Abstract
Background
The clinical significance of viral load and co-infections in children with respiratory infections is not clear.
Objective
To evaluate the correlation of viral load as well as viral and bacterial co-infections with disease severity in hospitalized children with lower respiratory tract infections (LRTIs).
Study design
This is a prospective study conducted in children admitted for LRTIs for two seasons. To determine viral and bacterial load of respiratory pathogens we performed multiplex real-time polymerase chain reaction and semiquantitative bacterial cultures on nasopharyngeal aspirates (NPA).
Results
During the study period 244 (60%) children were hospitalized for LRTI with acute virus-induced wheezing and 160 (40%) for radiologic confirmed pneumonia. In the first NPA, viruses were identified in 315 (78%) of the 404 samples and bacteria in 198 (63.3%) of 311 samples. The viral load significantly decreased between the first and second NPA sample in most single and viral co-infections, except rhinovirus and human bocavirus infections. Viral load was inversely related to CRP in RSV infections, whereas a positive correlation was observed in adenovirus infections. Duration of hospitalization was significantly longer in RSV single infections compared to rhinovirus single infections whereas in the latter, leucocytosis and use of systemic steroids was more common. In RSV viral co-infections the presence of fever, leucocytosis, and the use of antibiotics was significantly more frequent. Positive cultures of Haemophilus influenzae dominated in RSV and rhinovirus single infections and Moraxella catarrhalis in RSV viral co-infections.
Conclusions
Specific viral single and co-infections as well as viral load contribute to disease severity in children with LRTIs.
Keywords: Respiratory viruses, Co-infection, Viral load, Pneumonia, Acute virus-induced wheezing
1. Background
The majority of infections of the lower respiratory tract (LRTI) in children are caused by viruses.1 A great variety of respiratory viruses have repeatedly been detected in acute LRTI including influenza virus types A and B, parainfluenza virus type 1, 2 and 3, respiratory syncytial virus (RSV), rhinovirus, enterovirus and adenovirus.2, 3 This panel of viruses has recently been expanded by newly discovered viruses like human metapneumovirus (HMPV),4 coronavirus,5 and human bocavirus (hBoV). 6
Several previous studies have shown that the virus type and viral load may influence the clinical characteristics of infection.7, 8 The presence of more than one type of virus in the respiratory specimen may also affect the clinical presentation of LRTI.7, 9 However, many aspects of the relationship between the type of virus, the number of viruses detected, their quantity in respiratory secretions and the severity of illness in children remain unclear.
Many of the bacteria detected during a respiratory infection in cultures from respiratory secretions are commensals of the upper respiratory tract. They often contaminate respiratory samples making the assessment of their causal role for lower respiratory tract infection difficult.10 Interactions between viruses and bacteria have been described.1, 3 Pneumococcal conjugate vaccination has been found to reduce the risk of virus-associated pneumonia, pointing to clinically relevant interactions between bacterial pathogens and viral pneumonia.11 A correlation between bacterial colonization and viral infection in LRTI merits further investigation.
2. Objective
To perform a prospective clinical study to investigate the correlation between the type, number and quantity of respiratory viruses and bacteria in the nasopharyngeal aspirate by multiplex real-time (RT)-PCR and semiquantitative bacterial culture and the clinical characteristics of the infection in pediatric patients hospitalized for acute virus-induced wheezing or pneumonia.
3. Study design
The prospective clinical study was conduced at the University Children's Hospital Düsseldorf and at the Children's Hospital of Evangelisches Krankenhaus Düsseldorf during a 2-year period from November 2006 until October 2008. Children aged 0–16 years were recruited who were consecutively admitted to the two hospitals for LRTI. Patients with primary or secondary immunosuppression were excluded. The clinical respiratory diagnosis acute virus-induced wheezing or pneumonia was based on the diagnosis given by physicians in charge, attributed to a combination of clinical, laboratory and radiological findings. Initiation of an antibiotic therapy was also based on these parameters. A chest radiogram was not a study requirement. Sample collection and routine diagnostic procedures were conducted in adherence to the guidelines of good clinical practice under approval of the Institutional Review Board of the University Hospital Düsseldorf. Written informed consent was obtained from all parents prior to any study procedures being performed.
Nasopharyngeal aspirates (NPA) were collected on the day of admission (1. NPA) and on the 3rd or 4th day of hospitalization (2. NPA). The NPAs were submitted to the Institute of Virology of the University Hospital Düsseldorf and evaluated for RSV, rhinovirus, influenza types A and B, parainfluenza types 1, 2 and 3, enterovirus, adenovirus, human metapneumovirus, coronaviruses 229E, OC43 and NL63 and human bocavirus using quantitative real-time RT-PCR.12 Briefly, a combination of duplex- and triplex one-step-RNA- and DNA-PCRs was used. All positive results were confirmed in a monoplex-assay and quantified using plasmid standards. The sensitivity and specificity was as demonstrated as described elsewhere.12
The specimens were simultaneously submitted to the Institute of Medical Microbiology and Hospital Hygiene of the University Hospital Düsseldorf where standard bacterial cultures were performed. For standard bacterial cultures NPAs were plated on Columbia agar for aerobic and anaerobic cultures, chocolate agar and MacKonkey agar (bioMerieux, Nürtingen, Germany) and incubated at 36 °C for 48 h. Aerobic Columbia and chocolate agars were incubated in a humidified atmosphere of 5% CO2. Cultures were examined after 24 and 48 h. The results of the bacterial cultures were expressed in semiquantitative steps: “no bacterial growth”, “occasional pathogenic bacteria”, “moderate amount of pathogenic bacteria” and “abundant pathogenic bacteria”.
