Abstract
Summary. Chronic hepatitis C virus (HCV) infection is associated with chronic inflammation of liver, which leads to the development of cirrhosis and hepatocellular carcinoma (HCC). Because of severe side effects and only a 50–70% cure rate in genotype 1 HCV‐infected patients upon current standard treatment with pegylated interferon‐α plus ribavirin, new therapeutic regimens are still needed. San‐Huang‐Xie‐Xin‐Tang (SHXT) is a transitional Chinese herbal formula, composed of Rhei rhizoma, Scutellaria radix and Coptidis rhizome, and possesses anti‐inflammatory effect. Here, we describe a (+)‐catechin‐containing fraction extracted from SHXT, referred as SHXT‐frC, exhibited effective inhibition of HCV replication, with selectivity index value (SI; CC50/EC50) of 84, and displayed synergistic anti‐HCV effects when combined with interferon‐α, HCV protease inhibitor telaprevir or polymerase inhibitor 2′‐C‐methylcytidine. The activation of factor‐κB (NF‐κB) and cyclooxygenase‐2 (COX‐2) signalling pathway has particular relevance to HCV‐associated HCC. SHXT‐frC treatment also caused a concentration‐dependent decrease in the induction of COX‐2 and NF‐κB expression caused by either HCV replication or HCV NS5A protein. Collectively, SHXT‐frC could be an adjuvant treatment for patients with HCV‐induced liver diseases.
Keywords: cyclooxygenase‐2, hepatitis C virus, nuclear factor‐κB, San‐Huang‐Xie‐Xin‐Tang, synergistic
Abbreviations:
- CI
combination index
- COX‐2
cyclooxygenase‐2
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethylsulphoxide
- EC
effective concentration
- EA
ethyl acetate
- GAPDH
Glyceraldehyde 3‐phosphate dehydrogenase
- HCV
hepatitis C virus
- Huh‐7
human hepatoma cells
- HCVcc
cell culture HCV
- HPLC
high‐performance liquid chromatography
- IFN
interferon
- NMR
nuclear magnetic resonance
- PG
prostaglandins
- PGE2
prostaglandin E2
- RSV
respiratory syncytial virus
- SEAP
secreted alkaline phosphatase
- SHXT
San‐Huang‐Xie‐Xin‐Tang
Introduction
Approximately 170 million people worldwide are persistently infected with hepatitis C virus (HCV), which is associated with chronic inflammation of liver and has emerged as a leading cause of fibrosis, cirrhosis and hepatocellular carcinoma [1, 2]. No vaccine is effective against HCV. In addition, the severe side effects of the current treatments with pegylated interferon‐α (peg‐IFN‐α) plus ribavirin, including depression, fatigue, flu‐like symptoms and haemolytic anaemia, often lead to discontinuation of the treatment, thereby warranting the need to develop novel drugs with higher efficacy and more favourable side effect profiles to treat HCV infection.
Hepatitis C virus is an enveloped, positive‐stranded RNA virus belonging to the Hepacivirus genus within the Flaviviridae family [3]. It has a 9.6‐kb genome encoding a single polyprotein that is subsequently cleaved by both the host and virus proteases into at least 10 mature individual proteins: four structural proteins (C, E1, E2 and p7) and six nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A and NS5B) [4]. Among the latter, NS5A promotes improper upregulation of hepatic nuclear transcription factor‐kappaB (NF‐κB) and the cyclooxygenase‐2 (COX‐2) signalling pathway, leading to chronic inflammation or proliferation of hepatoma cells [5, 6, 7]. COX‐2 is an inducible enzyme involved in the production of various prostaglandins (PGs) upon exposure to multiple stimuli such as viral and bacterial products including lipopolysaccharides, cytokines, oncogenes, mitogens and growth factors [8]. However, aberrant upregulation of COX‐2 expression contributes to the progress of hepatic carcinogenesis because elevated PG concentrations promote cell proliferation, survival and inhibition of the pro‐apoptosis pathway, ultimately resulting in tumour formation [8]. Recently, various studies have reported that elevated COX‐2 protein levels favour increased HCV replication, although the exact mechanism remains to be elucidated [7, 9, 10]. On the basis of these findings, an attractive means of minimizing the HCV‐related liver malignancy is by eliminating the NF‐κB‐COX‐2‐dependent signalling pathway for simultaneously reducing viral infection and hepatocarcinogenesis.
San‐Huang‐Xie‐Xin‐Tang (SHXT) is a commonly used traditional Chinese medicine formula composed of Rhei rhizoma (Rheum officinale Baill), Scutellaria radix (Scutellaria baicalensis Georgi) and Coptidis rhizome (Coptis chinensis Franch). SHXT possesses a variety of bioactivities including hypotensive effects [11], anti‐inflammatory effects [12, 13], cardiovascular effects [14], gastric protection [15], antioxidant activity [16], neuronal protection [17] and immunomodulatory effects [18]. SHXT has been used in the clinical treatment of human diseases, such as hypertension, gastritis, gastric bleeding and peptic ulcers for several years [19, 20, 21]. Currently, a number of studies have demonstrated that SHXT and its herbal components possessed antihepatocellular carcinoma activity, suggesting that SHXT is a potential ‘hepatoprotector’ agent [22]. Several marker compounds in SHXT such as baicalin and wogonin possess antiviral activities against respiratory syncytial virus (RSV), hepatitis B virus, severe acute respiratory syndrome coronavirus, HIV‐1 infection or influenza virus [23, 24, 25, 26, 27, 28]. However, to date, the anti‐HCV activity of the constituents of SHXT has not been documented. Therefore, in the present study, we assessed its inhibitory effect on HCV replication and the synergistic anti‐HCV activity in combination with various inhibitors using a cell‐based HCV replicon system. As an extension of chemotherapeutic carcinogenesis, the inhibitory effects of SHXT extracts on the improper induction of NF‐κB and COX‐2 expression caused by HCV replication and aetiologic HCV NS5A protein were evaluated. These findings may contribute to a better treatment regimen for patients with chronic HCV infection.
