Abstract
Two studies were conducted in the Eimeria zuernii infection model in order to investigate the pathology of E. zuernii coccidiosis and the efficacy of toltrazuril (Baycox 5% suspension) in this infection. For this purpose, a total of 30 calves were infected experimentally with E. zuernii oocysts and faecal samples taken regularly from the rectum and examined for faecal consistency and oocyst excretion. Six of the calves underwent pathological examination at various points in time after infection. Significant macroscopic and microscopic changes were demonstrated and parasitic stages were identified in the intestinal mucosa of infected calves during the late prepatent and patent period. Inflammatory reactions revealed by light microscopy were confirmed by electron microscopical investigations. Treatment of calves with toltrazuril during the late prepatent period resulted in significantly lower frequencies of diarrhoea and levels of oocyst excretion, and weight gain was significantly higher than in shamtreated animals.
Keywords: Eimeria zuernii, Coccidiosis, Cattle, Treatment, Toltrazuril
1. Introduction
Eimeria zuernii is an important causal agent of clinical coccidiosis in housed calves. This disease is associated with massive diarrhoea and reduced weight gain [1], [2], [3] and is thus also of great economic importance. E. zuernii is characterised by a prepatent period of about 16–17 days [4]. This is followed by the patent period with oocyst excretion and clinical symptoms.
The purpose of these studies was to characterise experimental E. zuernii infection in clinical, parasitological and pathomorphological terms and to test the efficacy of treatment with toltrazuril against experimental E. zuernii infection on the basis of clinical and parasitological parameters, specifically faecal consistency, oocyst excretion and weight gain.
2. Materials and methods
For the studies, a total of 30 calves were kept in two sequences (Study I and Study II, study schedules see Table 1 ) under the same housing conditions (same stable, two calves per pen, air-conditioned rooms, feeding with milk replacer and roughage), including six calves for pathological examination and 24 for the analysis of efficacy of treatment with toltrazuril. All animals were healthy Holstein-Mix bull calves, aged between one and four weeks at the start of the study.
Table 1.
Schedule of the studies (dpi = day post infectionem)
| Study I | Group/no. | Sampling day | Parameters assessed (groups A–C) |
|---|---|---|---|
| Infection with 150,000 sporulated E. zuernii oocysts per calf (study day 0) | A/2 | 16 dpi (late prepatent period) | –clinical and parasitological parameters until pathological examination: see Study II |
| B/2 | 21 dpi (patent period) | –pathologic-anatomical and histopathological findings in the mucosa | |
| C/2 | 26 dpi (late patent period/early postpatency) | ||
| Study II | Group/no. | Treatment | Parameters assessed (groups A–C) |
| Infection with 150,000 sporulated E. zuernii oocysts per calf (study day 0) | D/12 | Toltrazuril (15 mg/kg, day 14 after infection) | –Clinical examination: daily |
| E/11 | -(sham dosing with water, day 14 after infection) | –Faecal consistency and oocyst excretion: on 0, 2, 5, 7, 9, 12 and 15–32 dpi | |
At the start of the studies, the animals were infected orally with 150,000 sporulated E. zuernii-oocysts each of field isolates from one commercial farm, used unpassaged for Study I (approximately one month after isolation) and passaged once approximately 2 weeks before use in Study II, respectively. This infective dose had been determined as suitable to provoke clinical coccidiosis in a preliminary study observing clinical effects in animals inoculated with dose rates of 50,000 and 150,000 sporulated E. zuernii-oocysts per calf. The infective material contained nearly 100% pure E. zuernii-oocysts with occasional contamination by E. ellipsoidalis-oocysts (verified by counting of the oocyst stock solution).
The calves' faeces (samples of approximately 50–100 g, taken by digital stimulation of the anus) were examined three times weekly for the first 14 days post infectionem (dpi) and then daily for consistency and oocyst excretion. The calves were also under continuous clinical surveillance, i.e. animal health observations (animal attitude and behaviour) were performed in all groups on 0–13 dpi, animal health examinations (animal behaviour, body temperature and skin turgidity) were performed daily from 14 dpi (study I) or 15 dpi (study II), respectively, to 28 dpi (or up to the day of sacrifice of the respective animal in study I). Complete clinical examinations of all calves were performed before infection and in study II additionally on day 14 directly prior to randomisation. The live weight of the animals was determined once a week.
