Abstract
Background:
Human metapneumovirus (hMPV), a recently identified virus, causes respiratory illness in children.
Objectives:
A real-time reverse transcription-polymerase chain reaction (RT-PCR) assay was developed and used to detect and quantify hMPV in respiratory specimens.
Study design:
The quantitative RT-PCR assay amplified an approximately 70 base pair fragment from the hMPV fusion protein gene. The assay was validated and used to test respiratory specimens obtained from children seen at a hospital in Seattle, Washington, from December 2002 through May 2003.
Results:
The assay detected 1000 hMPV copies/mL of specimen, did not detect 19 other respiratory viruses, and was able to detect and accurately quantify isolates from the four known hMPV genetic lineages in a proficiency panel of 20 previously tested samples. hMPV was detected in 52 (7.2%) of 719 pediatric respiratory specimens. The mean log 10 copies/mL of hMPV in the 52 positive specimens was 7.67 (range = 4.59–10.60). Children aged 7–12 months had a significantly higher hMPV prevalence (12.4%) than did children younger than 7 months (4.7%) (P < 0.005). Children in this age group also had significantly higher levels of hMPV in their respiratory specimens (mean log 8.43 copies/mL) than did the younger children (mean log 6.93 copies/mL) (P = 0.0025).
Conclusions:
The rapid real-time RT-PCR assay described here is a sensitive test for clarifying the epidemiology of and diseases associated with hMPV.
Keywords: Human metapneumovirus, Reverse transcription-polymerase chain reaction, Quantitative
Abbreviations: bp, base pair; DNA, deoxyribonucleic acid; mL, milliliter; nm, nanometer; nM, nanomolar; RNA, ribonucleic acid; RT-PCR, reverse transcription-polymerase chain reaction; μL, microliter
1. Introduction
Human metapneumovirus (hMPV), a member of the genus Metapneumovirus in the Paramyxoviridae family, was first isolated in 2001 from nasopharyngeal specimens obtained from young children (van den Hoogen et al., 2001) and since then has been detected in respiratory specimens collected from patients in several countries throughout the world (Peret et al., 2002, Stockton et al., 2002, Mackay et al., 2003, Freymuth et al., 2003, Peiris et al., 2003, Maggi et al., 2003, Viazov et al., 2003, Madhi et al., 2003, Ebihara et al., 2004, Galiano et al., 2004). Both upper and lower respiratory tract infections have been associated with hMPV in young children and adults and the clinical symptoms are similar to those of respiratory syncytial virus (RSV) infection (Van den Hoogen et al., 2001, Peret et al., 2002, Freymuth et al., 2003, Viazov et al., 2003, Boivin et al., 2002).
hMPV cannot be reliably cultivated in many commonly used laboratory cell lines (Van den Hoogen et al., 2001, Boivin et al., 2002) and there is no commercially available antigen detection test. However, hMPV genomic ribonucleic acid (RNA) can be detected in clinical specimens using conventional (Stockton et al., 2002, Freymuth et al., 2003, Peiris et al., 2003, Maggi et al., 2003, Viazov et al., 2003, Madhi et al., 2003, Ebihara et al., 2004, Galiano et al., 2004, Falsey et al., 2003, Bastien et al., 2003, Van den Hoogen et al., 2003, Williams et al., 2004, Mullins et al., 2004, Esper et al., 2004) or real-time reverse transcription-polymerase chain reaction (RT-PCR) (Mackay et al., 2003, Boivin et al., 2003). Genetic analysis has identified two major subgroups of hMPV, with two minor genetic clusters within each subgroup (Van den Hoogen et al., 2004, Boivin et al., 2004). Reliable diagnostic tests that can detect hMPV strains from all four genetic lineages are needed for accurate diagnosis of hMPV infections.
This study describes the design and application of a real-time RT-PCR assay using TaqMan techniques for the detection and quantification of hMPV in respiratory specimens from children. To rule out false negative results due to the presence of amplification inhibitors or poor quality of the RNA extraction, specimens were evaluated by detection and quantification of an exogenous, unrelated RNA control. Detection of hMPV using this rapid and sensitive assay will provide important information about the role of hMPV in respiratory tract disease among all patient groups.
