Summary
Background
Despite large reductions in under-5 lower respiratory infection (LRI) mortality in many locations, the pace of progress for LRIs has generally lagged behind that of other childhood infectious diseases. To better inform programmes and policies focused on preventing and treating LRIs, we assessed the contributions and patterns of risk factor attribution, intervention coverage, and sociodemographic development in 195 countries and territories by drawing from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017 (GBD 2017) LRI estimates.
Methods
We used four strategies to model LRI burden: the mortality due to LRIs was modelled using vital registration data, demographic surveillance data, and verbal autopsy data in a predictive ensemble modelling tool; the incidence of LRIs was modelled using population representative surveys, health-care utilisation data, and scientific literature in a compartmental meta-regression tool; the attribution of risk factors for LRI mortality was modelled in a counterfactual framework; and trends in LRI mortality were analysed applying changes in exposure to risk factors over time. In GBD, infectious disease mortality, including that due to LRI, is among HIV-negative individuals. We categorised locations based on their burden in 1990 to make comparisons in the changing burden between 1990 and 2017 and evaluate the relative percent change in mortality rate, incidence, and risk factor exposure to explain differences in the health loss associated with LRIs among children younger than 5 years.
Findings
In 2017, LRIs caused 808 920 deaths (95% uncertainty interval 747 286–873 591) in children younger than 5 years. Since 1990, there has been a substantial decrease in the number of deaths (from 2 337 538 to 808 920 deaths; 65·4% decrease, 61·5–68·5) and in mortality rate (from 362·7 deaths [330·1–392·0] per 100 000 children to 118·9 deaths [109·8–128·3] per 100 000 children; 67·2% decrease, 63·5–70·1). LRI incidence declined globally (32·4% decrease, 27·2–37·5). The percent change in under-5 mortality rate and incidence has varied across locations. Among the risk factors assessed in this study, those responsible for the greatest decrease in under-5 LRI mortality between 1990 and 2017 were increased coverage of vaccination against Haemophilus influenza type b (11·4% decrease, 0·0–24·5), increased pneumococcal vaccine coverage (6·3% decrease, 6·1–6·3), and reductions in household air pollution (8·4%, 6·8–9·2).
Interpretation
Our findings show that there have been substantial but uneven declines in LRI mortality among countries between 1990 and 2017. Although improvements in indicators of sociodemographic development could explain some of these trends, changes in exposure to modifiable risk factors are related to the rates of decline in LRI mortality. No single intervention would universally accelerate reductions in health loss associated with LRIs in all settings, but emphasising the most dominant risk factors, particularly in countries with high case fatality, can contribute to the reduction of preventable deaths.
Funding
Bill & Melinda Gates Foundation.
Introduction
Lower respiratory infections (LRIs) are the leading infectious cause of death among children younger than 5 years globally, and mortality due to LRIs has declined substantially since the 1990s.1 Accelerating and maintaining these declines is essential to meeting Sustainable Development Goals for under-5 childhood mortality and ensuring that children everywhere have the opportunity to live a full, healthy life. Yet, no country has a national pneumonia control strategy and pneumonia attracts a small fraction of international development assistance and research and development funding.2 Several global initiatives have sought to fill this gap and provide guidance on the most efficient interventions to avert illness and mortality and to champion LRI as a preventable cause of death.2, 3, 4, 5 These programmes have typically categorised risk factors and interventions into groups that are defined by the stage in the morbidity pathway at which they occur, including protection against illness, prevention of infection, and treatment of disease.4, 5
Research in context.
Evidence before this study
Lower respiratory infections (LRIs) haven previously been identified as the leading infectious cause of death among children younger than 5 years. Several prominent global burden estimation groups, the WHO Maternal and Child Epidemiology Estimation group, and the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) have iteratively quantified the morbidity and mortality associated with LRIs. Based on these findings, several initiatives have sought to give guidance about effective ways to reduce health loss due to LRIs, including the Global Action Plan for Pneumonia and Diarrhoea, The Missing Piece, and a 2013 Lancet Series about effective ways to reduce child mortality. We have previously published estimates of LRI mortality from GBD 2015 and 2016 and in those Articles, have looked at risks and interventions. We conducted a search in PubMed on April 30, 2019, using the search terms “(“lower respiratory infection” OR pneumonia) AND mortality AND global AND risk AND trend*)”. After removing publications using GBD results, we found 49 articles, many of which reported on single risk factors or countries. These manuscripts have been primarily cross-sectional and, to our knowledge, no other study has attempted to evaluate changes in LRI disease burden over time due to demographic changes and changes in risk factor exposure.
Added value of this study
Here we report findings from GBD 2017, which builds on previous iterations of GBD with additional data and modelling improvements. We use estimates for 13 risk factors or interventions for LRI morbidity or mortality, produced for GBD, to evaluate changes in LRI mortality among children younger than 5 years. We use a conceptual framework to group these risk factors into categories of those that primarily prevent initial LRI episodes (such as the pneumococcal conjugate vaccine) and those that primarily protect children with LRIs from dying (such as antibiotic therapy). A major component of GBD is producing internally consistent and externally comparable estimates for all locations and over time, which allows us to identify countries where the incidence or mortality has changed most rapidly and to evaluate the risk factors or interventions are most associated with these changes. We provide cross-sectional and longitudinal estimates of the reasons for which children are dying from LRIs, how this varies, and where specific interventions might have the greatest impact.
Implications of all the available evidence
The incidence and mortality due to LRIs among HIV-negative children younger than 5 years has declined in many parts of the world, particularly because of decreased exposure to household air pollution, reductions in prevalence of childhood wasting, and increased vaccine coverage. However, there is variation by country, suggesting that there is no single intervention that will substantially reduce LRI mortality in every country. Individual countries or regions must consider their specific context to identify strategies to reduce LRI disease burden. Our results, while being mindful of the limitations of modelled estimates, can help provide the evidence needed to develop plans to give children everywhere a chance at a life free from LRIs.
The decline in under-5 LRI mortality has not been universal and has varied between countries.6 Understanding why it declined faster in some countries than in others provides specific, actionable evidence to further reduce disease burden. The Global Burden of Diseases, Injuries, and Risk Factors Study 2017 (GBD 2017) is a systematic, scientific effort to quantify morbidity and mortality, including LRIs and their risk factors. We used results from GBD 2017 to assess which countries have performed best in reducing under-5 LRI mortality and compare countries on the basis of mortality rates, case fatality, and changes in risk factor exposure. This Article identifies countries where the change in under-5 LRI mortality has been largest, and uses the expansive set of estimates produced for GBD 2017 to analyse these changes, aiming to assess how and why they have occurred and to provide a roadmap for strategies to accelerate declines in mortality.
Methods
Overview
Detailed methods on GBD and on LRI estimation in GBD have been previously published.1, 6, 7, 8, 9 We describe these methods briefly. There were no substantial modelling changes between GBD 2016 and GBD 2017. LRIs are defined as diseases of the lower airways including pneumonia and bronchiolitis. Uncertainty in the LRI estimates are maintained through the modelling process using draws and is reflected as 2·5th and 97·5th percentiles of the posterior distribution. In compliance with the Guidelines for Accurate and Transparent Health Estimates Reporting, data and code for GBD 2017 are publicly available. There are four main components of the analysis that we share here: LRI mortality estimation; LRI morbidity estimation; estimation of LRI mortality attributable to the independent effects of risk factors; and an analysis of trends in LRI mortality.
LRI mortality and morbidity estimation
Most causes of death in GBD 2017, including LRI, are modelled with the Cause of Death Ensemble model tool.1, 10 This statistical tool is designed to create a wide variety of models using a covariate selection algorithm and then to weight these models on the basis of their out-of-sample predictive validity. We combined these models into an ensemble that predicts LRI mortality by age, sex, year, and location from 1980 to 2017. The model for LRI used vital registration data, demographic surveillance data, and verbal autopsy data. Covariates included childhood growth failure, ambient and household air pollution, nutritional deficiency, Socio-Demographic Index (SDI), and maternal education, among others (appendix pp 6). Causes of death in the GBD study are mutually exclusive and each death has one cause. Importantly, any LRI death among people with HIV is considered to have HIV as the underlying cause of death, therefore our results represent LRI mortality among HIV-negative children younger than 5 years (appendix p 2).
The incidence and prevalence of LRI were modelled using DisMod-MR 2.1 (DisMod), a Bayesian meta-regression tool.7 One of the primary advantages of DisMod is that it enforces consistency between incidence, prevalence, recovery, and mortality by solving a series of ordinary differential equations. Input data for this model are from population-representative surveys, health-care utilisation records, and scientific literature. We used two covariates to help predict in areas with little or no data coverage: a composite indicator of the cumulative risk exposure for LRI, called the summary exposure variable and developed for GBD, and the SDI (appendix pp 9, 10).
LRI trend analysis
We applied the results of the aforementioned models to spatiotemporal patterns. We compared estimates of LRI mortality and incidence in 1990 and 2017. To group countries into categories of similar burden, we identified country groupings on the basis of the burden in 1990. We split countries into four groups on the basis of the median mortality rate and incidence in 1990 and defined them as: high mortality and high incidence, high mortality and low incidence, low mortality and high incidence, and low mortality and low incidence.
Case fatality ratio
The case fatality ratio is defined as the ratio of number of deaths to number of incident cases. We fit a log-normal regression using SDI to predict the expected change in LRI case fatality ratio. This was considered the baseline change in case fatality ratio that is explained by SDI.
Risk factor attribution
Risk factors in GBD 2017 are causally related to LRI incidence or mortality.8 In this study, we analysed 13 of the risk factors for LRI identified in GBD 2017 (ambient air pollution, household air pollution, low Haemophilus influenzae type b [Hib] vaccine coverage, low pneumococcal conjugate vaccine [PCV] coverage, no handwashing, second-hand smoking, zinc deficiency, breastfeeding, low antibiotic coverage, low birthweight and short gestation, stunting, underweight, and wasting; appendix pp 13–15). The estimation strategy for risk factors involved a counterfactual approach that quantifies the level of exposure to the risk factor in a population and the relative risk of LRI given exposure. Typically, the exposure in a population is modelled on the basis of surveys and scientific literature and the risk of LRI is derived from published meta-analyses. Childhood growth failure risks were estimated as a continuous exposure of the height or weight Z scores. Likewise, air pollution was considered a continuous exposure of the amount of fine particulate matter smaller than 2·5 μm in diameter. Other risk factors, such as low vaccine coverage, are modelled when the exposure is a population prevalence of being exposed to that risk factor (eg, the population prevalence of being unvaccinated for low vaccine coverage). Descriptions of the risk-factor exposure models and relative risks are provided in the appendix (pp 13–66). Risk factors in GBD are part of a comparative risk assessment framework and are modelled independently.8 Therefore, in our study, the burden associated with each risk factor can be considered as the LRI mortality that could be averted if exposure to that risk factor was eliminated. Since they were modelled independently, our analysis does not quantify the potential impact of combined interventions and combining risk-factor burden by summing risk factors is not appropriate and could lead to greater attribution than disease burden.
Intervention efficiency assessment
To assess the efficiency of targeted interventions for each risk factor among children younger than 5 years, we took advantage of the counterfactual definition of risk-factor burden such that the LRI mortality rate attributable to each risk factor was equivalent to the reduction expected given complete absence of the risk factor.8 For example, for vaccines, the risk exposure was defined as no vaccination, so the counterfactual was full vaccine coverage.
We classified risk factors into two categories based on their biological mechanism of risk and modelled after a conceptualisation proposed by the Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea.3 Conceptually, prevention risks are those that increase the probability of developing a LRI and include ambient air pollution, household air pollution, low Hib vaccine coverage, low PCV coverage, no access to a handwashing station with soap and water, second-hand smoke exposure, and zinc deficiency (appendix p 12). Protection risks are those that increase the probability of dying once a child developed an LRI and include suboptimal breastfeeding, low antibiotic coverage, low birthweight and short gestation, childhood stunting, childhood underweight, and childhood wasting (appendix p 13–15). We decomposed the effect of the change in exposure to each risk factor on the LRI mortality rate between 1990 and 2017, accounting for the independent effects of population growth, population ageing, and other drivers of LRI mortality. This process has been described in detail elsewhere.6, 8
Role of the funding source
The funder of the study played no role in study design, data collection, data analysis, data interpretation, or writing of the report. All collaborators had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
Globally, LRIs were the leading infectious cause of death among children younger than 5 years in 2017 (808 920 deaths, 95% uncertainty interval [UI] 747 286–873 591; table), responsible for 15·0% (14·0–16·0) of all under-5 deaths. There was no substantial difference in the under-5 LRI mortality rate between boys (118·2 deaths, 108·2–129·6, per 100 000 boys) and girls (119·5 deaths, 109·6–129·6, per 100 000 girls; data are available on GBD-Compare). Since 1990, there has been a substantial decrease in the number of deaths (65·4% decrease, 61·5–68·5; from 2 337 538 deaths to 808 920 deaths), the mortality rate (67·2% decrease, 63·6–70·2; from 362·7 deaths, 330·1–392·0, per 100 000 children to 118·9 deaths, 109·8–128·3, per 100 000 children; table), and the percent of under-5 deaths that were due to LRIs (24·6% decrease, 17·2–30·4; from 19·9%, 18·1–21·4, to 15·0%, 14·0–16·0) among children younger than 5 years.
Table.