Faecal consistency was assessed on the basis of a scoring system (1: normal to pasty, 2: semiliquid to liquid, 3: watery, 4: haemorrhagic and/or with tissue). Oocyst excretion was determined quantitatively in the faecal samples using the McMaster method [5], modified by using 4 g of fresh faeces per 60 ml of saturated NaCl solution and mixing the suspension with a magnetic stirrer for about 2 min at the highest setting before transferring it into the McMaster slide. The quantitative results obtained were documented as absolute opg (oocysts per gram faeces) value and also analysed in terms of an opg score (0: no oocysts detectable, 1: ≤ 100 opg, 2: ≤ 1,000 opg, 3: ≤ 10,000 opg, 4: ≥ 10,000 opg). Faecal samples were also sent for differential diagnosis to an extern laboratory (BioCheck, Leipzig, Germany) and examined in regard of pathogenic bacteria (salmonella, Escherichia coli, coliform germs, Yersinia enterocolitica, Clostridium perfringens), viruses (rota- and corona virus) and yeasts.
Two animals each were sacrificed at various time points (Table 1), i.e. during the prepatent period (Group A, day 16 post infection [16 dpi]), in the patent period (Group B, 21 dpi), and in the late patent period (Group C, 26 dpi). On the scheduled necropsy days, the calves were euthanised by complete exsanguination after anesthesia by captive bolt.
At necropsy, the intestine was removed, opened, rinsed, and examined for eventual macroscopic findings. Samples were collected from different localisations and fixed in 10% buffered formaldehyde solution. They were as follows: jejunum (mid part and caudal part, approximately 30 cm cranial of the ileocaecal junction), ileum (caudal part at the ileocaecal junction), caecum, colon (proximal and second loop of the spiral colon). Additionally, samples of the ileocaecal and the mesenteric lymph nodes were collected and fixed. Specimens of every localisation mentioned above were trimmed, embedded in paraffin wax, and cut at a thickness of approximately 5 μm. All slides were stained routinely with hemalum and eosin (H&E).
Samples from the terminal ileum, caecum and colon were taken for scanning electron microscopic evaluation. The samples were clamped on cork plates, flushed with cacodylate buffer (pH 7.2) and fixed in phosphate buffered glutaraldehyde (2%). They were post fixed in osmium tetroxide solution (pH 7.0), dehydrated with increasing ethanol and hexamethyledisilazan (HMDS), mounted on specimen holders and sputtered with gold (Balzers AG, Balzers, Liechtenstein). The evaluation was carried out using a Scanning Electron Microscope (ESEM, Quanta 400, FEI Company, Hillsboro, USA).
The aim of Study II was to investigate the efficacy of toltrazuril following metaphylactic treatment (Table 1), i.e. the study animals were treated during the prepatent period (before the start of oocyst excretion). The calves were assigned randomly to two groups on 14 dpi for the determination of efficacy (one calf was excluded from the study because of premature E. zuernii excretion). Twelve calves were treated with toltrazuril at a dose rate of 15 mg toltrazuril/kg live weight, eleven were shamtreated with water as negative controls. Following treatment, the calves were examined clinically and coproscopically daily for 18 days and weighed weekly.
2.1. Statistical methods
The results of the pathological investigations in Study I (in calf groups A, B and C) were analysed descriptively. The clinical and parasitological results from Study I were used to characterise the infection and determine the sampling times (for Groups B and C).
The results from Study II (Groups D and E) were analysed statistically using the SAS program (version 8.02.02M0P012301, SAS Institute Inc., Cary, USA). The mean values for the individual animals over the study period (14–32 dpi), concerning faecal score, opg score and weight gain were used as a basis for statistical analysis to compare both study groups statistically, using the one-way analysis of variance.
