Abstract
Despite efforts in prevention worldwide including recent advances in vaccine therapy, childhood community acquired pneumonia (CAP) remains a major cause of morbidity and mortality both in the developed and the developing world.
Traditionally, qualifying the aetiology of CAP proved to be fraught with challenges particularly due to low yields from blood and sputum specimens. In recent years however, new advances in techniques such as enzyme-linked immunosorbent assay and polymerase chain reaction have dramatically improved detection rates of both bacteria and viruses. In addition to qualifying the true burden of disease by known organisms such as Streptococcus pneumoniae it has led to the identification of organisms such as human bocavirus which have not previously been associated with CAP.
This article aims to provide a brief update to the clinician on the current epidemiology of CAP in this post-vaccination era. It is based on a combination of recommendations from existing clinical practice guidelines, recent systematic reviews and the current literature.
Keywords: children, community acquired pneumonia, epidemiology, guidelines, infection, prevalence
Definition
Childhood community acquired pneumonia (CAP) can be defined as an infection affecting an individual under the age of 16 years whose lungs are inflicted by a spectrum of pathogens acquired outside a hospital setting. This subsequently results in inflammation of the affected lung tissue. Clinically, it can be defined as the presence of signs and symptoms of pneumonia such as fever, tachypnoea and cough in a previously well child. In young children these symptoms may be non-specific.
Currently in spite of the limitations in its accuracy, chest X-rays remain the mainstay for diagnosis of childhood CAP. Radiographic findings of consolidation are typically used to verify the diagnosis in developed countries. This is not absolute, as good clinical assessment may precipitate empirical treatment for CAP with no further investigations. In the developing world, with difficulties in obtaining an X-ray, more emphasis is placed on clinical assessment.
Whilst the inclusion of a responsible pathogen is ideal in the definition of CAP, current techniques have yet to obtain sufficient sensitivity to detect all relevant organisms. Consequently, a significant proportion of cases have no pathogen identified, findings mirrored in many prospective epidemiological studies involving numerous specimen types such as blood and sputum. This raises assumptions and poses challenges on adopting an evidence-based approach to managing CAP, in particular on appropriate antibiotic and/or antiviral use. Resultantly, measurements such as blood white cell count and C - reactive protein are utilized as proxies to guide us on the nature and severity of the infection. Whilst these are regarded as useful measurements they do not necessarily allow us to direct our treatment towards specific pathogens. This commonly results in viral pneumonia being treated unnecessarily with antibiotics. This stratifies a clear need for better diagnostic methods.
Background
In spite of recent advances in vaccination therapies, environmental modification and treatment of underlying diseases that have been promoted extensively over the past decade, childhood CAP continues to be attributable to significant morbidity and mortality worldwide. CAP affects up to 450 million people a year in all regions of the world. Disproportionally, childhood CAP affects up to 156 million children per year and leads up to 4 million deaths per year.
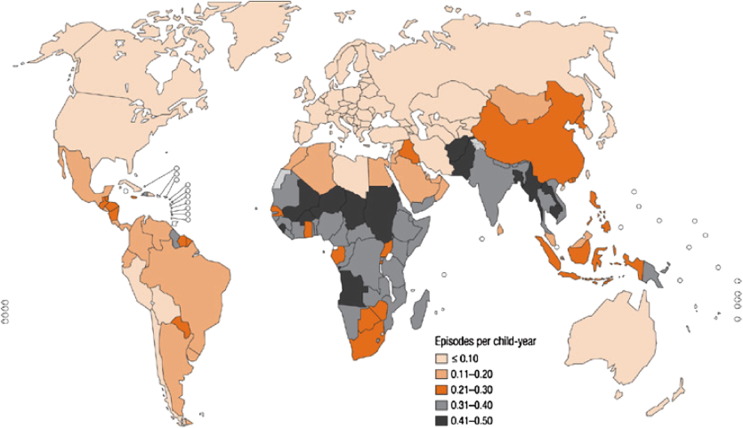
CAP has continued to be estimated as the leading single cause of childhood mortality in the world with recent data suggesting that the number of children with severe CAP is increasing worldwide. Difficulties continue to persist in producing evidence-based estimates, due in part to the absence of a simple investigation that has full accuracy to identify all children with CAP, variances in CAP case definition, the low specificity of verbal autopsies in CAP, similarity in clinical picture between pneumonia and malaria, difficulties in differentiation between CAP and neonatal sepsis and diagnosis based on clinical assessment and no verification by chest X-rays (Figure 1 ).
