Abstract
The causes of multiple sclerosis and amyotrophic lateral sclerosis have long remained elusive. A new category of pathogenic components, normally dormant within human genomes, has been identified: human endogenous retroviruses (HERVs). These represent ∼8% of the human genome, and environmental factors have reproducibly been shown to trigger their expression. The resulting production of envelope (Env) proteins from HERV-W and HERV-K appears to engage pathophysiological pathways leading to the pathognomonic features of MS and ALS, respectively. Pathogenic HERV elements may thus provide a missing link in understanding these complex diseases. Moreover, their neutralization may represent a promising strategy to establish novel and more powerful therapeutic approaches.
Keys to Understanding the Etiopathogenesis of Inflammatory and Degenerative Neurological Diseases May Lie in the Hidden Half of the Human Genome
A new comprehensive approach to the etiopathogenesis of some diseases is emerging from studies on genomic remnants of mobile genetic elements (see Glossary) that are known for their role in the molecular evolution of genomes 1, 2, 3, 4, with a particular focus on human endogenous retroviruses (HERVs). This domain cannot be appropriately understood through classical virology or genetics because it deals with entities that are neither viruses nor physiological genes [5].
In the present review we discuss recent scientific discoveries of these long-misunderstood elements, previously considered to be junk DNA but which, in some instances, are now known to contribute to physiological functions (‘domesticated copies’) 5, 6, 7 or to remain as dormant functional copies encoding retroviral proteins 5, 8, 9. We attempt to explain how these peculiar genetic elements may provide missing keys to understanding complex multifactorial diseases. The best-studied diseases where consistent scientific data support an involvement of HERV genetic elements in their pathogenesis are MS (Box 1 ) and amyotrophic lateral sclerosis (ALS), but we also introduce chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). In addition, we discuss novel HERV-targeted therapeutic avenues that are starting to be evaluated in MS and ALS. Moreover, HERVs have also been associated with other diseases such as schizophrenia and bipolar disorder [10], as well as with type 1 diabetes [11], but much less data are available for these disorders.
Box 1. Viruses in MS.
An infectious origin of MS was suggested for the first time by Pierre Marie in 1884, but was rejected by the contemporary medical community. The evidence that viruses may contribute to MS comes from the accumulation of immune cells within the brain and CSF, local immune reactivity to specific viruses, and the presence of oligoclonal bands. MS epidemiology indicates some triggers during adolescence, an unusual geographic distribution (a gradient with latitude, but with exceptions: e.g., contrasting patterns in Sardinia and Japan), and epidemic clusters in previously isolated islands. Over time, several viruses have been proposed as causative agents of MS. From the 1940s onward they included rabies virus, herpes simplex virus (HSV), scrapie prion, an unidentified MS-associated agent, parainfluenza virus 1, measles virus, simian virus 5, chimpanzee cytomegalovirus, coronavirus, EBV, an unidentified SMON-like virus (subacute myelo-opticoneuropathy virus), tick-borne encephalitis virus, HTLV-1, HSV-1, VZV, and HHV-6. The proposed mechanisms were direct brain or peripheral infection, activation of autoreactive T cells against nerve myelin, bystander activation, epitope spreading, molecular mimicry, and virus–virus interactions. However, the link to MS was shown to be weak for the majority of the above viruses. The most consistent and independently confirmed studies for viral involvement in MS are for EBV. They appear to be confirmed, but only with indirect links, by history of infectious mononucleosis (IM; primary infection with EBV causes IM), and high anti-EBNA-1 (EBV nuclear antigen 1) IgG titers before MS onset. However, a new concept arose with the discovery that HERVs express pathogenic proteins in disease, and the best evidence of an association and pathogenic involvement is for HERV-W/MSRV (detected in MS blood, spinal fluid, and brain, in parallel with MS stages, active/remission phases, and therapy outcome). EBV is known to activate HERV-W/MSRV in vitro and in vivo (in IM patients and in healthy humans with high anti-EBNA1 IgG titers). This suggests that EBV could be an initial trigger, and that HERV-W/MSRV is a direct neuropathogenic contributor, before and during MS, in addition to its known contribution to promoting autoreactive T cells, immunoinflammation, and remyelination blockade.
Alt-text: Box 1
Endogenous Retroviruses Originate from Ancestral Germline Infections by Exogenous Elements
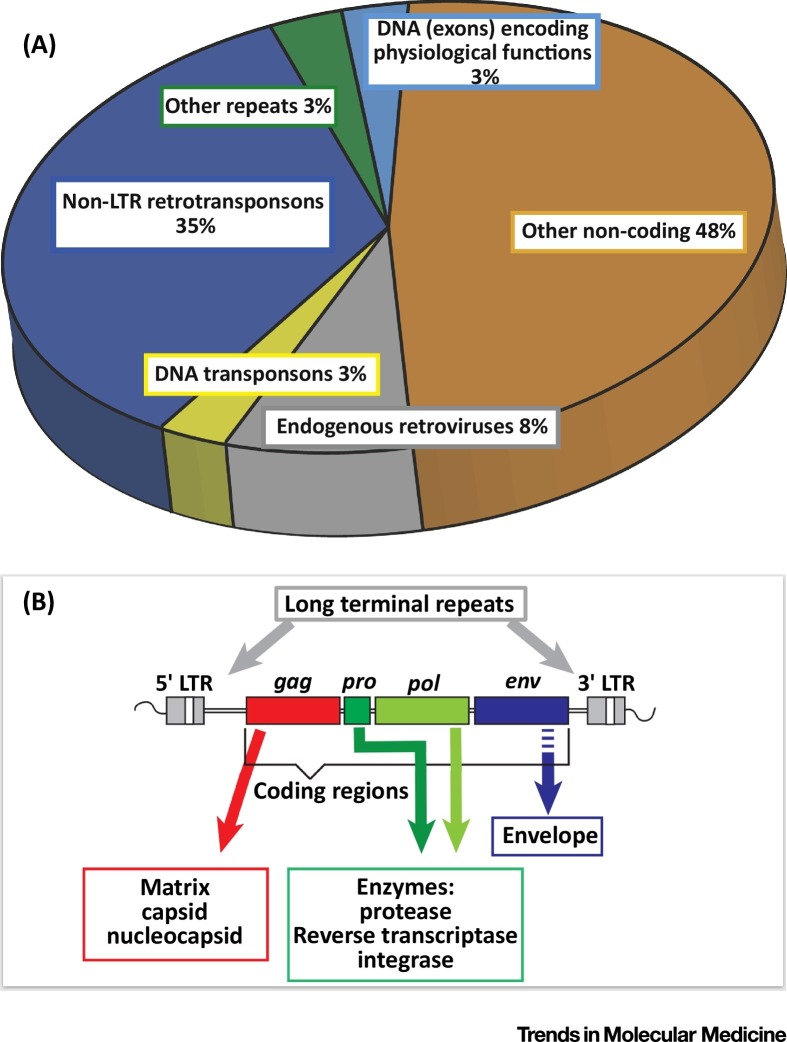
The eukaryotic genome is composed of a large set of DNA sequences, many of which derive from mobile genetic elements 1, 12. These were estimated to account for about 50% of the human genome 13, 14, 15 (Figure 1 A), if not more [16]. Their detection is particularly complex, which may explain why their proportion within the genome has been largely underestimated and why data evolve with technological improvements [12], among which are those allowing the sequencing of constitutive heterochromatin regions that long remained inaccessible [17].
Figure 1.
Relative Proportions of Different Types of DNA Sequences within the Human Genome, and the Prototypic DNA Sequence of Complete Human Endogenous Retrovirus (HERV) Genomes. (A) DNA sequences representing remnants of mobile genetic machinery represent nearly 50% of the human genome. These are predominantly retrotransposons, that use RNA intermediates and a ‘copy-paste’ mechanism for retrointegration into chromosomes, and DNA transposons that use a ‘cut and paste’ mechanism; these represent 42% and 3% of the human genome, respectively [3]. Among retrotransposons, an important group is represented by the endogenous retroviruses (ERVs; HERVs for human ERVs) that entered the genome of species through infections of germline cells during evolution leading to subsequent endogenization – integration into the genome and presence in the DNA of every cell of the offspring [5]. About 8% of the human genome is constituted by HERV sequences; complete sequencing has revealed that intact coding sequences for functional HERV proteins represent <3%, if not 1% 101, 102. The remaining sequences comprise non-coding DNA, often within introns of ‘classical’ genes, with a potential role in generating non-coding RNAs [103]. (B) Endogenous HERV DNA sequences are often truncated, and contain mutations, insertions, or deletions, but complete copies are also present. The gag (group-specific antigen), pol (polymerase), and env (envelope) genes encode structural proteins and are flanked by two inverted repeats of non-coding regions comprising many regulatory functions (promoter, enhancer, primer-binding site for reverse transcription, and others). Abbreviation: LTR, long terminal repeat.