2. Methods
2.1. Clinical specimens
From December 2002 through May 2003, 719 pediatric specimens (including 673 nasal washes, 27 nasal swabs, 13 tracheal aspirates, and 6 bronchoalveolar lavage (BAL) specimens) that were submitted to the University of Washington Virology Laboratory for respiratory virus fluorescent antibody assay (FA) or FA and culture, and contained sufficient residual material, were tested for hMPV by real-time RT-PCR. Specimens were submitted from both hospitalized (76%) and non-hospitalized (24%) patients. The age of the 681 patients from whom samples were collected ranged from 1 day to 20 years (median age = 10 months). Fifty-five percent of samples were from male and 45% were from female patients. Thirty-six patients provided more than one specimen (mean interval = 40 days, range = 14–97 days). The 719 specimens represent approximately one-third of the total pediatric specimens submitted during this time period to the laboratory for respiratory virus FA analysis. There was no significant difference between the median ages of the patients whose samples were tested by hMPV RT-PCR and those whose samples had insufficient volume for testing.
2.2. Respiratory virus antigen detection (FA)
Specimens were tested for RSV, parainfluenza virus types 1, 2, and 3, influenza virus types A and B, and adenovirus using an indirect fluorescent antibody assay. In brief, cells obtained from patients’ samples by centrifugation were suspended in buffer and spotted onto slides, air-dried, fixed in acetone, and incubated with a specific mouse anti-respiratory virus monoclonal antibody (Chemicon, Temecula, CA). After washing, goat anti-mouse fluorescein-conjugated monoclonal antibodies (ICN, Biomedicals, Inc., Costa Mesa, CA) were applied to the sample, and the slides were incubated, washed, and read using a fluorescent microscope. The presence of bright green fluorescence within intact cells was considered positive.
2.3. RT-PCR sample preparation
Total nucleic acids were isolated from 200 μL of each respiratory specimen as previously described (Kuypers et al., 2004). One low positive control with 2 × 104 copies/mL to 1 × 105 copies/mL (200–1000 copies/RT-PCR reaction) of hMPV harvested from cell culture and diluted in minimal essential medium (MEM), and one negative control consisting of cultured, uninfected human epithelial cells, were extracted with each batch of clinical specimens.
2.4. Design of primers and probes
The hMPV RT-PCR primer and probe sequences, shown in Table 1 , were designed using Primer Express software (Applied Biosystems, Foster City, CA) from 16 aligned hMPV fusion protein gene sequences obtained from the NCBI database. Four TaqMan primers and two probes were designed to amplify 69 bp and 74 bp fragments of the fusion protein genes from the two major hMPV genetic groups A and B, respectively. Each gene sequence in the alignment matched at least one of the primer/probe sets with no more than one base mismatch per oligonucleotide. The probes were labeled on the 5′ ends with the fluorescent dye 6FAM and on the 3′ ends with a minor groove binder non-fluorescent quencher (MGBNFQ) (Applied Biosystems). A second primer set and VIC-labeled probe (Limaye et al., 2001) were added to a separate RT-PCR reaction to amplify and detect the exogenously added EXO RNA molecules (Kuypers et al., 2004).
Table 1.
Real-time RT-PCR primer and probe sequences designed to detect hMPV
| Function | Sequence | Tm (°C) |
|---|---|---|
| Forward primers | GCC GTT AGC TTC AGT CAA TTC AAa | 59 |
| GCT GTC AGC TTC AGT CAA TTC AAb | 58 | |
| Reverse primers | TCC AGC ATT GTC TGA AAA TTG Ca | 59 |
| GTT ATC CCT GCA TTG TCT GAA AAC Tb | 59 | |
| Probes | 6FAM-CAA CAT TTA GAA ACC TTC T-MGBNFQa | 68 |
| 6FAM-CGC ACA ACA TTT AGG AAT CTT CT-MGBNFQb | 70 |
Primers and probe were designed from aligned sequences of hMPV subtype A.
Primers and probe were designed from aligned sequences of hMPV subtype B.