Figure 1.
Incidence of childhood clinical pneumonia at the country level.
Reproduced with permission from Rudan, I. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ.
Whilst no country is spared from the morbidity and mortality of CAP, disproportionally 95% of these episodes occur in developing countries (Figure 1). It remains the leading cause of death amongst children in low-income countries with just 15 countries of the world accounting for 74% (115.3 million episodes) of these episodes. Currently, more than half of the world's annual new CAP cases are concentrated in India (43 million), China (21 million), Pakistan (10 million), Bangladesh, Indonesia and Nigeria (6 million each).
CAP is attributable to a combination of exposure to risk factors related to the host, the environment and the resulting infection (Table 1 ). Some risk factors such as parental smoking are common to both developed and developing countries whilst others such as malnutrition and lack of immunizations which are specific to developing countries have exacerbated the disparity in the worldwide burden of CAP.
Table 1.
Risk factors related to the host and the environment that affects incidence of childhood clinical pneumonia in the community
| Definite | Malnutrition Low-birth-weight Non-exclusive breastfeeding (during the first 4 months of life) Lack of measles immunization (within the first 12 months of life) Indoor air pollution Crowding |
| Likely | Parental smoking Zinc deficiency Mother's experience as a caregiverConcomitant diseases (e.g. diarrhoea, heart disease, asthma) |
| Possible | Mother's education Day-care attendance Rainfall (humidity) High altitude (cold air) Vitamin A deficiency Birth order Outdoor air pollution |
Reproduced with permission from Rudan, I. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ.
Though the incidence of CAP varies widely amongst developing countries, developed regions such as North America and Europe share similar incidence figures. For example, the annual incidence of CAP in children under 5 years of age in North America is 34–40 cases per 1000 per year. This strongly correlates with the incidence in Europe, where in Finland the incidence is 36 cases per 1000 children per year. In the United Kingdom (UK), the incidence figures are lower at 1.44 cases per 1000 per year in children over 1 year of age. Whilst our incidence may seem small in comparison to Europe and North America, note that these rates are derived from a regional hospital-based audit excluding children under 1 year of age and those treated in the community where a significant proportion of cases may lie but where data is more difficult to collect.
CAP continues to be seen commonly in children younger than 5 years of age. To illustrate, in Finland the incidence falls from 36 to 16.2 cases per 1000 per year in children older than 5 years of age. Worldwide, CAP is attributable to 28–34% of all deaths in children in this age group. The World Health Organization estimates that one in three newborn infant deaths occur due to pneumonia. Alongside this, other risk factors for long term morbidity and mortality from CAP include prematurity, low-birth-weight, immunocompromised children and those known to have underlying conditions such as cystic fibrosis, congenital heart disease and asthma. Incidentally, previous upper respiratory tract infections (URTIs) are also independently associated with childhood CAP with a strong correlation of an increasing number of URTIs leading to a rise in CAP episodes, possibly due to higher infection susceptibility.
Aetiology
CAP is caused by a wide variety of organisms both bacterial and viral. Due to the absence of accurate, fast and widely available affordable diagnostic tools, the identification of the causative organism at presentation that would allow for effective direct treatment remains difficult.
Epidemiological studies conducted to delineate the aetiology of CAP can be segregated into two study forms. Traditionally these were prospective hospital-based studies that have relied on blood cultures and occasionally percutaneous lung aspiration and bronchoalveolar lavage (BAL). This approach lacks sensitivity due in part to the low yield of blood cultures, difficulty in obtaining adequate sputum samples and reluctance in performing lung aspiration and BAL in children.
Furthermore, even with rigorously conducted studies, the generalizability of these findings are questioned due to numerous confounders such as seasonality, setting, age of children, local criteria for hospital admission and whether it coincided with a particular epidemic which are difficult to control. These type of studies have not been discontinued as in recent years the use of newer methods and improvement of previous techniques such as indirect immunofluorescence, enzyme-linked immunosorbent assay, polymerase chain reaction (PCR), examination of nasopharyngeal specimens or by serology in paired samples have improved respiratory viral identification and pneumococcal detection in blood.