3. Results
3.1. Clinical symptoms and oocyst excretion (Studies I and II)
As a result of infection, the animals in Groups B, C and E (infected, untreated, not sacrificed in the prepatent period) developed significant symptoms of clinical coccidiosis, characterised by severe diarrhoea and oocyst excretion after the prepatent period that varied from 17 to 24 days.
Study I: In group B (sacrificed 21 dpi) diarrhoea started on 16 dpi, one calf developed up to watery (score 3 on 18 and 20 dpi) and the other calf up to haemorrhagic diarrhoea (score 4 on 20 dpi). In group C (sacrificed 26 dpi) both calves developed diarrhoea from 17 to 24 dpi, up to haemorrhagic quality (score 4 on 22 or 23 dpi, respectively). Oocyst excretion in groups B and C reached values of up to 50,450 opg or 53,200 opg, respectively (see Table 2 ).
Table 2.
Oocyst excretion of groups B (n = 2), C (n = 2), D (n = 12) and E (n = 11) of Studies I and II, characterized by median of the opg (oocysts per gram), minimum opg and maximum opg in the respective group (data for previous faecal oocyst counts is not given in this table, in all faecal examinations on 0–14 dpi (day post infectionem) no oocysts were detected); *: na = not applicable (animals sacrificed)
| Study | dpi | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study I | Median opg of group B | 0 | 0 | 1200 | 1650 | 3500 | 35,925 | na* | na | na | na | na | na | na | na | na | na | na | na |
| Minimum opg of group B | 0 | 0 | 0 | 0 | 900 | 21,400 | na | na | na | na | na | na | na | na | na | na | na | na | |
| Maximum opg of group B | 0 | 0 | 2400 | 3300 | 6100 | 50,450 | na | na | na | na | na | na | na | na | na | na | na | na | |
| Median opg of group C | 150 | 1475 | 5025 | 8700 | 10,425 | 5200 | 27,350 | 8150 | 5200 | 5000 | 25 | na | na | na | na | na | na | na | |
| Minimum opg of group C | 0 | 0 | 0 | 0 | 1200 | 750 | 1500 | 6750 | 550 | 400 | 0 | na | na | na | na | na | na | na | |
| Maximum opg of group C | 300 | 2950 | 10,050 | 17,400 | 19,650 | 9650 | 53,200 | 9550 | 9850 | 9600 | 50 | na | na | na | na | na | na | na | |
| Study II | Median opg of group D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minimum opg of group D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Maximum opg of group D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Median opg of group E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 100 | 450 | 650 | 300 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Minimum opg of group E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Maximum opg of group E | 0 | 0 | 8850 | 9950 | 20,550 | 2750 | 77,850 | 6750 | 18,400 | 5400 | 13,800 | 3300 | 123,100 | 32,600 | 200 | 200 | 0 | 50 |
Study II: The diarrhoea observed in group E of Study II was of liquid to haemorrhagic quality, partly with tissue, and persisted for 4–11 days between 19 and 32 dpi. Oocyst excretion was observed in all animals of group E, persisting for 2–11 days (see Table 2). In three calves, there were additionally 1–2 days of zero opg within this period of oocyst excretion. The disease following infection in shamtreated calves also had marked depressing effects on weight gain, mainly at the end of the observation period (see Table 3 and Section 3.3).
Table 3.
Weight gain of groups D (n = 12) and E (n = 11) of Study II—arithmetic means (mean) and standard deviations (SD) of weekly live weight measurements
| Group | dpi | 0 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|---|
| D | mean ± SD | 54.9 ± 4.5 | 61.0 ± 5.3 | 65.7 ± 5.9 | 69.5 ± 6.1 | 75.3 ± 6.6 |
| E | mean ± SD | 52.1 ± 5.0 | 56.8 ± 5.4 | 62.2 ± 6.1 | 65.2 ± 5.8 | 68.3 ± 6.6 |
3.2. Pathological results (Study I)
The animals studied showed lesions of varying severity at all time points during the study with exception of one calf sacrificed in the prepatent period (Group A, 16 dpi). Concomitant diseases as causes for inflammatory reactions (e.g. other pathogens) could not be detected throughout clinical and pathological examinations.