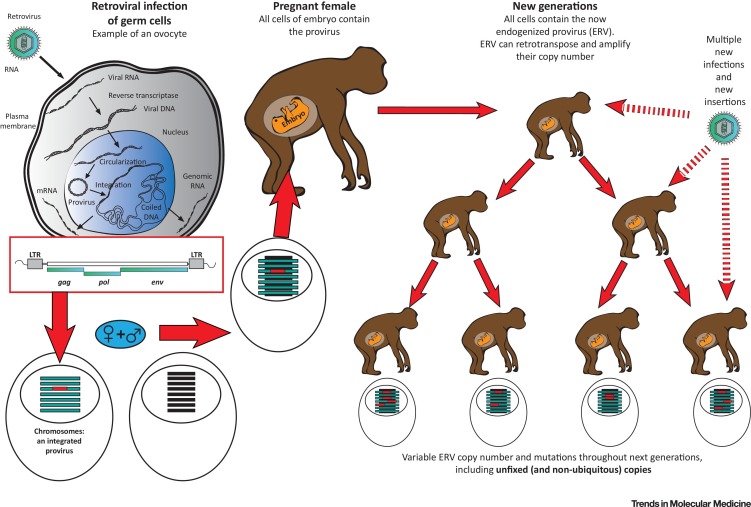
Two types of transposable elements can be distinguished: (i) elements that can be transposed via a DNA intermediate and a cut-and-paste mechanism (transposons) [18], and (ii) those using a RNA and a copy-paste mechanism (retrotransposons) [16]. Retrotransposons comprise endogenous retroviruses (ERVs), for which the current literature presents incomplete data as well as several classifications and nomenclatures, leading to ambiguities [15]. The term ‘HERV’ refers to the sequences of human ERV families. HERVs retain structural features of retroviral genomes (Figure 1B), and originally entered the genome of species through repeated infections of germ cells over millions of years (Figure 2 ) 5, 19.
Figure 2.
Successive Steps Leading to Endogenization of Retroviruses and to Multicopy Endogenous Retroviral (ERV) Families in Descendants. This illustration depicts the successive steps of retroviral endogenization, starting from infection of gametes, integration of a DNA retroviral copy (provirus) into a chromosome, giving birth to a viable individual inheriting and retaining this copy in the DNA of all cells and transmitting this to its offspring. Throughout successive generations and evolution, both endogenous retrotranspositions and reinfections of the germline of particular individuals (provided that the exogenous strain persists in the environment) generates multiple and variable copy numbers in the final population. This variability has been well known in some animal species and has recently also been evidenced in humans [91]. Abbreviations: env, envelope; gag, group-specific antigen; LTR, long terminal repeat; pol, polymerase.
Silent HERVs Can Be Activated by Environmental Triggers
The question of why and how HERV copies that have retained coding potential may become expressed is now addressed. The ability of HERVs to become activated is linked to the chromatin state where a given copy is located [20]. DNA methylation and histone modifications are essential to the epigenetic control of human genes, including HERV elements. The prerequisite for functional transcription is that HERV sequences must retain functional long terminal repeats (LTRs), or become controlled by another promoter, without deletions or nucleotide substitutions that disrupt their open reading frames (ORFs). Because the nuclear microenvironment including the chromatin accessibility of coding regions differs between tissues, the baseline predisposition of a HERV copy to be activated can be tissue-, cell-, or maturation stage-specific.
Inflammatory stimuli may activate HERVs via epigenetic dysregulation. For instance, transcription of HERV-W sequences has been reported to be upregulated by proinflammatory cytokines in cultured cells from MS patients [21], and this was shown to correlate with increased differentiation of peripheral blood mononuclear cells from MS patients [22]. By contrast, reductionof anti-Env antibody reactivity for HERV-H and HERV-W [23] and MS-associated retrovirus (MSRV) load [24] in the blood have been in seen in MS patients treated with IFN-β, suggesting efficacy of the therapy or low disease activity.
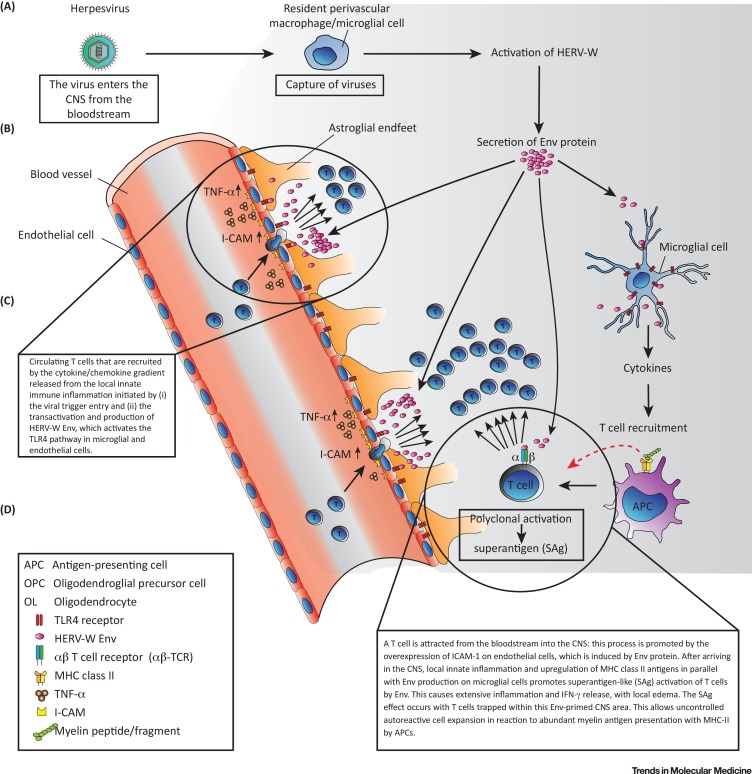
After an initial study, which first presented evidence that herpesviruses might trigger HERVs in patients with MS as part of the retrovirus hypothesis for MS etiology [25], several teams showed that transcription of HERV genes and/or reverse transcriptase (RT) activity is increased in various human cells in vitro by Herpesviridae with tropism for nervous cell. This includes herpes simplex virus type 1 (HSV-1) in lymphocytes from MS patients [26] and in neuronal or in brain endothelial cell lines [27]; varicella-zoster virus (VZV) in lymphocytes from MS patients [26]; cytomegalovirus (CMV) in kidney transplant recipients [28]; human herpes virus type 6 (HHV-6) in lymphocytes from MS patients [26] and in T cell leukemic cell lines [29]; and Epstein–Barr virus (EBV) in T cell lines [30] and in peripheral blood mononuclear cells from MS patients as well as in astrocyte cell lines [31]. Many of these viruses have been implicated in MS (reviewed in [32]). Herpesviridae may invade brain parenchyma and induce local proinflammatory responses, but these pathogens are normally intercepted by perivascular macrophages (Figure 3 ). Macrophages are not permissive for their replication but may allow expression of HERV-transactivating Herpesviridae immediate-early genes [33]. The EBV gp350 protein has been shown to activate HERV-W in vitro in B cells and monocytes, but not in T cells, whereas monocyte/macrophage cells appear to be most susceptible [31]. Because EBV has also been shown to potently modify epigenetic traits of host cell DNA (reviewed in [34]), the reported association with infectious mononucleosis, in addition to the elevated anti-EBV nuclear antigen-1 IgG titers in patients with MS [35], might support the hypothesis that EBV acts as a priming trigger. However, this has not been directly demonstrated. Nevertheless, Herpesviridae (or other environmental activating factors) are now suggested to upregulate HERV-W expression, with its Env protein acting as a pathogenic effector in MS [36].
Figure 3.
Hypothesized Human Endogenous Retrovirus (HERV)-Mediated Activation Cascades Leading to the Pathogenesis of Multiple Sclerosis (MS). This illustration depicts a hypothetical pathogenic pathway for MS involving HERV-W activation and envelope (Env) protein expression triggered by an environmental factor. This causes dysregulated inflammatory and immune responses accompanied by activation of microglial cells, which thereby also express pathogenic HERV-W Env protein (formerly called MSRV-Env). The proposed direct cytotoxic effect on endothelial cells through TLR4 activation could contribute to T cell homing through upregulated ICAM-1 expression in response to cytokines and chemokines secreted from the initial site of HERV-W expression.