The clinical data was prospectively collected during the hospital stay using a standard data collection form. Upon admission, the demographic characteristics and medical history of the children were systematically recorded, using standardized written questionnaires and, after a complete physical examination, children with a LRTI with the diagnosis of acute infectious wheezing, based on well-established criteria, or pneumonia were enrolled.13 Children known to have had previous similar episodes of wheezing related respiratory infections were diagnoses with recurrent wheezing. The diagnosis and classification of asthma was based on the criteria of the new 2002 Global Initiative for Asthma (GINA) guidelines.14 In patients who underwent chest radiography pneumonia was defined on WHO criteria.15
Beside age, gender, presence of chronic underlying conditions, clinical disease correlates were collected continuously during hospitalization and included supplemental oxygen requirement, use of bronchodilators, systemic corticosteroids, antibiotics, and performance of a chest radiography examination and the presence of fever. Infectious parameters were obtained as clinically indicated. Leucocytosis was defined as values above 15,000/μl and a significantly raised C-reactive protein (CRP) when raised above 5 mg/dl.
Values are expressed as percentages for discrete variables, or as mean and standard deviation for continuous variables, except age and days of hospitalization, which are described by their median. Clinical characteristics and laboratory variables are compared using the Pearson χ 2 test, Fisher's exact test, Kruskal–Wallis test, Wilcoxon signed rank test and Mann Whitney U-test as appropriate. A two sided p-value <0.05 was considered statistically significant.
4. Results
4.1. Patient characteristics
A total of 404 pediatric patients aged 0–16 years were recruited during the study period. Two hundred and fourty-four (60%) children had a discharge diagnosis of LRTI with acute virus-induced wheezing, 160 (40%) with radiologic confirmed pneumonia. The mean duration of clinical symptoms before admission was 3 days. Seventy-two percent of the study population were below the age of 2 (0–3 months 24%, 4–11 months 31%, 12–23 months 17%, 24–59 months 21%, 5–16 years 7%). The median age was 0.8 years. 60% were male. In the age group below 1 year, acute virus-induced wheezing was the predominant diagnosis whereas pneumonia was increasingly found in older children (Supplemental Fig. 1). In 196 (48%) cases an underlying condition was identified, most commonly prematurity (17%) followed by cardiac (7%), and pulmonary conditions (18%). Recurrent wheezing and asthma was identified in 14% and 1% cases, respectively.
4.2. Single and co-infections with respiratory viruses among children with LRTI
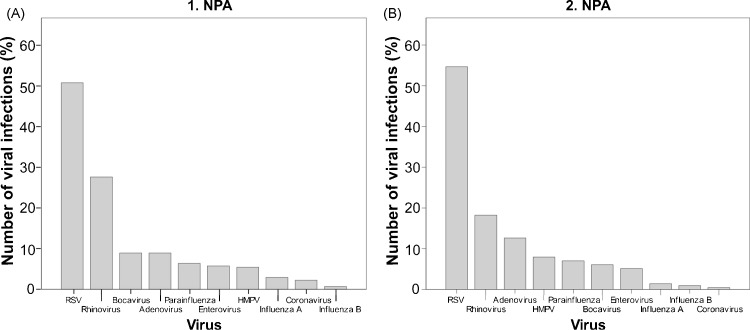
Respiratory viruses were detected in 315 (78%) of the collected specimen. The first NPA was collected in 100% of the cases, the second in 71% of the cases. The drop outs were due to earlier discharge or withdrawal of the approval by the parents. RSV type A and B were detected in 160 (51%) specimen followed by 87 detections of rhinovirus (28%) and 28 detections (9%) each of adenovirus and human bocavirus (Fig. 1A and B). An equal number of 80 RSV A and 80 RSV B infections were found. With all further analyses of clinical characteristics, viral load and co-infections there were no significant statistical difference between RSV A and RSV B infections (data not shown), therefore the results for the two RSV types were summarized.
Fig. 1.
Virus distribution in the nasopharyngeal aspirates. Virus distribution in the first nasopharyngeal aspirate (NPA) (A) and in the second NPA (B).
Viral single infections were detected in 66% and co-infections in 34% of all viral infections (Table 1 ). Two or more viruses were found in 67 cases (17%). A RSV viral co-infection with human bocavirus was the single most common observation (n = 14) (48%). The majority of the rhinovirus co-infections were in combination with enterovirus (n = 14) (47%). Overall, 30 viral co-infections were in combination with rhinovirus, 22 (73%) of these were dual infections, and 8 (27%) were infections with more than two viruses. Nineteen (68%) of the 28 detected infections with human bocavirus and adenovirus were viral co-infections. No viral co-infection between influenza A, influenza B, parainfluenza, RSV, and HMPV occurred.
Table 1.