The gross pathological examinations revealed no remarkable abnormal findings in the calves of group A (prepatent period, 16 dpi). In group B (patent period, 21 dpi) the mucosa of caecum and colon of both animals was slightly reddened. In group C (26 dpi), necropsy revealed slight reddening of the mucosa of the caecum of both and in the colon of one of the two calves.
The main characteristics under the light microscope of the intestinal sections taken from the animals examined during the late prepatent period (Group A, 16 dpi) were mild inflammatory infiltration of the mucosa and increased cellular debris (also containing merozoites and trophozoites) in crypts and glands from the caudal jejunum to the middle colon. The caudal jejunum of one animal contained some second-generation schizonts (Fig. 1a).
Fig. 1.
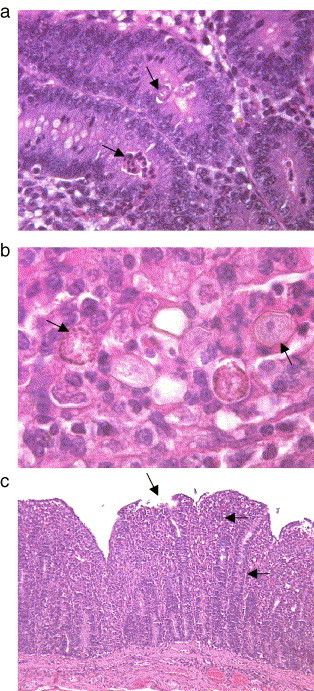
Light microscopical findings. (a) Crypt of the caudal jejunum with parasitic structures (second generation schizont, arrow above), crypt with granulocytic infiltration (arrow below) in animals during the prepatent period. H&E, Obj. 40 ×. (b) Cecal mucosa with second generation schizonts (left arrow) and oocysts (right arrow) in animals during the patent period. H&E, oil immersion, 100 ×. (c) Acute parasitic typhlitis, crypts filled with parasitic structures (right arrow), increased numbers of inflammatory infiltrates (middle arrow). Note loss of superficial epithelium (arrow above). H&E, Obj. 10 ×.
In group B animals which were sacrificed on 21 dpi, parasitic structures were identified from the middle jejunum to the middle caecum (Fig. 1b). The caecum and proximal colon showed particularly high levels of infection. In both calves acute typhlitis and partially necrotising colitis were found in addition to inflammatory granulocytic infiltration of the mucosa and cellular debris in most of the intestinal sections examined (Fig. 1c). While the superficial mucosa was already visibly repairing in one animal, the other animal also showed slight inflammatory demarcation of the submucosa.
In one of the group C calves subjected to pathological examination (sacrificed 26 dpi), the intestinal mucosa was characterised by slightly shortened villi in the middle jejunum, while slight re-epithelialisation was identifiable in the caecal mucosa. In both animals, regeneration and/or hyperplasia of the cryptal epithelium with proliferation of goblet cells was observed in the caecum and colon, and in one animal also in the terminal ileum. At most of the localisations examined, mixed cellular inflammatory infiltrates in the mucosa and cellular debris were still present.
The scanning electron microscopic evaluation of animals from the prepatent period (Group A, 16 dpi) revealed a normal appearance of the ileum (Fig. 2a) in both animals with some minor irregularities of the epithelial structure in one calf. In the caecum of one animal there was a superficial epithelial injury of slight/moderate degree and in the colon of this animal of minimal degree, corresponding to the inflammatory changes observed by light microscopy. In the caecum and colon of the other animal no abnormal findings could be detected.
Fig. 2.