Other viruses reported to transactivate HERVs are the exogenous retroviruses HTLV-1 and HIV-1. Specifically, the HTLV-1 Tax transactivator potently increased the transcriptional activity of HERV-W, HERV-H, HERV-K, and HERV-E families in T cells [37]. Moreover, effects of HIV on HERV-K and on HERV-W in astrocyte cell lines and peripheral blood cells in vitro have been reported to be mediated by the HIV Tat protein, which can indirectly activate HERV-W through Toll-like receptor-4 (TLR4) along with TNF-α and NF-κB. Thus, by this pathway, HIV Tat could influence non HIV-infected cells [22]. This indirect mechanism suggests that the HIV-driven activation pathway requires persisting Tat stimulation for HERV activation. This differs from Herpesviridae in that self-sustained HERV expression may be induced following specific triggering events, which could explain the lifelong progression of MS and the multiple triggering events that are required before a pathogenic threshold leading to disease onset can be passed.
Nonetheless, if HERV proteins are not expressed, HERV RNA expression (transcription alone) does not seem to have biological effects per se in humans. Moreover, when produced, HERV proteins are not implicitly pathogenic. An example is provided by the gag (group-specific antigen)-encoded capsid protein of HERV-W which had no immunopathogenic effect on peripheral blood lymphocyte cultures from healthy donors, whereas the Env protein of HERV-W particles (previously termed MSRV) induced proinflammatory and superantigen (SAg)-like effects [38].
Immune Cells Can Mediate Major Effects of Pathogenic HERV Expression
Abnormal activation of some HERVs is thought to have proinflammatory effects, leading to dysregulation of the immune system, as we will now illustrate with relevant examples.
HERV-K and HERV-W families share an interesting common feature in that the protein encoded by their env gene has been shown to trigger responses in T lymphocyte cells expressing a specific variable region of the T cell receptor (TCR) β chain in vitro 30, 38. Usually, T lymphocytes recognize their target antigen through a combination of variable domains in TCR chains that define the antigen-binding site of specific T cell clones. The interaction of these HERV Env proteins with another TCR region, which is known to be independent of the antigen-binding site and present on numerous T cells, is known to activate multiple clones irrespective of their antigen specificity. Molecules inducing such polyclonal activation are SAgs. An inflammatory loop also likely contributes to HERV pathogenicity: following initial priming and induction of specific HERV copies, macrophages and/or B cells produce HERV Env proteins that might fuel local innate inflammation and upregulate major histocompatibility complex (MHC) expression, causing further polyclonal activation of tissue-attracted T cells and therefore of B cells. This potential inflammatory loop could result in the development of devastating local lesions, while antigen-presenting cells stimulated via TLR and polyclonal activation of lymphocytes might promote breaks in immune tolerance that lead to autoimmunity, as was observed in a mouse model of experimental autoimmune encephalomyelitis (EAE) treated with HERV-W Env protein [39].
HERV-W and MS
MS is an inflammatory disease of the central nervous system (CNS) and a major cause of neurological impairment in young adults. There is no available cure for MS, and current therapies can only limit the number of relapses and slow disease progression. The most common symptoms include chronic fatigue, paresthesia with acute and chronic pain, optic neuritis, paresis, gait disturbance, incoordination, sphincter problems, and cognitive impairment, altogether leading to progressive disability. Histopathologically, MS is characterized by demyelinating lesions that predominantly expand in the white matter, causing destruction of myelin and oligodendrocytes and leading to axonal disruption in the brain and spinal cord. Lesions spread to cortical regions, affecting grey matter and neurons. Active lesions also show blood–brain barrier (BBB) breakdown, with infiltration of macrophages and lymphocytes, whereas activated microglia represent the hallmark of regions within active and chronic lesions, where ongoing demyelination and axonal loss are now understood to cause functional impairment (reviewed in [40]).
Immunological dysfunctions in MS are characterized by multifocal CNS hyperinflammatory reactions, and systemic autoimmune reactivity of B and/or T cells towards myelin autoantigens, with evidence of intrathecal chronic IgG production as revealed by the presence of oligoclonal bands in the cerebrospinal fluid (CSF) [41]. Whereas initial disease stages may present reversible phases with remission following relapse, evolution towards progressive forms generally follows [42]. The relapsing forms are dominated by aberrant inflammatory responses, whereas in progressive stages neurodegenerative features take precedence. This may be paralleled by repeated abnormal lymphocyte stimulation that is known to lead to T cell exhaustion, anergy, or depletion [43], and which implicitly downregulates T cell-driven activation of B cells [44].
The underlying etiology of MS is still not fully understood, but multiple disease-associated loci confer genetic predisposition to develop MS [45], and numerous environmental factors (e.g., infectious mononucleosis and smoking) 46, 47 appear to contribute to disease onset and progression. This led to the formulation of a pathogenic concept based on gene–environment interactions [48] with a partial, but elusive, role of viral infections [49].
In the search for etiological factors in MS, HERVs have been detected within the human genome, thus opening a new avenue of research because of their potential interactions with environmental factors. Diverse scientific and technical approaches have indicated that three human endogenous retroviruses, HERV-H, HERV-K, and HERV-W, may be abnormally represented or expressed in MS.
A genetic polymorphism in a single copy of HERV-Fc1 (a HERV-H-related element) and its relative distribution in MS patients, with the exception of primary progressive forms (PPMS), was reported 50, 51. HERV-K mRNA expression was found to be upregulated in postmortem brain tissue from MS patients [52], but no evidence of protein expression was provided. Retroviral sequences from RT-PCR with selected primers on particles produced by MS derived EBV-B lymphoblastoid cell lines identified sequence variants homologous to HERV-H elements (formerly named RTVL-H or RGH) [53]. Another study showed that B cells and monocytes from patients with active MS showed detectable surface expression of both HERV-H Env and HERV-W Env[54]. Taken together, this evidence suggests that activation of multiple HERV families might be linked to MS [55].
The most compelling evidence for an association between HERV expression and MS is for HERV-W and comes from a recent meta-analysis [56]. This line of research was prompted by the isolation of retroviral particles from MS patients in the early 1990s 57, 58. Subsequent studies over the following 25 years revealed that these particles originated from HERV elements, first termed MS-associated retrovirus (MSRV), whose sequences were determined from purified retrovirus-like particles isolated from MS cell culture supernatants 59, 60. The initial sequence identified from several MS isolates was obtained using a PCR protocol designed to detect unknown retroviral sequences flanked by conserved domains in most retroviral pol (polymerase) genes [61]. This prototype MSRV sequence identified a previously unknown HERV family, now named HERV-W because it uses a tryptophan (W) tRNA as a primer for reverse transcription, and that comprises multiple copies homologous to MSRV prototypic sequences 59, 62.
Independent groups subsequently reported that HERV-W env and pol expression could be detected in serum, peripheral blood mononuclear cells (PBMCs), and CSF from MS patients but not from healthy controls 60, 63, 64, 65, 66. HERV-W association with MS was further evidenced by studies showing that expression levels not only correlated with MS but also increased with disease activity and progression 24, 64, 65, 67. Further investigations indicated that HERV protein expression is mainly restricted to macrophages and microglia, a minor proportion of lymphocytes, and to a few endothelial and astrocyte cells in active lesion areas 31, 63, 68, 69. HERV-W Env protein was similarly detected in MS lesions by using different monoclonal antibodies (mAbs) directed against different epitopes [64]. Its association with areas of active demyelination from active to chronic brain lesions, with fairly intense expression until the death of the patient, as seen post-mortem, suggests an involvement in the long-term pathogenic progression of the disease [69].
HERV-W was further revealed to play functional roles in inflammatory processes. Proinflammatory cytokine expression was shown to be induced in both human and murine monocytes upon in vitro stimulation with HERV-W recombinant Env protein, a process that required TLR4 receptor activation 39, 70, 71, while MSRV Env-treated human dendritic cells were elicited to promote type 1 T helper cell (Th1)-like lymphocyte differentiation [71]. MSRV particles (HERV-W) were previously shown to induce superantigen-like T cell responses that were reproduced by the Env protein but not by the gag-encoded capsid protein [38]. A hypothetical scenario illustrating all these effects is presented in Figure 3. HERV-W envelope also promoted EAE in mouse and induced elevated autoimmune T cell reactivity [39].