Single and co-infections with respiratory viruses.
| Virus | Infections total | Single infections (number) | Co-infections (number) | Co-infections (%) | Influenza A | Influenza B | Parainfluenza | RSV | HMPV | Corona 229 E | Corona OC43 | Corona NL63 | Rhinovirus | Enterovirus | Adenovirus | HBoV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSV | 160 | 131 | 29 | 18,1 | 1 | 1 | 1 | 8 | 2 | 8 | 14 | |||||
| HMPV | 16 | 14 | 2 | 12,5 | 1 | 1 | ||||||||||
| Influenza A | 9 | 8 | 1 | 11,1 | 1 | |||||||||||
| Influenza B | 2 | 2 | 0 | 0,0 | ||||||||||||
| Parainfluenza | 20 | 15 | 5 | 25,0 | 2 | 3 | ||||||||||
| Corona 229E | 1 | 0 | 1 | 100,0 | 1 | |||||||||||
| Corona OC43 | 4 | 0 | 4 | 100,0 | 1 | 1 | 1 | 1 | ||||||||
| Corona NL63 | 2 | 1 | 1 | 50,0 | 1 | |||||||||||
| Rhinovirus | 87 | 57 | 30 | 34,5 | 2 | 8 | 1 | 1 | 14 | 7 | 5 | |||||
| Adenovirus | 28 | 9 | 19 | 67,9 | 3 | 8 | 7 | 2 | 4 | |||||||
| HBoV | 28 | 9 | 19 | 67,9 | 14 | 5 | 3 | 4 | ||||||||
| Enterovirus | 18 | 2 | 16 | 88,9 | 2 | 14 | 2 | 3 |
No viral co-infections between Influenza A, Influenza B, Parainfluenza, RSV, and HMPV occurred.
4.3. Clinical characteristics of viral single- and co-infections
The group of patients with RSV single infection (n = 131) was significantly younger than that with rhinovirus single infection (n = 57) (median age 0.5 vs. 1.4, p < 0.001) (Table 2 ). 68% of patients with rhinovirus single infection were male vs. 52% in RSV (p = 0.054). Underlying pulmonary diseases dominated in rhinovirus infections (12% vs. 4%, p = 0.04). The mean duration of hospitalization, was 1 day longer in RSV infection (p < 0.001). Use of systemic steroids (p = 0.02) and leucocytosis (p < 0.001) were more frequent in subjects with rhinovirus.
Table 2.
Comparisons of clinical characteristics among patients with RSV, rhinovirus, human bocavirus (HBoV) and adenovirus single infections, and RSV, rhinovirus, HBoV and adenovirus co-infections with other respiratory viruses.
| RSV single | Rhino single | HBoV single | Adenovirus single | RSV co-infection | Rhinovirus co-infection | HBoV co-infection | Adenovirus co-infection | |
|---|---|---|---|---|---|---|---|---|
| N | 131 | 57 | 9 | 9 | 29 | 30 | 19 | 19 |
| Age (years, median) | 0.5a,b | 1.4a | 1.2 | 1.1 | 0.8b | 1.0 | 1.1 | 1.0 |
| Male (%) | 68 (52)a | 39 (68)a | 5 (55) | 8 (89) | 19 (66) | 16 (53) | 12 (63) | 13 (68) |
| Pneumonia (%) | 38 (29)b | 18 (32) | 7 (78) | 7 (78) | 15 (52)b | 10 (33) | 10 (53) | 10 (53) |
| Supplemental O2 (%) | 56 (43) | 18 (32) | 2 (22) | 2 (22) | 12 (41) | 15 (50) | 7 (37) | 5 (26) |
| Antibiotics (%) | 53 (40)b | 21 (37) | 7 (78) | 8 (89) | 20 (69)b | 12 (40) | 6 (32) | 9 (47) |
| Bronchodilators (%) | 112 (85) | 52 (91) | 8 (89) | 3 (33) | 26 (90) | 27 (90) | 18 (95) | 16 (84) |
| Systemic corticosteroids (%) | 25 (19)a | 21 (37)a | 1 (11) | 2 (22) | 6 (21) | 11 (37) | 12 (63) | 9 (47) |
| Chest radiography (%) | 53 (40) | 28 (49) | 7 (78) | 8 (89) | 16 (55) | 15 (50) | 12 (63) | 13 (68) |
| Fever (≥38.5̊C) (%) | 50 (38)b | 21 (37) | 6 (67) | 7 (78) | 20 (69)b | 11 (37) | 12 (63) | 10 (53) |
| CRP ≥5 mg/dl | 12 (9) | 9 (16) | 4 (44) | 4 (44) | 4 (14) | 1 (3) | 2 (1) | 2 (1) |
| Leucocytosis (%) | 28 (21)a,b | 26 (46)a | 5 (55) | 4 (44) | 14 (48)b | 20 (67) | 8 (42) | 10 (53) |
| DOH (days, median) | 5a | 4a | 5 | 5 | 5 | 4 | 5 | 5 |
Other respiratory viruses were:
ap < .05, Rhino single vs. RSV single infections.
bp < .05, RSV-co-infection vs. RSV single infections.
Clinical characteristics did not differ significantly in HBoV and adenovirus single and co-infections.
Abbreviations: DOH: days of hospitalization and N: number.
Thirty-eight patients (29%) with a RSV single infection had pneumonia vs. 15 (52%) with a RSV viral co-infection (p = 0.05). The patients with viral co-infection were somewhat older (median age 0.8 vs. 0.5, p = 0.02). In RSV viral co-infections the use of antibiotics (p = 0.007) presence of fever (p = 0.003) and leucocytosis (p = 0.004) were significantly more frequent than in RSV single infections.
