Highlights
-
•
Cryptosporidium spp. were detected using PCR and ELISA in diarrheal feces from calves.
-
•
PCR and ELISA data showed good agreement in detecting C. parvum.
-
•
Multilocus typing with 18S rRNA, gp60, and hsp70 genes of C. parvum was performed.
-
•
Subtyping of C. parvum gp60 gene first revealed IIaA18G3R1 and IIaA16G3R1 in Asia.
-
•
PCR and sequencing of hsp70 gene clearly differentiated C. bovis and C. ryanae.
Keywords: Calves, Cryptosporidium spp., Diarrhea, Genotyping, Multilocus typing
Abstract
We assessed the prevalence and performed molecular analysis of Cryptosporidium spp. in diarrheal feces of calves in Korea. Diarrheal fecal samples were collected from 951 young calves (<3 months) on 425 farms. Cryptosporidium prevalence was assessed by PCR and ELISA, and molecular characterization was performed by targeting the 18S rRNA, heat-shock protein 70 (hsp70), and glycoprotein 60 (gp60) genes. Data were analyzed according to the sex, type of cattle, region, season, and type of diarrhea. PCR analysis revealed Cryptosporidium spp. in 9.9% (94/951) of diarrheal fecal samples. C. parvum and C. bovis/ryanae were present in 6.1% (58/951) and 4.1% (39/951) of diarrheal fecal samples, respectively. In addition, ELISA showed positive results for C. parvum in 9.7% (92/951) samples. Statistical analysis of the PCR and ELISA results revealed a lower prevalence of C. parvum in the hemorrhagic diarrheal samples (P < 0.05). For C. bovis/ryanae, seasonality and high prevalence in hemorrhagic diarrhea were observed (P < 0.05). Of the 951 samples tested for C. parvum, 903 samples showed agreement with a κ value of 0.65, indicating good agreement between the two tests. Although C. bovis and C. ryanae share highly similar 18S rRNA sequences, PCR based on hsp70 successfully distinguished C. bovis from C. ryanae. Sequence analysis of gp60 revealed that C. parvum belonged to the IIa families and was further subtyped as IIaA18G3R1 and IIaA16G3R1, which have not been previously reported in Asia. These findings indicate that Cryptosporidium spp. play an important role in diarrhea in young calves in Korea. Considering the zoonotic significance of C. parvum IIa subtype and dense rearing system of cattle in Korea, prevention and continuous monitoring of Cryptosporidium are required.
1. Introduction
Cryptosporidium is an apicomplexan protozoan parasite that is distributed worldwide (Ryan et al., 2014, Wang et al., 2014). The genus Cryptosporidium includes more than 30 species that affect different hosts (Ryan et al., 2014, Shrestha et al., 2014, Mirhashemi et al., 2015). For example, Cryptosporidium parvum, C. andersoni, C. bovis, C. hominis, and C. ryanae infect cattle, while C. parvum and C. hominis are responsible for human cryptosporidiosis (Smith et al., 2005, Ryan et al., 2014).
Cryptosporidium is transmitted via the fecal-oral route, and water plays an important role in the transmission (Karanis et al., 2007, Baldursson and Karanis, 2011, Moon et al., 2013). Cryptosporidium causes cryptosporidiosis, leading to diarrhea in various vertebrates. The clinical signs of cryptosporidiosis range from self-limiting to severe diarrhea in cattle and can cause death in small children or immunocompromised humans (Del Chierico et al., 2011, Mirhashemi et al., 2016). This disease affects 57,000 people annually in the United States (Scallan et al., 2011) and approximately 20% of children with diarrhea in developing countries (Mosier and Oberst, 2000).
In Korea, cryptosporidiosis was first identified in 1986 in chickens (Mo et al., 1988); subsequent studies identified Cryptosporidium infection in humans and animals (Rhee et al., 1991, Cho et al., 1993, Wee et al., 1996, Chai et al., 2001, Moon et al., 2013). A recent water-borne outbreak of cryptosporidiosis in humans prompted several studies on Cryptosporidium spp. in Korea (Moon et al., 2013). In calves, Cryptosporidium spp. were identified in the diarrheal feces of calves by using dimethyl sulfoxide-modified acid-fast staining, enzyme-linked immunosorbent assay (ELISA), and indirect immunofluorescence antibody assay (Wee et al., 1996). Previous studies on animals and humans in Korea focused on the regional prevalence, and only a few studies have evaluated the molecular characteristics of Cryptosporidium.