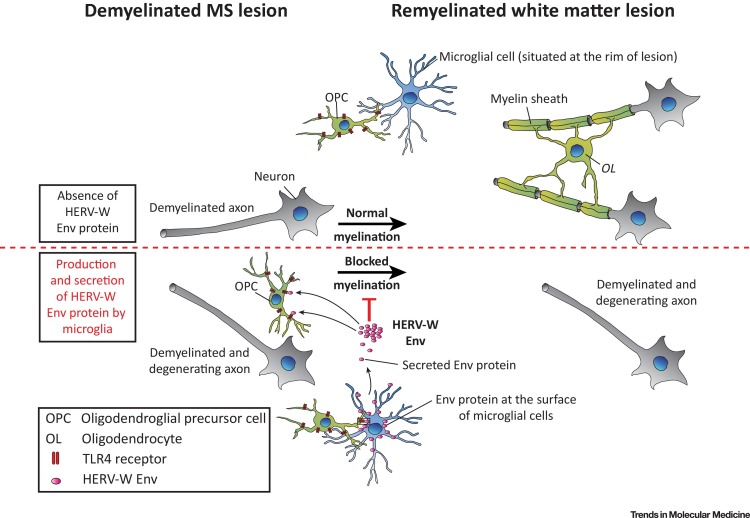
In addition to pathogenic effects targeting immune functions, HERV-W Env protein was also found to mediate TLR4-dependent effects on non-immune cells. Treatment of cultured primary oligodendroglial precursor cells (OPCs) with HERV-W Env was shown to result in an overall reduction of oligodendroglial differentiation via activation of TLR4 [68]. OPCs contribute to neuroregeneration and myelin repair processes in the adult CNS, and blocking their differentiation by HERV-W Env protein may therefore result in remyelination failure (Figure 4 ). The negative impact on myelin synthesis in primary oligodendroglial cells could be rescued using a specific Env-neutralizing humanized immunoglobulin termed GNbAC1 [72]. Different mAb versions of GNbAC1 have been assessed on a small scale in MSRV Env-induced experimental allergic encephalitis (EAE), an animal model of MS, and these appeared to inhibit and somehow reverse EAE clinical evolution [73]. In addition, exposure of the HCMEC/D3 brain endothelial cell line to HERV-W Env was shown to impair endothelial cell physiology by boosting ICAM-1 expression (allowing T cell homing into tissues) and proinflammatory cytokine release via TLR4 activation, thereby suggesting that Env might affect BBB integrity (Figure 3) [74].
Figure 4.
Normal Differentiation of Oligodendrocyte Precursor Cells (OPCs) versus Inhibition of Oligodendroglial Maturation by Human Endogenous Retrovirus HERV-W Envelope (Env) Protein. Activation of Toll-like Receptor 4 (TLR4) on OPCs by the pathogenic HERV-W Env protein blocks the differentiation of these cells, with potential impact on naturally occurring myelin repair. OPCs are targeted by pHERV-W Env either through contacts with microglia that present pHERV-W Env protein at the cell membrane surface, or through interaction with secreted or shed pHERV-W Env protein in the extracellular space.
Together, these data argue that pathogenic HERV-W Env protein (formerly MSRV-Env) is a potential therapeutic target in MS, and that the neutralizing humanized antibody GNbAC1 therefore warrants development; indeed, several early-phase trials have now been completed 73, 75, 76, 77. A Phase IIb multicenter clinical trial including 260 relapsing-remitting MS (RRMS) patients in 12 European countries is ongoing with this humanized IgG4 antibody. This trial is a 1 year study with a placebo arm and three treated groups receiving intravenous infusions every 4 weeks (6, 12, and 15 mg/kg) (ClinicalTrials.gov identifier: NCT02782858) [78].
Another approach, based on the hypothesis that antiretroviral drugs that are effective against exogenous HIV infections might also block HERV expression in MS [79], has led to a dedicated evaluation (ClinicalTrials.gov identifier: NCT01767701), but gave negative results for its primary endpoint (http://onlinelibrary.ectrims-congress.eu/ectrims/2016/32nd/146288/julian.gold.phase.2.baseline.versus.treatment.clinical.trial.of.the.hiv.drug.html?f=m3). This trial was a baseline-versus-treatment study with 20 patients with active RRMS defined as gadolinium-enhancing lesions on magnetic resonance imaging (MRI) at baseline. They were monitored for 3 months with monthly MRI and then treated with the integrase inhibitor raltegravir for 3 months. This trial did not reach its primary endpoint goal of significantly reducing either lesion count or lesion development during the treatment period versus baseline. Nonetheless, the tested drug is a known and effective inhibitor of HIV integrase which plays a role in chromosomal retrointegration of newly generated HIV DNA copies. Because this may not be a major aspect of HERV expression, and is unlikely to be a key issue for HERV Env production, the mode of action of this anti-HIV drug may not have had relevant effects on HERVs, thus explaining the negative results of the study.
Finally, following a study in which HERV-W Env protein was detected in sera and lymphoid cells of MS patients, but not in healthy controls nor in other neurological diseases, except for a few CIDP cases [64], a recent study confirmed upregulated HERV-W expression in blood cells and peripheral nerve lesions of patients suffering from CIDP [80]. This investigation also showed TLR4-mediated effects of HERV-W Env protein on primary human Schwann cells. Such direct effects on Schwann cells, and the ability of HERV-W Env protein to cause tissue inflammation and systemic autoimmunity, make it an interesting potential new target for the treatment of patients with CIDP and HERV-W upregulation. This evidence suggests that HERV-W is not specific for MS, but may be involved in different diseases through the pathogenic properties of its Env protein upon activation by variable factors in different conditions, tissues, and organs.
HERV-K and ALS
The first evidence that retroviral elements might be activated in ALS came from a study in which brain tissue extracts from two ALS patients in Guam were found to have RNA-directed DNA polymerase activity. This polymerase activity was RNase-sensitive [81], suggesting RT activity. However, no virus or transmissible agent was identified.
Subsequent studies in patients with ALS confirmed the presence of RT in serum and, although with limited numbers, showed that nearly 50% of the patients have detectable RT activity, whereas activity was only detected in a smaller number of first-degree relatives and an even smaller number of unrelated controls 82, 83, 84. However, several attempts to find an exogenous retrovirus in patients with ALS were unsuccessful 83, 85.
In 2011, the detection of RT encoded by the pol gene of HERV-K was reported in the brain of patients with sporadic ALS [86]; expression was specific for ALS because a protein resulting from HERV-K pol activation could not be detected in the brains of patients with Parkinson’s disease or in normal brains, despite occasional detection in patients with cancers. Sequencing of pol gene RNA transcripts suggested activation of multiple loci, with predominant homology to a copy in chromosome 7 [86]. A subsequent study showed expression of HERV-K pol, env, and gag genes in the brains of patients with sporadic ALS [87]. Expression levels of each gene correlated with each other, suggesting that an entire HERV-K genome is activated. HERV-K Env protein expression was also detected in cortical and spinal neurons of ALS patients, whereas no immunostaining was found in brains from healthy individuals or in patients with Alzheimer’s disease [87].
HERV-K virus is so-named because it uses lysine (K) tRNA as a primer. HERV-K represents thousands of insertions in the human genome, and 11 largely complete proviral sequences have been identified to date 88, 89. The number of insertions can vary between human populations, and copies are not necessarily found at a fixed chromosomal site in all carrier individuals 90, 91, while new insertions are thought to have occurred within the past 2 million years after the appearance of modern human [89]. Some of the recently endogenized HERV-K elements have complete ORFs and can form complete viral particles [92]. These are probably recent insertions into the human genome because evolution has not yet introduced major deletions or mutations, and epigenetic mechanisms are likely to be responsible for HERV-K gene silencing, which may be overcome by environmental triggers. Given this genetic complexity, it remains unknown whether the expression of HERV-K in patients with ALS derives from a single copy with a complete retroviral sequence or might represent trans-complementation between partially defective but complementary copies. Such a scenario may also be relevant for the activation of HERV-W in MS because it could also result in the expression of HERV pathogenic protein(s).
HERV-K also plays a role in early human embryonic development. It is expressed in the morula and blastocyst stage of the preimplantation embryo, but expression is silenced later in fetal development [93]. However, if HERV-K expression is forced in neurons, it causes cellular degeneration mediated by its Env protein [87]. Transgenic mice expressing HERV-K Env in neurons developed a clinical and pathological phenotype that resembles ALS, with upper and lower motor neuron degeneration [87].
What triggers the expression of HERV-K in adult neurons of patients with ALS remains unknown. In vitro studies showed that neuronal injury due to oxidative stress or excitotoxicity is insufficient to cause activation of HERV-K genes. Nonetheless, endogenous expression is modulated by transcription factor TDP-43, which has five binding sites on HERV-K LTR [87]. TDP-43 is known to be dysregulated in ALS and has been proposed as a biomarker of ALS lesions [94].