Materials and methods
Materials and reagents
The dry SHXT was offered by Sun Ten Pharmaceutical Co., Ltd (Taipei, Taiwan). Three traditional Chinese medicines were identified as R. officinale, S. baicalensis and Coptis chinesis with microscope and high‐performance liquid chromatography (HPLC) fingerprint profiles by Sun Ten Pharmaceutical Co., Ltd. The interferon‐alfa (IFN‐α) (Roferon©‐A) was purchased from Roche Ltd (San Francisco, CA, USA). 2′‐C‐methylcytidine (NM‐107) and telaprevir (VX‐950) were purchased from Toronto Research Chemicals Inc. and Acme Bioscience, Belmont, CA, USA, respectively, which were stored as 10 mm in 100% dimethylsulphoxide (DMSO). (+)‐catechin was purchased from Sigma, St Louis, MO, USA. The final concentration of DMSO in all reactions was maintained constantly at 0.1% in the experiments.
Extraction and isolation
The mixture of R. officinale, S. baicalensis and C. chinesis, blended in a 2:1:1 ratio (total 3.6 kg), was extracted three times using MeOH. The MeOH extract (500 g) was partitioned between H2O and ethyl acetate (EA) (1:1), which obtained H2O fraction (400 g) and EA fraction (96.7 g). Fifty grams of the EA fraction was subjected to normal‐phase flash column chromatography and eluted with a CH2Cl2‐EA‐MeOH (100‐0‐0, 0‐100‐0, 0‐80‐20, 0‐50‐50 and 0‐0‐100) gradient solvent system. Five subfractions (S1–5) were isolated by thin layer chromatography. Among them, the S2 subfraction (42.30 g) was further isolated by reverse‐phase chromatography and eluted with a MeOH/H2O (50/50) gradient solvent system, and seven subfractions (S1.1–S1.7) were separated by reverse‐phase chromatography. Finally, a (+)‐catechin‐containing fraction, termed SHXT‐frC (approximately 300 mg), was obtained from the S1.1 subfraction by reverse‐phase chromatography with MeOH/H2O (20/80). (+)‐catechin was elucidated by 1H and 13C nuclear magnetic resonance (NMR) spectra.
Chromatographic analysis of SHXT‐frC and isolation of SHXT‐frC‐C and SHXT‐frC‐R
A Jasco PU‐980 pump (Jaytee Biosciences Ltd, Herne Bay, Kent, UK), a Jasco UV‐970 detector (Jaytee Biosciences Ltd) and an Ascentis™ (Ascentis, Lancaster, UK) C18 (5 μm, 250 × 21.2 mm) column (Supelco) were employed for the SHXT‐frC HPLC analysis. Isocratic elution was performed with MeOH/H2O (25:75, v/v) at a flow rate of 4 mL/min. The solvents were filtered through a 0.45‐μm filter prior to being used. Eluents were detected at 254 nm, and data were analysed with the ‘Class‐LC10 series version 3.1’ software (Shimadzu, Kyoto, Japan). We further purified the SHXT‐frC fraction to verify the active components by the isolated condition as described previously. SHXT‐frC‐C and SHXT‐frC‐R were collected as shown in the Fig. 1A.
Figure 1.
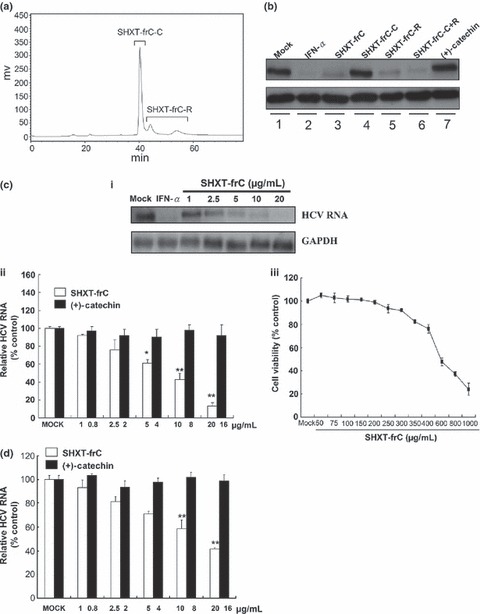
Inhibitory effect of derived fractions of San‐Huang‐Xie‐Xin‐Tang (SHXT) on hepatitis C virus (HCV) protein synthesis, replication and infection. (a) HPLC profile of the SHXT‐frC. The HPCL chromatogram showed the separation of two major components, designated as SHXT‐frC‐C and SHXT‐frC‐R. (b) Inhibition of HCV protein expression in HCV replicon cells by derived fractions of SHXT. Ava5 cells were treated with SHXT‐frC, SHXT‐frC‐C, SHXT‐frC‐R, SHXT‐frC‐C plus SHXT‐frC‐R and (+)‐catechin at 20 μg/mL for 4 days. Treatment with 100 U/mL IFN‐α and with 0.1% DMSO served as the positive control and the mock control, respectively. Western blotting was performed using anti‐NS5B and anti‐GAPDH (a loading control) antibodies. (c) Dose‐dependent reduction in HCV RNA replication by SHXT‐frC. After 3 days of treatment, HCV RNA levels were analysed by (i) Northern blot analysis and (ii) RT‐qPCR using HCV‐specific probe and primers, respectively. The relative HCV RNA levels were normalized by cellular gapdh mRNA. [, SHXT‐frC; , (+)‐catechin] (iii) Cellular toxicity was evaluated by the MTS assay. (d) Dose‐dependent inhibition of HCV JFH‐1 replication by SHXT‐frC. After 3 days of treatment, the levels of intracellular HCV RNA were determined by RT‐qPCR using HCV‐specific primers. The relative HCV RNA levels were normalized by cellular gapdh mRNA. [, SHXT‐frC; , (±)‐catechin]. Results are represented as means ± SD (error bar) for triplicate experiments. *P < 0.05; **P < 0.01.
Cell culture
Human hepatoma cells (Huh‐7) and Ava5 cells (kindly provided by Dr Charles Rice, Rockefeller University, New York, USA) were cultured in Dulbecco’s modified Eagle’s medium with 10% heat‐inactivated foetal bovine serum, 5% antibiotic–antimycotic and 5% nonessential amino acids. Ava5 cell lines harbouring HCV subgenomic replicon RNA were maintained in complete media containing 1 mg/mL G418 [29].