Cattle are one of the most important animals in the livestock industry in Korea. Approximately three million cattle, including dairy and beef cattle, are reared in Korea annually (Oh et al., 2016). According to the National Animal Health Monitoring System for U.S. dairy industries, diarrhea is responsible for 57% of deaths of weaning calves (US Department of Agriculture, 2008). Therefore, it is important to determine the cause of diarrhea in calves in order to prevent economic losses in the livestock industry.
The purposes of the present study were two-fold. First, we evaluated the prevalence of Cryptosporidium spp. in the diarrheal feces of calves reared in Korea, using PCR and ELISA. Second, genetic typing of Cryptosporidium spp. was conducted based on the gene sequences of 18S rRNA, heat-shock protein 70 (hsp70), and glycoprotein 60 (gp60).
2. Materials and methods
2.1. Study area and collection of diarrheal feces
Korea is located between 34°20′–37°11′ northern latitude and 126°07′–129°19′ eastern longitude and receives 1300 mm of precipitation annually (Jung et al., 2014). The annual mean temperature in Korea is 12.9 °C.
Between November 2013 and March 2016, 951 diarrheal fecal samples were collected from young calves (<3 months) reared in Korea (Fig. 1 ). The number of collected samples was determined using the following formula (Thrusfield, 2005b): , where n = required sample size, pexp = expected prevalence, and d = desired absolute precision. Expected prevalence and desired absolute precision were considered 15% and 5%, respectively, based on previously reported prevalence values in Korea (Wee et al., 1996). According to the formula, at least 196 samples were required; 951 samples were assessed in this study. The fecal samples were collected from 425 farms, and the mean number ± standard deviation of samples per farm was 2.2 ± 4.6.
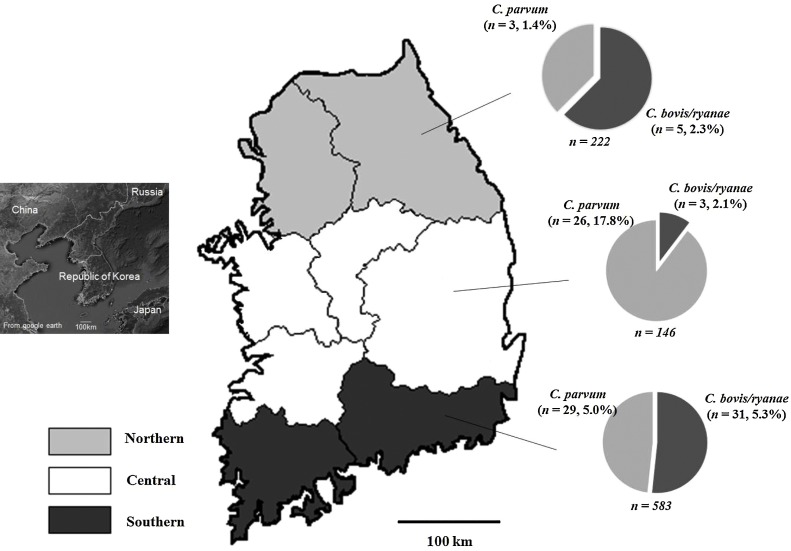
Fig. 1.
Map of Korea showing the sampling points at which diarrheal feces from calves were collected and the prevalence of Cryptosporidium spp. determined by PCR analysis. For statistical analysis, the samples were assigned to the northern, central, and southern groups according to administrative boundaries.
For this study, practicing veterinarians collected diarrheal fecal samples in sterilized containers stored in a cold box (0–4 °C) during treatment or regular medical checkup after receiving consent from the cattle owners. For the molecular identification of Cryptosporidium species, samples in the cold box were sent to the Laboratory of Veterinary Parasitology at Kyungpook National University, Daegu, Korea. Sample collection was neither harmful nor against animal welfare, and thus required no ethical approval from any authority.
For statistical analysis, data were collected for sex (male, female, or unknown), type of cattle (beef, dairy, or unknown), region [northern (Gyeonggi and Gangwon Provinces), central (Chungnam, Chungbuk, Gyeongbuk, and Jeonbuk Provinces), or southern (Jeonnam and Gyeongnam Provinces)], season [spring (March–May), summer (June–August), fall (September–November), or winter (December–February)], and type of diarrhea (hemorrhagic, watery, or pasty). When data were insufficient, the result was indicated as “unknown.”
