): Italy ex Jordan, contact cases. WHO; June 2, 2013. http://www.promedmail.org/direct.php?id=20130602.1750425 (accessed July 18, 2013). [Google Scholar]
- 22.ProMED-mail . MERS-CoV—Eastern Mediterranean (07): Tunisia ex Saudi Arabia/Qatar, fatal. WHO; May 22, 2013. http://www.promedmail.org/direct.php?id=20130522.1730663 (accessed July 18, 2013). [Google Scholar]
- 23.Corman VM, Eckerle I, Bleicker T. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17:20285. doi: 10.2807/ese.17.39.20285-en. [DOI] [PubMed] [Google Scholar]
- 24.Corman VM, Müller MA, Costabel U. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17:20334. doi: 10.2807/ese.17.49.20334-en. [DOI] [PubMed] [Google Scholar]
- 25.WHO Global alert and response (GAR): Middle East respiratory syndrome coronavirus (MERS-CoV)—update. July 21, 2013. http://www.who.int/csr/don/2013_07_21/en/index.html (accessed July 22, 2013).
- 26.Alqurashi KA, Aljabri KS, Bokhari SA. Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med. 2011;31:19–23. doi: 10.4103/0256-4947.75773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung GM, Hedley AJ, Ho LM. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141:662–673. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- 28.Hui DS, Chan PK. Severe acute respiratory syndrome and coronavirus. Infect Dis Clin North Am. 2010;24:619–638. doi: 10.1016/j.idc.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee N, Hui DS, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 30.Fan CK, Yieh KM, Peng MY, Lin JC, Wang NC, Chang FY. Clinical and laboratory features in the early stage of severe acute respiratory syndrome. J Microbiol Immunol Infect. 2006;39:45–53. [PubMed] [Google Scholar]
- 31.Booth CM, Matukas M, Tomlinson GA. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 32.Rainer TH, Lee N, Ip M. Features discriminating SARS from other severe viral respiratory tract infections. Eur J Clin Microbiol Infect Dis. 2007;26:121–129. doi: 10.1007/s10096-006-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu CL, Lu YT, Peng MJ. Clinical and laboratory features of severe acute respiratory syndrome vis-a-vis onset of fever. Chest. 2004;126:509–517. doi: 10.1378/chest.126.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christian MD, Poutanen SM, Loutfy MR, Muller MP, Low DE. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauch CT, Oraby T. Assessing the pandemic potential of MERS-CoV. Lancet. 2013 doi: 10.1016/S0140-6736(13)61504-4. published online July 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cauchemez S, Van Kerkhove M, Riley S, Donnelly C, Fraser C, Ferguson N. Transmission scenarios for Middle East respiratory syndrome coronavirus (MERS-CoV) and how to tell them apart. Euro Surveill. 2013;18:20503. [PMC free article] [PubMed] [Google Scholar]
- 37.Breban R, Riou J, Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013 doi: 10.1016/S0140-6736(13)61492-0. published online July 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maunder RG, Lancee WJ, Balderson KE. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerg Infect Dis. 2006;12:1924–1932. doi: 10.3201/eid1212.060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zumla A, Gant V, Bates M, Mwaba P, Maeurer M, Memish ZA. Rapid diagnostics urgently needed for killer infections. Lancet Resp Med. 2013;1:284–285. doi: 10.1016/S2213-2600(13)70099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palm D, Pereyaslov D, Vaz J, on behalf of the Joint ECDC-WHO Regional Office for Europe Novel Coronavirus Laboratory Survey participants. ECDC National Microbiology Focal Points. WHO European Region EuroFlu Network. European Network for Diagnostics of “Imported” Viral Diseases (ENIVD) Laboratory capability for molecular detection and confirmation of novel coronavirus in Europe, November 2012. Euro Surveill. 2012;17:20335. doi: 10.2807/ese.17.49.20335-en. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.