With the advances in vaccine therapy, estimation studies involving vaccines are now more commonplace. Here, the number of CAP cases prevented by a specific vaccine is presumed to be a minimum estimate of the prevalence of CAP due to the organism against which the vaccine is directed.
Causative organisms vary according to a child's age. Viruses are the commonest in children under 5 years old with bacterial causes being reported as being more common in older children. A significant number of cases of CAP continue to represent a mixed infection with comprehensive studies finding a mixed viral-bacterial infection in 23–33% of cases (Table 2 ).
Table 2.
Prospective studies of specific pathogens from developed countries
| Reference [evidence level] | Age | Year and setting | Tests | Total episodes | Viral (n) | Bacteria, % (n) | Mycoplasma, % (n) | Chlamydia, % (n) | Mixed, % (n) | Total diagnosed, % (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Wolf [Ib] | <5 years | ED | NPA hMPV PCR; NPIA | 1296 | RSV 23.1 hMPV 8.3 Adeno 3.4 Infl A 2.9 PIV 2.9 | |||||
| Cilla [Ib] | 1–35 months | 2004–6, Spain, IP+OP | NPIA + PCR, BC, serology, Binax pleural fluid | 338 | 67 (18 viral co-infection) RSV 19.8 HboV 14.2 RV 13.6 HMPV 11.5 Corona 6.5 | Spn 2.1 (7) | 1.8 (6) | ∗ | NA | NA |
| Haman [II] | 0–19 years | 2005–6, Japan | NPA PCR | 1700 | 27.9 (2.1% multiple) RV 14.5 RSV 9.4 hMPV 7.2 HboV 2.9 | a | 14.8 (251) | 1.4 (24) | 15.2 | NAa |
| Don [II] | 0.3–16 years | 2001–2, Italy, IP+OP | Serology (viral and bacterial) | 101 | 42 (3 dual) RSV 17 PIV 12 Infl 9 hMPV 5 | 44 Spn18 HI 3 Mcat 1 | 26.7 (27)<2 years: 12–5 years: 8>5 years: 18p<0.0001 | 7.9 (8) | 20 | 65 (66) |
| Lin [II] | 3 months–18 years | 2001–2, Taiwan, IP | NPIA, NPVC; hMPV PCR; BC; urine Spn ag; serology MP+CP | 116 | 38.8 (45) RSV 28.9 Adeno 28.9 hMPV 13.3 Infl 13.3 | a | 37.9 (44) | 4.3 (5) | NA | NAa |
| Michelow [Ib] | 6 weeks–18 years | 1999–2000, USA, IP | NPIA, NPVC; Spn BPCR; BC; serology viral, Spn, MP, CP | 154 | 45 (65) RSV 13 Infl 22 PIV 13 Adeno 7 | 60 (93)Spn 44 (68) GAS 1 (2) SA 1 (2) | 14 (21) | 9 (14) | 23 | 79 (122) |
| Macherel[Ib] | 2 months–5 years | 2003–5, Switzerland: IP | NPIA + PCR; Spn BPCR; BC; serology viral, Spn, MP, CP; | 99 | 67 RV 20h MPV 13 RSV 13 Infl 14 Paraflu 13 Adeno 7 Corona 7 | 53 (52)Spn 46 (45)GAS 1 (1) | 11 | 7 | 33 (33) | 86 (85) |
| Drummond[II] | 0–16 years | 1996–8, UK, IP | NPIA; NPVC; serology viral, Spn, MP, CP; urine Spn ag; | 136 | 37 (50) RSV 25 Infl A 5 CMV 3 Adeno 1.4 | 12.5 (17) GAS 7 (9) Spn 4 (5) | 2 (3) | 11 (15) | 51 (70) | |
| Laundy[II] | 0–5 years | 2001–2, UK, IP+OP | NPIA+PCR;BC; specifically viral testing | 51 | 43 (22) RSV 18 (9) Infl A 16 (8) Adeno 6 (3) PIV 6 (3) | 12 (6) Spn 6 | 4 (2) | NA | NA | 49 (25) |
| Tsolia[Ib] | 5–14 years | 2004, Greece, IP | NPA PCR; serology MP, CP, Spn, HI, Mcat; | 75 | 65 (49) RV 45 (34) Adeno 12 (9) PIV 8 (6) Infl 7 (5) RSV 3 (2) hMPV 1 (1) | 40 (30)Spn 7 (5) | 35 (26) | 3 (2) | 28 (21) | 77 (58) |
| Macherel[Ib] | 2 months–5 years | 2003–5, Switzerland: IP | NPIA + PCR; Spn BPCR; BC; serology viral, Spn, MP, CP; | 99 | 67 RV 20h MPV 13 RSV 13 Infl 14 Paraflu 13 Adeno 7 Corona 7 | 53 (52)Spn 46 (45)GAS 1 (1) | 11 | 7 | 33 (33) | 86 (85) |
| Drummond[II] | 0–16 years | 1996–8, UK, IP | NPIA; NPVC; serology viral, Spn, MP, CP; urine Spn ag; | 136 | 37 (50) RSV 25 Infl A 5 CMV 3 Adeno 1.4 | 12.5 (17)GAS 7 (9) Spn 4 (5) | 2 (3) | 11 (15) | 51 (70) | |
