Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is a newly emerging infectious disease caused by a novel bunyavirus with high mortality. Immune suppression is thought to be crucial in disease progression. However, data on immune responses during SFTS are scarce. This study aimed to evaluate the changes in CD4 T-cell subsets throughout the entirety of infection and analyse their relationships with disease severity in SFTS patients. In parallel with CD4 T-cell depletion, decreased Th1, Th2 and Treg numbers, but comparable Th17-cell numbers, were observed in deceased patients compared with those in surviving patients. Additionally, increased Th2 and Th17-cell percentages in the residual CD4 T-cell population led to aberrant Th2/Th1 and Th17/Treg ratios, which were positively correlated with disease severity. Collectively, our data indicated that CD4 T-cell deficiency, Th2 and Th17 bias were closely correlated with the severity of SFTS, indicating therapeutic potential of early immune interventions to ameliorate disease severity.
Keywords: Severe fever with thrombocytopenia syndrome, T lymphocytes, Effector TH cell subsets, Regulatory T cells, Disease severity
1. Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging febrile illness caused by a novel phlebovirus, SFTS bunyavirus (SFTSV). SFTS is characterized by sudden onset of hyperpyrexia, respiratory or gastrointestinal symptoms, leukocytopenia, thrombocytopenia, neurological symptoms and possibly even death within 7–14 days after the onset of the disease, with a reported fatality rate varying between 12% and 30% [1, 2]. To date, neither effective antiviral strategies nor conventional vaccines have achieved sustainable control of the spread of SFTSV. A better understanding of the mechanism underlying SFTS progression will be helpful in developing new preventive and therapeutic strategies.
Although the mechanism of SFTS is incompletely understood, it is accepted that the immune system may play an important role. Previous studies have demonstrated that immune cells, such as monocytes, myeloid and plasmacytoid dendritic cells in the peripheral blood are also dramatically decreased in acute SFTS patients, especially in deceased patients [3, 4]. A previous study demonstrates that the lymphocyte cellularity of the red pulp is decreased in the spleens of SFTSV-infected mice during the first week after inoculation [5]. Additional reports show that CD4 T cells are decreased in the acute phase and in patients who eventually die from the disease [[6], [7], [8], [9]]. However, almost all previous studies collected samples at one or two times points during the disease process rather than determining the dynamic changes in the surviving and the deceased patients. No studies on the correlation between CD4 T cells and disease severity exist. Additionally, the role of CD4 T-cell subsets in SFTS progression remains unclear.
CD4 T cells are subdivided into multiple subsets, including T helper 1 (Th1), Th2, Th17, and T regulatory cells (Tregs), as defined by their characteristic cytokine production profiles [10]. Th1 cells are defined by their ability to secrete the cytokines interferon-γ (IFN-γ) and tumour necrosis factor alpha (TNF-α) and play critical roles in the immune responses to many viral infections [10]. Th2 cells are critical for promoting anti-helminthic immunity and allergic inflammation through the production of interleukin 4 (IL-4), IL-5 and IL-13 [10]. Th17 cells are a more recently discovered population of T helper cells, implicated in driving harmful inflammation during autoimmunity. Th17 cells may contribute to immunologic injury during responses against viruses by producing the hallmark cytokines IL-17 and IL-22 [11, 12]. Tregs maintain immune homeostasis by limiting the magnitude of immune responses against pathogens and by controlling inflammatory reactions by secreting anti-inflammatory cytokines, such as TGF-β and IL-10 [13]. When the total counts of CD4 T-cell subsets are decreased or their functions are lost, the ability of these cells to mediate viral removal, provide help for B cells, and regulate immunopathology will be damaged. In addition, it is becoming increasingly clear that considerable plasticity exists within CD4 T-cell subsets in vivo, especially during responses to pathogens [14, 15]. Previous studies have suggested that the Th1/Th2 and Th17/Tregs balances are critical for the maintenance of immune T cell homeostasis in a number of different autoimmune diseases and viral infections [[16], [17], [18]]. A deep understanding of the changes in Th1, Th2, Th17 and Tregs in SFTS patients is needed to develop effective antiviral strategies and immune regulation methods to eliminate the virus and protect the host from excessive inflammatory damage.
In the present study, we measured the dynamic changes in the frequencies of T lymphocytes and CD4 T-cell subsets in 12 deceased and 30 surviving patients throughout the entire course of infection. Additionally, the correlations of T lymphocyte and CD4 T-cell subset frequencies with the SFTS index (SFTSI) were evaluated. The results suggested that T-lymphocyte deficiency and CD4 T-cell subset imbalance potentially play important roles in SFTS pathogenesis. This information will be useful for promoting a more in-depth investigation of the mechanisms of SFTSV pathogenesis and may provide an immune basis for new therapeutic strategies.
2. Materials and methods
2.1. Ethics statement
The Research and Ethics Committee of The Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) approved this study. Each enrolled participant provided informed written consent to enter the study prior to blood collection.
2.2. Patients
Between May 2015 and September 2016, patients with SFTSV infection according to the clinical guidelines on SFTS released by the Ministry of Health of the People's Republic of China in 2010 were admitted to Union Hospital in Wuhan City. Individuals with concurrent hepatitis C virus, hepatitis B virus, human immunodeficiency virus (HIV)-1 infections, or Epstein-Barr virus infections and patients who met the clinical or biological criteria for bacterial or fungal infection were excluded. All patients were admitted to the hospital on days 3–7 after the onset of the illness. The study protocol was approved by the ethics committee of our unit, and written informed consent was obtained from each subject. Patient details, including clinical history, physical examination findings, and routine haematological laboratory results were collected from medical records to conduct a retrospective analysis. The basic characteristics of these subjects at admission are listed in Table 1 .
Table 1.
Differences in clinical and laboratory characteristics of the surviving and deceased patients infected with SFTSV at admission.
| Characteristicsa | ALL cases (N = 42) | Non-fatal (N = 30) | Fatal (N = 12) | p-value |
|---|---|---|---|---|
| Age, years | 60 (23–81) | 58 (23–81) | 66 (42–80) | 0.0003b |
| Male, sex, n(%) | 20 (47.6) | 14 (46.7) | 6 (50) | 0.85c |
| Plasma RNA, Log10 | 4.15 (1.13–6.95) | 3.73 (1.13–6.2) | 4.39 (2.20–6.95) | <0.0001d |
| PLT, 109/l | 43 (11–115) | 42 (11–115) | 45.5 (18–84) | 0.48b |
| MNC, 109/l | 0.11 (0–1.89) | 0.14 (0.01–1.89) | 0.11 (0–0.83) | 0.34b |
| LYM, 109/l | 0.59 (0.11–2.31) | 0.62 (0.13–2.31) | 0.51 (0.11–1.89) | 0.098b |
| NEU, 109/l | 1.54 (0.15–10.85) | 1.75 (0.15–7.58) | 1.54 (0.18–10.85) | 0.50b |
| MNC % | 4.45 (0.2–42.2) | 4.8 (0.32–42.2) | 4.16 (0.2–19.4) | 0.035b |
| LYM % | 24.3 (7.3–76.8) | 25.5 (7.3–76.8) | 22.6 (7.3–64) | 0.9b |
| NEU % | 68.9 (3.73–91.47) | 66.8 (3.73–91.47) | 72.6 (24–86.73) | 0.19b |
| WBC, 109/l | 2.43 (0.81–12.63) | 2.73 (0.81–8.41) | 2.5 (0.75–12.63) | 0.35b |
| ALT, U/L | 76.5 (14–911) | 69.5 (18–431) | 97 (14–911) | 0.26d |
| AST, U/L | 223 (27–3876) | 176 (27–1410) | 274 (98–3876) | 0.002d |
| GGT, U/L | 35 (10–412) | 33 (10–412) | 37 (17–158) | 0.19d |
| CK, U/L | 625 (51–15,143) | 606 (51–15,143) | 636 (138–3020) | 0.018d |
| LDH, U/L | 717 (183–6894) | 852 (183–3092) | 909 (360–6894) | 0.34d |
| Cr, umol/L | 73.9 (42.4–217.5) | 69.4 (42.4–141.8) | 87.6 (43.4–217.5) | 0.028d |
| ALP, U/L | 66 (24–729) | 63.5 (24–729) | 75 (24–347) | 0.29d |
PLT, platelet; MNC, monocyte; LYM, lymphocyte; NEU, neutrophil; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; CK, creatine kinase; LDH, lactate dehydrogenase; Cr, creatinine; ALP, alkaline phosphatase.