4.4. Relationship of viral load with correlates of disease severity
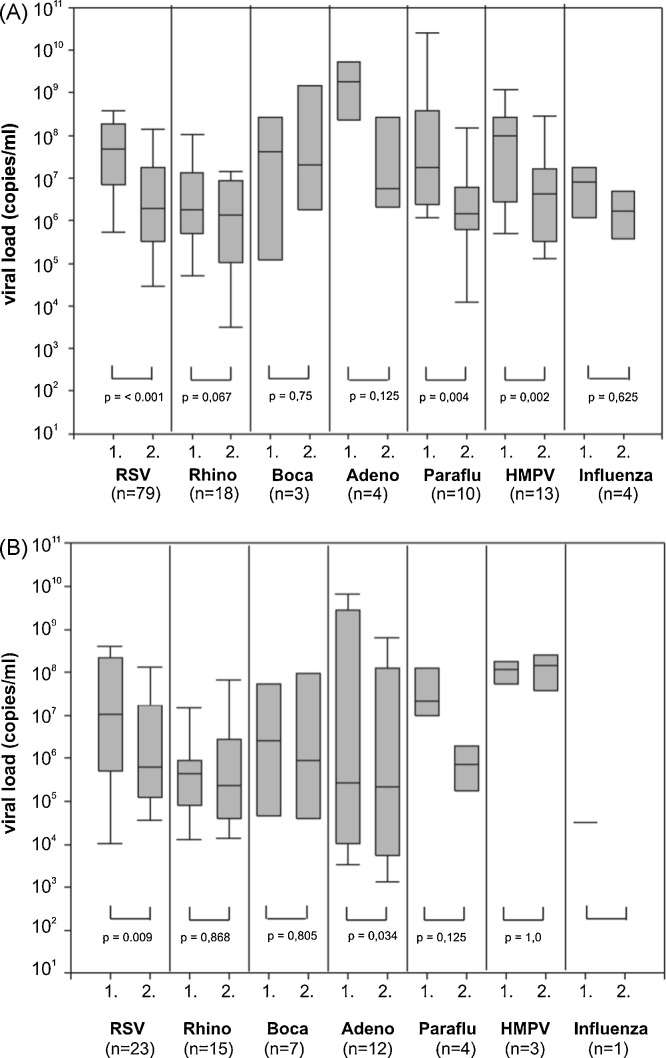
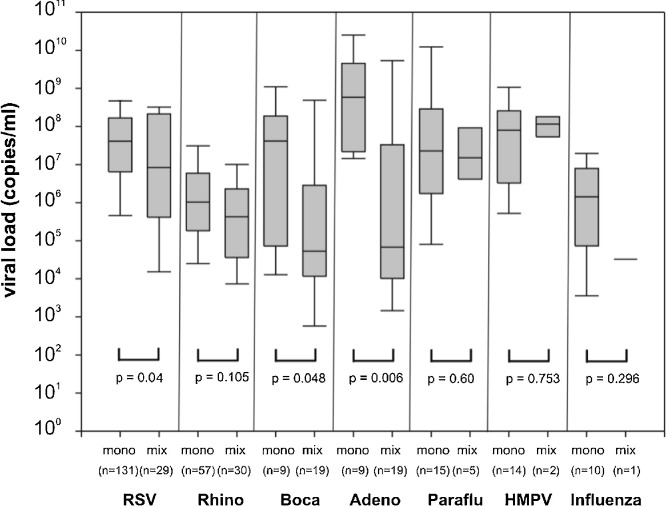
The viral load significantly decreased between the first and second NPA sample in single infections with RSV (p < 0.001), parainfluenza virus (p = 0.004), HMPV (p = 0.002) and viral co-infections with RSV (0 < 0.009), and adenovirus (p = 0.03) (Fig. 2 ). In RSV, bocavirus and adenovirus viral co-infections the viral load of these viruses was significantly lower than in single infections (respectively, p = 0.04, p = 0.048 and p = 0.006) (Fig. 3 ). In 24 of the 34 (70%) viral co-infections involving RSV, its viral load was higher than that of the concomitantly detected viruses (p = 0.03).
Fig. 2.
Viral load of respiratory viruses drops in the course of viral single- and co-infections. The figure shows the differences of viral load of paired 1 and 2. NPAs in viral single (A) and co-infections (B).
Fig. 3.
Viral load of respiratory viruses is lower in viral co-infections compared to single infections.
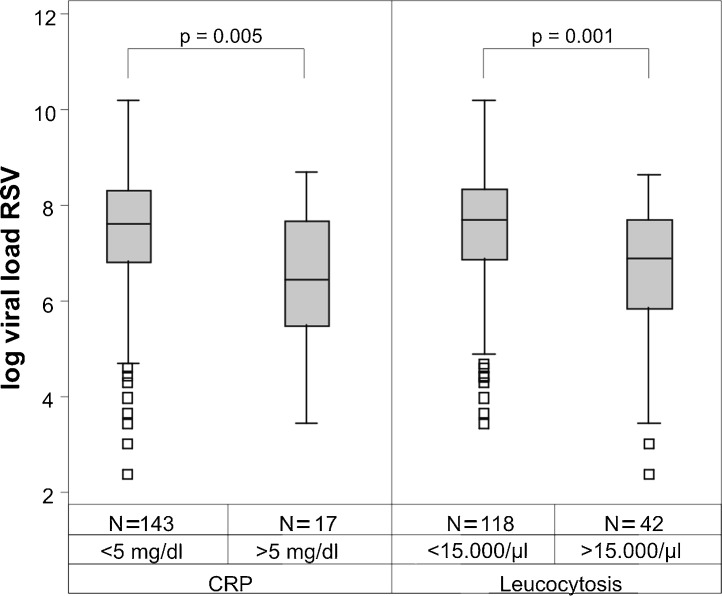
No statistically significant impact of the viral load in the NPA on the correlates of disease severity including duration of hospital stay, supplemental oxygen requirement, use of bronchodilators, use of systemic corticosteroids, use of antibiotics, use of chest radiography examination and presence of fever was found in RSV, rhinovirus, adenovirus or human bocavirus infections (data not shown). However, a significant negative correlation between the absolute viral load in RSV infections and infectious parameters in blood was detected for CRP (p = 0.005) and leucocyte count (p = 0.001) (Supplemental Fig. 2). In adenovirus single infections (n = 9) the presence of an elevated CRP above 5 mg/dl in serum correlated positively with viral load (p = 0.01) (data not shown).