Deaths and case fatality attributable to and incidence of lower respiratory infections among children younger than 5 years by Global Burden of Diseases, Injuries, and Risk Factors Study regions and super-regions, 2017
| Deaths (95% UI) | Mortality rate per 100 000 (95% UI) | Percentage change mortality rate (95% UI), 1990–2017 | Incidence per 100 000 (95% UI) | Percentage incidence change (95% UI), 1990–2017 | Case fatality ratio (95% UI) | Attributable fraction for all risks (95% UI) | Attributable fraction for prevention-associated risks (95% UI) | Attributable fraction for protection-associated risks (95% UI) | |
|---|---|---|---|---|---|---|---|---|---|
| Global | 808 920 (747 286 to 873 591) | 118·9 (109·8 to 128·3) | –67·2% (−70·2 to −63·6) | 12 197·8 (9 762·1 to 14 908·7) | –32·4% (−37·5 to −27·2) | 1·0% (0·9 to 1·1) | 93·4% (90·3 to 95·7) | 65·2% (50·2 to 77·6) | 82·0% (62·6 to 92·1) |
| Central Europe, eastern Europe, and central Asia | 16 040 (14 296 to 18 051) | 57·3 (51·0 to 64·4) | –66·8% (−70·7 to −62·3) | 9 219·6 (7 103·3 to 11 586·8) | –36·3% (−43·2 to −28·6) | 0·6% (0·6 to 0·7) | 84·5% (77·5 to 89·7) | 47·8% (33·5 to 62·2) | 75·8% (53·6 to 88·4) |
| Central Asia | 13 937 (12 246 to 15 922) | 145·4 (127·7 to 166·1) | –69·9% (−73·8 to −65·2) | 6 206·9 (5 003·0 to 7 601·2) | –46·9% (−53·8 to −39·2) | 2·3% (2·2 to 2·6) | 85·1% (78·1 to 90·1) | 47·5% (32·6 to 62·2) | 77·2% (54·6 to 89·5) |
| Central Europe | 707 (632 to 795) | 12·5 (11·2 to 14·1) | –84·2% (−86·1 to −82·1) | 8 700·5 (6 862·8 to 10 868·4) | –26·9% (−34·5 to −18·2) | 0·1% (0·1 to 0·2) | 80·5% (71·7 to 87·6) | 53·0% (39·9 to 66·1) | 71·4% (47·0 to 85·5) |
| Eastern Europe | 1 396 (1 277 to 1 509) | 10·9 (10·0 to 11·8) | –78·2% (−80·2 to −76·5) | 11 710·4 (8 712·6 to 15 012·1) | –32·5% (−41·5 to −23·2) | 0·1% (0·1 to 0·1) | 81·2% (72·5 to 87·8) | 49·0% (34·2 to 63·1) | 72·6% (50·7 to 85·1) |
| High income | 1 857 (1 702 to 2 027) | 3·2 (2·9 to 3·5) | –70·6% (−73·2 to −67·9) | 4 843·7 (3 772·5 to 6 137·4) | –19·5% (−24·9 to −13·4) | 0·1% (0·1 to 0·1) | 67·7% (55·8 to 78·1) | 28·8% (16·0 to 44·7) | 66·5% (42·0 to 82·1) |
| Australasia | 42 (32 to 53) | 2·3 (1·8 to 2·9) | –65·4% (−76·2 to −53·4) | 5 798·0 (4 448·5 to 7 449·4) | 2·2% (−6·7 to 11·5) | 0·0% (0·0 to 0·0) | 66·6% (53·8 to 78·1) | 23·0% (10·1 to 41·9) | 65·8% (40·8 to 82·1) |
| High-income Asia Pacific | 180 (163 to 197) | 2·4 (2·2 to 2·6) | –72·2% (−75·9 to −68·4) | 8 472·0 (6 595·5 to 10 872·2) | –3·9% (−14·8 to 8·2) | 0·0% (0·0 to 0·0) | 75·3% (63·3 to 84·6) | 33·3% (20·9 to 48·9) | 70·5% (43·5 to 85·9) |
| High-income North America | 684 (618 to 742) | 3·2 (2·9 to 3·5) | –60·6% (−65·9 to −56·2) | 4 791·2 (3 679·0 to 6 155·8) | –29·6% (−33·9 to −25·3) | 0·1% (0·1 to 0·1) | 57·1% (43·7 to 70·2) | 21·0% (9·1 to 37·6) | 61·0% (36·9 to 78·4) |
| Southern Latin America | 568 (466 to 696) | 11·1 (9·1 to 13·6) | –77·5% (−82·0 to −72·3) | 11 895·3 (9 444·5 to 14 789·3) | –11·2% (−23·8 to 2·8) | 0·1% (0·1 to 0·1) | 78·3% (67·7 to 86·7) | 37·1% (19·7 to 56·4) | 73·2% (48·2 to 87·0) |
| Western Europe | 383 (350 to 426) | 1·7 (1·6 to 1·9) | –72·0% (−75·6 to −68·8) | 1 940·4 (1 526·6 to 2 431·0) | –19·1% (−24·6 to −13·2) | 0·1% (0·1 to 0·1) | 67·4% (55·2 to 78·1) | 28·1% (16·0 to 43·6) | 64·5% (40·9 to 80·1) |
| Latin America and Caribbean | 21 606 (19 618 to 24 079) | 42·4 (38·5 to 47·3) | –79·1% (−81·9 to −75·8) | 12 192·4 (9 920·3 to 14 782·1) | –37·4% (−42·4 to −31·9) | 0·3% (0·3 to 0·4) | 78·8% (70·5 to 85·9) | 44·5% (30·5 to 58·4) | 72·4% (45·9 to 87·8) |
| Andean Latin America | 3 787 (2 988 to 4 694) | 56·5 (44·6 to 70·0) | –87·0% (−90·0 to −83·3) | 16 610·1 (14 120·4 to 19 324·6) | –40·3% (−46·5 to −33·0) | 0·3% (0·3 to 0·4) | 74·7% (64·8 to 83·0) | 40·0% (23·0 to 57·9) | 68·6% (40·4 to 86·2) |
| Caribbean | 3 932 (2 985 to 5 131) | 100·5 (76·3 to 131·2) | –51·8% (−63·9 to −35·7) | 11 164·6 (8 986·4 to 13 596·2) | –9·9% (−18·2 to 0·5) | 0·9% (0·8 to 1·0) | 89·3% (84·1 to 93·2) | 68·9% (53·4 to 81·9) | 76·7% (52·9 to 90·1) |
| Central Latin America | 9 257 (8 062 to 10 826) | 38·3 (33·3 to 44·7) | –73·6% (−77·4 to −68·5) | 15 259·9 (12 336·1 to 18 680·2) | –39·9% (−45·4 to −33·9) | 0·3% (0·2 to 0·3) | 78·9% (70·7 to 85·6) | 41·8% (26·9 to 56·8) | 72·5% (45·2 to 88·0) |
| Tropical Latin America | 4 630 (4 163 to 5 158) | 28·8 (25·9 to 32·0) | –85·8% (−88·6 to −83·7) | 5 990·7 (4 896·3 to 7 296·4) | –45·2% (−49·3 to −40·9) | 0·5% (0·4 to 0·5) | 73·2% (62·3 to 82·7) | 28·0% (17·2 to 41·5) | 70·6% (41·9 to 87·4) |
| North Africa and Middle East | 43 558 (37 550 to 49 735) | 67·7 (58·3 to 77·3) | –76·5% (−80·7 to −71·1) | 19 258·4 (15 414·9 to 23 501·0) | –25·6% (−32·2 to −19·0) | 0·4% (0·3 to 0·4) | 91·9% (87·6 to 94·9) | 62·3% (46·8 to 75·6) | 81·1% (58·5 to 92·3) |
| South Asia | 249 595 (225 643 to 275 313) | 143·1 (129·4 to 157·9) | –71·2% (−74·9 to −66·7) | 13 153·1 (10 465·5 to 16 238·0) | –22·7% (−28·7 to −16·2) | 1·1% (1·0 to 1·2) | 95·9% (93·9 to 97·4) | 65·4% (50·5 to 77·3) | 83·2% (66·8 to 92·1) |
| Southeast Asia, east Asia, and Oceania | 63 661 (58 190 to 69 821) | 45·0 (41·1 to 49·3) | –85·7% (−87·2 to −83·7) | 13 383·7 (10 686·7 to 16 401·7) | –38·8% (−44·3 to −32·3) | 0·3% (0·3 to 0·4) | 88·7% (83·6 to 92·8) | 61·2% (45·3 to 75·0) | 78·8% (56·2 to 90·8) |
| East Asia | 22 824 (20 743 to 25 438) | 27·1 (24·6 to 30·2) | –90·7% (−91·9 to −89·2) | 9 376·4 (7 387·3 to 11 625·0) | –54·8% (−59·6 to −49·2) | 0·3% (0·3 to 0·3) | 83·2% (76·5 to 88·9) | 61·5% (41·9 to 77·8) | 70·9% (46·3 to 85·7) |
| Oceania | 1 770 (1 295 to 2 325) | 99·5 (72·8 to 130·7) | –48·1% (−63·1 to −26·9) | 16 573·7 (13 249·1 to 20 596·4) | –12·5% (−22·0 to −1·8) | 0·6% (0·5 to 0·6) | 93·1% (89·9 to 95·6) | 68·3% (50·0 to 82·7) | 85·2% (64·2 to 94·7) |
| Southeast Asia | 39 066 (34 532 to 44 291) | 70·2 (62·1 to 79·6) | –80·7% (−83·5 to −77·1) | 19 344·3 (15 535·4 to 23 655·9) | –20·7% (−27·5 to −13·0) | 0·4% (0·3 to 0·4) | 91·7% (87·5 to 94·8) | 61·2% (46·0 to 74·3) | 82·6% (61·1 to 93·1) |
| Sub-Saharan Africa | 412 604 (357 299 to 471 442) | 252·5 (218·7 to 288·6) | –62·9% (−67·6 to −56·8) | 10 493·2 (8 558·0 to 12 858·7) | –34·5% (−39·0 to −29·4) | 2·4% (2·2 to 2·6) | 94·0% (90·9 to 96·2) | 68·0% (50·6 to 81·8) | 82·8% (61·9 to 93·2) |
| Central sub-Saharan Africa | 47 357 (37 232 to 58 184) | 239·7 (188·4 to 294·5) | –61·8% (−69·9 to −51·0) | 11 728·4 (9 490·0 to 14 347·2) | –28·4% (−36·3 to −19·7) | 2·0% (2·0 to 2·1) | 94·2% (91·0 to 96·4) | 68·5% (48·8 to 83·9) | 82·9% (58·6 to 94·3) |
| Eastern sub-Saharan Africa | 111 613 (99 529 to 124 670) | 176·3 (157·2 to 196·9) | –71·2% (−75·4 to −65·6) | 12 894·4 (10 363·6 to 15 813·8) | –33·7% (−38·7 to −28·6) | 1·4% (1·2 to 1·5) | 93·3% (90·0 to 95·7) | 66·7% (51·4 to 78·4) | 81·3% (59·0 to 92·6) |
| Southern sub-Saharan Africa | 10 513 (9 192 to 12 063) | 123·1 (107·7 to 141·3) | –54·7% (−61·5 to −46·6) | 7 357·1 (6 032·7 to 8 847·9) | –30·3% (−35·5 to −24·5) | 1·7% (1·6 to 1·8) | 87·4% (81·5 to 92·1) | 51·9% (36·5 to 66·3) | 77·0% (51·0 to 90·7) |
| Western sub-Saharan Africa | 243 122 (198 471 to 290 155) | 338·7 (276·5 to 404·3) | –60·2% (−67·1 to −50·7) | 8 408·3 (6 875·4 to 10 219·7) | –37·7% (−42·7 to −31·7) | 4·0% (4·0 to 4·0) | 94·5% (91·7 to 96·5) | 69·3% (49·0 to 84·9) | 83·8% (63·7 to 93·8) |
Estimates for every country are available in the appendix (pp 67–92). UI=uncertainty interval.