Plasmid construction
The coding sequence of NS5A was amplified by polymerase chain reaction (PCR) from pCon1 plasmid containing cDNA of HCV 1b strain and cloned into the expression vector pcDNA™4/myc‐His A (Invitrogen, Carlsbad, CA, USA), designed as pCMV‐NS5A‐Myc. COX‐2 promoter fragment was amplified from human genomic DNA as described [30] and inserted into the promoterless luciferase vector pGL3‐Basic (Promega Co, Madison, WI, USA), designed as pCOX‐2‐Luc. pNF‐κB‐Luc is the reporter vector to measure NF‐κB transcription activity (Stratagene, La Jolla, CA, USA). The cloned DNA fragments were verified by DNA sequencing.
Northern blotting analysis
Total cellular RNA was isolated using Total RNA Miniprep Purification Kit (Hopegen Biotechnology Development Enterprise, Taiwan) according to the manufacturer’s instructions. Northern hybridization was performed as previously described [31].
Quantification of hepatitis C virus RNAs
Quantitative real‐time RT‐PCR (RT‐qPCR) was performed to measure mRNAs of HCV and GAPDH as previously described [32].
Hepatitis C virus JFH‐1 infection assay
The infectious HCV JFH‐1 particles (HCVcc) were generated by transfection of in vitro‐transcribed genomic JFH‐1 RNA in Huh‐7.5 cells as described [33]. The Huh‐7 cells were seed at density of 4 × 104 cells/well in 24‐well culture plate and infected with 100 μL of HCVcc at a multiplicity of infection of 0.1 for 6 h followed by incubation with various concentrations of SHXT‐frC or (±)‐catechin for an additional 72 h. Subsequently, total RNAs were collected and subjected to RT‐qPCR for measuring mRNAs of HCV and GAPDH as described previously.
Western blotting assay
Western blotting was performed as described previously [32]. The membranes were probed with either anti‐NS5B antibody (Abcam, Cambridge, MA, USA), or anti‐GAPDH antibody (GeneTex, CA, USA) or anti‐C‐Myc antibody (GeneTex, Irvine, CA, USA).
Cytotoxicity assay
Cell viability was determined by MTS assay with The CellTiter 96® AQueous One Solution Cell Proliferation assay system (Promega) as described previously [32].
Analysis of drug synergism
Ava5 cells were treated with serially diluted SHXT‐frC (1, 2.5, 5, 10 and 20 μg/mL) in combination with serially diluted IFN‐α (7.5, 15, 30 and 60 U/mL), HCV RNA‐dependent RNA polymerase nucleoside inhibitor 2′‐C‐methylcytidine (NM‐107) [34] (0.75, 1.5, 3 and 6 μm) or HCV NS3/4A protease telaprevir (VX‐950) [35] (0.075, 0.15, 0.3 and 0.6 μm). After 3 days, the levels of HCV subgenomic RNA were measured by quantitative real‐time RT‐PCR (RT‐qPCR) as previously described [32]. Multiple drug combination data were determined using CalcuSyn2™ software based on the method of Chou and Talalay [36] (Biosoft, Cambridge, UK), which compares single and multiple drug dose‐effect and determines the presence of antagonism (combination index; CI > 1), additivity (CI = 1) or synergism (CI < 1). The CI for the calculated effective concentration (EC50), EC75 and EC90 is presented herein.
Transfection and luciferase activity assay
Ava5 cells were transfected with 0.5 μg of plasmid pNF‐κB‐Luc (BD Biosciences Clontech, Palo Alto, CA, USA) or pCOX‐2‐Luc using T‐Pro™ reagent (Ji‐Feng Biotechnology Co., Ltd., Taipei, Taiwan) in accordance with the manufacturers’ instructions. Huh‐7 parent cells were cotransfected with 0.5 μg of pCMV‐NS5A‐Myc together with 0.5 μg of pNFκ‐B‐Luc or pCOX‐2‐Luc. Each transfection complex contains 0.1 μg of secreted alkaline phosphatase (SEAP) reporter vector (pCMV‐SEAP) to serve as an internal control for normalization of the transfection efficiency. Then, the transfected cells were incubated with various concentrations of SHXT‐frC (1, 2.5, 5, 10 and 20 μg/mL). After 3 days, cell lysates were prepared for luciferase activity using the Bright‐Glo™ Luciferase assay system (Promega) and for Western blotting analysis following the manufacturer’s instructions.
Intracellular prostaglandin E2 (PGE2) measurements
Cells were seeded in 96‐well plates at a density of 5 × 103 and treated with SHXT‐frC at various concentrations. After 3 days of incubation, cells were harvested and cell membranes were lysed to release intracellular PGE2. PGE2 expression levels were detected by the PGE2 enzyme‐linked immunosorbent assay system (Biotrak, Amersham Bioscience) according to the manufacturer’s protocol.
Statistical analysis
Data are presented as mean ± SD for at least three independent experiments. Statistical analysis was carried out with Student’s t‐test, and P‐values of <0.01 were considered significant.
Results
Chromatographic analysis of SHXT‐frC
Based on bioassay‐guided fractionation and isolation, a subfraction of SHXT, designated here as SHXT‐frC, displayed an effective inhibitory action against HCV protein expression (Fig. 1B, lane 3). The HPLC trace of SHXT‐frC is shown in Fig. 1A. According to the analysis of HPLC profile and NMR spectra, the major component of the SHXT‐frC fraction was identified as (+)‐catechin. The retention time of (+)‐catechin in SHXT‐frC was found to be 24.5 min, which was identical to that of the standard of (+)‐catechin under same HPLC conditions. Subsequently, the SHXT‐frC‐C, a major peak harbouring (+)‐catechin, and SHXT‐frC‐R, a residue eluent harbouring two minor peaks, were individually collected to examine anti‐HCV activity. Based on the calculations of the peak areas by Class‐LC10 series version 3.1 software (Shimadzu), the amount of SHXT‐frC‐C and SHXT‐frC‐R represents 80% and 20% in SHXT‐frC, respectively. Principally, the standardized herbal extract of SHXT‐frC can be cautiously prepared based on (+)‐catechin as a fraction marker.