Data are the median (range) unless otherwise specified.
Determined by t-test.
Determined by Pearson's chi-square test.
Determined by Mann-Whitney U test.
2.3. Sample collection and processing
All SFTSV-infected patients received standard antiviral and support treatments after admission to the hospital based on SFTS treatment guidelines from the Chinese Ministry of Health. Sequential blood samples were collected at regular intervals (within 24 h of admission, every three days thereafter and on the day before discharge). Additionally, we considered the patients' blood test results on the first day of admission (within 7 days after disease onset) to be “acute phase” and the patients' blood test results on the day before discharge (two weeks after disease onset, clinical symptoms begin to resolve and laboratory measurements gradually revert to normal) to be “recovery phase”. Fifteen SFTSV-uninfected blood donors, who had no background diseases and matched the infected patients with regard to sex, age and ethnic background, were enrolled in the present study, and blood samples were collected at a single time point at the time of enrolment. All samples were processed within the first 4 h after collection. Peripheral blood mononuclear cells (PBMCs) were separated via density gradient centrifugation with Ficoll-Paque Plus (DAKEW Biotech, China) according to the manufacturer's instructions.
2.4. Flow cytometry
For the measurement of intracellular cytokines, 2 × 105 PBMCs were stimulated with PMA (50 ng/ml; Sigma-Aldrich, USA) and ionomycin (1 μg/ml; Sigma-Aldrich) in the presence of brefeldin A (10 mg/ml; eBioscience, USA) during 5 h of culture. After stimulation, the PBMCs were collected in tubes and surface stained with APC-Cy7-labelled CD3 (300,318, BioLegend, USA), PerCP-cy5.5-labelled CD4 (317,428, BioLegend) and PE-Cy7-labelled CD8 (300,914, BioLegend) antibodies as well as eFluor 506-labelled Fixable Viability Dye (65–0866-18, eBioscience) at 4 °C for 30 min. These cells were then treated with Fix and Perm Reagent (00–5523-00, eBioscience), followed by intracellular staining with PE-labelled IL-4 (12–7049-42, eBioscience), APC-labelled IL-17A (17–7179-42, eBioscience) and PE-Cy7-labelled IFN-γ (25–7319-82, eBioscience).
For the analysis of Tregs, PBMCs were collected in tubes without PMA, BFA or ionomycin stimulation and surface stained with APC-Cy7-labelled CD3 (300,318, BioLegend), PerCP-cy5.5-labelled CD4 (317,428, BioLegend), PE-Cy7-labelled CD127 (351,320, BioLegend) and APC-labelled-CD25 (302,610, BioLegend) antibodies as well as eFluor 506-labelled Fixable Viability Dye (65–0866-18, eBioscience) at 4 °C for 30 min and then washed twice with phosphate-buffered saline (PBS) (HyClone, USA). Subsequently, the samples were resuspended in 200 μl of PBS before acquisition on a FACSCalibur flow cytometer (BD Biosciences). Approximately 5 × 104–1 × 105 events per tube were acquired via flow cytometry to determine the frequencies of the circulating lymphocytes. The results were analysed with FlowJo software (Tree Star, Ashland, OR, USA). Absolute numbers were obtained by multiplying the number of lymphocytes (cells/μl of venous peripheral blood) by the percentages of CD4 T-cell subsets as follows [19, 20]: CD4 + IFN-γ + for Th1; CD4 + IL-4+ for Th2; CD4 + IL-17A+ for Th17; and CD4 + CD25+ CD127low for Tregs.
2.5. SFTS viral load assay
As described previously [3, 4], total RNA was extracted from every clinical patient serum specimen using a viral RNA kit from DAAN Gene (Guangzhou, China), and the viral load of SFTSV RNA copies in the SFTS patients was determined using a certified real-time PCR kit (SFDA Registration No. 340166, China) in accordance with the manufacturer's instructions.
2.6. Elisa
The concentrations of IL-6, IL-10 and IFN-γ (BioLegend, USA) as well as IL-17A (R&D, USA) in the serum of SFTS patients were measured by ELISA using commercial kits according to the manufacturer's instructions. The detection limit for each cytokine was as follows: IL-6, 7.8–500 pg/ml; IL-10, 3.9–250 pg/ml; IL-17A, 0.2–15 pg/ml; and IFN-γ, 15.6–1000 pg/ml.
2.7. Evaluating the severity of the SFTS patients
As reported previously [21], the SFTSI of patients at admission, where SFTSI = 5× neurological symptom level + 4× respiratory symptom level + 3× LG10 viral load - 2× LN monocyte% - 7, can be used to evaluate the severity of SFTS.
2.8. Statistical analysis
All results were analysed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6.01 (GraphPad Software Inc., San Diego, CA, USA). Continuous variables were summarized as the median (range) or median ± 95% CI, and dichotomous variables were specified using absolute values and percentages. For two independent groups, two-tailed Mann-Whitney U tests were performed, while for single comparisons of paired samples, two-tailed Wilcoxon matched pair tests were performed. Dichotomous variables in the tables were analysed using χ 2 or Fisher's exact tests. Correlation analysis of the continuous variables was performed using a non-parametric Spearman correlation test. For all tests, two-tailed p-values of <0.05 (95% confidence internal) were considered statistically significant.
3. Results
3.1. Characterization of the deceased and surviving SFTS patients at admission
A total of 42 hospitalized patients (30 surviving and 12 deceased) were enrolled in this study. The detailed laboratory parameters and clinical information for the patients included in this study are shown in Table 1. Consistent with the results of previous reports [22, 23], there was no sex bias in the deceased and surviving patients (p = .85). The median age of the deceased individuals was greater than that of the surviving individuals (p = .0003). Furthermore, the deceased SFTS patients showed significantly higher viral loads; higher levels of AST, CK and creatinine; and lower monocyte percentages compared with those in the surviving patients. There was no significant difference between the deceased and surviving SFTS patients with respect to their total platelet, monocyte, lymphocyte, neutrophil and leukocyte counts at admission.