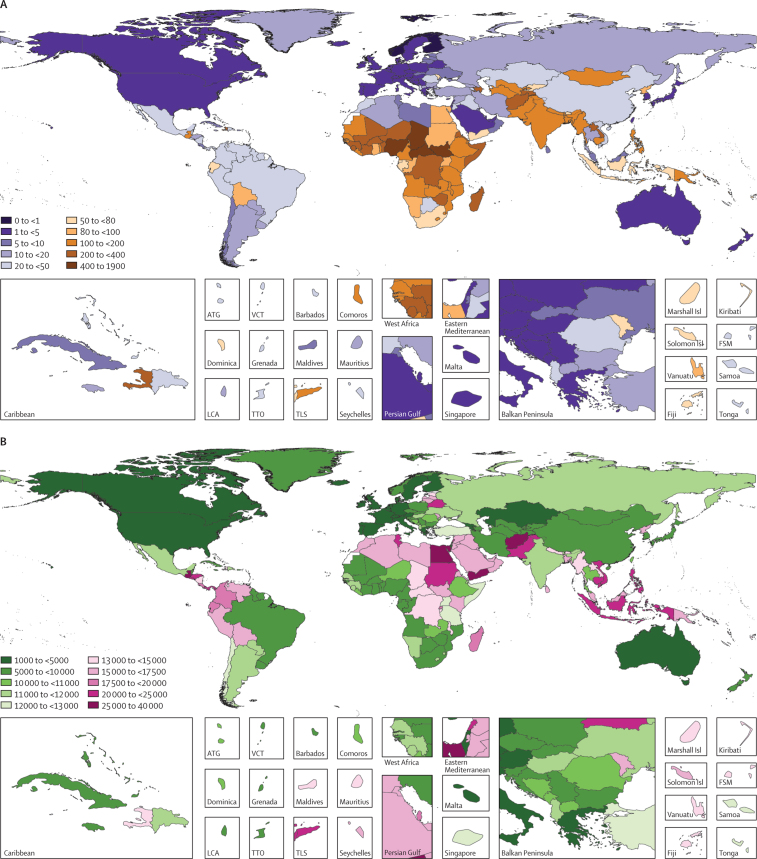
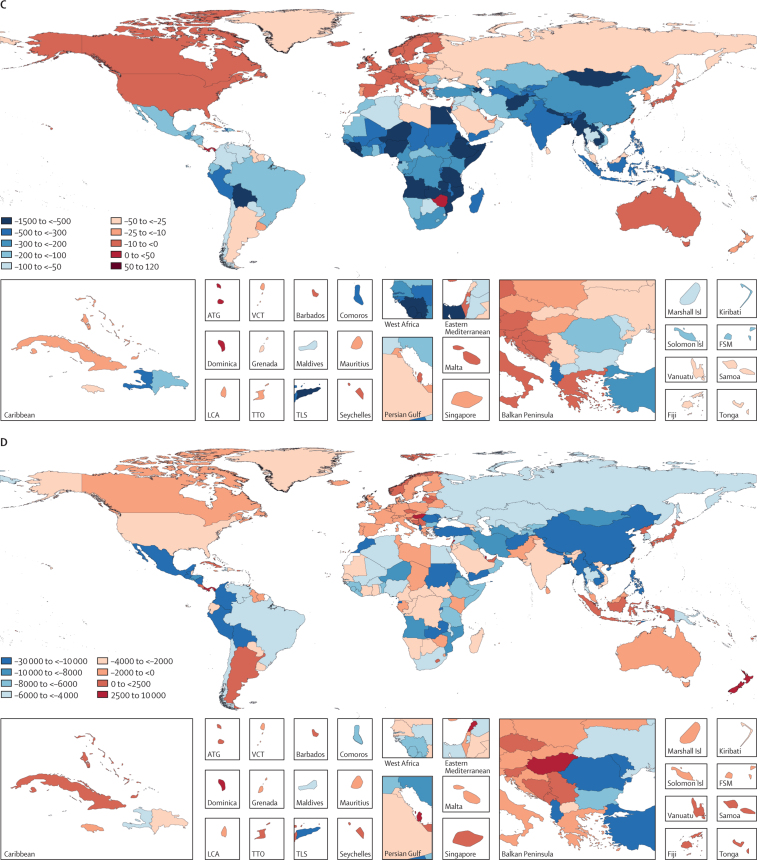
Most under-5 LRI deaths in 2017 occurred in India (185 429 deaths, 95% UI 167 676–204 328), Nigeria (153 069 deaths, 115 332–196 193), and Pakistan (40 480 deaths, 28 805–57 002; appendix pp 67–92). The highest LRI mortality rate occurred in South Sudan (527·7 deaths, 386·2–707·5, per 100 000 children; Figure 1, Figure 2; appendix pp 67–92). Likewise, reductions in LRI mortality rates have varied by location: Turkey (96·4% decline, 94·4–97·6) declined at the fastest rate whereas Niger experienced the largest absolute reduction in under-5 LRI mortality rate, from the highest mortality rate globally in 1990 (1349·0 deaths, 1027·0–1714·3, per 100 000 children) to 329·7 deaths (231·0–451·6) per 100 000 children in 2017 (ie, 1019·3 fewer deaths, 796·0–1262·7, per 100 000 children; Figure 1, Figure 2; appendix pp 67–92). Between 1990 and 2005, the fastest annualised rate of change in LRI mortality rate occurred in Oman (14·9% decrease per year) and the fastest annualised rate of change between 2000 and 2017 occurred in Saudi Arabia (12·7% decrease per year; data not shown, available on GBD-Compare).
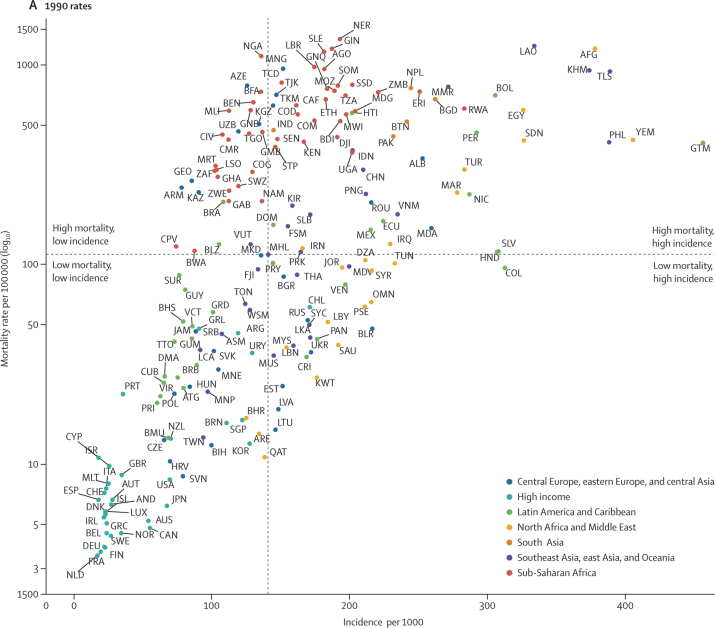
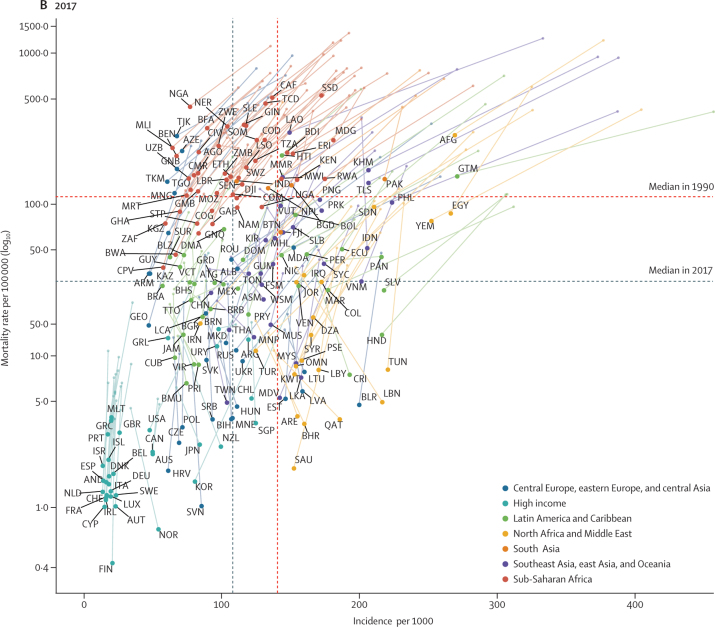
Figure 1.
Under-5 LRI incidence and mortality rates in 1990 (A) and 2017 (B)
Points represent countries (labelled according to the International Organization for Standardization 3166 alpha-3 codes) and the colour indicates the Global Burden of Diseases, Injuries, and Risk Factors Study super-region each of them belongs to. The vertical line indicates the median incidence among all countries and the horizontal line indicates the median mortality rate among all countries. The plots are therefore divided into four quadrants based on each country's relative incidence and mortality rate compared with all other countries in 1990 and in 2007.
Figure 2.
Global distribution of LRI burden among children younger than 5 years
(A) Under-5 LRI mortality rate in 2017; (B) LRI incidence per 100 000 child-years in 2017; (C) absolute difference in LRI mortality rate between 1990 and 2017; and (D) absolute difference in LRI incidence rate between 1990 and 2017. ATG=Antigua and Barbuda. FSM=Federated States of Micronesia. Isl=Islands. LCA=Saint Lucia. LRI=lower respiratory infection. TLS=Timor-Leste. TTO=Trinidad and Tobago. VCT=Saint Vincent and the Grenadines.
The global LRI incidence among children younger than 5 years was 12 197·8 new cases (95% UI 9762·1–14 908·7) per 100 000 child-years. LRI incidence was highest in Guatemala (27 126·3 new cases, 22 443·4–32 304·3, per 100 000 child-years; Figure 1, Figure 2; appendix pp 67–92). LRI incidence declined globally (from 18 054·0 new cases, 14 808·2–21 833·2, per 100 000 child-years, in 1990; 32·4% decrease, 27·2–37·5) with the fastest declines in Turkmenistan (58·0% decrease, 50·9–64·5), Mongolia (56·6% decrease, 48·9–63·9), and China (56·0% decrease, 50·5–60·6; figure 1; appendix pp 67–92). However, the incidence of LRI increased in some locations such as Norway (58·9% increase, 44·4–75·1; from 3406·1 new cases, 2659·4–4294·7, per 100 000 child-years to 5411·7 new cases, 4115·6–7016·1, per 100 000 child-years) and Lebanon (40·8% increase, 21·0–59·1; from 15 400·6 new cases, 12 300·8–19 254·4, per 100 000 child-years to 21 680·6 new cases, 16 166·6–28 291·3, per 100 000 child-years; Figure 1, Figure 2; appendix pp 67–92). Additional results by age, sex, location, and year from 1990 to 2017 are available on GBD-Compare.
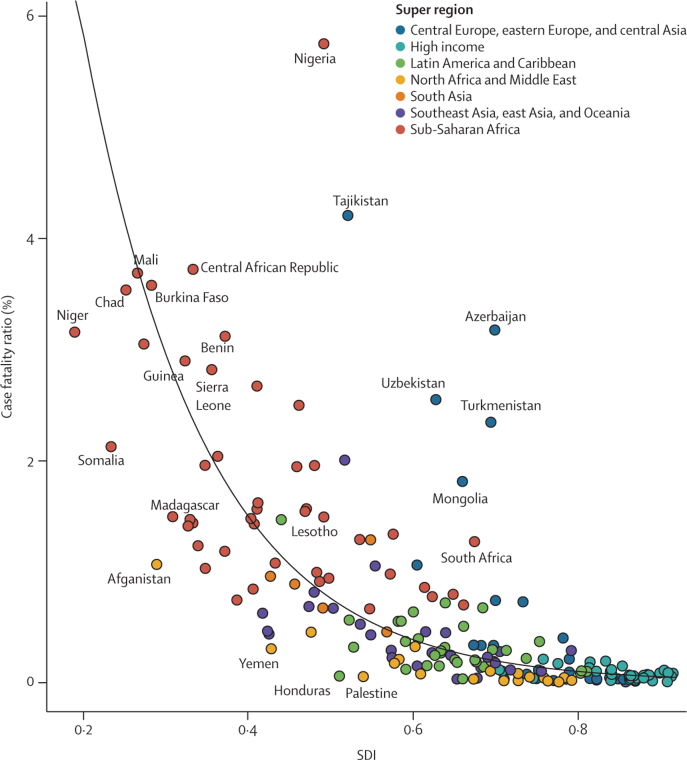
The global case fatality ratio for LRIs decreased from 2·0% (2·2–1·8) in 1990, to 1·0% (95% UI 0·9 to 1·1) in 2017. In 2017, the lowest case fatality ratios globally occurred in Saudi Arabia (<0·1%, <0·1 to <0·1) and Slovenia (<0·1%, <0·1 to <0·1), whereas the highest occurred in Nigeria (5·8%, 5·4 to 6·0) and Tajikistan (4·2%, 4·2 to 4·3; figure 3; appendix pp 67–92). In 2017, if all countries with a case fatality ratio exceeding the global average had been reduced to the global average, then 291 611 deaths would be averted. Some countries in central Asia (eg, Azerbaijan, Mongolia, and Tajikistan) and in western sub-Saharan Africa (eg, Guinea, Nigeria, and Sierra Leone) had case fatality ratios much higher than expected on the basis of SDI alone (figure 3). If these countries had experienced case fatality ratios corresponding with the average relationship between case fatality ratio and SDI, an additional 326 900 deaths, including 133 600 in Nigeria, could possibly have been averted in 2017.
Figure 3.
Case fatality ratio among children under-5 in 2017
We used the Socio-demographic Index as a predictor of the case fatality ratio by country. The solid black line indicates a log-linear curve for these values.
Overall, 93·4% (95% UI 90·3 to 95·7) of under-5 LRI mortality could be attributed to risk factors and interventions modelled by GBD in 2017 (table). Because of the counterfactual strategy in risk factor attribution, this suggests that 755 513 under-5 LRI deaths (691 459 to 819 746) would have been avertable if exposures to all risk factors had been reduced to their theoretical minimum levels. Risk factors in the GBD study are not mutually exclusive and so individual attributable fractions might sum to more than 100%. Protection-related risk factors were responsible for 82·0% (62·6 to 92·1) of under-5 LRI deaths in 2017 (table), including 52·6% (35·1 to 62·8) of under-5 LRI deaths attributable to wasting, 14·7% (1·6 to 34·7) to stunting, 11·5% (7·6 to 20·4) to underweight, and 7·4% (4·2 to 11·1) to non-exclusive breastfeeding (data available on GBD-Compare). Interventions to prevent risk exposure could have averted 65·2% (50·2 to 77·6) of under-5 LRI deaths in 2017 (table) including 11·2% (7·3 to 14·8) of deaths attributable to insufficient handwashing with soap, 28·5% (22·4 to 34·1) to household air pollution, 17·5% (13·2 to 22·6) to ambient particulate matter pollution, 19·2% (16·5 to 21·8) to low PCV coverage, and 9·6% (<0·1 to 20·6) due to low Hib vaccine coverage (data available on GBD-Compare).