The possibility that HERV-K plays a crucial role in the pathophysiology of ALS, as represented in Figure 5 , is attractive for several reasons. It would explain why several researchers have detected RT in ALS brain and blood samples, but have not been able to demonstrate human-to-animal or human-to-human transmission of the disease, because HERVs arise from the genome and not from the environment. Further, it may also explain the anatomical spread of the illness through paracrine activation of permissive autologous cells, which generally starts in one region of the body and then spreads along an anatomical pathway.
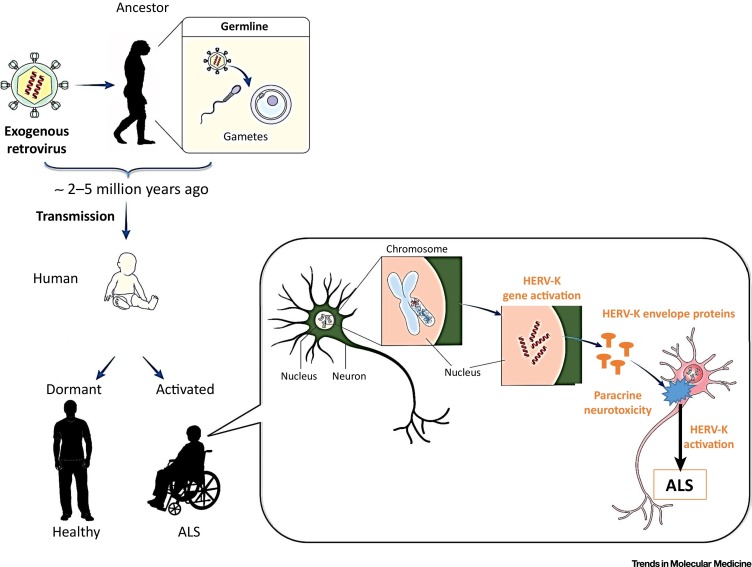
Figure 5.
Hypothetical Scenario Involving Human Endogenous Retrovirus HERV-K Activation Leading to Amyotrophic Lateral Sclerosis (ALS). This scenario depicts the hypothesized origin of retroviral endogenizations during the evolution of species millions of years ago, and the transmission of HERV copies to humans in which HERV-K copies may remain dormant (latent) or activated by still unknown mechanisms. This figure relates to the most recent HERV-K insertions (and not to previous HERV-K/HML-2 insertions at ∼30 million years ago), which are thought to be the most active copies because they are normally less mutated than earlier insertions. When activated in specific areas of the central nervous system, the resulting pathogenic pathway involves HERV-K expression and release of its envelope (Env) protein that causes neurotoxicity in targeted motor neurons.
In addition, this potential mechanism leads to new therapeutic perspectives for these patients. Because HERV-K, although endogenous, retains retroviral properties that underlie its pathogenic expression, an approach to drug development similar to that taken for HIV could be considered. A panel of antiretroviral drugs approved for treating HIV infection was screened, but elevated concentrations were found to be necessary to control HERV-K replication in HeLa cells in vitro [95]. A previous pilot clinical trial with indinavir, a protease inhibitor used for HIV antiretroviral therapy, failed to show any efficacy in ALS [96]. Notably, some patients with HIV infection can develop an ALS-like syndrome, which may show symptom regression and halted evolution under treatment with anti-HIV drugs [97]. These patients also showed expression of HERV-K in blood, the levels of which fell in all patients after the initiation of antiretroviral drugs [98]. As previously discussed in the context of HERV-W, it is possible that HIV proteins such as Tat may regulate the expression of HERV-K [99], and this could explain why the triggering pathways and expression feedback loops may differ in sporadic ALS that is not associated with HIV. It remains possible that anti-HIV drugs might indeed inhibit HERV-K, but that their efficacy is not as good as for HIV [95]. To further investigate the possibility that antiretroviral therapies might be of benefit in ALS, an open-label pilot study has been initiated in Australia and the UK. The trial is enrolling 40 patients with ALS, and will follow them for 3 months without treatment; then treat them for 6 months with triumeq – which includes two reverse transcriptase inhibitors (abacavir and amivudine) that have been shown to effectively inhibit HERV-K reverse transcriptase activity in vitro, and an integrase inhibitor dolutegravir (Clinicaltrials.gov identifier: NCT02868580).
Conclusion and Perspectives
The present review discusses the largely disregarded aspects of HERV multicopy elements which may be dysregulated by epigenetic changes, transactivated by environmental triggers, and contribute to the development of neurological diseases such as MS, ALS, and CIDP. Data accumulated over recent decades provide rationales to identify particular HERV families and their toxic proteins as potential therapeutic targets in these diseases. This has already prompted different groups to evaluate novel therapeutic approaches in patients with MS or ALS.
One potential strategy relies upon the analogy between endogenous HERVs and their ancestral exogenous retroviral origins, and proposes to test antiretroviral drugs that are effective in HIV-infected, but not in HTLV-infected patients with, for example, associated myelopathy.
Another strategy may be to use a humanized neutralizing antibody (GNbAC1) targeting the toxic Env protein that mediates HERV-W pathogenicity (illustrated in Figure 6 ).
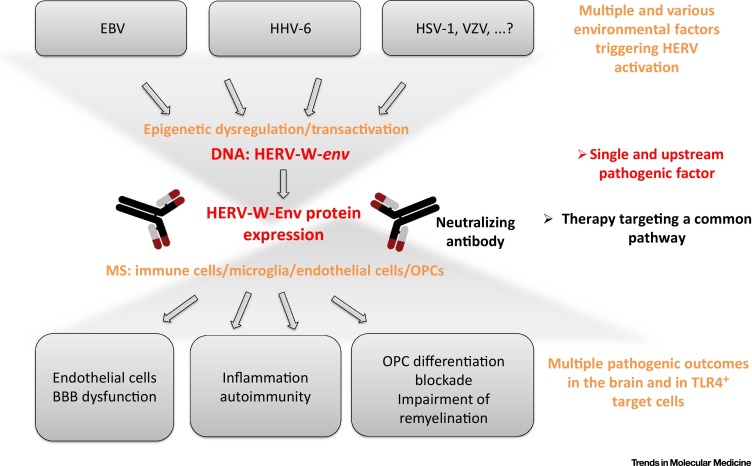
Figure 6.
Proposed Global Model: From Gene–Environment Interactions to a Targeted Therapy in Multiple Sclerosis (MS). The proposed model presents how, after being activated by variable and multiple environmental factors, HERV-W (formerly named MSRV) expresses a pathogenic envelope (Env) protein that appears to represent an unique agonist in the pathway. (i) Downstream HERV-W DNA expression and (ii) upstream pathogenic pathways that are activated in multiple direct and indirect target cells. An antibody neutralizing this pathogenic protein might block the pathogenic pathway at this level, independently of multiple environmental triggers and pathogenic downstream cascades, without interfering with host physiological functions. Abbreviations: EBV, Epstein–Barr virus; HHV-6, human herpes virus 6; HSV-1, herpes simplex virus 1; OPC, oligodendroglial precursor cell; VZV, varicella-zoster virus.
The perspectives offered by the emergence of this new category of ‘pathogens from within’, given consistent findings of their interplay with epidemiologically associated environmental factors [100] and their relevant pathogenic effects, are unlikely to be limited to the examples discussed in the present review. Although many issues remain unresolved (see Outstanding Questions), this scientific domain represents a rapidly evolving area of cutting-edge research (Box 2 ), and further studies may reveal that HERVs contribute to other complex and multifactorial human diseases. Therapeutic approaches targeting toxic HERV proteins or, more directly, HERV gene expression may thus create a change of paradigm with completely novel treatment perspectives. Finally, but not of the least interest, treatments targeting non-physiological HERV components would not dysregulate physiological functions and thus should have favorable safety profiles.
Outstanding Questions.
HERVs are evolutionarily acquired, mostly defective and inactive, and comprise epigenetically silenced genetic elements without assigned physiological functions. However, for what reasons are they are maintained and represent 8% of the human genome?
How are HERV dynamics in health and disease related to the transcriptional activity or protein expression, composition, or mutation of ancestral copies, as well as of unfixed non-ubiquitous copies?
How can we best neutralize endogenous proteins such as those encoded by HERVs? Potential approaches include vaccination, antibody-mediated neutralization of pathogenic components, and antiretroviral compounds.
What are the appropriate windows of opportunity for anti-HERV treatment? Can biomarkers be identified in peripheral blood that would permit routine assessment of HERV reactivation in CNS disease?