2.2. DNA extraction, PCR, and sequencing
DNA was extracted from the diarrheal feces (200 mg) by using the QIAamp® Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and stored at −20 °C until use. The quality and quantity of the extracted DNA were evaluated using the Infinite® 200 PRO NanoQuant (Tecan, Mannedorf, Switzerland). PCR was performed with 100 ng fecal DNA and 10 pmole of each primer by adjusting 20 μl reaction volume with distilled water in the AccuPower® HotStart PCR PreMix Kit (Bioneer, Daejeon, Korea).
To detect Cryptosporidium spp. in feces, 18S rRNA of Cryptosporidium spp. was amplified using the primer set 18SiF/18SiR as described previously (Cheun et al., 2007). For samples that yielded positive results for Cryptosporidium spp., species identification was performed using species-specific primers (Table 1 ). For molecular characterization, hsp70 and gp60 genes were amplified for Cryptosporidium spp. and C. parvum, respectively (Peng et al., 2001, Satoh et al., 2005).
Table 1.
Primers used to amplify Cryptosporidium spp. 18S rRNA, gp60, and hsp70 gene fragments in the diarrheal feces of young calves.
| Target species | Target gene | Primer name | Primer sequence (5′ to 3′) | Tm (°C) | Reference |
|---|---|---|---|---|---|
| Cryptosporidium spp. | 18S rRNA | 18SiF | AGT GAC AAG AAA TAA CAA TAC AGG | 55.8 | Cheun et al. (2007) |
| 18SiR | CCT GCT TTA AGC ACT CTA ATT TTC | 57.5 | |||
| hsp70 | CPHSPF | AGT GAT ATG ACT CAC TGG CCA TT | 58.8 | Satoh et al. (2005) | |
| CPHSPR | ACA ACA TCA TGT ACA GAT CTC TT | 55.3 | |||
| C. parvum | 18S rRNA | CpaFca | GAA ATA ACA ATA CAG GAC TTT TT | 51.7 | In this study |
| gp60 | AL3531 | ATA GTC TCC GCT GTA TTC | 51.4 | Peng et al. (2001) | |
| AL3534 | GCA GAG GAA CCA GCA TC | 55.1 | |||
| AL3532 | TCC GCT GTA TTC TCA GCC | 55.6 | Peng et al. (2001) | ||
| AL3533 | GAG ATA TAT CTT GGT GCG | 44.4 | |||
| C. andersoni | 18S rRNA | Can18Fa | ACG GAT CGC ATC TCT GAT GC | 59.3 | In this study |
| C. bovis/ryanae | 18S rRNA | Cbrx18Fa | CAA TAC AGA RCC TTA CGG TT | 56.4 | In this study |
18SiR was used as the reverse primer.
Because C. bovis and C. ryanae share highly similar 18S rRNA sequences, it is difficult to use PCR results to distinguish between C. bovis and C. ryanae (Mirhashemi et al., 2016). Therefore, primers were designed to amplify the 18S rRNA of both C. bovis and C. ryanae, and the positive results were indicated as C. bovis/ryanae.
For sequencing, we selected 9 C. parvum samples and 4 C. bovis/ryanae samples, all of which were PCR-positive. The factors of sex, type of cattle, region, season, type of diarrhea, and ELISA results were considered. The C. parvum 18S rRNA gene was sequenced using the primer set 18SiF/18SiR or CpaFc/18SiR. Sequencing was performed using the BigDye Terminator V.3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and analyzed with the ABI 3730XL DNA Analyzer (Applied Biosystems).
2.3. ELISA
A commercial ELISA kit (Cryptosporidium parvum Antigen Test Kit, IDEXX Laboratories, Inc., Westbrook, ME, USA) was used to detect C. parvum in the diarrheal feces. The diagnostic test was performed according to the manufacturer’s instructions, and in each experiment, positive and negative controls provided by the manufacturer were included.
To determine positivity, optical density (OD) was evaluated at 450 nm using the Infinite® 200 PRO NanoQuant plate reader (Tecan), and the sample-to-positive ratio (S/P) was calculated as manufacturer’s instruction: . The S/P greater than 20 was considered to be a positive result.
2.4. Phylogenetic analysis
For molecular characterization, phylogenetic analysis was performed using the 18S rRNA, hsp70, and gp60 of Cryptosporidium spp. Phylogenetic trees were constructed using MEGA 6.0 with the maximum likelihood method (Tamura et al., 2013). To support topology, 100 resamplings were performed.