At the global level, changes to all risk factors for LRI mortality accounted for a 12·2% decrease (95% UI 11·6–13·1) between 1990 and 2017 (figure 4; appendix pp 93–111). Globally, increased Hib vaccine coverage (11·4%, 0·0–24·5) and PCV coverage (6·3%, 6·1–6·3) were responsible for large decreases in LRI mortality among children younger than 5 years between 1990 and 2017 (figure 4; appendix pp 93–111). This effect was evident also in all subgroups of countries classified according to their mortality and incidence rates in 1990. Although decreased exposure to household air pollution reduced LRI mortality by 8·4% (6·8–9·2), increased exposure to ambient air pollution increased mortality by 4·1% (2·7–6·2; figure 4).
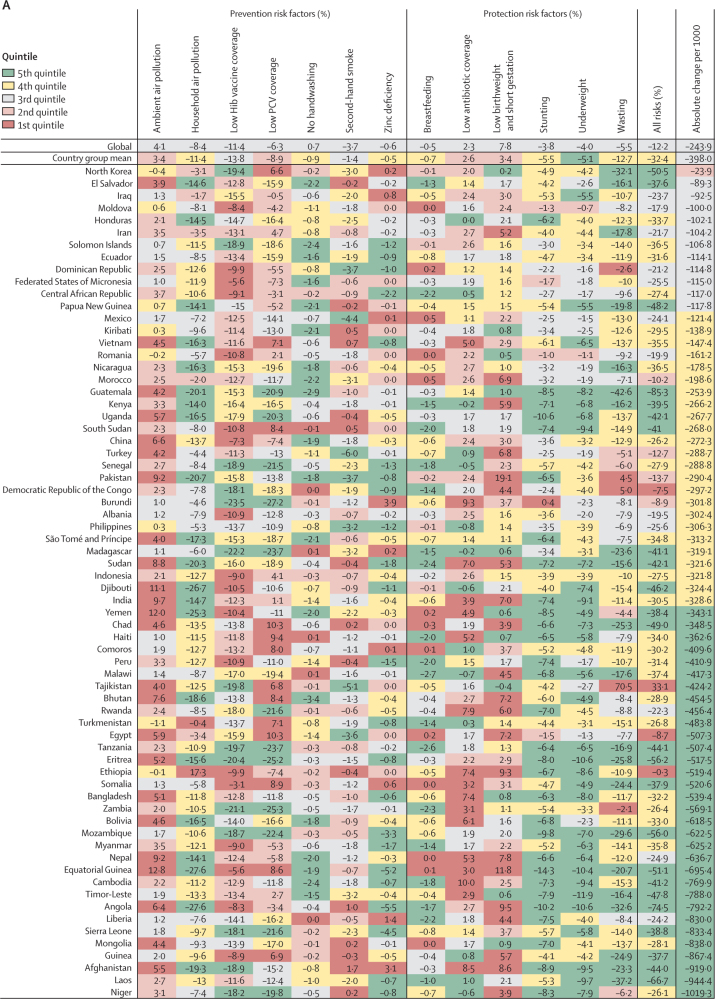
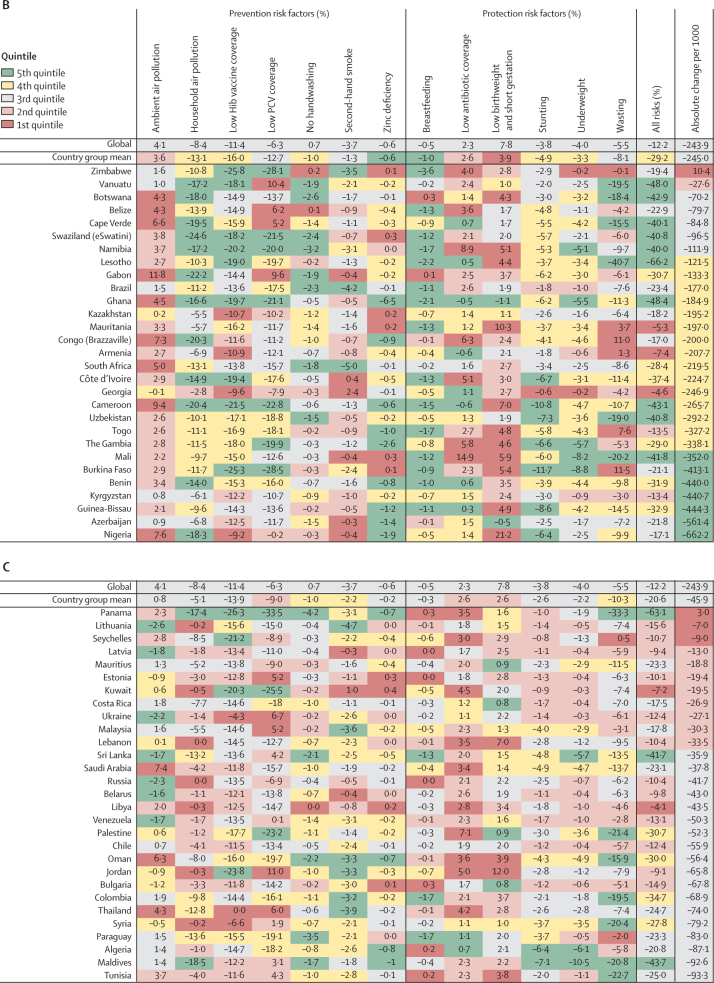
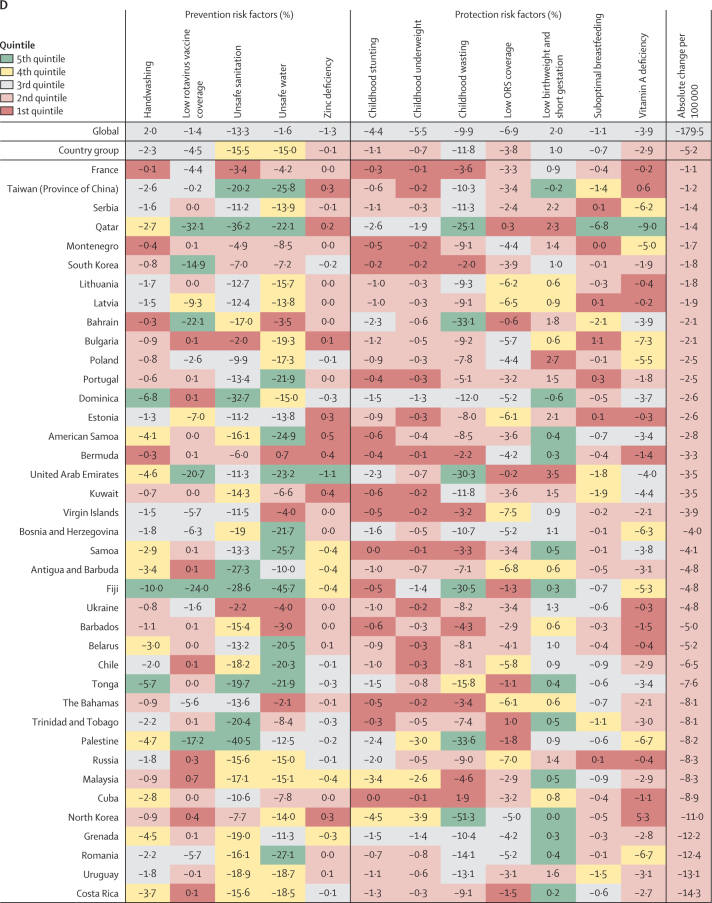
Figure 4.
Change in LRI mortality rate attributable to changes in risk factor exposure by country, 1990–2017
Countries are grouped by their mortality and incidence (higher or lower than the global median in 1990, as identified by the quandrants in figure 1A) and are ordered within each group from slowest absolute change in under-5 LRI mortality rate per 1000 children between 1990 and 2017. Colors indicate the quintile for the absolute change in each risk factor attributable fraction among all countries. Country groupings are: (A) high mortality, high incidence (n=68); (B) high mortality, low incidence (n=29); (C) low mortality, high incidence (n=29); and (D) low mortality, low incidence (n=69). Hib=Haemophilus influenzae type b. PCV=pneumococcal conjugate vaccine.
In 1990, both the mortality and incidence rates were higher than the corresponding country-group mean values in 68 countries, which were categorised as high mortality and high incidence (upper right quadrant of figure 1A; figure 4A). From 1990 to 2017, the under-5 LRI mortality rate declined by a greater amount than the global median in 50 (74%) of these 68 countries and the LRI mortality rate decreased by a mean of 398·0 deaths (95% UI 100·7–857·8) per 100 000 children in these countries (Figure 1, Figure 4). These countries tended to have large decreases in LRI mortality attributable to changes in childhood growth failure indicators, including a mean 12·7% (2·3–31·2) reduction due to childhood wasting, 5·5% (1·5–9·5) reduction due to childhood stunting, and 5·1% (1·6–10·2) reduction due to childhood underweight (figure 4A). Among the countries with the greatest magnitude change, childhood stunting accounted for a 14·3% (2·5–28·9) decrease in LRI mortality rate in Equatorial Guinea and a 10·6% (2·0–22·8) decrease in Uganda. Childhood underweight accounted for an 11·9% (7·5–19·7) decrease in LRI mortality rate in Timor-Leste and a 10·6% (6·8–18·0) decrease in Angola. Changes in childhood wasting were responsible for a 42·6% (26·3–55·3) decrease in LRI mortality rate in Guatemala and a 37·2% (29·0–42·2) decrease in Laos (figure 4A). The greatest absolute decline in LRI mortality rate occurred in Niger and the vaccine-related risk factors were responsible for the largest decrease in LRI mortality (18·2% [0·0–37·3] decrease due to increased Hib vaccine coverage and 19·8% [18·6–20·0] decrease due to increased PCV coverage). Some countries in this group had large reductions in LRI mortality due to household air pollution (27·6% [20·4–33·3] reduction in Angola) and vaccine coverage (23·5% [0·0–51·0] decline due to increased Hib vaccine coverage and 27·2% [23·0–30·5] decline due to increased PCV coverage in Burundi). The LRI mortality rate increased due to ambient air pollution in 64 (94%) of 68 countries in this group (median increase 3·4% [0·0–9·5]; figure 4A; appendix pp 93–111).
In 29 countries, the mortality rate was higher than the global median but the incidence was lower than the global median in 1990. We classified these countries as having high mortality and low incidence (upper left quadrant of figure 1; figure 4B). This group of countries had a mean decline in LRI mortality rate of 245·0 deaths (44·7–514·6) per 100 000 children during 1990–2017. Countries in this group tended to have large reductions in LRI mortality attributable to changes in household air pollution (mean decrease 13·1% [5·6–21·5]), including a 24·6% (17·9–30·6) decline in Swaziland (eSwatini). Increased Hib vaccine coverage also contributed to a substantial reduction in LRI mortality in this group of countries (16·0% decrease [10·0–23·8]). This group also had small declines in LRI mortality attributable to improved breastfeeding (mean 1·0% [0·0–2·2]) and zinc deficiency (mean 0·6% [0·2–2·3]). The LRI mortality rate decreased by 662·2 deaths (554·7–755·6) per 100 000 children in Nigeria, where the largest attributable changes were due to household air pollution (18·3% decrease [11·9–22·9]), childhood wasting (9·9% decrease [8·5–11·1]), and childhood stunting (6·4% decrease [1·4–13·5]; figure 4B; appendix pp 93–111).
In 29 countries, the mortality rate was lower than the global median in 1990 but the incidence was higher than the global median. We classified these countries as having low mortality and high incidence (lower right quadrant of figure 1; figure 4C). The LRI mortality rate decreased by 45·9 deaths (95% UI 7·8–90·4) per 100 000 children in these countries and the absolute change in the LRI mortality rate was in the 3rd quintile for 18 (62%) of 29 countries. Relative to other groups, this group of countries had greater reductions attributable to behavioural risk factors such as no handwashing (1·0% mean decline [0·2–3·0]), second-hand smoke exposure (2·2% mean decline [0·3–3·7]), and childhood wasting (10·3% mean decline [2·3–22·2]). Although vaccine coverage reduced LRI mortality in this country group, this reduction was similar to the all-country mean for Hib vaccine coverage (13·9% mean reduction [5·2–22·8]) and slower than the all-country mean for PCV coverage (9·0% mean reduction [6·4–24·6]). By contrast, these countries had mean increases in LRI mortality attributable to low antibiotic coverage (2·6% median increase [1·1–4·8]) and low birthweight and short gestation (2·6% median increase [0·1–5·7]). Many of these countries did not introduce the PCV, which was responsible for an increase in LRI mortality in ten countries (figure 4C), with Jordan (11·0% increase [6·2–15·9]) and Ukraine (6·7% increase [3·8–9·8]) having the largest increase in mortality rate due to low PCV coverage (appendix pp 93–111).