4.5. Association between positive bacterial culture and type or number of viruses
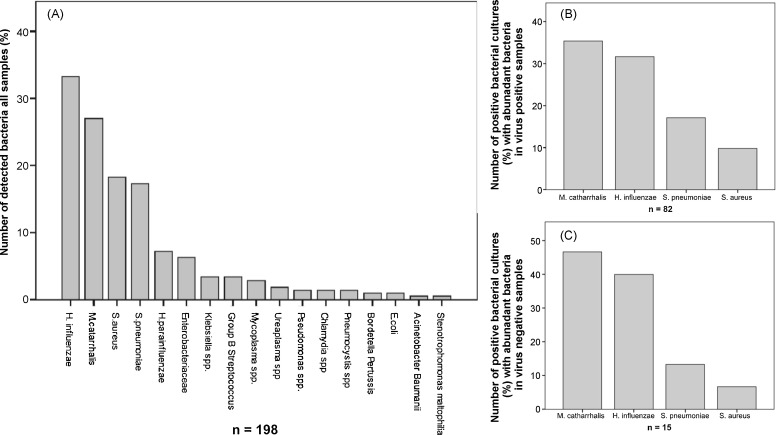
Bacterial cultures analyses for bacterial respiratory pathogens were performed in 311 samples from the 1. NPA. The missing 93 samples are mainly due to delayed start of bacterial culture sampling by approximately 6 weeks from the study beginning. A positive bacterial culture was detected in 198 (63.3%) NPAs and in 97 (31.1%) samples with the semiquantitative result “abundant pathogenic bacteria”. The samples with the result “abundant pathogenic bacteria” were considered for the viral co-infection analyses.
The pathogen distribution from the first NPA with a positive bacterial culture is shown in Fig. 4Aand those 1. NPAs with the result “abundant of pathogenic bacteria” in virus positive sample in Fig. 4B. Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae and S. aureus were the most frequently detected pathogens. Positive bacterial cultures were also found in 28 of 63 virus negative samples (44.4%), in which 15 (23.8%) samples had “abundant pathogenic bacteria” (Fig. 4C).
Fig. 4.
Pathogen distribution in bacterial cultures from nasopharyngeal aspirates. Pathogen distribution in all bacterial cultures from all 1. NPAs (A), pathogen distribution of positive bacterial cultures with the result “abundant pathogenic bacteria” in virus positive samples of the 1. NPAs (n = 97) (B) and from virus negative 1. NPAs (C) (n = 15).
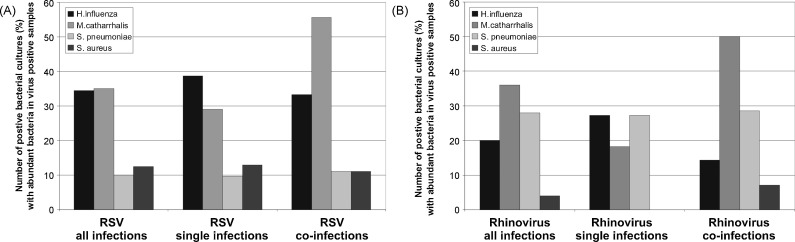
In RSV and rhinovirus single infections H. influenzae (39% and 27%, respectively) detections dominated while in viral co-infections the rate of M. catarrhalis positive cultures (56% in RSV and 50% in rhinovirus infections, respectively) were the most frequently detected bacterial pathogens (Fig. 5 ). S. pneumoniae was less frequently detected in RSV infections than in rhinovirus infections (28% vs. 10% of all infections). There was no significant difference in the rate of positive bacterial cultures between the two diagnosis groups acute virus-induced wheezing and pneumonia (32% vs. 26%, p = 0.32). Antibiotic treatment was initiated in 161 cases (40%). There was no positive correlation between a positive bacterial culture and initiation of an antibiotic treatment: 45 patients (46%) vs. 116 patients (50%) with and without a positive bacterial culture respectively received antibiotics during the hospital stay.
Fig. 5.
Predominance of Haemophilus influenzae in RSV and rhinovirus single infections, and Moraxellacatharralis in RSV and rhinovirus co-infections. Distribution of the most frequently detected bacteria in bacterial cultures from the 1. NPA with the semiquantitative result “abundant pathogenic bacteria” in all RSV (A) respectively rhinovirus (B) single infections and co-infections.