Antiviral effect of SHXT‐frC, SHXT‐frC‐C and SHXT‐frC‐R on hepatitis C virus replicon cells
To further identify the constituents in SHXT‐frC that contributed to anti‐HCV activity, Huh‐7 cells harbouring an HCV subgenomic RNA replicon, designated Ava5 cells [29], were treated with various elutants at concentration of 20 μg/mL for 4 days. Subsequently, cell lysates were subjected to Western blotting with anti‐HCV NS5B antibody; the level of GAPDH served as the loading control. As shown in Fig. 1B, the SHXT‐frC (lane 3) and SHXT‐frC‐R (lane 5) significantly inhibited HCV protein synthesis compared with the mock control (0.1% DMSO) (lane 1). The inhibitory effect was similar to the effectiveness of IFN‐α treatment (lane 2). In contrast, treatment with SHXT‐frC‐C‐containing (+)‐catechin and (+)‐catechin (Sigma) exhibited an insignificant decrease in HCV protein levels (lanes 4 and 7). Notably, the inhibitory effect on HCV protein synthesis was conserved when SHXT‐frC‐C was combined with SHXT‐frC‐R, (lane 6), confirming that the SHXT‐frC‐R contains bioactive constituents against HCV protein synthesis. As current data were unable to clearly discriminate the individual constituents in SHXT‐frC‐R, we used the SHXT‐frC mixture to investigate the anti‐HCV activity in this report. Treatment with an equal ratio of commercially available (+)‐catechin served as an internal control in each experiment and thus eliminated the biological interference of (+)‐catechin on anti‐HCV activity assay. To further confirm the antiviral activity of SHXT‐frC, Northern blotting and quantitative RT‐PCR (RT‐qPCR) were performed with a specific probe and primers corresponding to HCV NS5B, respectively, in which the levels of the cellular gapdh gene served as the loading control. As shown in Fig. 1C (a and b, white bars), a clear dose‐dependent decrease in HCV RNA levels occurred upon treatment with SHXT‐frC for 3 days compared to the treatment of mock control cells (0.1% DMSO). The calculated 50% effective concentration (EC50), defined as the compound concentration that reduced HCV RNA levels by 50%, was 7.0 ± 0.5 μg/mL as measured by RT‐qPCR analysis (b). At 20 μg/mL, more than 90% of HCV replication was decreased, and no toxicity was observed (b, white bars), while HCV RNA levels remained unchanged in the presence of (+)‐catechin at 16 μg/mL (b, black bars). The cytotoxic index of the SHXT‐frC was evaluated by MTS, and the 50% cytotoxic concentration (CC50), defined as the compound concentration that resulted in 50% cell death, was 589.0 ± 4.2 μg/mL (c), which yielded a high selectivity index value (SI; CC50/EC50) of 84. Similarly, the anti‐HCV activity of SHXT‐frC was also observed in HCV JFH‐1 infectious system [33], with an EC50 value of 12.3 ± 0.3 μg/mL (Fig. 1D, white bars). These data support the conclusion that the SHXT‐frC is a promising anti‐HCV agent and warrants further study.
Inhibition of hepatitis C virus RNA replication in combination of SHXT‐frC with INF‐α or hepatitis C virus enzyme inhibitors
Next, we investigated whether the combination of IFN‐α, the NS3/4A protease inhibitor Telaprevir (VX‐950) [35] and the NS5B polymerase inhibitor 2′‐C‐methylcytidine (NM‐107) [34] would produce a synergistic effect on the inhibition of HCV replication. Ava5 cells were treated with SHXT‐frC combined with anti‐HCV agents at various concentrations as described in the Materials and methods section. The effects of combination were calculated using the isobologram method and the CalcuSyn2™ computer program [36, 37]. As shown in Table 1, the combination of SHXT‐frC with the individual inhibitors synergistically inhibited HCV replication, as revealed by the CI values of <1 for EC50, EC75 or EC90 (ranging from 0.53 to 0.85), in which no apparent cytotoxicity was observed at the tested concentrations of combination by MTS analysis.
Table 1.
Inhibitory effects of SHXT‐frC combined with various inhibitors on HCV replication. Ava5 cells were treated with SHXT‐frC and IFN‐α, VX‐950 and NM‐107 at various concentrations either alone or in combinations for 3 days. Anti‐HCV activity was performed using RT‐qPCR analysis for measurement of HCV RNA levels
| Combination compound | CI values at | Influence | ||
|---|---|---|---|---|
| EC50 | EC75 | EC90 | ||
| IFN‐α | 0.85 | 0.81 | 0.78 | Synergistic |
| VX‐950 | 0.57 | 0.61 | 0.67 | Synergistic |
| NM‐107 | 0.53 | 0.55 | 0.58 | Synergistic |
The combination index (CI) value for an effective concentration of 50% (EC50), 75% (EC75) or 90% inhibition (EC90) was calculated using the CalcuSyn2™ program [36]. CI values indicate the degree of interaction of potential drugs; values of <1, =1 and >1 are indicative of synergistic, additive and antagonistic effects, respectively. HCV, hepatitis C virus; SHXT, San‐Huang‐Xie‐Xin‐Tang.
SHXT‐frC attenuates cyclooxygenase‐2 and NF‐κB activation in subgenomic hepatitis C virus‐replicating cells
The preventive effects of standardized SHXT have been reported to be able to inhibit lipopolysaccharides‐induced inflammatory‐associated gene expression, including NF‐κB, COX‐2 and prostaglandin E2 (PGE2) production [11, 17]. To test whether the HCV‐induced COX‐2 expression is influenced by SHXT‐frC, Ava5 cells were transfected with a plasmid (pCOX‐2‐Luc) carrying firefly luciferase, which is driven by the COX‐2 promoter, for 3 days in the presence of increasing concentrations of SHXT‐frC. As shown in Fig. 2A, concentration‐dependent reduction in the luminescent signal was observed in SHXT‐frC‐treated cells when compared with 0.1% DMSO‐treated Ava5 cells (white bars) and parental Huh‐7 cells (the fold of control), while treatment with (+)‐catechin had no significant effect on COX‐2 gene expression (black bars). Subsequently, Western blotting was performed to further confirm the effect of SHXT‐frC on the suppression of COX‐2 expression. Consistent with the results of a promoter‐based reporter assay, SHXT‐frC reduced the COX‐2 protein levels in a concentration‐dependent manner (Fig. 2Ba). In contrast, (+)‐catechin did not result in a significant reduction in COX‐2 protein levels (Fig. 2Bb). Additionally, COX‐2 activity in the presence of the SHXT‐frC was quantified using the Biotrak PGE2 enzyme immunoassay, and the results showed that the level of PGE2 decreased in a concentration‐dependent manner (Fig. 2C). NF‐κB activation is one of the most ubiquitous mediators of COX‐2 activation in a viral infection [38]. Therefore, we performed a promoter‐based assay to determine whether the suppression of COX‐2 was related to the modulation of transcription factor NF‐κB upon SHXT‐frC treatment. Ava5 and Huh‐7 cells were transiently transfected with the cis‐reporting plasmid pNF‐κB‐Luc, following which the transfected cells were incubated with or without SHXT‐frC for 3 days. As shown in Fig. 3, the luciferase activity assay revealed that SHXT‐frC significantly inhibited NF‐κB‐mediated transcriptional activity in a concentration‐dependent manner (white bars), which supports the possibility that the suppression of NF‐κB may be involved in the anti‐COX‐2 effect of SHXT‐frC. As expected, (+)‐catechin did not affect NF‐κB‐mediated transcriptional activity (black bars).