In 69 countries, both mortality and incidence were lower than the global median in 1990. We classified these countries as having low mortality and low incidence (lower left quadrant of figure 1; figure 4D). The mean decline in LRI mortality rate in these countries was 13·7 deaths (95% UI 1·7 to 39·9) per 100 000 children. Many of these countries reduced exposure to ambient air pollution (the greatest reduction was a 3·5% decline, 2·5 to 3·8, in the Czech Republic). The mean decline due to second-hand smoke exposure was 2·2% (−0·3 to 5·9) and was greatest in Greece (8·8% reduction, 7·3 to 10·9) and Iceland (7·5% reduction, 5·6 to 9·4; figure 4D). Countries in this group generally had decreases in LRI mortality attributable to greater vaccine coverage but these changes were similar to the decrease across all countries (appendix pp 93–111).
Discussion
At the global level, LRI mortality declined substantially among children younger than 5 years between 1990 and 2017. Despite these declines, such progress has not occurred equally across countries, and LRIs remained the leading infectious cause of death among children younger than 5 years in 2017. In most locations, LRI incidence declined more slowly than mortality, suggesting that improvements in protecting against death are probably outpacing improvements in reducing the underlying risk of infection. Although SDI is strongly associated with under-5 LRI mortality, changes in SDI were not strongly associated with changes in mortality rates between 1990 and 2017. At the global level, 93·4% of under-5 LRI deaths were attributable to a risk factor. Among risk factors estimated in GBD 2017, we have conceptualised the change in under-5 LRI burden into two components, the changes in risks that predispose children to disease (prevention risk factors) and those that increase the risk of mortality given disease (protection risk factors). These categories are likely to partly overlap. For example, childhood growth failure, via immunological mechanisms, might make children both more likely to get sick and die from an LRI. Such a distinction is broad but provides a conceptual framework with which to assess the relationship between trends in risk factor exposure, mortality, incidence, and case fatality.
One of the main findings from our study is that the drivers of change in LRI mortality are not universal and might be highly specific to each location and that strategies to reduce health loss due to LRI must be tailored to a given setting. For example, the three countries with the greatest absolute decline in LRI mortality rate were Niger (1019·3 fewer deaths per 100 000 children), Laos (944·4 fewer deaths per 100 000 children) and Afghanistan (919·0 fewer deaths per 100 000 children). The Hib and pneumococcal vaccines were responsible for the largest percent change in Niger, whereas changes in childhood wasting explained more than 20% of the decline in Laos and Afghanistan, and lower exposure to household air pollution in Afghanistan decreased LRI mortality rate by 19·3%. By contrast, the two countries that observed the largest relative decline in LRI mortality between 1990 and 2017, China and Turkey, had relatively small changes attributable to changes in risk factors. Under-5 LRI mortality in China decreased by 91·2% between 1990 and 2017, whereas the mortality rate decreased by 26·2% because of changes in risk factor exposure. This suggests that other factors might explain the change in some countries.
Other studies have identified economic development, health-care reform to provide government-sponsored health care in rural areas,11, 12 improved detection of pneumonia following the severe acute respiratory syndrome and influenza virus subtype H1N1 pandemics, and programmes to reduce household air pollution from solid fuels as the main drivers of reduction in LRI mortality in China.13 The decline in LRI mortality in China might also be related to a so-called nutritional transition—similar to a transition previously observed in the USA and other high-income countries, but occurring at lower gross national product14—and might be explained by agricultural policies (including subsidisation and subsequent increase in soya bean oil and soya consumption),15 urbanisation,16 liberalisation of food production,15 and rapid economic development (exceeding 8% annually) in China during this time.17 This study did not include covariates that would specifically capture changes in infrastructure or food production but such changes might be reflected in the rapid increase in SDI observed in China between 1990 and 2017, and these trends deserve further investigation. Interventions and structural policies that aim to improve childhood nutrition can play a substantial role in protecting children from dying because of infectious diseases, including LRIs.5
China and Turkey have some of the fastest improvements in the Healthcare Access and Quality Index, a composite metric of amenable mortality.18 This improvement in preventing amenable causes of death suggests that the health-care systems in these countries have improved and are likely to have contributed to the rapid declines in LRI mortality. Access to care, adherence to treatment protocols, maternal education, appropriate technology, and adequate health-care staffing have all been identified as predictors of under-5 LRI survival5 that were not quantified in this study and might be components of a comprehensive LRI treatment strategy.
Our study found that low antibiotic use for LRIs was responsible for an increase in LRI mortality, particularly among countries with high mortality in 1990. An analysis of appropriate antibiotic therapy for childhood pneumonia determined that the availability and accessibility of WHO-recommended antibiotics is not equitable in some locations with substantial variation within countries.19 Antibiotic use for symptoms of pneumonia varied greatly among low-income and middle-income countries,20 and was estimated at just 18·8% globally, in 2017, in this study. A cohort study consisting of eight sites and a mixture of urban and rural locations found that 61% of children aged 0–2 years with acute LRI received antibiotics, including 69% in rural Tanzania and 86% in urban Bangladesh.21 Community case management of acute respiratory infections could reduce pneumonia-related mortality by 32%, according to a systematic review,22 and effective treatments such as oxygen therapy, rehydration, and antibiotics can dramatically reduce LRI mortality.
Appropriate care for LRIs might also depend on the availability and utilisation of health care. Primary health care in some low-income and middle-income countries has experienced challenges in funding, with general underuse of facilities and neglected human resources. Nigeria had the highest case fatality ratio globally in 2017 (5·7%, 95% UI 5·4–6·0) but had average coverage of antibiotics, suggesting other factors might be driving the high rate. Nigeria is a country of complex demographic, geographic, and cultural characteristics with substantial variation in health and health-care utilisation and performance indicators.23 It has the highest population of any country in Africa and by far the most deaths due to LRI among children younger than 5 years. Yet, a review of published literature on child health interventions found only 18 papers from Nigeria.24 Building evidence from high-burden countries should be a priority and is essential to a complete understanding of health loss due to LRI. One qualitative study25 revealed demand-side barriers to health-care utilisation in Nigeria, suggesting that costs, physical distance, cultural considerations, and knowledge of symptoms and warning signs might all play a role in delayed care-seeking and could explain behaviours in other locations. In one study26 of six countries in sub-Saharan Africa, only 30% of care givers recognised fast breathing or difficulty in breathing as a symptom of LRI. Among several countries in western sub-Saharan Africa, the proportion of care givers who reported seeking treatment for symptoms of pneumonia ranged from 27·4% in Chad to 73·2% in Sierra Leone and was 41·9% in Nigeria.26
Appropriate care and treatment to protect against LRI mortality are important but the two risk factors responsible for the largest reduction in LRI mortality at the global level and for the largest prevention of incident episodes of LRI are Hib vaccine coverage and household air pollution. Vaccines have been an important part of LRI prevention in many countries. In 2011, 2% of US$30·6 billion in international assistance for health spending was spent on pneumonia, and about 82% of that was through Gavi, the Vaccine Alliance, suggesting that nearly all pneumonia funding was for vaccines.27 A different analysis found that between 2000 and 2015, pneumonia received approximately $3 billion in funding for research and development, compared with more than $38 billion that went towards HIV/AIDS research during the same time.28 Of that $3 billion, about $839 million went to treatment, another $164 million to diagnostics, and $858 million went to vaccines.28
Globally, Hib and pneumococcal vaccines were responsible for an estimated 11·4% (Hib) and 6·3% (PCV) decrease in LRI mortality. Although nearly every country has introduced the Hib vaccine in their immunisation programmes, several high-burden countries such as Nigeria, Chad, and Somalia have not introduced the pneumococcal vaccine. Studies have shown substantial reductions in hospital admissions for pneumonia, invasive Hib, and pneumococcal pneumonia following vaccine introduction.29, 30, 31, 32 Although more countries might introduce PCV with Gavi support, there is uncertainty regarding the sustainability of these vaccines among countries that approach graduation from Gavi.33 The continued and expanded use of these vaccines have substantial impacts on under-5 LRI mortality and should be prioritised as part of routine immunisation programmes to prevent these deaths.
Two other risk factors that are associated with preventing incident cases of LRI had opposite trends in burden attribution. Between 1990 and 2017, LRI mortality decreased by 8·4% globally due to reductions in household air pollution but increased by 4·1% due to ambient air pollution. A pair of systematic reviews and meta-analyses found that exposure to household air pollution from solid fuel use increased the risk of pneumonia among children younger than 5 years.34, 35 A pair of randomised controlled trials found little statistical evidence of a reduction in pneumonia incidence with provision of chimneys36 or cleaner burning cookstoves,37 and several more studies investigating household air pollution and childhood pneumonia are in progress.38, 39 This suggests that the provision of chimneys or cleaner burning practices does not result in a sufficient reduction of air pollution to translate into a reduction in health effects. This finding is consistent with the analysis and recommendations by the WHO guidelines. Rapid urbanisation in many countries and a shift from conventional heating sources, such as coal and wood, to natural gas have probably contributed to reduced exposure to household air pollution.40 Reduced exposure to household air pollution was strongly associated with decreased incidence and mortality, particularly in high-burden countries in our study. We have previously described the interplay between development and ambient air pollution that is occurring in many countries that are rapidly urbanising and growing economically.6 Preventing LRI cases and deaths should be a part of any policy conversation regarding the burning of fossil fuels, and focusing on health effects of carbon-based energy might be a powerful rhetorical tool in developing policy to reduce air pollution.41, 42, 43
There are several limitations in this study. We have previously described some of the data gaps associated with LRI mortality modelling, specifically among countries with the highest estimated LRI burden.6 All our estimates are reported with uncertainty intervals and these intervals are larger in areas where we have fewer data. We attempt to improve our models by using patterns from locations where we do have data and by the relationship between LRI mortality and covariates. Many of the covariates used in the mortality modelling are also part of the risk factor attribution. Risk factors in GBD are typically a combination of population-level exposure and the relative risk of an outcome. The exposure to each risk factor is a covariate in our model. This analysis is strengthened by our ability to use risk factors to predict time trends in LRI mortality. Yet, the risk factor exposure used at multiple points in this analysis are also modelled values, dependent on data availability. Although we have quantified several interventions for LRI health-loss and antibiotic use, we do not explicitly account for health-care seeking behaviours, primary health-care availability, and specific recommended diagnostics or treatments for LRI. Understanding patterns in diagnostics and the use of pulse oximetry, chest x-rays, and oxygen therapy might reveal additional information about the drivers in LRI mortality trends. We do not have a modelled covariate for influenza vaccine coverage. Additionally, our model quantifies LRI deaths in HIV-negative people only because any deaths due to select infectious diseases, including LRI, among people with HIV, are classified as HIV deaths in the International Classification of Diseases; these deaths are captured in the overall HIV-related mortality estimation in GBD.1 To date, we have not separately quantified LRI deaths in HIV-infected people. As GBD is an iterative endeavour, we can examine this in our future work.
The global reduction in under-5 LRI mortality from 1990 to 2017 should be viewed as simultaneously a major public health achievement and as a call for renewed efforts to continue to reduce the burden of disease. Declines in LRI burden over time have been highly specific by location and are not fully explained by improvements in sociodemographic status alone. These results illustrate the contribution of improvements in air pollution, Hib and PCV vaccine coverage, child nutrition, and effective treatment to reducing disease and death due to LRI. Quantitative estimates of LRI incidence, mortality, and risk factor exposure can serve as a tool to sustain the progress made since 1990 and to design targeted strategies for countries where high LRI burden remains.
Data sharing
In compliance with the Guidelines for Accurate and Transparent Health Estimates Reporting, data and code for GBD 2017 are publicly available.