3.2. Significant correlation of CD4, but not CD8 T cells, with SFTS severity
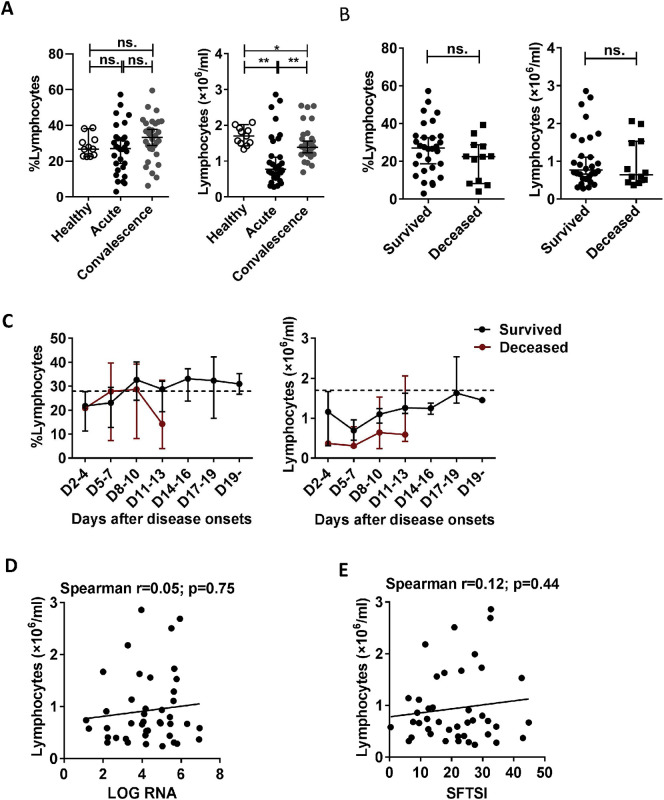
Although the percentages and numbers of lymphocytes were not significantly different between the deceased and surviving patients (Table 1, Supplementary Fig. 2B), the numbers of lymphocytes (but not the percentage) in surviving patients were dramatically lower during the acute and recovery phases compared with those in the healthy controls (Supplementary Fig. 2A).
Supplementary Fig. 2.
Changes in the percentage and absolute number of lymphocytes during SFTSV infection in humans. (A): The percentage and number of lymphocytes in the healthy controls (n = 11) and the surviving patients with SFTS in the acute phase (n = 30) and SFTS in the recovery phase (n = 30). (B): The percentage and number of lymphocytes at admission in the surviving patients (n = 30) and deceased patients (n = 12). (C): Dynamic changes in the percentage and number of lymphocytes in the surviving patients (n = 30) and deceased patients (n = 12). These parameters were monitored at the indicated time points for the entire hospital stay of the patients. The dashed line represents the median of the uninfected controls. (D) and (E): Correlation of the number of lymphocytes with the viral load and SFTSI in the SFTS patients (n = 42). Correlation analysis was performed using a non-parametric Spearman correlation test. In the graphs, r and p indicate the correlation coefficient and the p-value of significance, respectively. Data from (A), (B) and (C) are shown as the median ± 95% CI. Statistical analysis was performed using the Mann–Whitney U test or Wilcoxon matched pairs test. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001.
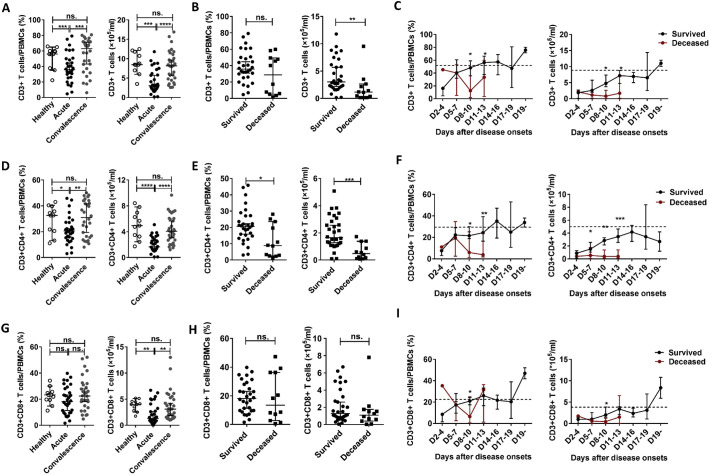
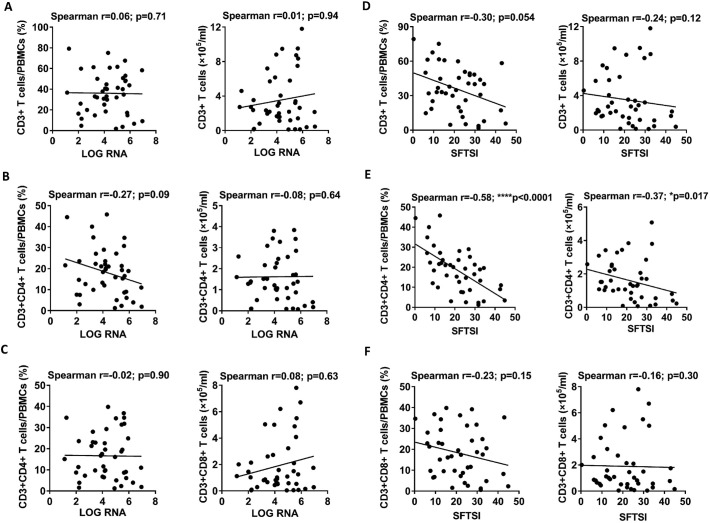
To evaluate the correlations of T lymphocyte levels (including CD4 T cells and CD8 T cells) with the viral loads and SFTSI, we analysed the percentages and numbers of T lymphocytes at various phases in the 42 SFTS patients via flow cytometry. Data from the surviving patients showed that the percentage and number of CD3 T cells were significantly decreased in the acute-phase patients compared with those in the healthy control and recovery-phase patients (Fig. 1A). Furthermore, compared with the surviving patients, the number of CD3 T cells but not the percentage at admission was significantly decreased in the deceased patients (Fig. 1B). However, there was no significant correlation between the number or percentage of CD3 T cells and the viral loads or SFTSI (Fig. 2A and D).
Fig. 1.
Changes in the percentages and absolute numbers of CD3, CD4 and CD8 T cells in PBMCs during SFTSV infection in humans.
(A), (D) and (G): The percentages and numbers of CD3, CD4 and CD8 T cells in PBMCs of the healthy controls (n = 11) and the surviving patients with SFTS in the acute phase (n = 30) and SFTS in the recovery phase (n = 30). (B), (E) and (H): The percentages and numbers of CD3, CD4 and CD8 cells in PBMCs at admission in the surviving patients (n = 30) and deceased patients (n = 12). (C), (F) and (I): Dynamic changes in the percentages and numbers of CD3, CD4 and CD8 cells in PBMCs of the surviving patients (n = 30) and deceased patients (n = 12). These parameters were monitored at the indicated time points for the entire hospital stay of the patients. The dashed line represents the median of the uninfected controls. The data are shown as the median ± 95% CI. The statistical analysis was performed using the Mann–Whitney U test or the Wilcoxon matched pair test. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001.
Fig. 2.
Correlations of the numbers of CD3, CD4 and CD8 T cells in PBMCs with the viral load and with the severity of SFTS.
(A), (B) and (C): Correlation of the percentages and numbers of CD3, CD4 and CD8 cells with viral load at admission in the 42 SFTS patients (including 30 surviving patients and 12 deceased patients). (D), (E) and (F): Correlation of the percentages and numbers of CD3, CD4 and CD8 cells with SFTSI at admission in the 42 SFTS patients (including 30 surviving and 12 deceased patients). Correlation analysis was performed via a non-parametric Spearman correlation test. In the graphs, r and p indicate the correlation coefficient and the p-value of significance, respectively. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001.
We further analysed the changes in CD4 T cells and CD8 T cells in the SFTS patients. Data from the surviving patients showed that the percentage and number of CD4 T cells were both significantly decreased in the acute-phase patients compared with those in the healthy control and recovery-phase patients (Fig. 1D). In addition, the number but not the percentage of CD8 T cells was significantly decreased in the acute-phase patients compared with that in the healthy controls and recovery-phase patients (Fig. 1G).