2.5. Real-time RT-PCR assay
One-step RT-PCR reaction mixtures (TaqMan One-Step RT-PCR Master Mix Reagents, Applied Biosystems) were prepared as previously described (Kuypers et al., 2004), using 200 nM of each of the two hMPV forward and two reverse primers and 50 nM of each of the two hMPV probes in one reaction mix. One hMPV RT-PCR reaction for each RNA sample was performed and analyzed in a 7000 Sequence Detection System (PRISM, Applied Biosystems). The threshold cycles of samples were compared to a standard curve generated by amplification of known numbers of hMPV or EXO RNA transcripts. Results were expressed as hMPV copies/mL of original sample. All samples with negative hMPV results (<1000 copies/mL) required detection of at least 200 EXO RNA copies/reaction to be valid. RNA extraction (if sufficient sample volume was available), and RT-PCR were repeated on all samples that were negative for both hMPV and EXO. Only specimens with satisfactory amplification of EXO were used in the analyses (719 (98.8%) of 728).
2.6. Preparation of RNA transcripts for standard curves
The hMPV PCR amplicons from several clinical specimens that were positive for hMPV were cloned and sequenced. Clones with sequences matching each of the two primer/probe sets were transcribed as previously described for an RSV RT-PCR assay (Kuypers et al., 2004) so that negative sense RNA transcripts for hMPV subtype A and subtype B were synthesized. The RNA was purified by two rounds of DNAse treatment (DNA free, Ambion, Inc., Austin, TX), phenol chloroform extraction, and ethanol precipitation, confirmed for size and purity on a bioanalyzer (Center for Expression Arrays, University of Washington, Seattle, WA), and quantified by absorbance at 260 nm. Contaminating deoxyribonucleic acid (DNA) was not detected by real-time PCR amplification of up to 107 copies of RNA transcript/reaction. Ten-fold serial dilutions containing 107–101 copies of RNA transcript were added to real-time RT-PCR reactions in duplicate. The results were used to generate standard curves for quantification of hMPV and EXO RNA in clinical samples.
2.7. Assay validation
The specificity of the hMPV RT-PCR assay was assessed by testing RNA or DNA purified from at least two isolates each of 19 viruses commonly found in respiratory specimens including respiratory syncytial virus, parainfluenza virus types 1, 2, and 3, influenza virus types A and B, rhinovirus, coronavirus, enterovirus, coxsackie B virus, adenovirus, and herpes viruses 1 through 8. In addition, a proficiency panel of 20 unknown specimens, prepared by MedImmune, Inc. (Mountain View, CA), was also tested. The content of the samples was not revealed until after results had been submitted to MedImmune. The samples were also tested at MedImmune using a quantitative RT-PCR assay with primers that target the hMPV nucleocapsid gene (Maertzdorf et al., 2004). The reproducibility of the RNA extraction method and the RT-PCR reaction was evaluated using two previously quantified nasal wash specimens that were diluted in varying amounts into previously tested, hMPV negative nasal wash specimens. RNA from four replicates each of eight specimens containing between 5000 hMPV copies/mL and 2 × 109 hMPV copies/mL was extracted and amplified.
2.8. Statistical analysis
Significant differences between groups were determined by the Wilcoxon rank sum test for comparison of medians, the t-test for comparison of means, and the Chi-squared test for comparison of proportions.
3. Results
3.1. hMPV RT-PCR performance
The RT-PCR assay consistently detected 10 hMPV subtype A or B RNA copies/reaction, which corresponds to a sensitivity of 1000 copies of hMPV/mL of specimen, and was linear to 108 copies/reaction. hMPV subtype A and subtype B RNA transcripts generated standard curves with nearly identical characteristics and regression coefficients ≥0.98. Nucleic acid extracted from at least 2 isolates each of 19 non-hMPV viruses commonly found in respiratory specimens was not detected by the assay. To evaluate the reproducibility of the assay, RNA was extracted and amplified from four aliquots each of eight nasal washes containing between 5000 hMPV copies/mL and 2 × 109 hMPV copies/mL. The mean within-specimen coefficient of variation (CV) for the eight samples was 2.2%. The mean hMPV copies/reaction of the low positive control that was extracted and amplified with each batch of specimens was 380 (range = 212–829) copies/reaction, with an interassay CV of 6.2% for 17 assays.