5. Discussion
The major strengths of this pediatric clinical study were beside the prospective study design over 2 complete seasons, the use of a highly sensitive quantitative multiplex RT-PCR for a wide range of respiratory viruses, the simultaneous detection of bacteria, the evaluation of important clinical characteristics, the high number of patients included in the study.
The availability of multiplex PCR means a major breakthrough for the diagnosis of respiratory pathogens and makes the detection of co-infections easier or even possible. By means of modern multiplex PCR techniques at least one respiratory virus is detected in 66–89% of respiratory samples of patients with acute LRTI.7, 19 The detection rate of 78% in our samples is comparable with these. In this study viral co-infections were detected in 34% of the cases. This rate is high, but also in accordance with the previously published data implicating the rate of dual and multiple infections to vary between 5% and 20% of all viral LRTIs.2, 7, 9 Human bocavirus, rhino- and enteroviruses and adenoviruses were the four pathogens most frequently involved in dual infections. Due to the close relationship of rhinoviruses and enteroviruses we cannot completely exclude that in some cases a crossreactivity in PCR testing mimics a co-infection between these two groups of viruses. Interestingly no viral co-infections between members of the orthomyxoviruses (influenza) and the paramyxoviruses (parainfluenza, RSV, HMPV) were seen.
In this study population RSV (51%) was the main reason for hospitalization for LRTI followed by rhinovirus (28%). Previously published findings have repeatedly confirmed RSV to be the main viral pathogen causing LRTI in children.1, 3, 16 Interestingly, twenty-two percent of our hospitalized children with LRTI had a single infection with rhinovirus, 32% of the rhinovirus single infections had pneumonia. Calvo et al. found only 3.5% of Spanish children infected with rhinovirus to be hospitalized for pneumonia, whereas others reported rates ranging between 16% and 24%.1, 17, 18 However, a relatively high rhinovirus prevalence of 12–20% in asymptomatic children has been reported.20, 21 According to our data rhinovirus is an important causative agent of lower respiratory tract infections in young children, second to RSV.
The comparison of clinical characteristics of RSV and rhinovirus infections revealed a shorter duration of hospitalization in rhinovirus infections, but a more frequent use of systemic steroids in rhinovirus infections. Physicians may tend to treat LRTI caused by rhinovirus with corticosteroids due to initial severity of presenting symptoms. A recently published controlled clinical trial demonstrated a benefit of systemic prednisolone in children with picornavirus (i.e. rhinovirus or enterovirus) LRTI.22 The high incidence of fever and leucocytosis might explain the higher rate of antibiotic use in RSV viral co-infections compared to RSV single infections in our study. However, viral factors may be responsible for RSV subgroup-related disease severity differences, including differences in the ability of certain viruses to promote a pathogenic inflammatory host response.23
Previous studies have investigated the relationship of viral load and viral dynamics to etiological role and disease severity in respiratory tract infections.24, 25 Gerna et al. found a significant drop in RSV viral load between admission and discharge in children hospitalized for RSV LRTI.8 In contrast, Bosis et al. did not find a significant impact of the RSV viral load on the disease severity in children admitted to hospital for the first episode of acute wheezing.16 There were also no significant correlation of viral load and disease severity in human bocavirus infections.26
This study is the first to investigate the influence of the viral load and viral dynamics of a broad range of respiratory viruses and different clinical correlates of disease severity and inflammatory parameters in blood in a multiplex PCR assay. We found a consistent fall of the viral load between admission and discharge in single infections with most of the viral pathogens (significant for RSV, parainfluenza virus and hMPV single and additionally adenovirus co-infections). Since patients were discharged from hospital when they were clinically improved, a relationship between viral load and disease severity can be assumed. Our findings are in agreement with a very recently published study in children with acute respiratory tract infections that has shown a correlation between clinical improvement and a reduction of viral quantity after 3 days of hospitalization.27 A high initial viral load at presentation may represent a recent infection, a low viral load a past infection, but an additional study powered to confirm this aspect has to be performed.
However, we hardly found a strong correlation of viral load with clinical characteristics. A significant negative correlation between the absolute viral load in RSV infections and an elevated C-reactive protein in serum and presence of a leucocytosis was detected. Accordingly leucocytosis occurred in RSV infections significantly less frequently than in rhinovirus infections. It is possible that RSV could depress the signs of infection in blood. Suppression of the induction of interferons by the NS1 and NS2 proteins of RSV has also been demonstrated in human epithelial cells and macrophages.28 Purcell et al. recently proposed that an abnormal white blood count is of limited use for the detection of concurrent bacterial infection in RSV infections.29 In contrast, adenovirus infections have been demonstrated to promote acute-phase response with elevated CRP in children.30
Respiratory infections with certain bacteria may be associated with predisposition for certain types of viral infection or vice versa. The colonization rates for M. catarrhalis, H. influenzae and S. pneumoniae in healthy children are high and depend on various factors.31, 32 A few population based studies suggest a relationship between RSV infections and invasive bacteria.33, 34 Increased numbers of non-typeable H. influenzae (NTHi) and Streptococcus pneumoniae adhering to human respiratory epithelial cells have been detected in RSV infections in vitro.35
In this study M. catarrhalis and H. influenzae were the most commonly detected bacterial agents. In RSV and rhinovirus single infections H. influenzae detections dominated while in RSV and rhinovirus co-infections M. catarrhalis was more frequently found. Therefore, a positive link between infection or colonization with certain bacteria and viral co-infections may be assumed.