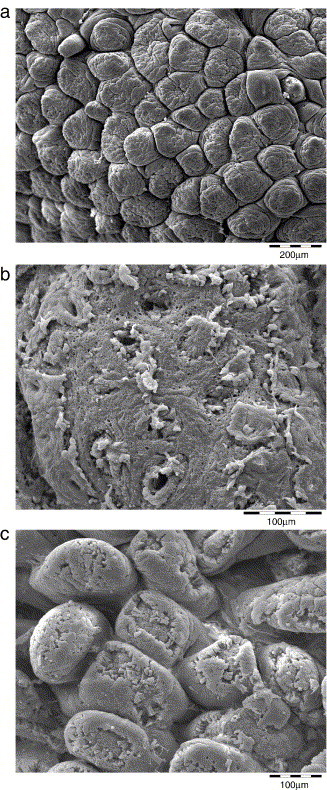
Electron microscopical findings. (a) Normal appearance of the ileum in animals during the prepatent period. (b) Superficial epithelial loss in the cecum of animals during the patent period. (c) Superficial epithelial injury in the ileum of animals during the late patent period.
The calves from the patent period (Group B, 21 dpi) showed some focal damage of the apical villous structures together with some bacteria focally incorporated. In the caecum (Fig. 2b) and the colon, distinct loss of the superficial epithelial layer occurred in both animals. Higher magnification of the lesions clearly demonstrated the disrupted epithelial layer with inflammatory cells in the deeper levels of the mucosa.
In the ileum of the animals sacrificed in the late patent or postpatent period (Group C, 26 dpi) the apical epithelium of the villi showed some injury in one of the two animals (Fig. 2c). This demonstrated the involvement also of the terminal ileum in the process at that time point. The findings in the colon and caecum of group C calves were consistent with the findings in group B in terms of quality, but were of a milder nature. No parasitic structures were detected by electron microscopal examination of the intestine, which was consistent with the findings of light microscopy showing parasitic structures only in deeper layers of the intestinal mucosa.
3.3. Efficacy of toltrazuril (Study II)
3.3.1. Diarrhoea
Treatment with toltrazuril resulted in a significant reduction in diarrhoea, both in terms of the number of scour days as well as in the severity of the diarrhoea (evaluated on the basis of the scoring system; p < 0.0001 for LS mean values). In group E, all eleven shamtreated animals developed diarrhoea which persisted for between 4 and 11 days (7.2 days on average) and which corresponded to an average score of 1.5 on the severity scale, the maximum score (score 4) being attained repeatedly (Fig. 3a). In group D, four out of 12 toltrazuril treated animals developed diarrhoea which persisted for up to 3 days (0.6 scour days per animal on average in the group). The average faecal score in group D was 1.1, score 2 being the maximum score observed (Fig. 3b). The differential diagnosis of the faecal samples did not reveal any abnormal findings, i.e. the calves were obviously not infected by any other intestinal pathogenic agent.
Fig. 3.
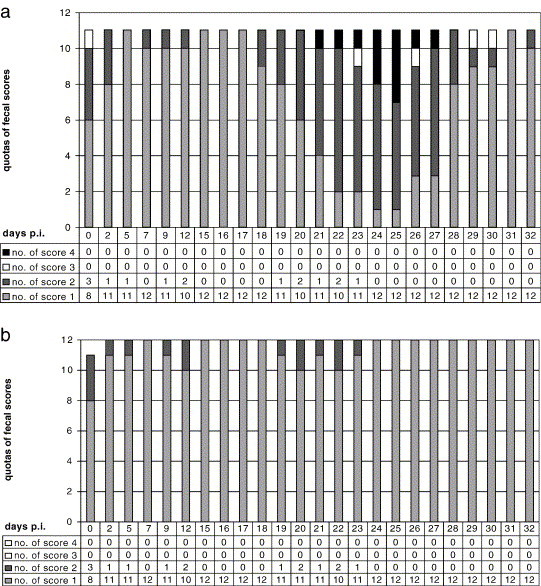
Distribution of the faecal scores in groups D and E (Study II)-respective quota of the total sample size (on day 0 post infectionem (dpi) one faecal sample was missing in group D). (a) group E (infected, shamtreated control). (b) group D (infected, treated with 15 mg toltrazuril/kg liveweight on 14 dpi).