To further assess the accuracy of our assay, a proficiency panel of 20 coded specimens from MedImmune, Inc., containing samples of all 4 known hMPV genetic lineages, was tested in a blinded fashion. The results obtained with our assay were compared with those obtained by the MedImmune RT-PCR assay (Maertzdorf et al., 2004) (Table 2 ). There was 100% concordance between the methods for detection of hMPV, and good correlation of quantification (R 2 = 0.864) (Fig. 1 ).
Table 2.
Detection of hMPV in 20 hMPV positive and negative samples by two laboratories
| Specimena | hMPV strain | hMPV subtypeb | hMPV quantity (copies/mL) |
|
|---|---|---|---|---|
| MedImmune, Inc. | University of Washington | |||
| Medium | NA | NA | No Ctc | Negative |
| Medium | NA | NA | No Ct | Negative |
| RSV | NA | NA | No Ct | Negative |
| RSV | NA | NA | No Ct | Negative |
| RSV | NA | NA | No Ct | Negative |
| hPIV3 | NA | NA | No Ct | Negative |
| hPIV3 | NA | NA | No Ct | Negative |
| hPIV3 | NA | NA | No Ct | Negative |
| hMPV | NL/1/00 | A1 | 3.66E + 07 | 1.22E + 07 |
| hMPV | NL/1/00 | A1 | 2.29E + 04 | 4.53E + 05 |
| hMPV | NL/1/00 | A1 | 3.53E + 08 | 2.09E + 08 |
| hMPV | NL/17/00 | A2 | 1.94E + 06 | 5.15E + 06 |
| hMPV | NL/17/00 | A2 | 7.50E + 06 | 4.42E + 07 |
| hMPV | NL/17/00 | A2 | 3.22E + 07 | 3.65E + 08 |
| hMPV | NL/1/99 | B1 | 2.03E + 07 | 9.12E + 07 |
| hMPV | NL/1/99 | B1 | 1.38E + 06 | 9.00E + 06 |
| hMPV | NL/1/99 | B1 | 1.22E + 05 | 8.99E + 05 |
| hMPV | NL/1/94 | B2 | 1.82E + 07 | 9.68E + 07 |
| hMPV | NL/1/94 | B2 | 2.56E + 03 | 5.49E + 04 |
| hMPV | NL/1/94 | B2 | 1.56E + 04 | 1.55E + 06 |
Specimens were tested in random order.
Subtype designation according to Maertzdorf et al. (2004).
Sample signal did not cross threshold during PCR amplification.
Fig. 1.
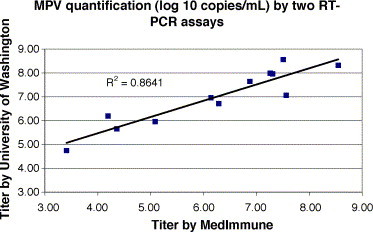
The titer of hMPV obtained from the 12 hMPV positive samples listed in Table 2 using the RT-PCR assay developed at the University of Washington is plotted against the titer obtained using an RT-PCR assay performed at MedImmune, Inc. The linear regression trend line and R2-value of the line are shown.
3.2. Detection of hMPV in respiratory specimens
hMPV was detected in 52 (7.2%) of 719 pediatric respiratory specimens overall, including 45 (6.7%) of 673 nasal washes, 5 (18.5%) of 27 nasal swabs, 1 (7.7%) of 13 tracheal aspirates, and 1 (16.7%) of 6 BAL. The proportion of hMPV positive nasal swabs was significantly higher than the proportion of hMPV positive nasal washes (P = 0.025). hMPV was detected in 18 (15.8%) of 114 specimens collected in April and May compared to 34 (5.6%) of 605 specimens collected from December through March (P < 0.005) (Fig. 2 ). Among the 36 children who provided more than one specimen, 32 were consistently hMPV negative, and 4 were positive for 1 of the 2 specimens submitted. The median age of the 52 hMPV positive children was 11.5 months, while the median age of the children providing the 667 MPV negative specimens was 9 months. Fifty percent of the hMPV positive versus 55.5% of hMPV negative specimens were from males, and 69% of the hMPV positive versus 76.7% of the hMPV negative patients were hospitalized.
Fig. 2.