Figure 2.
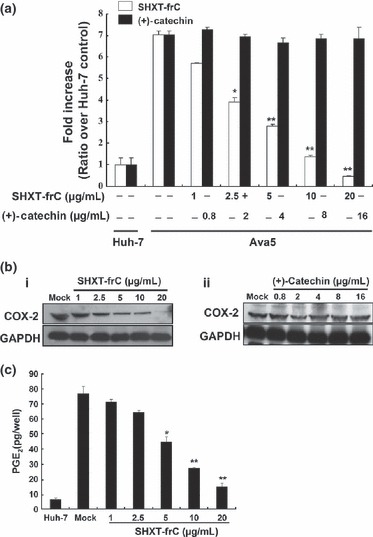
Effect of San‐Huang‐Xie‐Xin‐Tang (SHXT)‐frC on hepatitis C virus (HCV)‐induced cyclooxygenase‐2 (COX‐2) gene promoter, protein expression and activity in HCV replicon cells. (a) Dose‐dependent reduction in COX‐2 promoter activity by SHXT‐frC. The pCOX‐2‐Luc‐transfected Ava5 cells were treated with indicated concentrations of SHXT‐frC and (+)‐catechin for 3 days. Subsequently, cell lysates were subjected to luminescence detection using the Steady‐Glo Luciferase Assay kit (Promega). The luciferase activity in the pCOX‐2‐Luc‐transfected Huh‐7 cells without drugs treatment served as the basal control of induced COX‐2 promoter activity, which was defined as 1. [□, SHXT‐frC; , (+)‐catechin] (b) Dose‐dependent reduction in COX‐2 expression by SHXT‐frC. Ava5 cells were incubated for 3 days with indicated concentrations of (i) SHXT‐frC and (ii) (+)‐catechin. Cell lysates were subjected to Western blotting with anti‐COX2 and anti‐GAPDH (a loading control) antibodies. (c) Dose‐dependent reduction in prostaglandin E2 (PGE2) production by SHXT‐frC. After 3 days of treatment, the intercellular PGE2 levels were assayed with the Biotrak PGE2 enzyme immunoassay system (Amersham). ‘Mock’ indicates a treatment of 0.1% dimethylsulphoxide. Each value represents the mean ± SD from triplicate experiments. The * symbol represents statistical significance of P‐value ≤0.05. The ** symbol represents statistical significance of P‐value ≤0.01.
Figure 3.
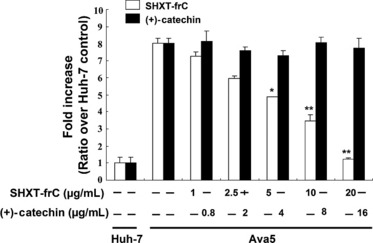
Inhibitory effect of San‐Huang‐Xie‐Xin‐Tang (SHXT)‐frC on hepatitis C virus (HCV)‐induced NF‐κB promoter activity in HCV replicon cells. The pNF‐κB‐Luc‐transfected Ava5 cells were treated with indicated concentrations of either SHXT‐frC or (+)‐catechin for 3 days, and cell lysates were subjected to luciferase activity assay. The luciferase activity in pNF‐κB‐Luc‐transfected Huh‐7 cells without drugs treatment served as the basal control of induced NF‐κB promoter activity, which was defined as 1. [, SHXT‐frC; , (+)‐catechin] Each value represents the mean ± SD from triplicate experiments. The * symbol represents statistical significance of P‐value ≤0.05. The ** symbol represents statistical significance of P‐value ≤0.01.
SHXT‐frC attenuates COX‐2 and NF‐κB activation in hepatitis C virus NS5A‐expressing cells
Hepatitis C virus NS5A protein has shown oncogenic potential linked to the stimulation of COX‐2 and NF‐κB expression through different mechanisms [5, 6, 39]. To examine whether SHXT‐frC was able to block NS5A‐induced COX‐2 expression for the prevention of hepatocarcinogenesis, an NS5A expression vector (pCMV‐NS5A‐Myc) was cotransfected with the COX‐2 reporter vector (pCOX‐2‐Luc) into the parental Huh‐7 cells in the absence and presence of SHXT‐frC or (+)‐catechin, in which transfection of pCOX‐2‐Luc alone was served as basal promoter activity of COX‐2. After 3 days, luciferase activity was measured. As shown in Fig. 4A, SHXT‐frC markedly inhibited NS5A‐induced COX‐2 promoter activity in a concentration‐dependent manner (a, white bars), while (+)‐catechin did not inhibit COX‐2 promoter activity induced by NS5A (black bars). Subsequently, we then extended our study to examine whether the inhibitory effect of NS5A‐induced COX‐2 by SHXT‐frC was mediated by NF‐κB activation. Plasmid pCMV‐NS5A‐Myc was cotransfected with NF‐κB promoter plasmid (pNF‐κB‐Luc) into Huh‐7 cells in the absence and presence of SHXT‐frC or (+)‐catechin for 3 days, in which transfection of pNF‐κB‐Luc alone served as basal promoter activity of NF‐κB. The result of the luciferase assay shows that NS5A‐induced NF‐κB promoter activities are markedly suppressed by SHXT‐frC in a concentration‐dependent manner (Fig. 4Ba, white bars). In contrast, (+)‐catechin did not inhibit the NS5A‐stimulated NF‐κB promoter activity (black bars). The equal expression of NS5A‐Myc in each condition was confirmed by Western blotting analysis using anti‐Myc antibody (Fig. 4A,B; b,c). These data showed that SHXT‐frC effectively targets the NF‐κB and COX‐2 expression, implying that SHXT‐frC has the potential to prevent virus‐induced hepatocarcinogensis.