According to our results the clinical course of viral LRTI in children may be affected by virus type, viral load and viral and bacterial co-infections. Clinical improvement was associated for most respiratory viruses with a reduction of viral quantity. The viral load had a significant impact on the infectious parameters in blood in case of RSV and adenovirus, but no effect on clinical correlates of disease severity. The multiplex PCR is an excellent diagnostic tool that helps both to improve the clinical management and expand the understanding of the pathogenesis of viral LRTI.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to thank all participating physicians, nurses and technical staff for recruiting and treating the patients, obtaining the NPAs and performing the assays.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2010.05.007.
Appendix A. Supplementary data
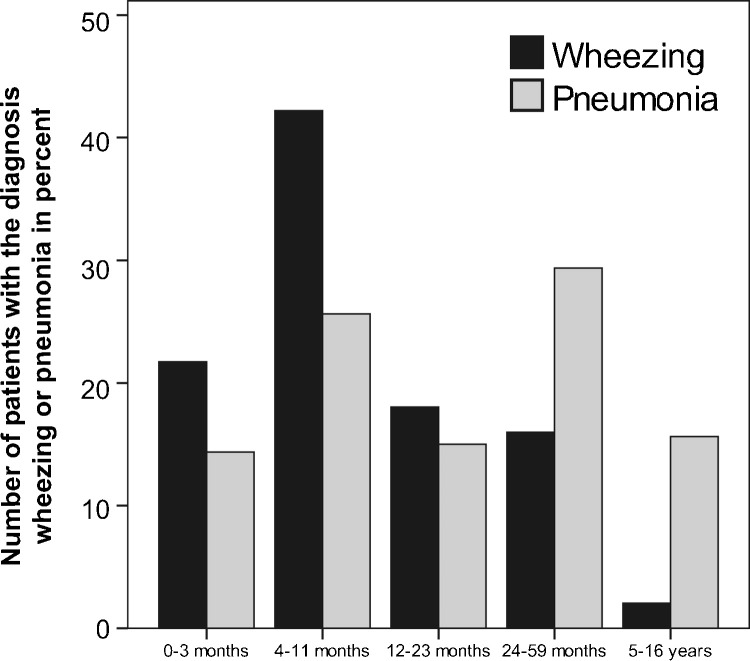
Supplementary Fig. 1.
Age distribution of the diagnoses pneumonia and acute virus-induced wheezing in the defined age groups. While the incidence of wheezing decreases, the incidence of pneumonia is similar in the different age groups.
Supplementary Fig. 2.
RSV viral load correlates with infection parameters. A higher RSV viral load negatively correlated with an increases CRP-value (>5 mg/dl) and a leukocytes above 15,000/μl.
References
- 1.Juvén T., Mertsola J., Waris M., Leinonen M., Meurman O., Roivainen M. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Legg J.P., Warner J.A., Johnston S.L., Warner J.O. Frequency of detection of picornaviruses and seven other respiratory pathogens in infants. Pediatr Infect Dis J. 2005;24:611–616. doi: 10.1097/01.inf.0000168747.94999.aa. [DOI] [PubMed] [Google Scholar]
- 3.Weigl J.A., Puppe W., Belke O., Neusüss J., Bagci F., Schmitt H.J. The descriptive epidemiology of severe lower respiratory tract infections in children in Kiel, Germany. Klin Padiatr. 2005;217:259–267. doi: 10.1055/s-2004-820352. [DOI] [PubMed] [Google Scholar]
- 4.van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pyrc K., Berkhout B., van der Hoek L. Identification of new human coronaviruses. Expert Rev Anti Infect Ther. 2007;5:245–253. doi: 10.1586/14787210.5.2.245. [DOI] [PubMed] [Google Scholar]
- 6.Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvo C., García-García M.L., Blanco C., Vázquez M.C., Frías M.E., Pérez-Breña P. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol. 2008;42:268–272. doi: 10.1016/j.jcv.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerna G., Campanini G., Rognoni V., Marchi A., Rovida F., Piralla A. Correlation of viral load as determined by real-time RT-PCR and clinical characteristics of respiratory syncytial virus lower respiratory tract infections in early infancy. J Clin Virol. 2008;41:45–48. doi: 10.1016/j.jcv.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Aberle J.H., Aberle S.W., Pracher E., Hutter H.P., Kundi M., Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24:605–610. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 10.Nohynek H., Eskola J., Kleemola M., Jalonen E., Saikku P., Leinonen M. Bacterial antibody assays in the diagnosis of acute lower respiratory tract infection in children. Pediatr Infect Dis J. 1995;14:478–484. doi: 10.1097/00006454-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Madhi S.A., Klugman K.P. Vaccine trialist group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonzel L., Tenenbaum T., Schroten H., Schildgen O., Schweitzer-Krantz S., Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J. 2008;27:589–594. doi: 10.1097/INF.0b013e3181694fb9. [DOI] [PubMed] [Google Scholar]
- 13.Feigin R.D., Cherry J.D., editors. Textbook of pediatric infectious diseases. 6th edn. W.B. Saunders Company; Philadelphia, PY: 2009. [Google Scholar]
- 14.Global Initiative for Asthma . 2008. Global strategy for asthma management and prevention. http://www.ginasthma.org/Guidelineitem.asp??11=2&12=1&intId=60 [accessed 02.03.2010]. [Google Scholar]
- 15.World Health Organization Pneumonia Vaccine Trial Investigators’ Group. Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. Available at: http://www.who.int/vaccines-documents/DocsPDF01/www616.pdf.