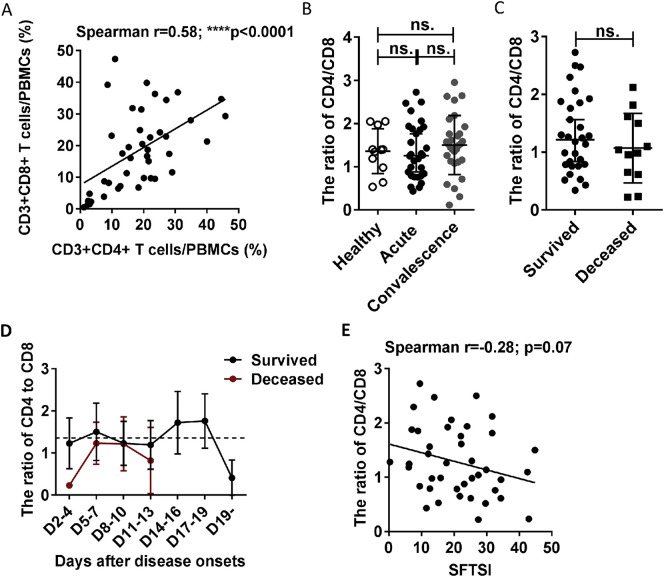
In addition, compared with the surviving patients, the deceased patients displayed lower percentage and number of CD4 T cells, but not that of CD8 T cells, at admission (Fig. 1E and H), which may suggest that CD4 T-cell depletion could be the major cause of T-lymphocyte deficiency in the deceased patients. Additionally, although the ratio of CD4/CD8 was lower in the deceased patients, there was no significant difference compared with that in the surviving patients (Supplementary Fig. 3C), and the CD4/CD8 ratio showed no significant correlation with disease severity (Supplementary Fig. 3E).
Supplementary Fig. 3.
No significantly difference in the ratio of CD4/CD8 between the deceased and the surviving patients. (A): Correlation analysis between the CD4 T cells and CD8 T cells of the 42 SFTS patients (30 survived and 12 deceased) at admission. Correlation analysis was done via a non-parametric Spearman correlation test. (B): The ratio of CD4/CD8 in the healthy controls (n = 11) and the surviving patients with SFTS in the acute phase (n = 30) and SFTS in the recovery phase (n = 30). (C): The ratio of CD4/CD8 at admission in the surviving patients (n = 30) and deceased patients (n = 12). (D): Dynamic changes in the ratio of CD4/CD8 in the surviving patients (n = 30) and the deceased patients (n = 12). These parameters were monitored at the indicated time points for the entire hospital stay of the patients. The dashed line represents the median of the uninfected controls. (E): Correlation analysis between the ratio of CD4/CD8 and SFTSI in the 42 SFTS patients at admission. Correlation analysis was done via a non-parametric Spearman correlation test. Data from (B), (C), (D) are shown as the median ± 95% CI. The statistical analysis was performed using the Mann-Whitney U test or Wilcoxon matched pairs text. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001.
Linear regression analyses showed no significant correlations of the percentages and numbers of CD4 T cells or CD8 T cells with the viral loads of the SFTS patients (Fig. 2B and C). However, the percentage and number of CD4, but not CD8, T cells were negatively correlated with SFTSI (Fig. 2E and F).
As shown in Fig. 1F, in the surviving patients, the percentage and number of CD4 T cells returned to normal levels on days 11–13 and days 17–19, respectively. In the deceased patients, the percentage and number of CD4 T cells were significantly decreased on days 8–13 after disease onset compared with those in the surviving patients and did not return to normal levels before death.
3.3. Differential changes in CD4 T-cell subsets (including Th1, Th2, Th17 and Tregs) in SFTSV-infected patients
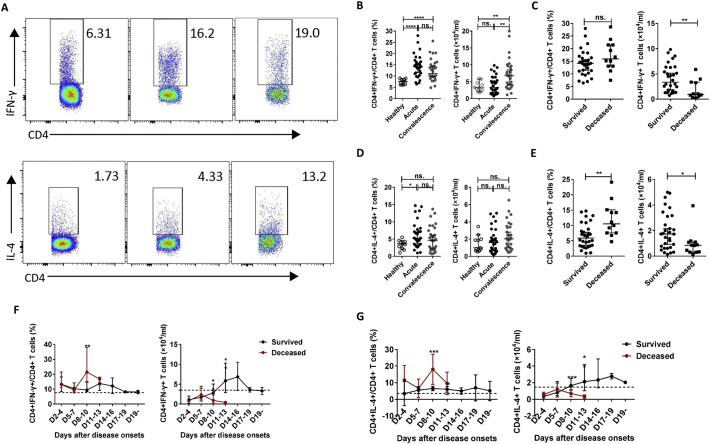
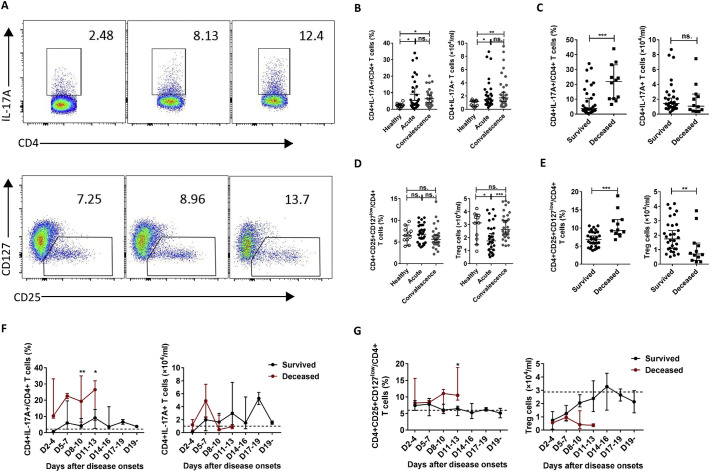
As the number and percentage of CD4 T cells were significantly correlated with the severity of SFTS, we further examined the frequencies of the CD4 T-cell subsets in the 42 SFTS patients during infection via flow cytometry. We then evaluated the correlation between the frequencies of CD4 T-cell subsets and SFTSI at admission via linear regression analyses. Data from the surviving patients showed that the percentages of Th1, Th2, and Th17 cells but not Tregs were increased in the acute-phase patients compared with those in the healthy controls (left panels of Figs. 3B, D and 4B, D ). Additionally, as shown in the right panel of Fig. 3B, the number of Th1 cells was increased in the recovery-phase patients compared with those in the acute-phase patients and healthy controls, but no significant difference between the acute-phase patients and the healthy controls was noted. The number of Th17 cells was increased in the acute- and recovery-phase patients compared with that in the healthy controls (Fig. 4B, right). However, the number of Tregs was lower in the acute-phase patients compared with those in the healthy control and recovery-phase patients (Fig. 4D, right).
Fig. 3.
Changes in the percentages and absolute numbers of Th1 and Th2 cells in SFTS patients.