Acknowledgments
Acknowledgments
Research reported in this publication was supported by the Bill & Melinda Gates Foundation. AA acknowledges support by the Department of Science and Technology, Government of India (New Delhi, India) through the INSPIRE Faculty program. SA acknowledges the International Centre for Casemix and Clinical Coding, the Faculty of Medicine, National University of Malaysia, and the Department of Health Policy and Management, Faculty of Public Health, Kuwait University for the approval and support to participate in this research project. ABad acknowledges support from the Public Health Agency of Canada. ABar acknowledges support for research from the Project of Ministry of Education, Science and Technology of the Republic of Serbia (number III45005). FC acknowledges funding support from Foundation for Science and Technology/Minister of Science, Technology, and Higher Education through national funds (UID/MULTI/04378/2019 and UID/QUI/50006/2019). AJC acknowledges support by the Health Effects Institute, Boston, MA, USA. MMSM acknowledges the support from the Ministry of Education, Science and Technological Development, Republic of Serbia (Contract number 175087). AMS was supported by the Egyptian Fulbright Mission Program (EFMP). RS-S acknowledges support from Applied and Environmental Sciences University (Bogota, Colombia). AS acknowledges support from Health Data Research UK.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
GBD 2017 Lower Respiratory Infections Collaborators
Christopher E Troeger, Ibrahim A Khalil, Brigette F Blacker, Molly H Biehl, Samuel B Albertson, Stephanie R M Zimsen, Puja C Rao, Degu Abate, Amha Admasie, Alireza Ahmadi, Mohamed Lemine Cheikh Brahim Ahmed, Chalachew Genet Akal, Fares Alahdab, Noore Alam, Kefyalew Addis Alene, Vahid Alipour, Syed Mohamed Aljunid, Rajaa M Al-Raddadi, Nelson Alvis-Guzman, Saeed Amini, Mina Anjomshoa, Carl Abelardo T Antonio, Jalal Arabloo, Olatunde Aremu, Hagos Tasew Atalay, Suleman Atique, Euripide F G A Avokpaho, Samah Awad, Ashish Awasthi, Alaa Badawi, Kalpana Balakrishnan, Joseph Adel Mattar Banoub, Aleksandra Barac, Quique Bassat, Neeraj Bedi, Derrick A Bennett, Krittika Bhattacharyya, Zulfiqar A Bhutta, Ali Bijani, Corey B Bills, Josip Car, Félix Carvalho, Carlos A Castañeda-Orjuela, Kate Causey, Devasahayam J Christopher, Aaron J Cohen, Lalit Dandona, Rakhi Dandona, Ahmad Daryani, Feleke Mekonnen Demeke, Shirin Djalalinia, Manisha Dubey, Eleonora Dubljanin, Eyasu Ejeta Duken, Maysaa El Sayed Zaki, Aman Yesuf Endries, Eduarda Fernandes, Florian Fischer, Joseph Frostad, Nancy Fullman, William M Gardner, Birhanu Geta, Keyghobad Ghadiri, Giuseppe Gorini, Alessandra C Goulart, Yuming Guo, Gessessew Bugssa Hailu, Arvin Haj-Mirzaian, Arya Haj-Mirzaian, Samer Hamidi, Hamid Yimam Hassen, Chi Linh Hoang, Nobuyuki Horita, Mihaela Hostiuc, Zakir Hussain, Seyed Sina Naghibi Irvani, Spencer L James, Ravi Prakash Jha, Jost B Jonas, André Karch, Amir Kasaeian, Tesfaye Dessale Kassa, Nicholas J Kassebaum, Adane Teshome Kefale, Yousef Saleh Khader, Ejaz Ahmad Khan, Gulfaraz Khan, Md Nuruzzaman Khan, Young-Ho Khang, Abdullah T Khoja, Ruth W Kimokoti, Adnan Kisa, Sezer Kisa, Niranjan Kissoon, Luke D Knibbs, Sonali Kochhar, Soewarta Kosen, Parvaiz A Koul, Ai Koyanagi, Barthelemy Kuate Defo, G Anil Kumar, Dharmesh Kumar Lal, Cheru Tesema Leshargie, Sonia Lewycka, Shanshan Li, Rakesh Lodha, Erlyn Rachelle King Macarayan, Marek Majdan, Abdullah A Mamun, Helena Manguerra, Varshil Mehta, Addisu Melese, Ziad A Memish, Desalegn Tadese Mengistu, Tuomo J Meretoja, Tomislav Mestrovic, Bartosz Miazgowski, Erkin M Mirrakhimov, Babak Moazen, Karzan Abdulmuhsin Mohammad, Shafiu Mohammed, Lorenzo Monasta, Catrin E Moore, Lidia Morawska, Jonathan F Mosser, Seyyed Meysam Mousavi, Srinivas Murthy, Ghulam Mustafa, Javad Nazari, Cuong Tat Nguyen, Huong Lan Thi Nguyen, Long Hoang Nguyen, Son Hoang Nguyen, Katie R Nielsen, Muhammad Imran Nisar, Molly R Nixon, Felix Akpojene Ogbo, Anselm Okoro, Andrew T Olagunju, Tinuke O Olagunju, Eyal Oren, Justin R Ortiz, Mahesh P A, Smita Pakhale, Maarten J Postma, Mostafa Qorbani, Reginald Quansah, Alireza Rafiei, Fakher Rahim, Vafa Rahimi-Movaghar, Rajesh Kumar Rai, Marissa Bettay Reitsma, Mohammad Sadegh Rezai, Aziz Rezapour, Maria Jesus Rios-Blancas, Luca Ronfani, Dietrich Rothenbacher, Salvatore Rubino, Zikria Saleem, Evanson Zondani Sambala, Abdallah M Samy, Milena M Santric Milicevic, Rodrigo Sarmiento-Suárez, Benn Sartorius, Miloje Savic, Monika Sawhney, Sonia Saxena, Alyssa Sbarra, Seyedmojtaba Seyedmousavi, Masood Ali Shaikh, Aziz Sheikh, Mika Shigematsu, David L Smith, Chandrashekhar T Sreeramareddy, Jeffrey D Stanaway, Mu'awiyyah Babale Sufiyan, Mohamad-Hani Temsah, Belay Tessema, Bach Xuan Tran, Khanh Bao Tran, Afewerki Gebremeskel Tsadik, Irfan Ullah, Rachel L Updike, Tommi Juhani Vasankari, Yousef Veisani, Fiseha Wadilo Wada, Yasir Waheed, Katie Welgan, Kirsten E Wiens, Charles Shey Wiysonge, Ebrahim M Yimer, Naohiro Yonemoto, Zoubida Zaidi, Heather J Zar, Stephen S Lim, Theo Vos, Ali H Mokdad, Christopher J L Murray, Hmwe Hmwe Kyu, Simon I Hay*, Robert C Reiner Jr*.
*Joint last authors.
Affiliations
Department of Health Metrics Sciences, School of Medicine (I A Khalil MD, Prof B Sartorius PhD, Prof D L Smith PhD, J D Stanaway PhD, Prof S S Lim PhD, Prof T Vos PhD, Prof A H Mokdad PhD, Prof C J L Murray DPhil, H H Kyu PhD, Prof S I Hay FMedSci, R C Reiner Jr PhD), Department of Anesthesiology & Pain Medicine (N J Kassebaum MD), Department of Global Health (S Kochhar MD, K R Nielsen MD, J R Ortiz MD), Department of Pediatrics (K R Nielsen), Institute for Health Metrics and Evaluation (C E Troeger MPH, I A Khalil, B F Blacker MPH, M H Biehl MPH, S B Albertson BS, S R M Zimsen MA, P C Rao MPH, K Causey BS, A J Cohen DSc, Prof L Dandona MD, Prof R Dandona PhD, J Frostad MPH, N Fullman MPH, W M Gardner AB, S L James MD, N J Kassebaum, H Manguerra BS, J F Mosser MD, M R Nixon PhD, M B Reitsma BS, A Sbarra MPH, Prof D L Smith, J D Stanaway, R L Updike MPH, K Welgan BS, K E Wiens PhD, Prof S S Lim, Prof T Vos, Prof A H Mokdad, Prof C J L Murray, H H Kyu, Prof S I Hay, R C Reiner Jr), University of Washington, Seattle, WA, USA (Prof E Oren PhD); Department of Medical Laboratory Sciences, Haramaya University, Harar, Ethiopia (D Abate MSc); Department of Medicine (F W Wada MSc), School of Public Health (A Admasie MPH), Wolaita Sodo University, Addis Ababa, Ethiopia; Department of Anesthesiology (A Ahmadi PhD), Kermanshah University of Medical Sciences, Kermanshah, Iran (K Ghadiri BEP); Department of Biology, University Mohammed V, Rabat, Morocco (M L C B Ahmed PhD); National Institute of Researches on Public Health, Ministry of Health, Nouakchott, Mauritania (M L C B Ahmed); Research Department, Prince Mohammed Bin Abdulaziz Hospital, Ministry of Health, Riyadh, Saudi Arabia (Prof Z A Memish MD); Department of Medical Laboratory Science, Bahir Dar University, Bahir Dar, Ethiopia (C G Akal MSc, F M Demeke MSc, A Melese MSc); Evidence Based Practice Center, Mayo Clinic Foundation for Medical Education and Research, Rochester, MN, USA (F Alahdab MD); Prevention Division, Queensland Health, Herston, QLD, Australia (N Alam MPH); Department of Medical Microbiology (B Tessema PhD), Institute of Public Health (K A Alene MPH), University of Gondar, Gondar, Ethiopia; Research School of Population Health, Australian National University, Canberra, ACT, Australia (K A Alene); Health Management and Economics Research Center, Iran University of Medical Sciences, Tehran, Iran (V Alipour PhD, J Arabloo PhD, A Rezapour PhD); Department of Health Policy and Management, Kuwait University, Safat, Kuwait (Prof S M Aljunid PhD); International Centre for Casemix and Clinical Coding, National University of Malaysia, Bandar Tun Razak, Malaysia (Prof S M Aljunid); Department of Family and Community Medicine, King Abdulaziz University, Jeddah, Saudi Arabia (Prof R M Al-Raddadi PhD); Research Group in Health Economics, Universidad de Cartagena, Cartagena, Colombia (Prof N Alvis-Guzman PhD); Research Group in Hospital Management and Health Policies, Universidad de la Costa, Barranquilla, Colombia (Prof N Alvis-Guzman); Department of Pediatrics (J Nazari PhD), Health Services Management Department (S Amini PhD), Arak University of Medical Sciences, Arak, Iran; Social Determinants of Health Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran (M Anjomshoa PhD); Department of Health Policy and Administration (C A T Antonio MD), Development and Communication Studies (E R K Macarayan PhD), University of the Philippines Manila, Manila, Philippines; Department of Applied Social Sciences, Hong Kong Polytechnic University, Hong Kong, China (C A T Antonio); School of Health Sciences, Birmingham City University, Birmingham, UK (O Aremu PhD); Department of Nursing, Aksum University, Aksum, Ethiopia (H T Atalay MSc); University Institute of Public Health, The University of Lahore, Lahore, Pakistan (S Atique PhD); Public Health Department, University of Hail, Hail, Saudi Arabia (S Atique); Bénin Clinical Research Institute, Abomey-Calavi, Benin (E F G A Avokpaho MD); Contrôle des Maladies Infectieuses, Laboratory of Studies and Research-Action in Health, Porto Novo, Benin (E F G A Avokpaho); Department of Pediatrics and Neonatology (S Awad MD), Department of Public Health and Community Medicine (Prof Y S Khader PhD), Jordan University of Science and Technology, Ramtha, Jordan; Indian Institute of Public Health, Gandhinagar, India (A Awasthi PhD); Public Health Foundation of India, Gurugram, India (A Awasthi, Prof L Dandona, Prof R Dandona, G A Kumar PhD, D K Lal MD); Public Health Risk Sciences Division, Public Health Agency of Canada, Toronto, ON, Canada (A Badawi PhD); Department of Nutritional Sciences (A Badawi), The Centre for Global Child Health, Hospital for Sick Children (Prof Z A Bhutta PhD), University of Toronto, Toronto, ON, Canada; Department of Environmental Health Engineering, Sri Ramachandra Medical College and Research Institute, Chennai, India (Prof K Balakrishnan PhD); Faculty of Medicine, Alexandria University, Alexandria, Egypt (J A M Banoub MD); Clinic for Infectious and Tropical Diseases, Clinical Center of Serbia, Belgrade, Serbia (A Barac PhD); Centre School of Public Health and Health Management (Prof M M Santric Milicevic PhD), Faculty of Medicine (A Barac, E Dubljanin PhD), University of Belgrade, Belgrade, Serbia; Barcelona Institute for Global Health, University of Barcelona, Barcelona, Spain (Prof Q Bassat MD); Catalan Institution for Research and Advanced Studies, Barcelona, Spain (Prof Q Bassat, A Koyanagi MD); Department of Community Medicine, Gandhi Medical College Bhopal, Bhopal, India (Prof N Bedi MD); Jazan University, Jazan, Saudi Arabia (Prof N Bedi); Big Data Institute, Nuffield Department of Medicine (C E Moore PhD), Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine (S Lewycka PhD), Nuffield Department of Population Health (D A Bennett PhD), University of Oxford, Oxford, UK; Department of Statistical and Computational Genomics, National Institute of Biomedical Genomics, Kalyani, India (K Bhattacharyya MSc); Department of Statistics, University of Calcutta, Kolkata, India (K Bhattacharyya); Center of Excellence in Women and Child Health (Prof Z A Bhutta), Department of Pediatrics & Child Health (M Nisar MSc), Aga Khan University, Karachi, Pakistan; Social Determinants of Health Research Center, Babol University of Medical Sciences, Babol, Iran (A Bijani PhD); Department of Emergency Medicine and Institute for Global Health Sciences, University of California San Francisco, San Francisco, CA, USA (C B Bills MD); Centre for Population Health Sciences, Nanyang Technological University, Singapore, Singapore (J Car PhD); Global eHealth Unit (J Car), School of Public Health (Prof S Saxena MD), Imperial College London, London, UK; Applied Molecular Biosciences Unit (Prof F Carvalho PhD), Institute of Public Health (Prof F Carvalho), Centre of Excellence in Green Chemistry, Chemistry and Technology Network (Prof E Fernandes PhD), University of Porto, Porto, Portugal; Colombian National Health Observatory, National Institute of Health, Bogota, Colombia (C A Castañeda-Orjuela MD); Epidemiology and Public Health Evaluation Group, National University of Colombia, Bogota, Colombia (C A Castañeda-Orjuela); Department of Pulmonary Medicine, Christian Medical College and Hospital, Vellore, India (Prof D J Christopher MD); Health Effects Institute, Boston, MA, USA (A J Cohen); Department of Immunology (Prof A Rafiei PhD), Invasive Fungi Research Center (Prof S Seyedmousavi PhD), Molecular and Cell Biology Research Center (Prof A Rafiei), Pediatric Infectious Diseases Research Center (M S Rezai MD), Toxoplasmosis Research Center (Prof A Daryani PhD), Mazandaran University of Medical Sciences, Sari, Iran; Deputy of Research and Technology, Ministry of Health and Medical Education, Tehran, Iran (S Djalalinia PhD); UN World Food Programme, New Delhi, India (M Dubey PhD); Department of Health Sciences, Wollega University, Nekemte, Ethiopia (E E Duken