| Laundy[II] | 0–5 years | 2001–2, UK, IP+OP | NPIA+PCR;BC; specifically viral testing | 51 | 43 (22) RSV 18 (9)Infl A 16 (8) Adeno 6 (3) PIV 6 (3) | 12 (6) Spn 6 | 4 (2) | NA | NA | 49 (25) |
| Tsolia[Ib] | 5–14 years | 2004, Greece, IP | NPA PCR; serology MP, CP, Spn, HI, Mcat; | 75 | 65 (49) RV 45 (34) Adeno 12 (9) PIV 8 (6) Infl 7 (5) RSV 3 (2) hMPV 1 (1) | 40 (30) Spn 7 (5) | 35 (26) | 3 (2) | 28 (21) | 77 (58) |
Reproduced with permission from the British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011.Case definitions include clinical findings compatible with CAP together with radiological changes. In the columns the percentage indicates the percentage of all CAP cases in which that organism was detected. Where both viral and bacterial isolates were detected, it was classified as mixed and indicated in a separate column. In some studies it was not possible to determine whether infections were single or mixed (as indicated). Bacterial isolates are not included if isolated from a sputum or upper respiratory tract specimen in the absence of other evidence of significance for example, a rise in antibody concentrations.
No serological tests were performed for Chlamydia pneumonia.
∗ Adeno, adenovirus; ag, antigen; BC, blood culture; BPCR, blood PCR; Corona, coronavirus; CP, Chlamydia pneumoniae; ED, emergency department; GAS, group A streptococcus; HboV, human bocavirus; HI, Haemophilus influenzae; hMPV, human metapneumovirus; Infl, influenza A and B virus; IP, inpatients; Mcat, Moraxella catarrhalis; MP, mycoplasma; NA, not available; NPA PCR, nasopharyngeal PCR; NPIA, nasopharyngeal immunoassay; NPVC, nasopharyngeal viral culture; OP, outpatients; PIV, parainfluenza virus 1–3; PC, pharyngeal culture; RSV, respiratory syncytial virus; RV, rhinovirus; Spn, Streptococcus pneumoniae.
All bacterial cases identified by NPA PCR so difficult to distinguish carriage from pathogen.
Viruses
Viruses remain the commonest cause of CAP in young children. Overall viruses account for 30–67% of cases and are most frequent in children under 1 year of age. In a recent vaccine probe study, only 6% of children under 2 years of age with a clinical diagnosis of CAP had Pneumococcus identified.
Respiratory syncytial virus (RSV): RSV remains the leading viral cause, being identified in 15–40% of CAP or bronchiolitis cases admitted to hospital in developing countries. This is in spite of difficulties in detection due to its fragility. It is found in significant frequency in all climatic and geographical areas, with sharp peaks of activity over a period of 2–4 months. It seasonality varies considerably between regions. Peaks typically occur in the winter season in temperate climates and in the rainy season in tropical climates.
A primary respiratory infection caused by RSV is attributable to increased risk of a secondary bacterial infection with viral or bacterial co-infection a common finding in approximately 20–30% of childhood CAP episodes. Additionally episodes of wheezing due to reactive airways are commonly seen. The risk of CAP or bronchiolitis caused by RSV is highest among children aged less than 2 years with the majority of episodes occurring in the first year of life, more commonly in boys. The most severe disease occurs in infants aged 3 weeks to 3 months.