Figure 4.
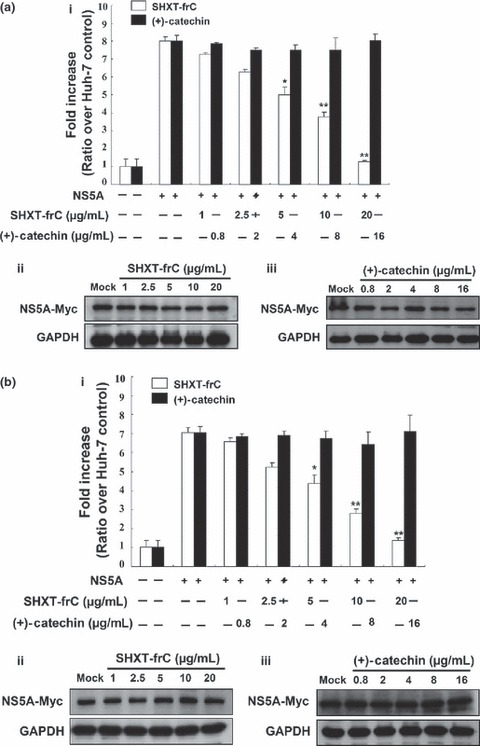
Inhibitory effect of San‐Huang‐Xie‐Xin‐Tang (SHXT)‐frC on NS5A‐induced cyclooxygenase‐2 (COX‐2) and NF‐κB transcriptional activities in Huh‐7 cells. Reporter plasmid (a) pCOX‐2‐Luc or (b) pNF‐κB‐Luc was cotransfected with pCMV‐NS5A‐Myc into parental Huh‐7 cells. Subsequently, the transfected cells were treated with indicated concentrations of either SHXT‐frC or (+)‐catechin for 3 days, and the cell lysates were subjected to (i) the luciferase activity assay and (ii and iii) Western blotting with anti‐Myc and anti‐GAPDH (a loading control) antibodies, respectively. The luciferase activity in reporter plasmid transfected Huh‐7 cells without pCMV‐NS5A‐Myc transfection and drugs treatment served as the basal control of induced promoter activity, which was defined as 1. The reductive luminescence compared to the signal of mock‐treated (0.1% dimethylsulphoxide) cells is represented as the mean fold of reduction ±SD from triplicate experiments. The * symbol represents statistical significance of P‐value ≤0.05. The ** symbol represents statistical significance of P‐value ≤0.01.
Discussion
In this study, we extend the bioactivity of SHXT on HCV, a causative agent of hepatocarcinogensis, and demonstrated that the most active extract SHXT‐frC, derived from the herbal medicine SHXT, possesses effective anti‐HCV activity (Fig. 1B). We further analysed the bioactive constituents in SHXT‐frC to find that (+)‐catechin, a major component in SHXT‐frC, has no significant anti‐HCV activity at a concentration of 16.0 μg/mL (Fig. 1Cb), which was consistent with those results recently obtained by Li et al., who demonstrated that a single (+)‐catechin or (−)‐epicatechin showed negligible inhibitory effect on HCV replication, even at a high concentration of 200 μm, approximately 59.2 μg/mL. The formation of dimer in (+)‐catechin and (−)‐epicatechin, designated procyanidin B1, is a requisite anti‐HCV activity [40]. However, this observation is controversial when taken with observations reported by Takeshita et al., [41] who demonstrated that catechin extracted from blueberry leaf suppressed HCV RNA synthesis with an EC50 of 16.18 μg/mL. These divergent results may be attributable to different geometric isomers of catechin [(+)‐catechin and (−)‐catechin] isolated in our study and the Takeshita et al. study.
To develop more effective treatment options for improving the sustained virological response, tolerability and treatment duration, several direct‐acting antiviral agents such as telaprevir (VX‐950, an HCV protease inhibitor) [42] and 2′‐C‐methylcytidine (NM‐107, a nucleoside HCV polymerase inhibitor) [34] have been examined in clinical trials. However, increasing drug resistance has become an obstacle for successful therapeutic intervention [43]. In the present study, we provide an effective and safe SHXT‐frC extract to synergistically inhibit HCV replication in combination with chemotherapeutic agents (interferon‐α, VX‐950 and NM‐107) in the HCV replicon system (Table 1), which could greatly reduce the potential resistance and side effects when included in combination therapy.
The aetiologic proteins of HCV, including the structural protein, core and nonstructural proteins, particularly NS3 and NS5A, markedly modulate the activation of NF‐κB and COX‐2, which appears to in turn promote HCV replication [5, 10, 44]. Recently, downregulation of HCV‐induced COX‐2 expression was considered a molecular mechanism involved in the inhibitory effect of HCV replication by using selective COX‐2 inhibitors, including acetylsalicylic acid, NS398, COX‐2 inhibitor III and SC‐560 [10, 45, 46]. Whether the anti‐HCV activity of SHXT‐frC depended on the downregulation of COX‐2 and NF‐κB will be further investigated. Additionally, modulation of the activation of NF‐κB and COX‐2 has been shown to increases the replication of a number of viruses, such as cytomegalovirus [47], herpesvirus [48], RSV [49] and enterovirus 71 [50], although the detailed mechanism is not clearly defined. Moreover, we also found that tumour necrosis factor (TNF)‐alpha‐induced activation of COX‐2 and NF‐κB promoters was also inhibited by SHXT‐frC (data not shown). Consequently, blocking the NF‐κB‐mediated COX‐2 pathway by SHXT‐frC (Fig. 4) also may provide potential agent in the chemoprevention of other virus infections.
In conclusion, we first demonstrated that SHXT‐frC effectively inhibits HCV replication and displays synergistic anti‐HCV activity in combination with various HCV inhibitors. Moreover, SHXY‐frC inhibits the improper induction of NF‐κB and COX‐2 expression caused by either HCV replication or aetiologic NS5A protein. Therefore, SHXT‐frC may serve as a potential supplementary treatment for patients with chronic hepatitis C by simultaneous attenuation of viral replication, inflammation and carcinogenesis, which are closely correlated with an aberrant activation of NF‐κB and COX‐2 expression.