- 16.Bosis S., Esposito S., Niesters H.G., Zuccotti G.V., Marseglia G., Lanari M. Role of respiratory pathogens in infants hospitalized for a first episode of wheezing and their impact on recurrences. Clin Microbiol Infect. 2008;14:677–684. doi: 10.1111/j.1469-0691.2008.02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvo C., García-García M.L., Blanco C., Pozo F., Flecha I.C., Pérez-Breña P. Role of rhinovirus in hospitalized infants with respiratory tract infections in Spain. Pediatr Infect Dis J. 2007;26:904–908. doi: 10.1097/INF.0b013e31812e52e6. [DOI] [PubMed] [Google Scholar]
- 18.Miller E.K., Lu X., Erdman D.D., Poehling K.A., Zhu Y., Griffin M.R. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie J.K., Roy-Burman A., Guardia-Labar L., Boston E.J., Kiang D., Padilla T. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr Infect Dis J. 2009;28:337–339. doi: 10.1097/INF.0b013e31818ffc1b. [DOI] [PubMed] [Google Scholar]
- 20.van der Zalm M.M., Uiterwaal C.S., Wilbrink B., de Jong B.M., Verheij T.J., Kimpen J.L. Respiratory pathogens in respiratory tract illnesses during the first year of life: a birth cohort study. Pediatr Infect Dis J. 2009;28:472–476. doi: 10.1097/inf.0b013e318195e26e. [DOI] [PubMed] [Google Scholar]
- 21.van Benten I., Koopman L., Niesters B., Hop W., van Middelkoop B., de Waal L. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol. 2003;14:363–370. doi: 10.1034/j.1399-3038.2003.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jartti T., Lehtinen P., Vanto T., Vuorinen T., Hartiala J., Hiekkanen H. Efficacy of prednisolone in children hospitalized for recurrent wheezing. Pediatr Allergy Immunol. 2007;18:326–334. doi: 10.1111/j.1399-3038.2007.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinello R.A., Chen M.D., Weibel C., Kahn J.S. Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis. 2002;186:839–842. doi: 10.1086/342414. [DOI] [PubMed] [Google Scholar]
- 24.Campanini G., Percivalle E., Baldanti F., Rovida F., Bertaina A., Marchi A. Human respiratory syncytial virus (hRSV) RNA quantification in nasopharyngeal secretions identifies the hRSV etiologic role in acute respiratory tract infections of hospitalized infants. J Clin Virol. 2007;39:119–124. doi: 10.1016/j.jcv.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allander T., Jartti T., Gupta S., Niesters H.G., Lehtinen P., Osterback R. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brieu N., Guyon G., Rodière M., Segondy M., Foulongne V. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J. 2008;27:969–973. doi: 10.1097/INF.0b013e31817acfaa. [DOI] [PubMed] [Google Scholar]
- 27.Jansen R.R., Schinkel J., Dek I., Koekkoek S.M., Visser C.E., de Jong M.D. Quantitation of respiratory viruses to clinical course in children with acute respiratory tract infections. Pediatr Infect Dis J. 2009;(October) doi: 10.1097/INF.0b013e3181b6de8a. [DOI] [PubMed] [Google Scholar]
- 28.Spann K.M., Tran K.C., Chi B., Rabin R.L., Collins P.L. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected] J Virol. 2004;78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. Erratum in: J Virol 2005;78:6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell K., Fergie J. Lack of usefulness of an abnormal white blood cell count for predicting a concurrent serious bacterial infection in infants and young children hospitalized with respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2007;26:311–315. doi: 10.1097/01.inf.0000258627.23337.00. [DOI] [PubMed] [Google Scholar]
- 30.Mistchenko A.S., Diez R.A., Mariani A.L., Robaldo J., Maffey A.F., Bayley-Bustamante G. Cytokines in adenoviral disease in children: association of interleukin-6, interleukin-8, and tumor necrosis factor alpha levels with clinical outcome. J Pediatr. 1994;124:714–720. doi: 10.1016/s0022-3476(05)81360-5. [DOI] [PubMed] [Google Scholar]
- 31.García-Rodríguez J.A., Fresnadillo Martínez M.J. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother. 2002;50(Suppl. S2):59–73. doi: 10.1093/jac/dkf506. [DOI] [PubMed] [Google Scholar]
- 32.Rückinger S., van der Linden M., Reinert R.R., von Kries R., Burckhardt F., Siedler A. Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine. 2009;27:4136–4141. doi: 10.1016/j.vaccine.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 33.Jansen A.G., Sanders E.A., Van Der Ende A., Loon VAN.F A.M., Hoes A.W., Hak E. Invasive pneumococcal and meningococcal disease: association with influenza virus and respiratory syncytial virus activity? Epidemiol Infect. 2008;136:1448–1454. doi: 10.1017/S0950268807000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson M., Gilmour R., Menzies R., Ferson M., McIntyre P. New South Wales Pneumococcal Network. The association of respiratory viruses, temperature, and other climatic parameters with the incidence of invasive pneumococcal disease in Sydney, Australia. Clin Infect Dis. 2006;42:211–215. doi: 10.1086/498897. [DOI] [PubMed] [Google Scholar]
- 35.Avadhanula V., Rodriguez C.A., Devincenzo J.P., Wang Y., Webby R.J., Ulett G.C. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 2006;80:1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]