Box 2. Clinician’s Corner.
The involvement of HERV expression in MS and ALS provides a shift in paradigm not only for understanding their complex pathogenesis but also as for the development of therapeutic strategies to prevent or treat such diseases.
Although positive results in the first therapeutic attempts to target these HERV elements or their pathogenic proteins would create an immediate breakthrough, the emerging domain of HERVs and human disease is likely to remain cutting-edge science for many years.
The perspectives of future research in this domain at the frontiers of science are expected to unravel etiopathogenic cascades leading to many of the chronic, complex, and multifactorial diseases for which present knowledge remains obscure or partial, and where appropriate therapeutic approaches are not yet available.
Alt-text: Box 2
Acknowledgments
We acknowledge Peter Göttle for artwork in Figures 2, 3, and 4. Research on myelin repair and HERVs in the laboratory of P.K. is supported by the French societies ARSEP (Fondation pour l’Aide à la Recherche sur la Sclérose en Plaques) and AFM (Association Française Contre les Myopathies). The MS Center at the Department of Neurology in Düsseldorf (P.K. and H-P.H.) is additionally supported by the Walter and Ilse Rose Foundation and the Peek & Cloppenburg Düsseldorf Foundation. The studies of P.N.M. have been supported in part by the Agence Nationale de la Recherche (ANR-08-MNPS-041) and by the French patient association ARSEP.
Acknowledgments
Disclaimer Statement
P.K. has performed consultancy work for Geneuro. A.C. has received departmental research grants from Biogen-Idec, CSL-Behring, Geneuro, Novartis, and Octapharma, and has provided expert testimony to Novartis, CSL-Behring, and Genzyme. P.N.M. is inventor on a relevant patent. G.G. has received honoraria from AbbVie, Bayer HealthCare, Biogen, Canbex, FivePrime, Genzyme, GlaxoSmithKline, GW Pharma, Merck Serono, Novartis, Protein Discovery Laboratories, Roche, Synthon, Teva Neuroscience, UCB, and Vertex; research grant support from Biogen, Ironwood, Merck Serono, Merz, and Novartis; and compensation from Elsevier as co-chief editor of Multiple Sclerosis and Related Disorders. J.G. declares no conflicts of interest. H.P.H. has received fees for consulting, speaking, and serving on steering committees from Bayer Healthcare, Biogen, Geneuro, MedImmune, Merck, Novartis, Opexa, Receptos Celgene, Roche, Sanofi Genzyme, and Teva. H.P. receives compensation for his work by Geneuro and is inventor on patents owned by BioMérieux, INSERM, or Geneuro, but has transferred all his rights to BioMérieux or to Geneuro under applicable laws for employed inventors.
Glossary
- Amyotrophic lateral sclerosis (ALS)
a neurodegenerative disease characterized by loss of both upper and lower motor neurons. The symptoms usually start in one anatomical region and then spread, causing motor paralysis, dysphagia, and ultimately affecting respiratory function. In some cases ALS can also involve the prefrontal lobes, leading to cognitive dysfunction. The vast majority of the cases are sporadic, but in 10–20% patients genetic abnormalities are associated with familial forms of ALS.
- Chronic inflammatory demyelinating polyneuropathy (CIDP)
a peripheral nervous system disease and the commonest chronic immune-mediated peripheral neuropathy that takes either a relapsing or progressive course. Clinically it manifests by the development of weakness and sensory disturbance that lead to marked disability. Multifocal inflammation and stripping of myelin sheaths by macrophages are thought to result from aberrant immune responses, mediated by T and/or B lymphocytes, against peripheral nerve antigens.
- Human endogenous retroviruses (HERVs)
sequences belonging to human ERV families retaining structural features of retroviral genomes that have become integrated into the genome through repeated infections during evolution.
- Immune tolerance
unresponsiveness of the immune system to substances that normally elicit an immune response.
- Major histocompatibility complex (MHC)
cell-surface proteins crucial for the recognition of foreign molecules by the immune system. They bind to antigens derived from pathogens and display them for recognition by appropriate T cells.
- Mobile genetic elements
these represent nearly 50% of the human genome, and consist of retrotransposons and DNA transposons.
- Myelin
a major component of white matter that consists of a lipid-rich structure organized into multilayered sheaths around axons. It provides axonal integrity, trophic and metabolic support, and accelerated nerve conduction. Its structural integrity is of vital importance for CNS function.
- Neuroregeneration
there are substantial differences in the extent and cellular origin of neuroregenerative responses between the CNS and the peripheral nervous system (PNS). Whereas injured axons and lost myelin sheaths can be restored in injured peripheral nerves, repair processes in the CNS are scarce and are limited to partial remyelination. Demyelinated CNS axons are functionally impaired, highly vulnerable, and degenerate over time. In contrast to peripheral neurons, such injured central neurons lack the capacity to regrow axons, and this further contributes to irreversible functional deficits. Nonetheless, in chronic PNS diseases such as CIDP the extent of repair and functional recovery is also largely impaired.
- Oligoclonal bands (OCBs)
bands of specific immunoglobulins that can be detected in patient blood serum or cerebrospinal fluid. They have diagnostic value in MS.
- Superantigens (SAgs)
molecules inducing antigen-independent and hence polyclonal activation of lymphocytes.
References
- 1.Hayes M., et al. Pathological and evolutionary implications of retroviruses as mobile genetic elements. Genes. 2013;4:573–582. doi: 10.3390/genes4040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babatz T.D., Burns K.H. Functional impact of the human mobilome. Curr. Opin. Genet. Dev. 2013;23:264–270. doi: 10.1016/j.gde.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pace J.K., 2nd, Feschotte C. The evolutionary history of human DNA transposons: evidence for intense activity in the primate lineage. Genome Res. 2007;17:422–432. doi: 10.1101/gr.5826307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katzourakis A., Gifford R.J. Endogenous viral elements in animal genomes. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feschotte C., Gilbert C. Endogenous viruses: insights into viral evolution and impact on host biology. Nat. Rev. Genet. 2012;13:283–296. doi: 10.1038/nrg3199. [DOI] [PubMed] [Google Scholar]
- 6.Chuong E.B., et al. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science. 2016;351:1083–1087. doi: 10.1126/science.aad5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnaud B., et al. Evidence of selection on the domesticated ERVWE1 env retroviral element involved in placentation. Mol. Biol. Evol. 2004;21:1895–1901. doi: 10.1093/molbev/msh206. [DOI] [PubMed] [Google Scholar]
- 8.Engel M.E., Hiebert S.W. The enemy within: dormant retroviruses awaken. Nat. Med. 2010;16:517–518. doi: 10.1038/nm0510-517. [DOI] [PubMed] [Google Scholar]
- 9.Volkman H.E., Stetson D.B. The enemy within: endogenous retroelements and autoimmune disease. Nat. Immunol. 2014;15:415–422. doi: 10.1038/ni.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perron H., et al. Molecular characteristics of human endogenous retrovirus type-W in schizophrenia and bipolar disorder. Transl. Psychiatry. 2012;2:e201. doi: 10.1038/tp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levet S., et al. An ancestral retroviral protein identified as a therapeutic target in type-1 diabetes. JCI Insight. 2017;2 doi: 10.1172/jci.insight.94387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criscione S.W., et al. Transcriptional landscape of repetitive elements in normal and cancer human cells. BMC Genomics. 2014;15:583. doi: 10.1186/1471-2164-15-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang P., Tang W. Database documentation of retrotransposon insertion polymorphisms. Front. Biosci. 2012;4:1542–1555. doi: 10.2741/479. [DOI] [PubMed] [Google Scholar]
- 14.Hedges D.J., Belancio V.P. Restless genomes: humans as a model organism for understanding host–retrotransposable element dynamics. Adv. Genet. 2011;73:219–262. doi: 10.1016/B978-0-12-380860-8.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina J., Perron H. DNA sequences from mobile genetic elements, a hidden half of the human genome. Med. Sci. 2017;33:151–158. doi: 10.1051/medsci/20173302010. [DOI] [PubMed] [Google Scholar]
- 16.Hancks D.C., Kazazian H.H., Jr. Roles for retrotransposon insertions in human disease. Mob. DNA. 2016;7:9. doi: 10.1186/s13100-016-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishibuchi G., Dejardin J. The molecular basis of the organization of repetitive DNA-containing constitutive heterochromatin in mammals. Chromosome Res. 2017;25:77–87. doi: 10.1007/s10577-016-9547-3. [DOI] [PubMed] [Google Scholar]