MSc); Mycobacteriology Research Center, Jimma University, Jimma, Ethiopia (E E Duken); Department of Clinical Pathology, Mansoura University, Mansoura, Egypt (Prof M El Sayed Zaki PhD); Public Health Department, Saint Paul's Hospital Millennium Medical College, Addis Ababa, Ethiopia (A Y Endries MPH); Department of Public Health Medicine, Bielefeld University, Bielefeld, Germany (F Fischer PhD); Department of Pharmacy, Wollo University, Dessie, Ethiopia (B Geta MSc); Occupational and Environmental Epidemiology Section, Cancer Prevention and Research Institute, Florence, Italy (G Gorini MD); Center for Clinical and Epidemiological Research, University Hospital and Internal Medicine Department, University of São Paulo, São Paulo, Brazil (A C Goulart PhD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof Y Guo PhD, S Li PhD); Department of Epidemiology and Biostatistics, College of Public Health, Zhengzhou University, Zhengzhou, China (Prof Y Guo); Biomedical Sciences Division (G B Hailu MSc), Clinical Pharmacy Unit (T D Kassa MSc), School of Medicine (D T Mengistu MSc), School of Pharmacy (A G Tsadik MSc, E M Yimer MSc), Mekelle University, Mekelle, Ethiopia; Center of Expertise in Microbiology (Prof S Seyedmousavi), Department of Health Management and Economics (S Mousavi PhD), Department of Pharmacology (Arv Haj-Mirzaian MD, Ary Haj-Mirzaian MD), Endocrinology and Metabolism Molecular-Cellular Sciences Institute (F Rahim PhD), Hematologic Malignancies Research Center (A Kasaeian PhD), Hematology-Oncology and Stem Cell Transplantation Research Center (A Kasaeian), Non-communicable Diseases Research Center (S S N Irvani MD), Sina Trauma and Surgery Research Center (Prof V Rahimi-Movaghar MD), Tehran University of Medical Sciences, Tehran, Iran; Obesity Research Center (Arv Haj-Mirzaian), Research Institute for Endocrine Sciences (S S N Irvani), Shahid Beheshti University of Medical Sciences, Tehran, Iran; Department of Health Policy and Management (A T Khoja MD), Department of Radiology (Ary Haj-Mirzaian), Johns Hopkins University, Baltimore, MD, USA; School of Health and Environmental Studies, Hamdan Bin Mohammed Smart University, Dubai, United Arab Emirates (Prof S Hamidi DrPH); Pharmacy Department (A T Kefale MSc), Public Health Department (H Y Hassen MPH), Mizan-Tepi University, Teppi, Ethiopia; Unit of Epidemiology and Social Medicine, University Hospital Antwerp, Wilrijk, Belgium (H Y Hassen); Center for Excellence in Behavioral Medicine, Nguyen Tat Thanh University, Ho Chi Minh, Vietnam (C L Hoang BMedSc, L H Nguyen PhD, S H Nguyen BS); Department of Pulmonology, Yokohama City University, Kanazawa-ku, Yokohama, Japan (N Horita PhD); National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA (N Horita); Department of General Surgery, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania (M Hostiuc PhD); Department of Internal Medicine, Bucharest Emergency Hospital, Bucharest, Romania (M Hostiuc); Provincial TB Control Program, Department of Health, Gilgit, Pakistan (Z Hussain MPH); Department of Community Medicine, Banaras Hindu University, Varanasi, India (R P Jha MSc); Department of Ophthalmology (Prof J B Jonas MD), Institute of Public Health (B Moazen MSc, S Mohammed PhD), Heidelberg University, Mannheim, Germany; Beijing Institute of Ophthalmology, Beijing Tongren Hospital, Beijing, China (Prof J B Jonas); Institute for Epidemiology and Social Medicine, University of Münster, Münster, Germany (A Karch MD); Epidemiology and Biostatistics Department, Health Services Academy, Islamabad, Pakistan (E A Khan MPH); Department of Medical Microbiology & Immunology, United Arab Emirates University, Al Ain, United Arab Emirates (Prof G Khan PhD); Department of Population Sciences, Jatiya Kabi Kazi Nazrul Islam University, Mymensingh, Bangladesh (M N Khan MSc); Department of Public Health, University of Newcastle, Newcastle, NSW, Australia (M N Khan); Department of Health Policy and Management and Institute of Health Policy and Management, Seoul National University, Seoul, South Korea (Prof Y Khang MD); Department of Public Health, Imam Muhammad Ibn Saud Islamic University, Riyadh, Saudi Arabia (A T Khoja); Department of Nutrition, Simmons College, Boston, MA, USA (R W Kimokoti MD); Department of Health Management and Health Economics, Kristiania University College, Oslo, Norway (Prof A Kisa PhD); Department of Health Services Policy and Management, University of South Carolina, Columbia, SC, USA (Prof A Kisa); Nursing and Health Promotion, Oslo Metropolitan University, Oslo, Norway (S Kisa PhD); Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada (Prof N Kissoon MD, S Murthy MD); Institute for Social Science Research (A A Mamun PhD), School of Public Health (L D Knibbs PhD), The University of Queensland, Herston, QLD, Australia; Department of Public Health, Erasmus University Medical Center, Rotterdam, Netherlands (S Kochhar); Independent Consultant, Jakarta, Indonesia (S Kosen MD); Independent Consultant, Karachi, Pakistan (M A Shaikh MD); Department of Internal and Pulmonary Medicine, Sheri Kashmir Institute of Medical Sciences, Srinagar, India (Prof P A Koul MD); Biomedical Research Networking Center for Mental Health Network, San Juan de Dios Sanitary Park, Sant Boi de Llobregat, Spain (A Koyanagi); Department of Demography and Department of Social and Preventive Medicine, University of Montreal, Montreal, QC, Canada (Prof B Kuate Defo PhD); Department of Public Health, Debre Markos University, Debre Markos, Ethiopia (C T Leshargie MPH); Oxford University Clinical Research Unit, Wellcome Trust Asia Programme, Hanoi, Vietnam (S Lewycka); Department of Paediatrics, All India Institute of Medical Sciences, New Delhi, India (Prof R Lodha MD); Ariadne Labs (E R K Macarayan), Division of General Internal Medicine and Primary Care (Prof A Sheikh MD), Harvard University, Boston, MA, USA; Department of Public Health, Trnava University, Trnava, Slovakia (M Majdan PhD); Department of Internal Medicine, SevenHills Hospital, Mumbai, India (V Mehta MD); College of Medicine, Alfaisal University, Riyadh, Saudi Arabia (Prof Z A Memish, M Temsah MD); Breast Surgery Unit, Helsinki University Hospital, Helsinki, Finland (T J Meretoja MD); University of Helsinki, Helsinki, Finland (T J Meretoja); Clinical Microbiology and Parasitology Unit, Dr Zora Profozic Polyclinic, Zagreb, Croatia (T Mestrovic PhD); University Centre Varazdin, University North, Varazdin, Croatia (T Mestrovic); Center for Innovation in Medical Education, Pomeranian Medical University, Szczecin, Poland (B Miazgowski MD); Faculty of General Medicine, Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan (Prof E M Mirrakhimov MD); Department of Atherosclerosis and Coronary Heart Disease, National Center of Cardiology and Internal Disease, Bishkek, Kyrgyzstan (Prof E M Mirrakhimov); Institute of Addiction Research, Frankfurt University of Applied Sciences, Frankfurt, Germany (B Moazen); Department of Biology, Salahaddin University, Erbil, Iraq (K A Mohammad PhD); ISHIK University, Erbil, Iraq (K A Mohammad); Department of Community Medicine (M B Sufiyan MD), Health Systems and Policy Research Unit (S Mohammed), Ahmadu Bello University, Zaria, Nigeria; Clinical Epidemiology and Public Health Research Unit, Burlo Garofolo Institute for Maternal and Child Health, Trieste, Italy (L Monasta DSc, L Ronfani PhD); International Laboratory for Air Quality and Health, Queensland University of Technology, Brisbane, QLD, Australia (Prof L Morawska PhD); Department of Pediatric Medicine, Nishtar Medical University, Multan, Pakistan (Prof G Mustafa MD); Department of Pediatrics & Pediatric Pulmonology, Institute of Mother & Child Care, Multan, Pakistan (Prof G Mustafa); Iranian Ministry of Health and Medical Education, Iran (J Nazari); Institute for Global Health Innovations, Duy Tan University, Hanoi, Vietnam (C T Nguyen MPH, H L T Nguyen MPH); Translational Health Research Institute, Western Sydney University, Penrith, NSW, Australia (F A Ogbo PhD); Department of Research, Measurement, and Results, Society for Family Health Nigeria, Abuja, Nigeria (A Okoro MPH); Department of Pathology and Molecular Medicine (T O Olagunju MD), Department of Psychiatry and Behavioural Neurosciences (A T Olagunju MD), McMaster University, Hamilton, ON, Canada; Department of Psychiatry, University of Lagos, Lagos, Nigeria (A T Olagunju); Graduate School of Public Health, San Diego State University, San Diego, CA, USA (Prof E Oren); Center for Vaccine Development, University of Maryland, Baltimore, MD, USA (J R Ortiz); Department of TB & Respiratory Medicine, Jagadguru Sri Shivarathreeswara University, Mysore, India (Prof M P A DNB); Department of Medicine, Ottawa Hospital Research Institute, Ottawa, ON, Canada (S Pakhale MD); Department of Economics and Business and University Medical Center Groningen, University of Groningen, Groningen, Netherlands (Prof M J Postma PhD); Non-communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran (M Qorbani PhD); School of Public Health, University of Ghana, Accra, Ghana (R Quansah PhD); Thalassemia and Hemoglobinopathy Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (F Rahim); Society for Health and Demographic Surveillance, Suri, India (R K Rai MPH); Department of Economics, University of Göttingen, Göttingen, Germany (R K Rai); Center for Health Systems Research, National Institute of Public Health, Cuernavaca, Mexico (M J Rios-Blancas MPH); Institute of Epidemiology and Medical Biometry, Ulm University, Ulm, Germany (Prof D Rothenbacher MD); Department of Biomedical Sciences, University of Sassari, Sassari, Italy (Prof S Rubino PhD); Rashid Latif College of Pharmacy, Lahore, Pakistan (Z Saleem PharmD); Cochrane South Africa, South African Medical Research Council, Cape Town, South Africa (E Z Sambala PhD, Prof C S Wiysonge MD); Department of Entomology, Ain Shams University, Cairo, Egypt (A M Samy PhD); Department of Health and Society, Faculty of Medicine, University of Applied and Environmental Sciences, Bogotá, Colombia (Prof R Sarmiento-Suárez MPH); Faculty of Infectious and Tropical Diseases, London School of Hygiene & Tropical Medicine, London, UK (Prof B Sartorius); GSK Biologicals, Wavre, Belgium (M Savic PhD); Department of Public Health Sciences, University of North Carolina at Charlotte, Charlotte, NC, USA (M Sawhney PhD); Usher Institute of Population Health Sciences and Informatics, University of Edinburgh, Edinburgh, UK (Prof A Sheikh); National Institute of Infectious Diseases, Tokyo, Japan (M Shigematsu PhD); Division of Community Medicine, International Medical University, Kuala Lumpur, Malaysia (C T Sreeramareddy MD); Department of Pediatrics, King Saud University, Riyadh, Saudi Arabia (M Temsah); Department of Health Economics, Hanoi Medical University, Hanoi, Vietnam (B X Tran PhD); Molecular Medicine and Pathology, University of Auckland, Auckland, New Zealand (K B Tran MD); Clinical Hematology and Toxicology, Military Medical University, Hanoi, Vietnam (K B Tran); Gomal Center of Biochemistry and Biotechnology, Gomal University, Dera Ismail Khan, Pakistan (I Ullah PhD); TB Culture Laboratory, Mufti Mehmood Memorial Teaching Hospital, Dera Ismail Khan, Pakistan (I Ullah); UKK Institute, Tampere, Finland (Prof T J Vasankari MD); Psychosocial Injuries Research Center, Ilam University of Medical Sciences, Ilam, Iran (Y Veisani PhD); Department of Microbial Cellular and Molecular Biology, Addis Ababa University, Addis Ababa, Ethiopia (F W Wada); Foundation University Medical College, Foundation University, Rawalpindi, Pakistan (Y Waheed PhD); Department of Global Health, Stellenbosch University, Cape Town, South Africa (Prof C S Wiysonge); Department of Psychopharmacology, National Center of Neurology and Psychiatry, Tokyo, Japan (N Yonemoto MPH); Department of Epidemiology, University Hospital of Setif, Setif, Algeria (Prof Z Zaidi PhD); and Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa (Prof H J Zar PhD).
Contributors
CET prepared the first draft. IAK and RCR provided overall guidance. BFB and MHB managed the project. CET, IAK, and RCR finalised the manuscript on the basis of comments from other authors and reviewer feedback. All other authors provided data, developed models, reviewed results, provided guidance on methods, or reviewed the manuscript.
Declaration of interests
SLJ reports grants from Sanofi Pasteur during the conduct of the study. MJP reports grants from Merck Sharp & Dohme, GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Novavax, Bayer, Bristol-Myers Squibb, Astra Zeneca, Sanofi, Asc Academics, IQVIA, and BioMerieux; personal fees from Merck Sharp & Dohme, GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Novavax, Quintiles, Bristol-Myers Squibb, Astra Zeneca, Sanofi, Novartis, Pharmerit, Ingress Health, Asc Academics, and IQVIA; stocks in Ingress Health, Pharmacoeconomics Advice Groningen, and Asc Academics; and non-financial support from Asc Academics, outside of the submitted work. MS reports employment by and shares in the GSK group of companies. All other authors declare no competing interests.