In spite of new vaccine development, RSV continues to be a very significant and potentially deadly pathogen that causes childhood CAP, both alone and through mixed infections with bacterial causes.
Influenza A: followed closely by RSV in causing significant morbidity and mortality in childhood CAP is Influenza A. It has raised to the forefront of epidemiological indices partly due to improved detection rates. Previously it was detected relatively infrequently in paediatric pneumonia using immunofluorescence. With viral PCR, the diagnostic yield of the virus has been doubled. It is causative in more than one-third of all viral CAP cases, a rate comparable with that of RSV CAP. In a prospective epidemiological study in the UK during a 6-month winter influenza season, 16% of children diagnosed with CAP were noted to have influenza A. Measured by the duration of fever, hospital stay and total duration of illness; the clinical burden of influenza A CAP is comparable with that of RSV CAP.
Human immunodeficiency virus (HIV): over the past decade, the HIV epidemic has been partly to blame in increasing the incidence and mortality from childhood CAP with bacterial super-infections the mainstay cause of childhood mortality. In comparison to uninfected children pathogens such as Pneumocystis jiroveci and Mycoplasma tuberculosis remain important causes of childhood CAP.
Though routine vaccinations provide lower efficacy in HIV infected children, they are still able to mount a sufficient immune response to protect a substantial proportion of CAP caused by those specific pathogens. Antiretroviral treatment and use of co-trimoxazole prophylaxis have been further noted to reduce the incidence and severity of HIV-associated CAP.
Other viruses: increasingly more common are the newly identified viruses such as human metapneumovirus and human bocavirus which are found in 8–12% and approximately 5% of CAP cases respectively. Other viruses isolated in children with diagnosed with CAP include adenovirus, rhinovirus, varicella zoster virus, cytomegalovirus, herpes simplex virus and enteroviruses. It will be some time before any of these causes are preventable by routine immunization.
Bacteria
Streptococcus pneumoniae (S. pneumoniae): S pneumoniae is a major cause of mortality and morbidity in both developing and industrialized countries, especially among young children and in both immunocompromised and immunocompetent individuals. It is implicated in both invasive (e.g. meningococcal disease and septicaemia) as well as noninvasive disease (CAP and otitis media).
The burden of pneumococcal disease is considerable, with incidence rates of both invasive and non-invasive disease peaking in children under 5 years of age. In a recent systematic review and meta analyses, S. pneumoniae was the most frequently isolated agent (11.08%; 95% confidence interval (CI) 7.63–15.08) with pneumococcal serotype 14 being the most prevalent (33.00%; 95% CI 25.95–40.45). This has been confirmed in numerous prospective, microbiology-based studies, with pneumococcus identified in 30–50% of CAP cases diagnosed.
Prior to the introduction of the pneumococcal vaccine the annual incidence of childhood CAP was 33 per 10 000 children aged 0–5 years and 14.5 per 10,000 children aged 0–16 years evidenced from remarkably consistent prospective studies both in Norway and the UK. With the introduction of these protein-polysaccharide vaccines, the burden of disease caused by S. pneumoniae have fallen dramatically. This falls have also applied in other encapsulated bacteriae such as Haemophilus influenzae, Neisseria meningitidis and Salmonella Typhi.
In the UK where infant vaccination with the seven-valent pneumococcal conjugate vaccination (PCV 7) started in 2007, a national time trends study has shown a 19% decrease in admission rates between 2006 and 2008. In countries such as the United States of America (USA) where PCV 7 has been available for longer, a decrease in hospital admissions of approximately 30% have been reported. With the introduction of the thirteen-valent pneumococcal conjugate vaccine (PCV 13) worldwide over the coming years the clinical suspicion of bacterial CAP in a fully vaccinated child is likely to fall further.
With the advent of the pneumococcal vaccine, there have been several equivocal findings. Whilst the incidence of invasive pneumococcal disease including bacterial CAP has declined changes in noninvasive pulmonary disease have not shown a similar decline. Furthermore rates of local complications associated with CAP including the prevalence of empyema have been surprisingly increasing in all age groups. This has been hypothesized to be due to changes in circulating pneumococcal strains which may be producing differential changes in disease incidence and disease presentation in several populations.