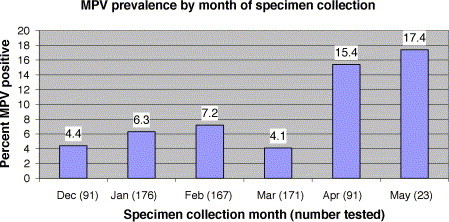
The proportion of specimens positive for hMPV by RT-PCR was determined among the total number of respiratory specimens collected during each of the 6 months of the study period (December, 2002 through May, 2003).
Eight (15%) of 52 hMPV positive specimens were positive by FA for another respiratory virus (5 RSV, 2 influenza A, and 1 adenovirus). Among the 414 specimens that were negative by FA for 7 other respiratory viruses, 44 (10.6%) were hMPV positive. Among the 44 patients with only hMPV detected, 63% were hospitalized compared to 87.5% of the 8 patients with dual respiratory virus infections. All eight of the dually infected samples were collected in January, February, or March.
3.3. Quantification of hMPV in respiratory specimens
The mean log 10 hMPV copies/mL for the 52 positive specimens was 7.67 (range = 4.59–10.60). The mean log 10 copies/mL of hMPV in the 44 specimens for which hMPV was the only respiratory virus detected was 7.97 compared to a mean log 10 hMPV copies/mL of 6.03 in the 8 dually infected samples (P = 0.001).
The hMPV prevalence and mean viral load for each of five patient age groups are shown in Fig. 3 . Children aged 7–12 months had a significantly higher prevalence of hMPV positive specimens (12.4%) than did children younger than 7 months (4.7%) (P < 0.005), but not those older than 12 months (7.4%). Children in this 7–12 months age group also had significantly higher levels of hMPV in their respiratory specimens (mean log 8.43 copies/mL) than did children younger than 7 months (mean log 6.93 copies/mL) (P = 0.0025). There were no significant differences in viral load between male and female patients or between hospitalized and non-hospitalized patients.
Fig. 3.
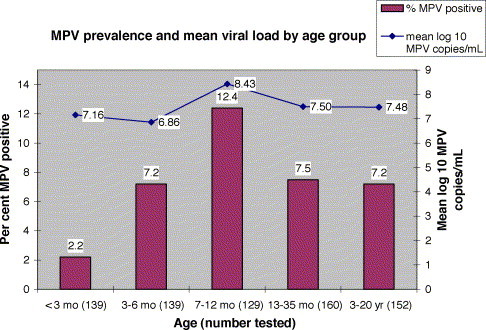
The proportion of specimens positive for hMPV by RT-PCR and the mean number of hMPV, expressed as log 10 copies/mL, detected in the positive specimens were determined among the total number of respiratory specimens collected from patients belonging to each of five age groups.
4. Discussion
The one-step, real-time RT-PCR assay described in this report for the detection and quantification of hMPV was rapid, sensitive, specific among the viruses tested, and reproducible. Several technical aspects of this assay warrant discussion. One of the strengths of our study was that, by testing every specimen for an external amplification control, false negative specimens due to inefficient RNA extraction or the presence of PCR inhibitors could be ruled out. However, we could not control for other factors that could lead to false negative results, such as stage of the patient's illness, or possible degradation of viral RNA during specimen transport. A previous report has suggested that RT-PCR primers that target the hMPV nucleoprotein or polymerase genes may be more suitable for hMPV detection than are primers that target the matrix, phosphoprotein, or fusion protein genes (Côté et al., 2003). As part of our study, we compared primers and probes designed to detect the hMPV fusion protein gene to an RT-PCR assay with primers that target the nucleoprotein gene (Maertzdorf et al., 2004). The hMPV fusion protein-based assay accurately detected and quantified hMPV isolates belonging to all four known genetic lineages. Detection of all the known subtypes of hMPV was achieved by using two primer/probe sets in the PCR reaction. Each set was designed to amplify isolates belonging to subtype A or B, but used together in one reaction mix. Whether specific hMPV subtypes are associated with the severity of illness, as has been shown for RSV (Martinello et al., 2002), has not been determined. Future work using the primer/probe sets from this assay in separate RT-PCR reactions will provide information about this question.