Declaration of funding interests
This work was supported the National Science Council of Taiwan (grant number: NSC 97‐2311‐B‐037‐001‐MY3).
Acknowledgements
We are grateful to Dr Charles Rice at Rockefeller University and Aapth, LCC, USA, for kindly supporting Con1b replicon plasmid, Human hepatoma cell; Huh‐7, Huh‐7.5 and HCV subgenomic replicon‐containing cell line; Ava5. We also thank Dr Takija Wakita (National Institute of Infectious Diseases, Tokyo, Japan) for kindly providing the plasmid pJFH‐1 containing full‐length cDNA of HCV genotype 2a.
References
- 1. Alter MJ. Epidemiology of hepatitis C. Hepatology 1997; 26(3 Suppl. 1): 62S–65S. [DOI] [PubMed] [Google Scholar]
- 2. Kenny‐Walsh E. The natural history of hepatitis C virus infection. Clin Liver Dis 2001; 5(4): 969–977. [DOI] [PubMed] [Google Scholar]
- 3. Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature 2005; 436(7053): 933–938. [DOI] [PubMed] [Google Scholar]
- 4. Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology 2004; 39(1): 5–19. [DOI] [PubMed] [Google Scholar]
- 5. Lu L, Wei L, Peng G et al. NS3 protein of hepatitis C virus regulates cyclooxygenase‐2 expression through multiple signaling pathways. Virology 2008; 371(1): 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nunez O, Fernandez‐Martinez A, Majano PL et al. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: role of viral core and NS5A proteins. Gut 2004; 53(11): 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waris G, Siddiqui A. Hepatitis C virus stimulates the expression of cyclooxygenase‐2 via oxidative stress: role of prostaglandin E2 in RNA replication. J Virol 2005; 79(15): 9725–9734. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Giannitrapani L, Ingrao S, Soresi M et al. Cyclooxygenase‐2 expression in chronic liver diseases and hepatocellular carcinoma: an immunohistochemical study. Ann N Y Acad Sci 2009; 1155: 293–299. [DOI] [PubMed] [Google Scholar]
- 9. Kanda T, Yokosuka O, Nagao K, Saisho H. State of hepatitis C viral replication enhances activation of NF‐kB‐ and AP‐1‐signaling induced by hepatitis B virus X. Cancer Lett 2006; 234(2): 143–148. [DOI] [PubMed] [Google Scholar]
- 10. Trujillo‐Murillo K, Rincon‐Sanchez AR, Martinez‐Rodriguez H et al. Acetylsalicylic acid inhibits hepatitis C virus RNA and protein expression through cyclooxygenase 2 signaling pathways. Hepatology 2008; 47(5): 1462–1472. [DOI] [PubMed] [Google Scholar]
- 11. Lo YC, Tsai PL, Huang YB et al. San‐Huang‐Xie‐Xin‐Tang reduces lipopolysaccharides‐induced hypotension and inflammatory mediators. J Ethnopharmacol 2005; 96(1–2): 99–106. [DOI] [PubMed] [Google Scholar]
- 12. Lo YC, Lin YL, Yu KL et al. San‐Huang‐Xie‐Xin‐Tang attenuates inflammatory responses in lipopolysaccharide‐exposed rat lungs. J Ethnopharmacol 2005; 101(1–3): 68–74. [DOI] [PubMed] [Google Scholar]
- 13. Shih YT, Wu DC, Liu CM, Yang YC, Chen IJ, Lo YC. San‐Huang‐Xie‐Xin‐Tang inhibits Helicobacter pylori‐induced inflammation in human gastric epithelial AGS cells. J Ethnopharmacol 2007; 112(3): 537–544. [DOI] [PubMed] [Google Scholar]
- 14. Tsai HH, Chen IJ, Lo YC. Effects of San‐Huang‐Xie‐Xin‐Tang on U46619‐induced increase in pulmonary arterial blood pressure. J Ethnopharmacol 2008; 117(3): 457–462. [DOI] [PubMed] [Google Scholar]
- 15. Lin WC, Tan TW. The role of gastric muscle relaxation in cytoprotection induced by san‐huang‐xie‐xin‐tang in rats. J Ethnopharmacol 1994; 44(3): 171–179. [DOI] [PubMed] [Google Scholar]
- 16. Shia CS, Hou YC, Juang SH et al. Metabolism and pharmacokinetics of San‐Huang‐Xie‐Xin‐Tang, a polyphenol‐rich chinese medicine formula, in rats and ex‐vivo antioxidant activity. Evid Based Complement Alternat Med 2009; doi: 10.1093/ecam/nep124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shih YT, Chen IJ, Wu YC, Lo YC. San‐Huang‐Xie‐Xin‐Tang protects against activated microglia‐ and 6‐OHDA‐induced toxicity in neuronal SH‐SY5Y cells. Evid Based Complement Alternat Med 2009; doi: 10.1093/ecam/nep025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li CY, Hou YC, Lee Chao PD, Shia CS, Hsu IC, Fang SH. Potential ex vivo immunomodulatory effects of San‐Huang‐Xie‐Xin‐Tang and its component herbs on mice and humans. J Ethnopharmacol 2010; 127(2): 292–298. [DOI] [PubMed] [Google Scholar]
- 19. Chen HC, Hsieh MT. Two‐year experience with “San‐Huang‐Hsieh‐Hsin‐Tang” in essential hypertension. Am J Chin Med 1986; 14(1–2): 51–58. [DOI] [PubMed] [Google Scholar]
- 20. Chen HC, Hsieh MT. Hemodynamic effects of “san‐huang‐hsieh‐hsin‐tang” in patients with essential hypertension. Am J Chin Med 1986; 14(3–4): 153–156. [DOI] [PubMed] [Google Scholar]
- 21. Zhang L, Yang L, Zheng X. A study of Helicobacterium pylori and prevention and treatment of chronic atrophic gastritis. J Tradit Chin Med 1997; 17(1): 3–9. [PubMed] [Google Scholar]
- 22. Cheng WY, Wu SL, Hsiang CY et al. Relationship Between San‐Huang‐Xie‐Xin‐Tang and its herbal components on the gene expression profiles in HepG2 cells. Am J Chin Med 2008; 36(4): 783–797. [DOI] [PubMed] [Google Scholar]
- 23. Hu JZ, Bai L, Chen DG, Xu QT, Southerland WM. Computational investigation of the anti‐HIV activity of Chinese medicinal formula Three‐Huang Powder. Interdiscip Sci 2010; 2(2): 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu G, Dou J, Zhang L, Guo Q, Zhou C. Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum. Biol Pharm Bull 2010; 33(2): 238–243. [DOI] [PubMed] [Google Scholar]