Haemophilus influenzae (H. Influenzae): H. influenzae type B (HiB) currently remains the second most common bacterial organism responsible for CAP. Numerous studies, particularly blood culture based have isolated the organism in up to 30% of childhood CAP cases. Controversy surrounds the role of non-typable H. Influenzae (NTHI) with several studies in Pakistan and Papua New Guinea noting it as a common blood culture or lung aspirate. Of note, these findings have yet to replicated elsewhere.
Staphylococcus aureus (S. Aureus): after S. pneumoniae and H. influenzae, S. aureus is the third most common bacterial organism isolated in CAP. In the USA, S. aureus has increased in frequency over the past decade, many of which were attributable to community acquired Methicillin-resistant S. Aureus (MRSA) and USA300 isolates. In approximately 15% of these cases, viral co-infection was attributed to causing respiratory failure.
Worldwide, in a recently completed WHO study of severe CAP, S. aureus was identified in 47 of the 112 cases (42% of cases), making it the second largest cause in the study. Furthermore, in an epidemiological study involving lung aspiration in Chile utilizing a sample size of over 500 children including normal controls, S. aureus was identified as the main pathogen.
Others: other organisms, such as Salmonella spp. Mycoplasma pneumoniae, Mycobacterium tuberculosis, Chlamydia spp., Pseudomonas spp., Escherichia coli, and measles, varicella, influenza, histoplasmosis and toxoplasmosis, also cause CAP.
Whilst infections from many of these organisms are yet to be preventable, worldwide immunization efforts against measles, influenza, bacille Calmette–Guérin (BCG) and now pneumococcus have been associated in contributing to the decline of the worldwide CAP burden.
The causes of neonatal pneumonia in developing countries have yet to be studied extensively, however several studies of neonatal sepsis have suggested that these include Gram-negative enteric organisms in particular Klebsiella spp and Gram-positive organisms such as pneumococcus, Group B Streptococcus and S. aureus.
Assessment and management
The investigation and management of CAP is beyond the scope of this article and readers should refer to the current BTS guideline on the management of childhood CAP for further reading. As a summary however, we've aimed to highlight several key learning points as an important preclude for further reading.
Clinical features
Though clinical features of CAP are not specific for aetiology, there are several key features which increase the clinical suspicion that a child is presenting with CAP. These are listed out by the National Institute for Health and Clinical Excellence (NICE) in the table above (Table 3 ).
Table 3.
Clinical features of pneumonia
|
Investigation
In a recent Cochrane review, it is suggested that investigation of all children with clinical features of CAP is unnecessary and treatment may be started empirically based on medical assessment.
Whilst chest X-ray findings do not help in specifying aetiology, the World Health Organisation has produced a method for standardizing the interpretation of chest radiographs in children. Previously, sequential rise in acute phase reactants has been suspected as a means of differentiating aetiology and/or severity of CAP, however new evidence have noted that they are not of clinical utility in distinguishing viral from bacterial infections and should not utilized as a routine test.
Management
Currently, evidence states that fully vaccinated children less than 2 years old presenting with mild symptoms of LRTI do not necessarily need to be treated with antibiotics however they should be reviewed if symptoms persist.
As bacterial and viral pneumonia may not be reliably distinguished on clinical features, all children with a clear clinical diagnosis of CAP should receive antibiotics. Oral amoxicillin remains the antibiotic of choice as it is effective, well tolerated and cheap.
Current statistics note that approximately one in four children with CAP require hospitalization. Whilst it has been noted that there is a high rate of intravenous antibiotic administration in children admitted to hospital with CAP, oral amoxicillin is the antibiotic of choice and should be administered as first line treatment. Intravenous antibiotics should be reserved for children who are unable to absorb oral medication or those presenting with complicated CAP or septicaemia.
Evidence also notes that chest physiotherapy although still practiced in the United Kingdom, is of no benefit and should not be performed in children with CAP. Simple oral antibiotics and supportive care are likely to be effective for the majority of children with CAP.
Conclusion
Approximately 150 million new episodes of childhood CAP occurred globally per year of which more than 95% of them occurred in developing countries. In spite of advances undertaken to reach the Millenium Development Goals, approximately 2 million deaths from CAP occur each year in children aged less than 5 years of age, mainly in the African and South-East Asia Regions.