(A): Th1 cells (CD4 + IFN-γ+) and Th2 cells (CD4 + IL-4+) by flow cytometry in the healthy controls, surviving SFTS patients, and deceased SFTS patients, as defined by flow cytometry. The cells were gated on the CD3 + CD4+ population within the single-cell lymphocyte gate. (B), (D): The percentages and numbers of Th1 and Th2 cells in the healthy controls (n = 11) and the surviving patients with SFTS in the acute phase (n = 30) and SFTS in the recovery phase (n = 30). (C), (E): The percentages and numbers of Th1 and Th2 cells at admission in the surviving patients (n = 30) and the deceased patients (n = 12). (F), (G): Dynamic changes in the percentages and numbers of Th1 and Th2 cells in the surviving patients (n = 30) and the deceased patients (n = 12). These parameters were monitored at indicated time points for the entire hospital stay of the patients, and the dashed line represents the median of the uninfected controls. The data are shown as the median ± 95% CI. Statistical analysis was performed using the Mann-Whitney U test or the Wilcoxon matched pair test. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001;
Fig. 4.
Changes in the percentages and absolute numbers of Th17 and Treg cells in SFTS patients.
(A): Th17 cells (CD4 + IL-17A+) and Treg cells (CD4 + CD25 + CD127low) by flow cytometry in the healthy controls, surviving SFTS patients, and deceased SFTS patients, as defined by flow cytometry. The cells were gated on the CD3 + CD4+ population within the single-cell lymphocyte gate. (B), (D): The percentages and numbers of Th17 and Treg cells in the healthy controls (n = 11) and the surviving patients with SFTS in the acute phase (n = 30) and SFTS in the recovery phase (n = 30). (C), (E): The percentages and numbers of Th17 and Treg cells at admission in the surviving patients (n = 30) and the deceased patients (n = 12). (F), (G): Dynamic changes in the percentages and numbers of Th17 and Treg cells in the surviving patients (n = 30) and the deceased patients (n = 12). These parameters were monitored at the indicated time points for the entire hospital stay of the patients, and the dashed line represents the median of the uninfected controls. The data are shown as the median ± 95% CI. Statistical analysis was performed using the Mann-Whitney U test or the Wilcoxon matched pair test. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001;
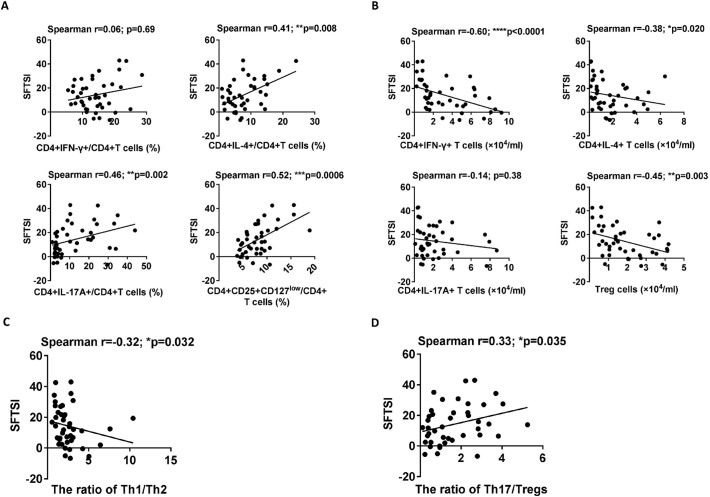
In addition, compared with the surviving patients, the deceased patients displayed increased percentages of Th2, Th17 and Tregs, but not Th1 cells, at admission (left panels of Figs. 3C, E and 4C, E). However, the numbers of Th1, Th2 and Tregs, but not Th17 cells, measured at admission were significantly decreased in the deceased patients compared with those in the surviving patients (right panels of Figs. 3C, E and 4C, E). Furthermore, the percentages of Th2, Th17 and Tregs were significantly positively correlated with SFTSI (Fig. 6A) and the numbers of Th1, Th2 and Tregs were significantly inversely correlated with SFTSI (Fig. 6B).
Fig. 6.
Correlation analysis of the percentages and counts of CD4 T-cell subsets with the severity of SFTS.
(A) and (B): Correlation analysis of the percentages and numbers of CD4+ T-cell subsets with SFTSI in the 42 SFTS patients (30 surviving and 12 deceased) at admission. (C) and (D): Correlation analysis of Th1/Th2 and Th17/Treg ratios with SFTSI in the 42 SFTS patients (30 surviving and 12 deceased) at admission. Statistical analysis was performed via a non-parametric Spearman correlation test. In the graphs, r and p indicate the correlation coefficient and the p-value of significance, respectively. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001.
Similar results were observed in the dynamic data for the percentages and numbers of CD4 T-cell subsets in the deceased and surviving patients. Compared with the surviving patients, the percentages of Th1 and Th2 were significantly increased on days 8–10, while the percentages of Th17 and Treg cells were significantly increased at days 8–13 and days11–13, respectively, in the deceased patients (left panels of Figs. 3F, G and 4F, G). Furthermore, in agreement with CD4-T cell deficiency, the numbers of Th1, Th2, Th17 and Tregs were decreased at days 8–13 after disease onset in the deceased patients compared with those in the surviving patients (right panels of Figs. 3F, G and 4F, G).
3.4. Significant correlation of Th1/Th2 and Th17/Treg ratios with disease severity in SFTS patients
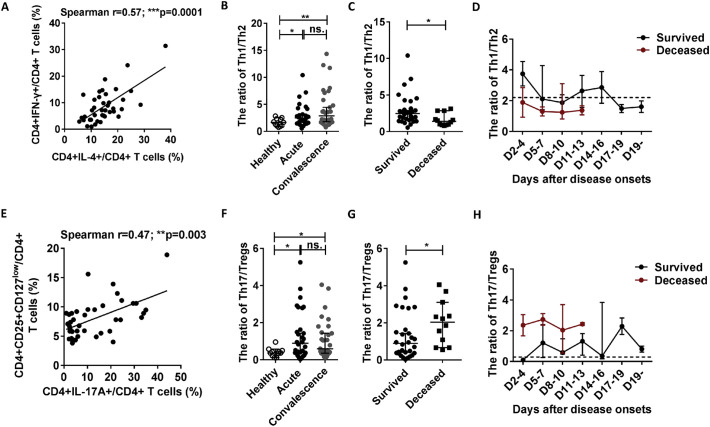
As the fine balances between Th1 and Th2 cells and that between Th17 and Tregs play important roles in the development of infectious diseases, we investigated the Th1/Th2 and Th17/Treg ratios in the 42 SFTS patients at admission. As shown in Fig. 5A and E, the percentages of Th1 and Th17 cells were positively correlated with the percentages of Th2 and Tregs, respectively. However, the data from the surviving patients showed that the Th1/Th2 and Th17/Treg ratios were markedly increased in the acute- and recovery- phase SFTS patients compared with those in the healthy controls (Fig. 5B and F, respectively).
Fig. 5.
Th1/Th2 and Th17/Treg imbalances are associated with SFTS patient outcomes.
(A) and (D): Correlation of Th1 with Th2 and Th17 with Tregs in the 42 SFTS patients (30 surviving and 12 deceased) at admission. Correlation analysis was performed via the non-parametric Spearman correlation test. (B) and (E): The Th1/Th2 and Th17/Treg ratios in the healthy controls (n = 11) and the surviving patients with SFTS in the acute phase (n = 30) and SFTS in the recovery phase (n = 30). (C) and (F): theTh1/Th2 and Th17/Treg ratios at admission in the surviving patients (n = 30) and the deceased patients (n = 12). (G) and (H): Dynamic changes in the Th1/Th2 and Th17/Treg ratios in the surviving patients (n = 30) and the deceased patients (n = 12). These parameters were monitored at the indicated time points for the entire hospital stay of the patients, and the dashed line represents the median of the uninfected controls. Data from (B), (C), (E), (F), (G) and (H) are shown as the median ± 95% CI. The statistical analysis was performed using the Mann-Whitney U test. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001;
In addition, compared with the surviving patients, the deceased patients exhibited lower Th1/Th2 ratio and higher Th17/Treg ratio (Fig. 5C and G, respectively).