- 18.Campos-Sanchez R., et al. Genomic landscape of human, bat, and ex vivo DNA transposon integrations. Mol. Biol. Evol. 2014;31:1816–1832. doi: 10.1093/molbev/msu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belshaw R., et al. Long-term reinfection of the human genome by endogenous retroviruses. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4894–4899. doi: 10.1073/pnas.0307800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Lagemaat L.N., et al. Multiple effects govern endogenous retrovirus survival patterns in human gene introns. Genome Biol. 2006;7:R86. doi: 10.1186/gb-2006-7-9-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serra C., et al. In vitro modulation of the multiple sclerosis (MS)-associated retrovirus by cytokines: implications for MS pathogenesis. J. Neurovirol. 2003;9:637–643. doi: 10.1080/13550280390246462. [DOI] [PubMed] [Google Scholar]
- 22.Uleri E., et al. HIV Tat acts on endogenous retroviruses of the W family and this occurs via Toll-like receptor 4: inference for neuroAIDS. AIDS. 2014;28:2659–2670. doi: 10.1097/QAD.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 23.Petersen T., et al. Effects of interferon-beta therapy on innate and adaptive immune responses to the human endogenous retroviruses HERV-H and HERV-W, cytokine production, and the lectin complement activation pathway in multiple sclerosis. J. Neuroimmunol. 2009;215:108–116. doi: 10.1016/j.jneuroim.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Mameli G., et al. Inhibition of multiple-sclerosis-associated retrovirus as biomarker of interferon therapy. J. Neurovirol. 2008;14:73–77. doi: 10.1080/13550280701801107. [DOI] [PubMed] [Google Scholar]
- 25.Perron H., et al. Herpes simplex virus ICP0 and ICP4 immediate early proteins strongly enhance expression of a retrovirus harboured by a leptomeningeal cell line from a patient with multiple sclerosis. J. Gen. Virol. 1993;74:65–72. doi: 10.1099/0022-1317-74-1-65. [DOI] [PubMed] [Google Scholar]
- 26.Brudek T., et al. Activation of endogenous retrovirus reverse transcriptase in multiple sclerosis patient lymphocytes by inactivated HSV-1, HHV-6 and VZV. J. Neuroimmunol. 2007;187:147–155. doi: 10.1016/j.jneuroim.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Ruprecht K., et al. Regulation of human endogenous retrovirus W protein expression by herpes simplex virus type 1: implications for multiple sclerosis. J. Neurovirol. 2006;12:65–71. doi: 10.1080/13550280600614973. [DOI] [PubMed] [Google Scholar]
- 28.Bergallo M., et al. CMV induces HERV-K and HERV-W expression in kidney transplant recipients. J. Clin. Virol. 2015;68:28–31. doi: 10.1016/j.jcv.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Tai A.K., et al. HHV-6A infection induces expression of HERV-K18–encoded superantigen. J. Clin. Virol. 2009;46:47–48. doi: 10.1016/j.jcv.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Sutkowski N., et al. Epstein–Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity. 2001;15:579–589. doi: 10.1016/s1074-7613(01)00210-2. [DOI] [PubMed] [Google Scholar]
- 31.Mameli G., et al. Expression and activation by Epstein–Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leibovitch E.C., Jacobson S. Viruses in chronic progressive neurologic disease. Mult. Scler. 2018;24:48–52. doi: 10.1177/1352458517737392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morahan P.S., et al. Molecular localization of abortive infection of resident peritoneal macrophages by herpes simplex virus type 1. J. Virol. 1989;63:2300–2307. doi: 10.1128/jvi.63.5.2300-2307.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammerschmidt W. The epigenetic life cycle of Epstein–Barr virus. Curr. Top. Microbiol. Immunol. 2015;390:103–117. doi: 10.1007/978-3-319-22822-8_6. [DOI] [PubMed] [Google Scholar]
- 35.Ascherio A., Munger K.L. Epstein–Barr virus infection and multiple sclerosis: a review. J. Neuroimmune Pharmacol. 2010;5:271–277. doi: 10.1007/s11481-010-9201-3. [DOI] [PubMed] [Google Scholar]
- 36.Mameli G., et al. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein–Barr virus latency: the missing link with multiple sclerosis? PLoS One. 2013;8 doi: 10.1371/journal.pone.0078474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toufaily C., et al. Activation of LTRs from different human endogenous retrovirus (HERV) families by the HTLV-1 tax protein and T-cell activators. Viruses. 2011;3:2146–2159. doi: 10.3390/v3112146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perron H., et al. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vbeta16 T-lymphocyte activation. Virology. 2001;287:321–332. doi: 10.1006/viro.2001.1045. [DOI] [PubMed] [Google Scholar]
- 39.Perron H., et al. Human endogenous retrovirus protein activates innate immunity and promotes experimental allergic encephalomyelitis in mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dendrou C.A., et al. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 41.Petereit H.F., Reske D. Expansion of antibody reactivity in the cerebrospinal fluid of multiple sclerosis patients – follow-up and clinical implications. Cerebrospinal Fluid Res. 2005;2:3. doi: 10.1186/1743-8454-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahad D.H., et al. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 43.Rocha B., et al. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J. Exp. Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Illingworth J., et al. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J. Immunol. 2013;190:1038–1047. doi: 10.4049/jimmunol.1202438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollenbach J.A., Oksenberg J.R. The immunogenetics of multiple sclerosis: a comprehensive review. J. Autoimmun. 2015;64:13–25. doi: 10.1016/j.jaut.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belbasis L., et al. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14:263–273. doi: 10.1016/S1474-4422(14)70267-4. [DOI] [PubMed] [Google Scholar]
- 47.Hedstrom A.K., et al. Environmental factors and their interactions with risk genotypes in MS susceptibility. Curr. Opin. Neurol. 2016;29:293–298. doi: 10.1097/WCO.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 48.Renz H., et al. Gene–environment interactions in chronic inflammatory disease. Nat. Immunol. 2011;12:273–277. doi: 10.1038/ni0411-273. [DOI] [PubMed] [Google Scholar]
- 49.McKay K.A., et al. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: a systematic review. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/817238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nissen K.K., et al. No additional copies of HERV-Fc1 in the germ line of multiple sclerosis patients. Virol. J. 2012;9:188. doi: 10.1186/1743-422X-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansen B., et al. Genetic association of multiple sclerosis with the marker rs391745 near the endogenous retroviral locus HERV-Fc1: analysis of disease subtypes. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muradrasoli S., et al. Development of real-time PCRs for detection and quantitation of human MMTV-like (HML) sequences HML expression in human tissues. J. Virol. Methods. 2006;136:83–92. doi: 10.1016/j.jviromet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Christensen T., et al. Molecular characterization of HERV-H variants associated with multiple sclerosis. Acta Neurol. Scand. 2000;101:229–238. doi: 10.1034/j.1600-0404.2000.101004229.x. [DOI] [PubMed] [Google Scholar]
- 54.Brudek T., et al. B cells and monocytes from patients with active multiple sclerosis exhibit increased surface expression of both HERV-H Env and HERV-W Env, accompanied by increased seroreactivity. Retrovirology. 2009;6:104. doi: 10.1186/1742-4690-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christensen T. Human endogenous retroviruses in neurologic disease. APMIS. 2016;124:116–126. doi: 10.1111/apm.12486. [DOI] [PubMed] [Google Scholar]
- 56.Morandi E., et al. The association between human endogenous retroviruses and multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perron H., et al. Leptomeningeal cell line from multiple sclerosis with reverse transcriptase activity and viral particles. Res. Virol. 1989;140:551–561. doi: 10.1016/s0923-2516(89)80141-4. [DOI] [PubMed] [Google Scholar]
- 58.Perron H., et al. Isolation of retrovirus from patients with multiple sclerosis. Lancet. 1991;337:862–863. doi: 10.1016/0140-6736(91)92579-q. [DOI] [PubMed] [Google Scholar]
- 59.Komurian-Pradel F., et al. Molecular cloning and characterization of MSRV-related sequences associated with retrovirus-like particles. Virology. 1999;260:1–9. doi: 10.1006/viro.1999.9792. [DOI] [PubMed] [Google Scholar]
- 60.Perron H., et al. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. The Collaborative Research Group on Multiple Sclerosis. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7583–7588. doi: 10.1073/pnas.94.14.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tuke P.W., et al. Development of a pan-retrovirus detection system for multiple sclerosis studies. Acta Neurol. Scand. Suppl. 1997;169:16–21. doi: 10.1111/j.1600-0404.1997.tb08145.x. [DOI] [PubMed] [Google Scholar]