The 7.2% prevalence of hMPV found in specimens obtained from children with respiratory illness in Seattle is similar to the 4–18% prevalence reported by others in different parts of the world, using both conventional and real-time RT-PCR methods (Peiris et al., 2003, Viazov et al., 2003, Ebihara et al., 2004, Boivin et al., 2003, Mullins et al., 2004). The prevalence of hMPV in nasal swab specimens was significantly higher than in nasal wash specimens. The reason for collection of a swab rather than a wash from a particular patient is not known, making it difficult to draw conclusions about the relative utility of each of these samples for hMPV detection. However, these data show hMPV can readily be detected from swab specimens. Interestingly, hMPV was detected in specimens submitted from symptomatic children in all months surveyed and ranged from 4.4% to 17.4% of specimens submitted. The peak months of April and May for hMPV detection in our population agree with some published reports that the prevalence of hMPV is higher in spring (March, April, or May) compared to winter months (December, January, or February) (Peiris et al., 2003, Galiano et al., 2004, Boivin et al., 2003, Williams et al., 2004, Mullins et al., 2004, Esper et al., 2004). The median age of 11.5 months for hMPV positive children is also similar to that reported by others (Williams et al., 2004, Mullins et al., 2004). Of note, the patient age group with the highest prevalence of hMPV (7–12 months) was older than the age group with the highest prevalence of RSV infection (0–6 months) reported in a previous study (Kuypers et al., 2004).
Among the 15% of hMPV positive specimens that were co-infected with another respiratory virus as determined by FA, RSV, and influenza were the most common co-infections, similar to findings reported by others (Viazov et al., 2003, Boivin et al., 2003). More importantly, hMPV was detected in 10.6% of specimens for which no other viral pathogen was detected by FA.
This is the first report of quantification of hMPV in pediatric respiratory specimens, although interpretation of these results must take into account the variability between patients in collection of respiratory specimens. In our collection and testing methods, both inter and extra-cellular viral RNA were detected by the assay so that the specimen volume, more than the number of cells in a sample, determined the viral load. Volumes were recorded for each specimen and varied across the study by a factor of four. While the viral load comparisons in this paper are based on respiratory specimens that do vary in volume and inferences should be made with caution, a 10-fold difference in hMPV copies/mL between specimens is most likely a real difference. The mean number of hMPV in these specimens (7.67 log 10 copies/mL) was similar to the mean number of RSV (7.30 log 10 copies/mL) detected in pediatric respiratory specimens (Kuypers et al., 2004). For RSV infections, the virus load was significantly higher for patients in the lowest (0–6 months) age group compared to older RSV positive children (Kuypers et al., 2004), while for hMPV, children 7–12 months old had significantly higher levels of hMPV than did younger children. Ongoing investigations will help determine any associations between viral load and clinical signs and symptoms.
The one-step, quantitative, real-time RT-PCR assay described here for the detection and quantification of hMPV in respiratory specimens is rapid, sensitive, and accurate when compared to another PCR assay. It will be a useful tool for further investigations on the epidemiology of and diseases associated with hMPV.
Acknowledgements
The authors thank Dr. Guy Boivin, a Centre de Recherche en Infectiologie investigator, Canada, and the US Centers for Disease Control and Prevention for providing a human metapneumovirus isolate for initial testing of the assay, and MedImmune, Inc., for providing the proficiency panel specimens and the results of their assay.