Similar results were observed in the dynamic data for the Th1/Th2 and Th17/Treg ratios in the deceased and surviving patients (Fig. 5D and H). Additionally, linear regression analyses showed that the Th1/Th2 and Th17/Treg ratios were negatively and positively correlated with SFTSI, respectively (Fig. 6C and D).
3.5. Significant correlation between the Th17/Treg ratio and the IL-6 level in SFTS patients
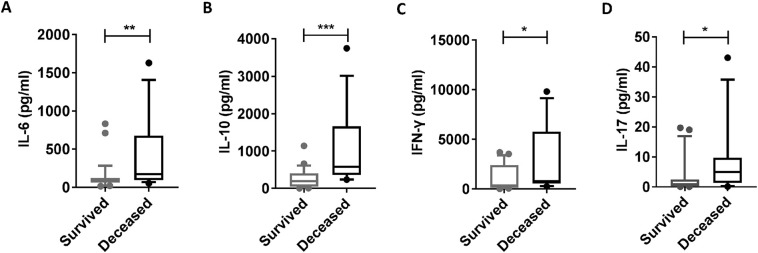
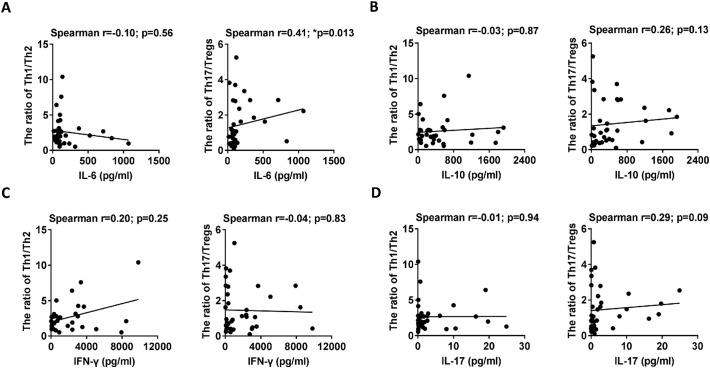
A previous study [23] demonstrates that host cytokine storm is associated with the severity of SFTS. Thus, we further evaluated the correlations of the Th1/Th2 and Th17/Treg ratios with the cytokines (including IL-6, IL-10, IL-17A and IFN-γ) at admission via linear regression analyses. Consistent with the previous studies [23, 24], the deceased patients displayed increased levels of IL-6, IL-10, IFN-γ and IL-17A at admission compared with the surviving patients (Supplementary Fig. 4A–D). Additionally, the linear regression analyses showed that the level of IL-6, but not IL-10, IL-17A and IFN-γ, was positively correlated with the Th17/Treg ratio (right panels of Fig. 7A–D). However, there was no significant correlation between the levels of IL-6, IL-10, IL-17A and IFN-γ and the Th1/Th2 ratio (left panels of Fig. 7A–D).
Supplementary Fig. 4.
The levels of IL-6, IL-10, IL-17A and IFN-γ were increased in the deceased patients compared with those in the surviving patients at admission. A, B, C and D: The plasma cytokine levels of IL-6, IL-10, IL-17A and IFN-γ were evaluated in the 42 SFTS patients (30 surviving and 12 deceased) at admission. The statistical analysis was performed using the Mann-Whitney U test. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001.
Fig. 7.
Correlation analysis of the levels of IL-6, IL-10, IL-17A and IFN-γ with the Th1/Th2 and Th17/Treg ratios.
Left panels of (A) - (D): Correlation analysis of the Th1/Th2 ratio with the levels of IL-6, IL-10, IL-17A and IFN-γ in the 42 SFTS patients (30 surviving and 12 deceased) at admission. Right panels of (A) - (D): Correlation analysis of the Th17/Treg ratio with the levels of IL-6, IL-10, IL-17A and IFN-γ in the 42 SFTS patients (30 surviving and 12 deceased) at admission. Statistical analysis was performed via a non-parametric Spearman correlation test. In the graphs, r and p indicate the correlation coefficient and the p-value of significance, respectively. The level of significance is indicated as follows: ns, not significant; *p < .05; **p < .01; ***p < .001; ****p < .0001.
4. Discussion
Although there have been several studies on immune cells and mediators in both humans and mice, the mechanisms by which SFTSV causes severe illness remain to be elucidated [[3], [4], [5], 7]. Our data indicated that the decreased percentage and number of CD4 T cells, and decreased numbers of Th1, Th2 and Tregs were associated with an increased severity of SFTS. Additionally, increased percentages of Th2, Th17 and Tregs in residual CD4 T cells, as well as skewing of Th17/Treg and Th2/Th1 ratios may cause poor outcomes in SFTS patients. Moreover, the Th17/Treg ratio was positively correlated with the level of IL-6.
Our data demonstrated that T lymphopenia—and CD4 T-cell depletion in particular— was commonly observed during the early course of SFTSV infection, especially in patients who die. This finding was consistent with the results of previous reports on SFTS viral infection [6, 8]. In addition, the percentage and number of CD4 T cells but not CD8 T cells were negatively correlated with the severity of SFTS. Previous studies have shown that the depletion of CD4 T cells (but not CD8 T cells) results in delayed pulmonary viral clearance, and CD4 T cells are required for the generation of optimal antibody responses following infection with coronavirus or vaccinia virus [25, 26]. Moreover, an absence of CD4 T cells has been shown to compromise the production of primary cytotoxic T lymphocyte (CTL) responses following infection with vaccinia virus and HSV [27, 28]. Overall, the dramatic loss of CD4 T cells in the deceased patients may limit the initiation and maintenance of effective humoral and cytotoxic T-cell immunity, leading to immunosuppression.
In line with the observed depletion of CD4 T cells, the numbers of Th1, Th2 and Tregs were also lower in the deceased patients compared with those in the surviving patients. Th1 cells play a significant role in antibacterial and antiviral immune defence and in protective humoral responses [10]. In addition, several studies have shown that adoptive transfer of either Th1 or Th2 cells efficiently boost the generation of neutralizing antiviral IgG responses [29, 30]. Accordingly, a separate study in SFTS patients suggests that patients with severe disease produce lower levels of IgG and IgM antibodies during early infection than patients with a mild form of the disease [7]. Although the percentage of Th1 and Th2 cells was increased in the acute phase and in patients who eventually died from the disease, decreased counts of Th1 and Th2 cells may impact the function of cellular and humoral immune responses, thus affecting viral removal. Additionally, Tregs have been shown to limit pathology during viral infection, including West Nile virus and respiratory syncytial viral infections [31, 32]. However, data from an observational clinical study [33] indicated that an increased Treg cell ratio in bronchoalveolar lavage fluid (BALF) obtained from acute respiratory distress syndrome (ARDS) patients at the time of admission is an independent risk factor for 30-day mortality. Additionally, a decreased number of Tregs in the deceased patients may decrease their function and thus limit the magnitude of the immune response against pathogens and control of inflammatory reactions. Interestingly, our dynamic data suggested that, unlike other CD4 T-cell subsets, the number of Th17 cells was higher during early infection in the deceased patients than that in the surviving patients. Previous studies have indicated that Th17cells up-regulate anti-apoptotic molecules and thus increase persistent infection by inhibiting the apoptosis of infected cells and desensitizing target cell killing by T effector cells [34]. Additional studies [35, 36] suggest that Th17 cells mediate tissue inflammation by supporting neutrophil recruitment and survival and by inducing the expression of proinflammatory cytokines. In a word, lower numbers of Th1, Th2 and Treg cells and relatively higher numbers of Th17 cells may affect the direct antiviral functions of these cells in providing assistance to B cells, regulating immunopathology and mediating the cytotoxic killing of virus-infected cells.