- 25. Cheng Y, Ping J, Xu HD, Fu HJ, Zhou ZH. Synergistic effect of a novel oxymatrine‐baicalin combination against hepatitis B virus replication, alpha smooth muscle actin expression and type I collagen synthesis in vitro. World J Gastroenterol 2006; 12(32): 5153–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen F, Chan KH, Jiang Y et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol 2004; 31(1): 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo Q, Zhao L, You Q et al. Anti‐hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res 2007; 74(1): 16–24. [DOI] [PubMed] [Google Scholar]
- 28. Ma SC, Du J, But PP et al. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J Ethnopharmacol 2002; 79(2): 205–211. [DOI] [PubMed] [Google Scholar]
- 29. Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science 2000; 290(5498): 1972–1974. [DOI] [PubMed] [Google Scholar]
- 30. Tazawa R, Xu XM, Wu KK, Wang LH. Characterization of the genomic structure, chromosomal location and promoter of human prostaglandin H synthase‐2 gene. Biochem Biophys Res Commun 1994; 203(1): 190–199. [DOI] [PubMed] [Google Scholar]
- 31. Lee JC, Shih YF, Hsu SP, Chang TY, Chen LH, Hsu JT. Development of a cell‐based assay for monitoring specific hepatitis C virus NS3/4A protease activity in mammalian cells. Anal Biochem 2003; 316(2): 162–170. [DOI] [PubMed] [Google Scholar]
- 32. Lee JC, Tseng CK, Chen KJ, Huang KJ, Lin CK, Lin YT. A cell‐based reporter assay for inhibitor screening of hepatitis C virus RNA‐dependent RNA polymerase. Anal Biochem 2010; 403(1–2): 52–62. [DOI] [PubMed] [Google Scholar]
- 33. Wakita T, Pietschmann T, Kato T et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 2005; 11(7): 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bassit L, Grier J, Bennett M, Schinazi RF. Combinations of 2′‐C‐methylcytidine analogues with interferon‐alpha2b and triple combination with ribavirin in the hepatitis C virus replicon system. Antivir Chem Chemother 2008; 19(1): 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin K, Perni RB, Kwong AD, Lin C. VX‐950, a novel hepatitis C virus (HCV) NS3‐4A protease inhibitor, exhibits potent antiviral activities in HCv replicon cells. Antimicrob Agents Chemother 2006; 50(5): 1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chou TC, Talalay P. Quantitative analysis of dose‐effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22: 27–55. [DOI] [PubMed] [Google Scholar]
- 37. Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther 2001; 298(3): 865–872. [PubMed] [Google Scholar]
- 38. Mann EA, Stanford S, Sherman KE. Prevalence of mutations in hepatitis C virus core protein associated with alteration of NF‐kappaB activation. Virus Res 2006; 121(1): 51–57. [DOI] [PubMed] [Google Scholar]
- 39. Kasprzak A, Adamek A. Role of hepatitis C virus proteins (C, NS3, NS5A) in hepatic oncogenesis. Hepatol Res 2008; 38(1): 1–26. [DOI] [PubMed] [Google Scholar]
- 40. Li S, Kodama EN, Inoue Y et al. Procyanidin B1 purified from Cinnamomi cortex suppresses hepatitis C virus replication. Antivir Chem Chemother 2010; 20(6): 239–248. [DOI] [PubMed] [Google Scholar]
- 41. Takeshita M, Ishida Y, Akamatsu E et al. Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J Biol Chem 2009; 284(32): 21165–21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perni RB, Almquist SJ, Byrn RA et al. Preclinical profile of VX‐950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3‐4A serine protease. Antimicrob Agents Chemother 2006; 50(3): 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarrazin C, Zeuzem S. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology 2010; 138(2): 447–462. [DOI] [PubMed] [Google Scholar]
- 44. Trujillo‐Murillo K, Alvarez‐Martinez O, Garza‐Rodriguez L et al. Additive effect of ethanol and HCV subgenomic replicon expression on COX‐2 protein levels and activity. J Viral Hepat 2007; 14(9): 608–617. [DOI] [PubMed] [Google Scholar]
- 45. Gretton S, Hughes M, Harris M. Hepatitis C virus RNA replication is regulated by Ras‐Erk signalling. J Gen Virol 2010; 91(Pt 3): 671–680. [DOI] [PubMed] [Google Scholar]
- 46. Okamoto M, Sakai M, Goto Y et al. Anti‐bovine viral diarrhoea virus and hepatitis C virus activity of the cyclooxygenase inhibitor SC‐560. Antivir Chem Chemother 2009; 20(1): 47–54. [DOI] [PubMed] [Google Scholar]
- 47. Zhu H, Cong JP, Yu D, Bresnahan WA, Shenk TE. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc Natl Acad Sci USA 2002; 99(6): 3932–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reynolds AE, Enquist LW. Biological interactions between herpesviruses and cyclooxygenase enzymes. Rev Med Virol 2006; 16(6): 393–403. [DOI] [PubMed] [Google Scholar]
- 49. Liu T, Zaman W, Kaphalia BS, Ansari GA, Garofalo RP, Casola A. RSV‐induced prostaglandin E2 production occurs via cPLA2 activation: role in viral replication. Virology 2005; 343(1): 12–24. [DOI] [PubMed] [Google Scholar]
- 50. Tung WH, Hsieh HL, Yang CM. Enterovirus 71 induces COX‐2 expression via MAPKs, NF‐kappaB, and AP‐1 in SK‐N‐SH cells: role of PGE(2) in viral replication. Cell Signal 2010; 22(2): 234–246. [DOI] [PubMed] [Google Scholar]