Contributor Information
GBD 2017 Lower Respiratory Infections Collaborators:
Christopher E Troeger, Ibrahim A Khalil, Brigette F Blacker, Molly H Biehl, Samuel B Albertson, Stephanie R M Zimsen, Puja C Rao, Degu Abate, Amha Admasie, Alireza Ahmadi, Mohamed Lemine Cheikh Brahim Ahmed, Chalachew Genet Akal, Fares Alahdab, Noore Alam, Kefyalew Addis Alene, Vahid Alipour, Syed Mohamed Aljunid, Rajaa M Al-Raddadi, Nelson Alvis-Guzman, Saeed Amini, Mina Anjomshoa, Carl Abelardo T Antonio, Jalal Arabloo, Olatunde Aremu, Hagos Tasew Atalay, Suleman Atique, Euripide F G A Avokpaho, Samah Awad, Ashish Awasthi, Alaa Badawi, Kalpana Balakrishnan, Joseph Adel Mattar Banoub, Aleksandra Barac, Quique Bassat, Neeraj Bedi, Derrick A Bennett, Krittika Bhattacharyya, Zulfiqar A Bhutta, Ali Bijani, Corey B Bills, Josip Car, Félix Carvalho, Carlos A Castañeda-Orjuela, Kate Causey, Devasahayam J Christopher, Aaron J Cohen, Lalit Dandona, Rakhi Dandona, Ahmad Daryani, Feleke Mekonnen Demeke, Shirin Djalalinia, Manisha Dubey, Eleonora Dubljanin, Eyasu Ejeta Duken, Maysaa El Sayed Zaki, Aman Yesuf Endries, Eduarda Fernandes, Florian Fischer, Joseph Frostad, Nancy Fullman, William M Gardner, Birhanu Geta, Keyghobad Ghadiri, Giuseppe Gorini, Alessandra C Goulart, Yuming Guo, Gessessew Bugssa Hailu, Arvin Haj-Mirzaian, Arya Haj-Mirzaian, Samer Hamidi, Hamid Yimam Hassen, Chi Linh Hoang, Nobuyuki Horita, Mihaela Hostiuc, Zakir Hussain, Seyed Sina Naghibi Irvani, Spencer L James, Ravi Prakash Jha, Jost B Jonas, André Karch, Amir Kasaeian, Tesfaye Dessale Kassa, Nicholas J Kassebaum, Adane Teshome Kefale, Yousef Saleh Khader, Ejaz Ahmad Khan, Gulfaraz Khan, Md Nuruzzaman Khan, Young-Ho Khang, Abdullah T Khoja, Ruth W Kimokoti, Adnan Kisa, Sezer Kisa, Niranjan Kissoon, Luke D Knibbs, Sonali Kochhar, Soewarta Kosen, Parvaiz A Koul, Ai Koyanagi, Barthelemy Kuate Defo, G Anil Kumar, Dharmesh Kumar Lal, Cheru Tesema Leshargie, Sonia Lewycka, Shanshan Li, Rakesh Lodha, Erlyn Rachelle King Macarayan, Marek Majdan, Abdullah A Mamun, Helena Manguerra, Varshil Mehta, Addisu Melese, Ziad A Memish, Desalegn Tadese Mengistu, Tuomo J Meretoja, Tomislav Mestrovic, Bartosz Miazgowski, Erkin M Mirrakhimov, Babak Moazen, Karzan Abdulmuhsin Mohammad, Shafiu Mohammed, Lorenzo Monasta, Catrin E Moore, Lidia Morawska, Jonathan F Mosser, Seyyed Meysam Mousavi, Srinivas Murthy, Ghulam Mustafa, Javad Nazari, Cuong Tat Nguyen, Huong Lan Thi Nguyen, Long Hoang Nguyen, Son Hoang Nguyen, Katie R Nielsen, Muhammad Imran Nisar, Molly R Nixon, Felix Akpojene Ogbo, Anselm Okoro, Andrew T Olagunju, Tinuke O Olagunju, Eyal Oren, Justin R Ortiz, Mahesh P A, Smita Pakhale, Maarten J Postma, Mostafa Qorbani, Reginald Quansah, Alireza Rafiei, Fakher Rahim, Vafa Rahimi-Movaghar, Rajesh Kumar Rai, Marissa Bettay Reitsma, Mohammad Sadegh Rezai, Aziz Rezapour, Maria Jesus Rios-Blancas, Luca Ronfani, Dietrich Rothenbacher, Salvatore Rubino, Zikria Saleem, Evanson Zondani Sambala, Abdallah M Samy, Milena M Santric Milicevic, Rodrigo Sarmiento-Suárez, Benn Sartorius, Miloje Savic, Monika Sawhney, Sonia Saxena, Alyssa Sbarra, Seyedmojtaba Seyedmousavi, Masood Ali Shaikh, Aziz Sheikh, Mika Shigematsu, David L Smith, Chandrashekhar T Sreeramareddy, Jeffrey D Stanaway, Mu'awiyyah Babale Sufiyan, Mohamad-Hani Temsah, Belay Tessema, Bach Xuan Tran, Khanh Bao Tran, Afewerki Gebremeskel Tsadik, Irfan Ullah, Rachel L Updike, Tommi Juhani Vasankari, Yousef Veisani, Fiseha Wadilo Wada, Yasir Waheed, Katie Welgan, Kirsten E Wiens, Charles Shey Wiysonge, Ebrahim M Yimer, Naohiro Yonemoto, Zoubida Zaidi, Heather J Zar, Stephen S Lim, Theo Vos, Ali H Mokdad, Christopher J L Murray, Hmwe Hmwe Kyu, Simon I Hay, and Robert C Reiner
Supplementary Material
References
- 1.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.JustActions . JustActions; New York, NY: 2018. The Missing Piece. Why continued neglect of pneumonia threatens the achievement of health goals. [Google Scholar]
- 3.WHO/The United Nations Children's Fund . World Health Organization; Geneva: 2013. End preventable deaths: Global Action Plan for Prevention and Control of Pneumonia and Diarrhoea. [Google Scholar]
- 4.International Vaccine Access Center. Johns Hopkins Bloomberg School of Public Health Pneumonia and diarrhea progress report 2018. 2018. https://www.jhsph.edu/ivac/wp-content/uploads/2018/11/Pneumonia-and-Diarrhea-Progress-Report-2018-1.pdf
- 5.Bhutta ZA, Das JK, Walker N. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. 2013;381:1417–1429. doi: 10.1016/S0140-6736(13)60648-0. [DOI] [PubMed] [Google Scholar]
- 6.GBD 2016 Lower Respiratory Infections Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2015 LRI Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang S, Meng Q, Chen L, Bekedam H, Evans T, Whitehead M. Tackling the challenges to health equity in China. Lancet. 2008;372:1493–1501. doi: 10.1016/S0140-6736(08)61364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Bai J, Na H. The history of China's maternal and child health care development. Semin Fetal Neonatal Med. 2015;20:309–314. doi: 10.1016/j.siny.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Guan X, Silk BJ, Li W. Pneumonia incidence and mortality in mainland China: systematic review of Chinese and English literature, 1985–2008. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drewnowski A, Popkin BM. The nutrition transition: new trends in the global diet. Nutr Rev. 1997;55:31–43. doi: 10.1111/j.1753-4887.1997.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhai F, Fu D, Du S, Ge K, Chen C, Popkin BM. What is China doing in policy-making to push back the negative aspects of the nutrition transition? Public Health Nutr. 2002;5:269–273. doi: 10.1079/phn2001303. [DOI] [PubMed] [Google Scholar]
- 16.Du S, Mroz TA, Zhai F, Popkin BM. Rapid income growth adversely affects diet quality in China—particularly for the poor! Soc Sci Med. 2004;59:1505–1515. doi: 10.1016/j.socscimed.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Du S, Lu B, Zhai F, Popkin BM. A new stage of the nutrition transition in China. Public Health Nutr. 2002;5:169–174. doi: 10.1079/PHN2001290. [DOI] [PubMed] [Google Scholar]
- 18.Fullman N, Yearwood J, Abay SM. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. The Lancet. 2018;391:2236–2271. doi: 10.1016/S0140-6736(18)30994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues CMC. Challenges of empirical antibiotic therapy for community-acquired pneumonia in children. Curr Ther Res Clin Exp. 2017;84:e7–e11. doi: 10.1016/j.curtheres.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruk ME, Gage AD, Arsenault C. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6:E1196–E1252. doi: 10.1016/S2214-109X(18)30386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogawski ET, Platts-Mills JA, Seidman JC. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ. 2017;95:49–61. doi: 10.2471/BLT.16.176123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das JK, Lassi ZS, Salam RA, Bhutta ZA. Effect of community based interventions on childhood diarrhea and pneumonia: uptake of treatment modalities and impact on mortality. BMC Public Health. 2013;13:S29. doi: 10.1186/1471-2458-13-S3-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abegunde D, Orobaton N, Shoretire K. Monitoring maternal, newborn, and child health interventions using lot quality assurance sampling in Sokoto State of northern Nigeria. Glob Health Action. 2015;8 doi: 10.3402/gha.v8.27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kana MA, Doctor HV, Peleteiro B, Lunet N, Barros H. Maternal and child health interventions in Nigeria: a systematic review of published studies from 1990 to 2014. BMC Public Health. 2015;15:334. doi: 10.1186/s12889-015-1688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bedford KJA, Sharkey AB. Local Barriers and solutions to improve care-seeking for childhood pneumonia, diarrhoea and malaria in Kenya, Nigeria and Niger: a qualitative study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noordam AC, Sharkey AB, Hinssen P, Dinant G, Cals JWL. Association between caregivers' knowledge and care seeking behaviour for children with symptoms of pneumonia in six sub-Saharan African Countries. BMC Health Serv Res. 2017;17:107. doi: 10.1186/s12913-017-2060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Institute for Health Metrics and Evaluation Development Assistance for Health Database, 1990–2011. 2012. http://ghdx.healthdata.org/record/ihme-data/development-assistance-health-database-1990-2011
- 28.Brown R, Head M. Research Investments in Global Health; Southampton: 2018. Sizing up pneumonia research: assessing global investments in pneumonia research 2000–2015. [Google Scholar]
- 29.Sgambatti S, Minamisava R, Bierrenbach AL. Early impact of 10-valent pneumococcal conjugate vaccine in childhood pneumonia hospitalizations using primary data from an active population-based surveillance. Vaccine. 2016;34:663–670. doi: 10.1016/j.vaccine.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Swingler G, Fransman D, Hussey G. Conjugate vaccines for preventing Haemophilus influenzae type B infections. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD001729.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Morris SK, Moss WJ, Halsey N. Haemophilus influenzae type b conjugate vaccine use and effectiveness. Lancet Infect Dis. 2008;8:435–443. doi: 10.1016/S1473-3099(08)70152-X. [DOI] [PubMed] [Google Scholar]
- 32.Theodoratou E, Johnson S, Jhass A. The effect of Haemophilus influenzae type b and pneumococcal conjugate vaccines on childhood pneumonia incidence, severe morbidity and mortality. Int J Epidemiol. 2010;39:i172–i185. doi: 10.1093/ije/dyq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tricarico S, McNeil HC, Cleary DW. Pneumococcal conjugate vaccine implementation in middle-income countries. Pneumonia Nathan Qld. 2017;9:6. doi: 10.1186/s41479-017-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86:390–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruce NG, Dherani MK, Das JK. Control of household air pollution for child survival: estimates for intervention impacts. BMC Public Health. 2013;13:S8. doi: 10.1186/1471-2458-13-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith KR, McCracken JP, Weber MW. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 37.Mortimer K, Ndamala CB, Naunje AW. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. Lancet. 2017;389:167–175. doi: 10.1016/S0140-6736(16)32507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack DW, Asante KP, Wylie BJ. Ghana randomized air pollution and health study (GRAPHS): study protocol for a randomized controlled trial. Trials. 2015;16:420. doi: 10.1186/s13063-015-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagel CL, Kirby MA, Zambrano LD. Study design of a cluster-randomized controlled trial to evaluate a large-scale distribution of cook stoves and water filters in Western Province, Rwanda. Contemp Clin Trials Commun. 2016;4:124–135. doi: 10.1016/j.conctc.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kara E, Özdilek HG, Kara EE. Ambient air quality and asthma cases in Niğde, Turkey. Environ Sci Pollut Res Int. 2013;20:4225–4234. doi: 10.1007/s11356-012-1376-0. [DOI] [PubMed] [Google Scholar]
- 41.Watts N, Adger WN, Agnolucci P. Health and climate change: policy responses to protect public health. Lancet. 2015;386:1861–1914. doi: 10.1016/S0140-6736(15)60854-6. [DOI] [PubMed] [Google Scholar]
- 42.Wolf J, Moser SC. Individual understandings, perceptions, and engagement with climate change: insights from in-depth studies across the world. Wiley Interdiscip Rev Clim Change. 2011;2:547–569. [Google Scholar]
- 43.Maibach EW, Nisbet M, Baldwin P, Akerlof K, Diao G. Reframing climate change as a public health issue: an exploratory study of public reactions. BMC Public Health. 2010;10:299. doi: 10.1186/1471-2458-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In compliance with the Guidelines for Accurate and Transparent Health Estimates Reporting, data and code for GBD 2017 are publicly available.