In addition to the observed CD4 T-cell depletion, the cellular microenvironment was altered in the deceased patients. Given the non-synchronous changes in the Th, Th2, Th17 and Treg cells, we used Th1/Th2 and Th17/Treg ratios to define the relationship between the inflammation status and the regulatory condition of the immune system. Our study demonstrated that in the peripheral blood, the Th1/Th2 ratio decreased and the Th17/Treg ratio increased in the deceased patients compared with those in the patients who survived. Previous studies have suggested that Th2 bias is related to the pathogenesis of respiratory syncytial virus (RSV) bronchiolitis and to infection severity in both humans and mice [18, 37]. However, no studies have shown that the Th1/Th2 ratio has the potential to predict death. In addition, several studies indicated that Th17/Tregs imbalance is significantly correlated with the severity of traumatic haemorrhagic shock and ARDS in patients [16, 38]. Furthermore, similar to previous studies that related to hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACHBLF) patients and ARDS patients [38, 39], an increased Treg/Th17 ratio was associated with the survival of SFTS patients. Moreover, our data showed that increased ratio of Th17/Tregs was associated with an increased level of IL-6. Previous studies [40, 41] suggest that the Th17/Treg imbalance that occurs in rheumatoid arthritis (RA) patients could be corrected by tocilizumab (TCZ)-mediated blockade of IL-6 signals, which is associated with improved clinical outcomes. Additionally, the Th17 response can be suppressed by Treg cells from patients who respond to treatment with adalimumab (a TNF-α inhibitor), via the control of monocyte-derived IL-6 production [42]. Given that decreases in CD4 T cells and Th1/Th2 ratio and increases in Th17/Tregs ratio were associated with disease severity, increasing the number of CD4 T cells and rectifying the Th2 and Th17 bias may help to maintain the immune system in a steady state, thus producing a therapeutic benefit.
To the best of our knowledge, this study provides the first kinetic data on the subsets of CD4 T cells during SFTSV infection. It is also the first study to emphasize that CD4 T-cell loss and Th2 and Th17 bias are significantly correlated with the severity of SFTS. Our data shed some light on the development of the disease and contribute to a better understanding of the mechanism underlying the progression of SFTS. These data may be of major significance for developing therapeutic strategies in the near future. Elucidating other potential factors leading to the decrease in CD4 T-cell numbers and to the Th2 and Th17 bias observed in deceased SFTS patients in the present study will require additional investigation.
The following are the supplementary data related to this article.
Supplementary Fig. 1.
(A): Gating strategy for the identification of CD4+ T cells and CD8+ T cells from peripheral blood samples.
Author contributions
All authors listed made a substantial, direct, and intellectual contribution to the work and approved it for publication. LMM, ZWJ, PC, and ZX had a substantial contribution to the conception and design of the project and its interpretation. LMY, ZYF, XY, XSE, ZCC, XLQ and LBY were responsible for the acquisition, analysis, and interpretation of the data, and LJ, LMJ and YDL guided the work. All authors contributed to revising of the manuscript and approved the final version.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank all of the donors and patients for participating in this research. This study was supported by the National Natural Science Foundation of China (81271884) and by Wuhan Union Hospital Faculty Research Funding, Huazhong University of Science and Technology (No. 000003385). The funding source played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Xiu-Fang Weng, Email: wengxiufang@hust.edu.cn.
Meng-ji Lu, Email: mengjilu@uni-due.de.
Dong-Liang Yang, Email: dlyang@hust.edu.cn.
Cheng Peng, Email: drpengcheng@hust.edu.cn.
Xin Zheng, Email: xin11@hotmail.com.
References
- 1.Yu X.J., Liang M.F., Zhang S.Y., Liu Y., Li J.D., Sun Y.L., Zhang L., Zhang Q.F., Popov V.L., Li C., Qu J., Li Q., Zhang Y.P., Hai R., Wu W., Wang Q., Zhan F.X., Wang X.J., Kan B., Wang S.W., Wan K.L., Jing H.Q., Lu J.X., Yin W.W., Zhou H., Guan X.H., Liu J.F., Bi Z.Q., Liu G.H., Ren J., Wang H., Zhao Z., Song J.D., He J.R., Wan T., Zhang J.S., Fu X.P., Sun L.N., Dong X.P., Feng Z.J., Yang W.Z., Hong T., Zhang Y., Walker D.H., Wang Y., Li D.X. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011;364(16):1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gai Z.T., Zhang Y., Liang M.F., Jin C., Zhang S., Zhu C.B., Li C., Li X.Y., Zhang Q.F., Bian P.F., Zhang L.H., Wang B., Zhou N., Liu J.X., Song X.G., Xu A., Bi Z.Q., Chen S.J., Li D.X. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J. Infect. Dis. 2012;206(7):1095–1102. doi: 10.1093/infdis/jis472. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W., Li M., Xiong S., Wang H., Xiong Y., Li M., Lu M., Yang D., Peng C., Zheng X. Decreased myeloid dendritic cells indicate a poor prognosis in patients with severe fever with thrombocytopenia syndrome. Int. J. Infect. Dis. 2017;54:113–120. doi: 10.1016/j.ijid.2016.11.418. [DOI] [PubMed] [Google Scholar]
- 4.Peng C., Wang H., Zhang W., Zheng X., Tong Q., Jie S. Decreased monocyte subsets and TLR4 mediated functions in patients with acute severe fever with thrombocytopenia syndrome (SFTS) Int. J. Infect. Dis. 2016;43:37–42. doi: 10.1016/j.ijid.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Jin C., Liang M., Ning J., Gu W., Jiang H., Wu W., Zhang F., Li C., Zhang Q., Zhu H., Chen T., Han Y., Zhang W., Zhang S., Wang Q., Sun L., Liu Q., Li J., Wang T., Wei Q., Wang S., Deng Y., Qin C., Li D. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc. Natl. Acad. Sci. U. S. A. 2012;109(25):10053–10058. doi: 10.1073/pnas.1120246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng Y., Chen N., Han Y., Xing Y., Li J. Clinical and laboratory characteristics of severe fever with thrombocytopenia syndrome in Chinese patients. Braz. J. Infect. Dis. 2014;18(1):88–91. doi: 10.1016/j.bjid.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Q.B., Cui N., Hu J.G., Chen W.W., Xu W., Li H., Zhang X.A., Ly H., Liu W., Cao W.C. Characterization of immunological responses in patients with severe fever with thrombocytopenia syndrome: a cohort study in China. Vaccine. 2015;33(10):1250–1255. doi: 10.1016/j.vaccine.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 8.Sun L., Hu Y., Niyonsaba A., Tong Q., Lu L., Li H., Jie S. Detection and evaluation of immunofunction of patients with severe fever with thrombocytopenia syndrome. Clin. Exp. Med. 2014;14(4):389–395. doi: 10.1007/s10238-013-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J., Wang L., Feng Z., Geng D., Sun Y., Yuan G. Dynamic changes of laboratory parameters and peripheral blood lymphocyte subsets in severe fever with thrombocytopenia syndrome patients. Int. J. Infect. Dis. 2017;58:45–51. doi: 10.1016/j.ijid.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Swain S.L., McKinstry K.K., Strutt T.M. Expanding roles for CD4(+) T cells in immunity to viruses. Nat. Rev. Immunol. 2012;12(2):136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowe C.R., Chen K., Pociask D.A., Alcorn J.F., Krivich C., Enelow R.I., Ross T.M., Witztum J.L., Kolls J.K. Critical role of IL-17RA in immunopathology of influenza infection. J. Immunol. 