Whilst evidence based estimates of the importance of causative organisms vary in different settings the main causes of CAP worldwide are RSV, S. pneumonia and HiB. Future studies, with further advances in current techniques and development of better methods to detect infections from a wider range of pathogens, will broaden our understanding of the cause of CAP further elucidating new vaccine development.
Practice points.
-
•
Community Acquired Pneumonia remains the major cause of morbidity and mortality in the developed and developing world.
-
•
Newer techniques have aided in qualifying the true burden of CAP aetiology
-
•
Viruses remain the commonest cause
-
•
Commonest viruses implicated are RSV and Influenza A whilst the commonest bacteria implicated are S. pneumoniae, H. influenzae and S. aureus
-
•
In the absence of isolation of a causative organism, oral amoxicillin is the antibiotic of choice
Further reading
- 1.Drummond P., Clark J., Wheeler J. Community acquired pneumonia – a prospective UK study. Arch Dis Child. 2000;83:408–412. doi: 10.1136/adc.83.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garenne M., Ronsmans C., Campbell H. The magnitude of mortality from acute respiratory infections in children under 5 years in developing countries. World Health Stat Q. 1992;45:180–191. [PubMed] [Google Scholar]
- 3.Gentile A., Bardach A., Ciapponi A. Epidemiology of community-acquired pneumonia in children of Latin America and the Caribbean: a systematic review and meta-analysis. Int J Infect Dis. 2012;16:5–15. doi: 10.1016/j.ijid.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Harris M., Clark J., Coote N. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66:927–928. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 5.Howidi M., Muhsin H., Rajah J. The burden of pneumococcal disease in children less than 5 years of age in Abu Dhabi, United Arab Emirates. Ann Saudi Med. 2011;31:356–359. doi: 10.4103/0256-4947.83214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabra S.K., Lodha R., Pandey R.M. Antibiotics for community-acquired pneumonia in children. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD004874.pub2. Issue 3. Art. No.:CD004874. [DOI] [PubMed] [Google Scholar]
- 7.Koshy E., Murray J., Bottle A., Sharland M., Saxena S. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997–2008. Thorax. 2011;65:770–774. doi: 10.1136/thx.2010.137802. [DOI] [PubMed] [Google Scholar]
- 8.Lakhanpaul M., Atkinson M., Stephenson T. Community acquired pneumonia in children: a clinical update. Arch Dis Child Educ Pract Ed. 2004;89:29–34. [Google Scholar]
- 9.Laundy M., Ajayi-Obe E., Hawrami K., Aitken C., Breuer J., Booy R. Influenza A community-acquired pneumonia in East London infants and young children. Pediatr Infect Dis J. 2003;22:S223–S227. doi: 10.1097/01.inf.0000092192.59459.8b. [DOI] [PubMed] [Google Scholar]
- 10.Lee G.E., Lorch S.A., Sheffler-Collins S., Kronman M.P., Shah S.S. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126:204–213. doi: 10.1542/peds.2009-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lexau C.A. The changing epidemiology of pneumococcal pulmonary disease in the era of the heptavalent vaccine. Curr Infect Dis Rep. 2008;10:229–235. doi: 10.1007/s11908-008-0038-3. [DOI] [PubMed] [Google Scholar]
- 12.NHS National Institute for Health and Clinical Excellence. Feverish illness in children. Available from: http://www.nice.org.uk/CG047 (accessed on 18 March 2012).
- 13.Rudan I., Tomaskovic L., Boschi-Pinto C., Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 14.Rudan I., Boschi-Pinto C., Biloglav Z., Mulholland K., Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:321–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teepe J., Grigoryan L., Verheij T.J. Determinants of community-acquired pneumonia in children and young adults in primary care. Eur Respir J. 2010;35:1113–1117. doi: 10.1183/09031936.00101509. [DOI] [PubMed] [Google Scholar]
- 17.United Nations. United Nations Millennium Development Goals. Available from: http://www.un.org/millenniumgoals/ (accessed on 18 March 2012).
- 18.Weber M.W., Mulholland K.E., Greenwood B.M. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Pneumonia. Available from: http://www.who.int/mediacentre/factsheets/fs331/en/index.html (accessed on 18 March 2012).