- 62.Blond J.L., et al. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J. Virol. 1999;73:1175–1185. doi: 10.1128/jvi.73.2.1175-1185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mameli G., et al. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not human herpesvirus 6. J. Gen. Virol. 2007;88:264–274. doi: 10.1099/vir.0.81890-0. [DOI] [PubMed] [Google Scholar]
- 64.Perron H., et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult. Scler. 2012;18:1721–1736. doi: 10.1177/1352458512441381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sotgiu S., et al. Multiple sclerosis-associated retrovirus and progressive disability of multiple sclerosis. Mult. Scler. 2010;16:1248–1251. doi: 10.1177/1352458510376956. [DOI] [PubMed] [Google Scholar]
- 66.Garson J.A., et al. Detection of virion-associated MSRV-RNA in serum of patients with multiple sclerosis. Lancet. 1998;351:33. doi: 10.1016/s0140-6736(98)24001-3. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Montojo M., et al. The DNA copy number of human endogenous retrovirus-W (MSRV-type) is increased in multiple sclerosis patients and is influenced by gender and disease severity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kremer D., et al. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann. Neurol. 2013;74:721–732. doi: 10.1002/ana.23970. [DOI] [PubMed] [Google Scholar]
- 69.van Horssen J., et al. Human endogenous retrovirus W in brain lesions: rationale for targeted therapy in multiple sclerosis. Mult. Scler. Relat. Disord. 2016;8:11–18. doi: 10.1016/j.msard.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Rolland A., et al. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 2006;176:7636–7644. doi: 10.4049/jimmunol.176.12.7636. [DOI] [PubMed] [Google Scholar]
- 71.Saresella M., et al. Multiple sclerosis-associated retroviral agent (MSRV)-stimulated cytokine production in patients with relapsing-remitting multiple sclerosis. Mult. Scler. 2009;15:443–447. doi: 10.1177/1352458508100840. [DOI] [PubMed] [Google Scholar]
- 72.Kremer D., et al. The neutralizing antibody GNbAC1 abrogates HERV-W envelope protein-mediated oligodendroglial maturation blockade. Mult. Scler. 2015;21:1200–1203. doi: 10.1177/1352458514560926. [DOI] [PubMed] [Google Scholar]
- 73.Curtin F., et al. Preclinical and early clinical development of GNbAC1, a humanized IgG4 monoclonal antibody targeting endogenous retroviral MSRV-Env protein. MAbs. 2015;7:265–275. doi: 10.4161/19420862.2014.985021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duperray A., et al. Inflammatory response of endothelial cells to a human endogenous retrovirus associated with multiple sclerosis is mediated by TLR4. Int. Immunol. 2015;27:545–553. doi: 10.1093/intimm/dxv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Curtin F., et al. Serum pharmacokinetics and cerebrospinal fluid concentration analysis of the new IgG4 monoclonal antibody GNbAC1 to treat multiple sclerosis: a Phase 1 study. MAbs. 2016;8:854–860. doi: 10.1080/19420862.2016.1168956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Derfuss T., et al. A phase IIa randomized clinical study testing GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis associated endogenous retrovirus in multiple sclerosis patients – a twelve month follow-up. J. Neuroimmunol. 2015;285:68–70. doi: 10.1016/j.jneuroim.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 77.Curtin F., et al. GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus: a first-in-humans randomized clinical study. Clin. Ther. 2012;34:2268–2278. doi: 10.1016/j.clinthera.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 78.Curtin F., et al. A placebo randomized controlled study to test the efficacy and safety of GNbAC1, a monoclonal antibody for the treatment of multiple sclerosis – rationale and design. MSARD. 2016;9:95–100. doi: 10.1016/j.msard.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Maruszak H., et al. Could antiretroviral drugs be effective in multiple sclerosis? A case report. Eur. J. Neurol. 2011;18:e110–e111. doi: 10.1111/j.1468-1331.2011.03430.x. [DOI] [PubMed] [Google Scholar]
- 80.Faucard R., et al. Human endogenous retrovirus and neuroinflammation in chronic inflammatory demyelinating polyradiculoneuropathy. EBioMedicine. 2016;6:190–198. doi: 10.1016/j.ebiom.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viola M.V., et al. RNA-instructed DNA polymerase activity in a cytoplasmic particulate fraction in brains from Guamanian patients. J. Exp. Med. 1975;142:483–494. doi: 10.1084/jem.142.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andrews W.D., et al. Detection of reverse transcriptase activity in the serum of patients with motor neurone disease. J. Med. Virol. 2000;61:527–532. doi: 10.1002/1096-9071(200008)61:4<527::aid-jmv17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 83.McCormick A.L., et al. Quantification of reverse transcriptase in ALS and elimination of a novel retroviral candidate. Neurology. 2008;70:278–283. doi: 10.1212/01.wnl.0000297552.13219.b4. [DOI] [PubMed] [Google Scholar]
- 84.Steele A.J., et al. Detection of serum reverse transcriptase activity in patients with ALS and unaffected blood relatives. Neurology. 2005;64:454–458. doi: 10.1212/01.WNL.0000150899.76130.71. [DOI] [PubMed] [Google Scholar]
- 85.Kim Y.J., et al. No evidence of HIV pol gene in spinal cord tissues in sporadic ALS by real-time RT-PCR. Amyotroph. Lateral Scler. 2010;11:91–96. doi: 10.3109/17482960902835988. [DOI] [PubMed] [Google Scholar]
- 86.Douville R., et al. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann. Neurol. 2011;69:141–151. doi: 10.1002/ana.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li W., et al. Human endogenous retrovirus-K contributes to motor neuron disease. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aac8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belshaw R., et al. Genomewide screening reveals high levels of insertional polymorphism in the human endogenous retrovirus family HERV-K(HML2): implications for present-day activity. J. Virol. 2005;79:12507–12514. doi: 10.1128/JVI.79.19.12507-12514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Subramanian R.P., et al. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology. 2011;8:90. doi: 10.1186/1742-4690-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marchi E., et al. Unfixed endogenous retroviral insertions in the human population. J. Virol. 2014;88:9529–9537. doi: 10.1128/JVI.00919-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wildschutte J.H., et al. Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E2326–E2334. doi: 10.1073/pnas.1602336113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee Y.N., Bieniasz P.D. Reconstitution of an infectious human endogenous retrovirus. PLoS Pathog. 2007;3:e10. doi: 10.1371/journal.ppat.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goke J., et al. Dynamic transcription of distinct classes of endogenous retroviral elements marks specific populations of early human embryonic cells. Cell Stem Cell. 2015;16:135–141. doi: 10.1016/j.stem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Igaz L.M., et al. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J. Clin. Invest. 2011;121:726–738. doi: 10.1172/JCI44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tyagi R., et al. Inhibition of human endogenous retrovirus-K replication by antiretroviral drugs. Retrovirology. 2017;14:21. doi: 10.1186/s12977-017-0347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scelsa S.N., et al. A pilot, double-blind, placebo-controlled trial of indinavir in patients with ALS. Neurology. 2005;64:1298–1300. doi: 10.1212/01.WNL.0000156913.24701.72. [DOI] [PubMed] [Google Scholar]
- 97.Alfahad T., Nath A. Retroviruses and amyotrophic lateral sclerosis. Antiviral Res. 2013;99:180–187. doi: 10.1016/j.antiviral.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bowen L.N., et al. HIV-associated motor neuron disease: HERV-K activation and response to antiretroviral therapy. Neurology. 2016;87:1756–1762. doi: 10.1212/WNL.0000000000003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez-Hernandez M.J., et al. Regulation of the human endogenous retrovirus K (HML-2) transcriptome by the HIV-1 Tat protein. J. Virol. 2014;88:8924–8935. doi: 10.1128/JVI.00556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perron H., et al. Endogenous retroviral genes, herpesviruses and gender in multiple sclerosis. J. Neurol. Sci. 2009;286:65–72. doi: 10.1016/j.jns.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 101.Makalowski W. The human genome structure and organization. Acta Biochim. Pol. 2001;48:587–598. [PubMed] [Google Scholar]
- 102.Grover D., et al. ALU-ring elements in the primate genomes. Genetica. 2005;124:273–289. doi: 10.1007/s10709-005-3086-8. [DOI] [PubMed] [Google Scholar]
- 103.Kulski J.K., Dawkins R.L. The P5 multicopy gene family in the MHC is related in sequence to human endogenous retroviruses HERV-L and HERV-16. Immunogenetics. 1999;49:404–412. doi: 10.1007/s002510050513. [DOI] [PubMed] [Google Scholar]