3.3.2. Oocyst excretion
In group D, the level of oocyst excretion, measured using the opg score, was significantly reduced (p = 0.0001). In group E, E. zuernii-oocyst excretion was observed in all animals, starting between 17 and 24 dpi, persisting for between 2 and 12 days and lasting up to 32 dpi. In three calves of group E oocyst excretion was not detectable for 1–2 days during the patent period, the other eight calves shed oocysts continuously over the patent period. The average oocyst excretion for group E over all calves and all study days following the treatment day (14 dpi) was 2023 opg. The median of the oocyst excretion over all faecal samples of group E with an opg higher than zero was 600 opg. On five of the 15 oocyst excretion days the median opg was higher than zero; on each day there was at least one calf without excretion of E. zuernii-oocysts (see Table 2). On 27 dpi, the maximum oocyst excretion of 123,100 opg was detected.
In the treated group D, however, only one calf showed excretion of 50 E. zuernii-oocysts per gram on 26 dpi, while the remaining calves excreted no oocysts. The average number of excretion days for the calves of group E was thus 5.5 days compared with 1 day for the single excreting calf in group D.
3.3.3. Weight gain
Weight gain in group D was significantly higher than in group E (p < 0.0001). The weight gain (arithmetic mean) was 9.9 kg live weight over the period of three weeks after treatment in group E compared with 14.6 kg liveweight in the calves of group D. The differences in weight gain showed mainly at the end of the patent period observed on day 28 pi (see Table 3).
4. Discussion
In the E. zuernii infection model, the changes in the intestinal mucosa in the course of the infection were examined using light and electron microscopy. The effectiveness of treatment with toltrazuril was also investigated.
It is known from the literature [4] that infection with E. zuernii causes significant coccidiosis after a prepatent period of about 16–17 days. Data on the duration of the prepatent period in E. zuernii infection in the literature are only sparse because most studies relate to natural infections. In the present studies, a prepatent period of between 15 and 24 days was observed in the infection model. All of the infected, untreated calves from both studies which were not sacrificed before the end of the prepatent period excreted E. zuernii oocysts during the patent period with a maximum output of 123,100 opg in one calf (27 dpi) and showed diarrhoea, partly haemorrhagic and with tissue. Differential diagnosis did not reveal any other pathogenic agents besides E. zuernii, so it is concluded that the clinical signs observed validate the presence of coccidiosis in the untreated calves. Contrary to the experiences described previously [3], it was possible to provoke clinically relevant coccidiosis regularly in healthy calves with the selected dose of 150,000 E. zuernii-oocysts per calf. This proved the validity of the infection model used and the selected infective dose. Because the study conditions were identical for all calves (housing, feed), the variability shown in the prepatent period was probably attributable to the nature of the pathogen, i.e. it is broader than previously [4] assumed. At the same time, a relatively high variability in the duration of the prepatent period of 15–24 days and in the duration and severity of the clinical symptoms was seen in both studies: diarrhoea varied from liquid to haemorrhagic diarrhoea with tissue, and calves showed normal to depressed animal behaviour. Maximum oocyst excretion differed from 350 opg (one animal of Study II) to 123,100 opg (one animal of Study II). Though all calves showed diarrhoea due to coccidial infection and clearly associated with oocyst excretion, a defined temporal correlation between the beginning of diarrhoea and oocyst excretion could not be observed for all calves, in group E of Study II the beginning of diarrhoea was between up to 4 days before and 1 day after the first oocyst excretion day. The correlation of the last day of diarrhoea and the last day of oocyst excretion was comparably broad: the diarrhoea ended up to 5 days before until up to 2 days after the end of oocyst excretion. In all calves diarrhoea was observed during the patent period, the mean number of days with concurrent oocyst excretion and occurrence of diarrhoea was 4.5 days over all calves of group E.
The use of an infection model was advantageous for the investigations on pathology of this disease under defined conditions (day of infection, oocyst dose given, feeding and housing) and for the evaluation of the efficacy of treatment with toltrazuril in comparison with shamtreated calves infected with the same quantity of E. zuernii oocysts of the same strain.