References
- Bastien N., Ward D., Van Caeseele P., Brandt K., Lee S.H.S., McNabb G. Human metapneumovirus infection in the Canadian population. J Clin Microbiol. 2003;41:4642–4646. doi: 10.1128/JCM.41.10.4642-4646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G., Abed Y., Pelletier G., Ruel L., Moisan D., Côté S. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:634–640. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- Boivin G., Serres G., Côté S., Gilca R., Abed Y., Rochette L. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:640–643. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G., Mackay I., Sloots T.P., Madhi S., Freymuth F., Wolf D. Global genetic diversity of human metapneumovirus fusion gene. Emerg Infect Dis. 2004;10:1154–1157. doi: 10.3201/eid1006.031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté S., Abed Y., Boivin G. Comparative evaluation of real-time PCR assays for detection of the human metapneumovirus. J Clin Microbiol. 2003;41:3631–3635. doi: 10.1128/JCM.41.8.3631-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T., Endo R., Kikuta H., Ishiguro N., Ishiko H., Hara M. Human metapneumovirus infection in Japanese children. J Clin Microbiol. 2004;42:126–132. doi: 10.1128/JCM.42.1.126-132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F., Martinello R.A., Boucher D., Weibel C., Ferguson D., Landry M.L. A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189:1388–1396. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R., Erdman D., Anderson L.J., Walsh E.E. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- Freymuth F., Vabret A., Legrand L., Eterradossi N., Lafay-Delaire F., Brouard J. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr Infect Dis J. 2003;22:92–94. doi: 10.1097/00006454-200301000-00024. [DOI] [PubMed] [Google Scholar]
- Galiano M., Videla C., Puch S.S., Martinez A., Echavarria M., Carballal G. Evidence of human metapneumovirus in children in Argentina. J Med Virol. 2004;72:299–303. doi: 10.1002/jmv.10536. [DOI] [PubMed] [Google Scholar]
- Kuypers J., Wright N., Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol. 2004;31:123–129. doi: 10.1016/j.jcv.2004.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye A.P., Huang M.-L., Leisenring W., Stensland L., Corey L., Boeckh M. Cytomegalovirus (CMV) DNA load in plasma for the diagnosis of CMV disease before engraftment in hematopoietic stem-cell transplant recipients. J Infect Dis. 2001;183:377–382. doi: 10.1086/318089. [DOI] [PubMed] [Google Scholar]
- Mackay I.M., Jacob K.C., Woolhouse D., Waller K., Syrmis M.W., Whiley D.M. Molecular assays for detection of human metapneumovirus. J Clin Microbiol. 2003;41:100–105. doi: 10.1128/JCM.41.1.100-105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S.A., Ludewick H., Abed Y., Klugman K.P., Boivin G. Human metapneumovirus-associated lower respiratory tract infections among hospitalized human immunodeficiency virus type 1 (HIV-1)-infected and HIV-1-uninfected African infants. Clin Infect Dis. 2003;37:1705–1710. doi: 10.1086/379771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertzdorf J., Wang C.K., Brown J.B., Quinto J.D., Chu M., de Graff M. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol. 2004;42:981–986. doi: 10.1128/JCM.42.3.981-986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi F., Pifferi M., Vatteroni M., Fornai C., Tempestini E., Anzilotti S. Human metapneumovirus associated with respiratory tract infections in a 3-year study of nasal swabs from infants in Italy. J Clin Microbiol. 2003;41:2987–2991. doi: 10.1128/JCM.41.7.2987-2991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinello R.A., Chen M.D., Weibel C., Kahn J.S. Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis. 2002;186:839–842. doi: 10.1086/342414. [DOI] [PubMed] [Google Scholar]
- Mullins J.A., Erdman D.D., Weinberg G.A., Edwards K., Hall C.B., Walker F.J. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Tang W.-H., Chan K.-H., Khong P.-L., Guan Y., Lau Y.-L. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peret T.C.T., Boivin G., Li Y., Couillard M., Humphrey C., Osterhaus A.D.M.E. Characterization of human metapneumovirus isolated from patients in North America. J Infect Dis. 2002;185:1660–1663. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton J., Stephenson I., Fleming D., Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis. 2002;9:897–901. doi: 10.3201/eid0809.020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoogen B.G., de Jong J.C., Groen J., Kiuken T., de Groot R., Fouchier R.A.M. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoogen B.G., van Doornum J.J., Fockens J.C., Cornelissen J.J., Beyer W.P.E., de Groot R. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- Van den Hoogen B.G., Herfst S., Sprong L., Cane P.A., Forleo-Neto E., de Swart R.L. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10:658–666. doi: 10.3201/eid1004.030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viazov S., Ratjen F., Scheidhauer R., Fiedler M., Roggendorf M. High prevalence of human metapneumovirus infection in young children and genetic heterogeneity of the viral isolates. J Clin Microbiol. 2003;41:3043–3045. doi: 10.1128/JCM.41.7.3043-3045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.V., Harris P.A., Tollefson S.J., Halburnt-Rush L.L., Pingsterhaus J.M., Edwards K.M. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]