2009;183(8):5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8(5):337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 13.Veiga-Parga T., Sehrawat S., Rouse B.T. Role of regulatory T cells during virus infection. Immunol. Rev. 2013;255(1):182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy K.M., Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat. Immunol. 2010;11(8):674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Shea J.J., Paul W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta D.L., Bhoi S., Mohan T., Galwnkar S., Rao D.N. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis. Cytokine. 2016;88:214–221. doi: 10.1016/j.cyto.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Noack M., Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014;13(6):668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Pinto R.A., Arredondo S.M., Bono M.R., Gaggero A.A., Diaz P.V. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics. 2006;117(5):e878–e886. doi: 10.1542/peds.2005-2119. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Qiu L., Wang Y., Aimurola H., Zhao Y., Li S., Xu Z. The circulating Treg/Th17 cell ratio is correlated with relapse and treatment response in pulmonary sarcoidosis patients after corticosteroid withdrawal. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arroyo Hornero R., Betts G.J., Sawitzki B., Vogt K., Harden P.N., Wood K.J. CD45RA distinguishes CD4+CD25+CD127−/low TSDR demethylated regulatory T cell subpopulations with differential stability and susceptibility to tacrolimus-mediated inhibition of suppression. Transplantation. 2017;101(2):302–309. doi: 10.1097/TP.0000000000001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong S., Zhang W., Li M., Xiong Y., Li M., Wang H., Yang D., Peng C., Zheng X. A simple and practical score model for predicting the mortality of severe fever with thrombocytopenia syndrome patients. Medicine (Baltimore) 2016;95(52) doi: 10.1097/MD.0000000000005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y.Z., He Y.W., Dai Y.A., Xiong Y., Zheng H., Zhou D.J., Li J., Sun Q., Luo X.L., Cheng Y.L., Qin X.C., Tian J.H., Chen X.P., Yu B., Jin D., Guo W.P., Li W., Wang W., Peng J.S., Zhang G.B., Zhang S., Chen X.M., Wang Y., Li M.H., Li Z., Lu S., Ye C., de Jong M.D., Xu J. Hemorrhagic fever caused by a novel Bunyavirus in China: pathogenesis and correlates of fatal outcome. Clin. Infect. Dis. 2012;54(4):527–533. doi: 10.1093/cid/cir804. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y., Jin C., Zhan F., Wang X., Liang M., Zhang Q., Ding S., Guan X., Huo X., Li C., Qu J., Wang Q., Zhang S., Zhang Y., Wang S., Xu A., Bi Z., Li D. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J. Infect. Dis. 2012;206(7):1085–1094. doi: 10.1093/infdis/jis452. [DOI] [PubMed] [Google Scholar]
- 24.Li J., Han Y., Xing Y., Li S., Kong L., Zhang Y., Zhang L., Liu N., Wang Q., Wang S., Lu S., Huang Z. Concurrent measurement of dynamic changes in viral load, serum enzymes, T cell subsets, and cytokines in patients with severe fever with thrombocytopenia syndrome. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010;84(3):1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sette A., Moutaftsi M., Moyron-Quiroz J., McCausland M.M., Davies D.H., Johnston R.J., Peters B., Rafii-El-Idrissi Benhnia M., Hoffmann J., Su H.P., Singh K., Garboczi D.N., Head S., Grey H., Felgner P.L., Crotty S. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008;28(6):847–858. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novy P., Quigley M., Huang X.P., Yang Y.P. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J. Immunol. 2007;179(12):8243–8251. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- 28.Smith C.M., Wilson N.S., Waithman J., Villadangos J.A., Carbone F.R., Heath W.R., Belz G.T. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat. Immunol. 2004;5(11):1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 29.Maloy K.J., Burkhart C., Junt T.M., Odermatt B., Oxenius A., Piali L., Zinkernagel R.M., Hengartner H. CD4(+) T cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability. J. Exp. Med. 2000;191(12):2159–2170. doi: 10.1084/jem.191.12.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahon B.P., Katrak K., Nomoto A., Macadam A.J., Minor P.D., Mills K.H. Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J. Exp. Med. 1995;181(4):1285–1292. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanteri M.C., O'Brien K.M., Purtha W.E., Cameron M.J., Lund J.M., Owen R.E., Heitman J.W., Custer B., Hirschkorn D.F., Tobler L.H., Kiely N., Prince H.E., Ndhlovu L.C., Nixon D.F., Kamel H.T., Kelvin D.J., Busch M.P., Rudensky A.Y., Diamond M.S., Norris P.J. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J. Clin. Invest. 2009;119(11):3266–3277. doi: 10.1172/JCI39387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee D.C., Harker J.A., Tregoning J.S., Atabani S.F., Johansson C., Schwarze J., Openshaw P.J. CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J. Virol. 2010;84(17):8790–8798. doi: 10.1128/JVI.00796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adamzik M., Broll J., Steinmann J., Westendorf A.M., Rehfeld I., Kreissig C., Peters J. An increased alveolar CD4 + CD25 + Foxp3 + T-regulatory cell ratio in acute respiratory distress syndrome is associated with increased 30-day mortality. Intensive Care Med. 2013;39(10):1743–1751. doi: 10.1007/s00134-013-3036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou W., Kang H.S., Kim B.S. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 2009;206(2):313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettelli E., Oukka M., Kuchroo V.K. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8(4):345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 36.Wu W., Li J., Chen F., Zhu H., Peng G., Chen Z. Circulating Th17 cells frequency is associated with the disease progression in HBV infected patients. J. Gastroenterol. Hepatol. 2010;25(4):750–757. doi: 10.1111/j.1440-1746.2009.06154.x. [DOI] [PubMed] [Google Scholar]
- 37.Christiaansen A.F., Knudson C.J., Weiss K.A., Varga S.M. The CD4 T cell response to respiratory syncytial virus infection. Immunol. Res. 2014;59(1–3):109–117. doi: 10.1007/s12026-014-8540-1. [DOI] [PubMed] [Google Scholar]
- 38.Yu Z.X., Ji M.S., Yan J., Cai Y., Liu J., Yang H.F., Li Y., Jin Z.C., Zheng J.X. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit. Care. 2015;19:82. doi: 10.1186/s13054-015-0811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang X.S., Li C.Z., Zhou Y., Yin W., Liu Y.Y., Fan W.H. Changes in circulating Foxp3(+) regulatory T cells and interleukin-17-producing T helper cells during HBV-related acute-on-chronic liver failure. World J. Gastroenterol. 2014;20(26):8558–8571. doi: 10.3748/wjg.v20.i26.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samson M., Audia S., Janikashvili N., Ciudad M., Trad M., Fraszczak J., Ornetti P., Maillefert J.F., Miossec P., Bonnotte B. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64(8):2499–2503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
- 41.Schinnerling K., Aguillon J.C., Catalan D., Soto L. The role of interleukin-6 signalling and its therapeutic blockage in skewing the T cell balance in rheumatoid arthritis. Clin. Exp. Immunol. 2017;189(1):12–20. doi: 10.1111/cei.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGovern J.L., Nguyen D.X., Notley C.A., Mauri C., Isenberg D.A., Ehrenstein M.R. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 2012;64(10):3129–3138. doi: 10.1002/art.34565. [DOI] [PubMed] [Google Scholar]