E. zuernii coccidiosis causes significant pathological changes in the target sections of the intestine which are small intestine, caecum and colon [6], [7] and is thus of great significance as a cause of diarrhoea [1], [2] and reduced weight gain [8]. The aim of the present studies was to confirm the light microscopical lesions described previously [6] and to corroborate the superficial findings by electron microscopy, for, to our knowledge, electron microscopical studies on E. zuernii have not been published previously.
Stockdale [6] who infected calves with 600,000 E. zuernii-oocysts each and used corticosteroids for immunosuppression showed partly complete loss of the epithelium of proximal colon and caecum during gross pathological examinations. By light microcopy he demonstrated presence of schizonts in the lamina propria of the small intestine with only little inflammatory reaction, and lesions in caecum and colon that were similar to each other and most extensive between 18 and 21 dpi with loss of the epithelium and presence of a thick layer of fibrin in which cellular debris and bacterial colonies were incorporated. After 21 dpi, the mucosal regeneration started. In contrast to those observations, there were under the light microscope no fibrinoid layers seen in our studies. The findings were of a milder nature and consistent with electron microscopical observations. In the calves examined during prepatent period (16 dpi), mild inflammation of the mucosa from caudal jejunum to middle colon in both animals and presence of second-generation schizonts in the caudal jejunum of one animal were demonstrated. Electron microscopical examination showed epithelial injury of slight to moderate degree.
The most pronounced changes were observed in the intestine of the calves sacrificed during the patent period (21 dpi) of the infection. In those calves acute typhlitis and partially necrotising colitis and parasitic infection, particularly high in the colon, were seen. Inflammatory infiltration of the mucosa with cellular debris characterised most of the intestinal sections examined on 21 dpi. The electron microscopy confirmed those results. Most conspicuous were the distinct loss of the superficial epithelial layer with inflammatory cells in the deeper levels of the mucosa in the caecum of the animals.
The mucosa of the large intestine was regenerating in calves killed during postpatency (26 dpi) but still showing signs of inflammation. Under the electron microscope, colon and caecum showed changes similar to the calves examined on 21 dpi, but milder.
Throughout all electron microscopical investigations, no parasitic structures were detected, corresponding to the light microscopical findings that showed parasitic stages only in deeper layers of the intestinal mucosa.
The less pronounced changes in the intestines of the calves examined in the course of Study I compared to earlier studies by Stockdale et al. [6] are possibly due to a lower dose of E. zuernii-occysts and the lacking use of corticosteroids. Pathogenicity of strains may vary [9] which may contribute to the differing observations reported previously [6].
In the present studies the disease was described in both clinical and histopathological terms over the course of the infection. The findings from the electron microscope investigations show that in some cases massive epithelial damage to the small and large intestine can still be present even after the end of oocyst excretion. This is also reflected clinically in the more sustained diarrhoea and lower weight gain in the shamtreated calves (see Study II).
Toltrazuril has been applied successfully to treat coccidiosis after natural Eimeria infections [2], [10], [11], [12] of calves. In the present study (Study II) these field experiences were validated under defined conditions in an experimental infection model. There are basically two options for the treatment of E. zuernii coccidiosis: either after the onset of clinical disease/oocyst excretion (therapeutic approach) or during the prepatent period, i.e. before in vivo diagnosis of infection is possible (metaphylactic approach). Both approaches have been studied comparatively in field studies [2] and caused a reduction in oocyst excretion and clinical symptoms, with metaphylaxis proving considerably more effective. In the present studies (Study II), metaphylactic treatment with toltrazuril was found to be very effective against massive E. zuernii infection. Significantly less diarrhoea in regard of duration and severeness, lower oocyst excretion and higher weight gain were found in the group treated with 15 mg toltrazuril/kg live weight compared to the shamtreated control group. While all shamtreated calves shed considerable numbers of oocysts and developed diarrhoea, mostly up to haemorrhagic quality, only four of the treated animals developed diarrhoea of liquid quality and only one of these calves shed a low amount of oocysts on 26 dpi. The diarrhoea observed in four of the toltrazuril treated calves was obviously not correlated with the coccidial infection, for no oocyst shedding was detected apart from one calf which shed such a low amount of oocysts (50 opg) that is not to be associated with clinical signs. The high effectiveness of treatment with toltrazuril during the prepatent period of coccidial infection is also consistent with experiences in calves [13] and other mammals [14], [15] already described.
Acknowledgements
We like to thank Dr. Michael Becka for the statistical analysis of the studies and Klaus Ide for technical assistance during electron microscopy evaluation.
References
- 1.Mage C., Reynal P. Epidemiological observations of coccidiosis in suckler calves in France. In: Yvore P., editor. Coccidia and intestinal coccidiomorphs, Vth International Coccidiosis Conference, Tours (France). 17–20 October 1989. INRA Publ.; Paris: 1989. pp. 457–460. [Google Scholar]
- 2.Staschen S. Kontrolle einer natürlichen Kälberkokzidiose. Vet-MedReport extra edition V3, 2-2 (2004).
- 3.Stockdale P.H.G., Bainborough A.R., Bailey C.B., Niilo L. Some pathophysiological changes associated with infection of Eimeria zuernii in calves. Can J Comp Med. 1981;45:34–37. [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst J.V., Benz G.W. Intestinal coccidiosis in cattle. Vet Clin North Am Food Anim Pract. 1986;2:283–291. doi: 10.1016/s0749-0720(15)31238-x. [DOI] [PubMed] [Google Scholar]
- 5.Thienpont D., Rochette F., Vanparijs O.F.J. Janssen Pharmaceutica; Beerse: 1990. Diagnose von Helminthosen durch koproskopische Untersuchung. [Google Scholar]
- 6.Stockdale P.H.G. The pathogenesis of the lesions produced by Eimeria zuernii in calves. Can J Comp Med. 1977;41:338–344. [PMC free article] [PubMed] [Google Scholar]
- 7.Stockdale P.H.G. Proposed life cycle of Eimeria zuernii. Br Vet J. 1977;133:471–473. doi: 10.1016/s0007-1935(17)33987-8. [DOI] [PubMed] [Google Scholar]
- 8.Stockdale P.H.G., Yates W.D.G. Resistance to Eimeria zuernii produced after chemotherapy of experimental infections in calves. Vet Parasitol. 1978;4:209–214. doi: 10.1016/0304-4017(82)90061-9. [DOI] [PubMed] [Google Scholar]
- 9.Fayer R. Epidemiology of protozoan infections. The coccidia. Vet Parasitol. 1980;6:75–106. [Google Scholar]
- 10.Bohrmann R. Treatment with toltrazuril in a natural outbreak of coccidiosis in calves. Dtsch Tierarztl. Wochenschr. 1991;98:343–345. [PubMed] [Google Scholar]
- 11.Emanuel C., Bianchi C., Biolatti B. Efficacy of toltrazuril in bovine coccidiosis. Vet Med Rev. 1988;59:90–91. [Google Scholar]
- 12.Pilarczyk B., Balicka-Ramisz A., Prost M. The dynamics of Eimeria spp. infection in calves treated and untreated with Baycox. Med Wet. 1999;55:523–526. [Google Scholar]
- 13.Mundt H.C., Daugschies A., Uebe F., Rinke M. Efficacy of toltrazuril against artificial infections with Eimeria bovis in calves. Parasitol Res. 2003;90(Suppl. 3):166–167. doi: 10.1007/s00436-003-0929-z. [DOI] [PubMed] [Google Scholar]
- 14.Mundt H.C., Daugschies A., Wustenberg S., Zimmermann M. Studies on the efficacy of toltrazuril, diclazuril and sulphadimidine against artificial infections with Isospora suis in piglets. Parasitol Res. 2003;90(Suppl. 3):160–162. doi: 10.1007/s00436-003-0927-1. [DOI] [PubMed] [Google Scholar]
- 15.Taylor S.M., Kenny J. Coccidiocidal efficacy of a single treatment of toltrazuril in naturally infected lambs. Vet Rec. 1988;123:573. doi: 10.1136/vr.123.22.573. [DOI] [PubMed] [Google Scholar]