2.5. Statistical analysis
To evaluate the association between prevalence and variables (sex, type of cattle, region, season, and type of diarrhea), the χ2 test of SPSS V.21.0 (IBM Corporation, Armonk, NY, USA) was used. “Unknown” data in the categories of sex and type of cattle were excluded from the statistical analysis. A P-value less than 0.05 was considered statistically significant, and 95% confidence intervals were calculated.
The κ statistic was used to evaluate the agreement between the PCR and ELISA results to detect C. parvum. The κ value was interpreted as follows (Thrusfield, 2005a): ≥0.81, very good agreement; 0.61–0.80, good agreement; 0.41–0.60, moderate agreement; 0.21–0.40, fair agreement; and ≤0.20, poor agreement.
3. Results
3.1. Prevalence based on PCR and ELISA
The overall prevalence of Cryptosporidium spp., based on the PCR results, was 9.9% (94/951; Table 2 ). At the species level, 6.1% (58) and 4.1% (39) of samples were positive for C. parvum and C. bovis/ryanae, respectively, whereas C. andersoni was not identified in any samples (Table 3 ). Both C. parvum and C. bovis/ryanae were present in 3 samples. At the genus level, a statistically significant difference in different regions was observed (P < 0.001). The C. parvum data revealed statistically significant differences in region (P < 0.001) and type of diarrhea (P = 0.025), while in C. bovis/ryanae, there were statistically significant differences in season (P < 0.001) and type of diarrhea (P = 0.008). At the farm level, PCR revealed that at least one Cryptosporidium spp. was present in 11.8% (50/425) of farms.
Table 2.
Detection of Cryptosporidium spp. at the genus level, using PCR analysis.
| Group |
No. tested |
Cryptosporidium spp. |
|||
|---|---|---|---|---|---|
| No. positive (%) | 95% CIa | P-value | |||
| Sex | Male | 433 | 34 (7.9) | 5.3–10.4 | 0.634 |
| Female | 248 | 17 (6.9) | 3.7–10.0 | ||
| Unknown | 270 | 43 (15.9) | 11.6–22.3 | ||
| Type of cattle | Beef | 909 | 91 (10.0) | 8.1–12.0 | 0.521 |
| Dairy | 21 | 3 (14.3) | 0–29.3 | ||
| Unknown | 21 | – | |||
| Region | Northern | 222 | 8 (3.6) | 1.2–6.1 | <0.001 |
| Central | 146 | 29 (19.9) | 13.4–26.3 | ||
| Southern | 583 | 57 (9.8) | 7.4–12.2 | ||
| Season | Spring (Mar–May) | 252 | 17 (6.8) | 3.7–9.8 | 0.105 |
| Summer (Jun–Aug) | 240 | 31 (12.9) | 8.7–17.2 | ||
| Fall (Sep–Nov) | 250 | 28 (11.2) | 7.3–15.1 | ||
| Winter (Dec–Feb) | 209 | 18 (8.6) | 4.8–12.4 | ||
| Type of diarrhea | Hemorrhagic | 105 | 10 (9.5) | 3.9–15.1 | 0.987 |
| Watery | 419 | 42 (10.0) | 7.2–12.9 | ||
| Pasty | 427 | 42 (9.8) | 7.0–12.7 | ||
| Total | 951 | 94 (9.9) | 8.0–11.8 | ||
CI: Confidence interval.
Table 3.
Detection of Cryptosporidium parvum and C. bovis/ryanae using PCR analysis and ELISA.
| Group |
No. tested |
C. parvum |
C. bovis/ryanae |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR |
ELISA |
PCR |
|||||||||
| No. positive (%) | 95% CIa | P-value | No. positive (%) | 95% CIa | P-value | No. positive (%) | 95% CIa | P-value | |||
| Sex | Male | 433 | 21 (4.8) | 2.8–6.9 | 0.312 | 42 (9.7) | 6.9–12.5 | 0.370 | 15 (3.5) | 1.7–5.2 | 0.911 |
| Female | 248 | 8 (3.2) | 1.0–5.4 | 19 (7.7) | 4.4–11.0 | 9 (3.6) | 1.3–6.0 | ||||
| Unknown | 270 | 29 (10.7) | 7.1–14.4 | 31 (11.5) | 7.7–15.3 | 15 (5.6) | 2.8–8.3 | ||||
| Type of cattle | Beef | 909 | 56 (6.2) | 4.6–7.7 | 0.529 | 90 (9.9) | 8.0–11.8 | 0.954 | 38 (4.2) | 2.9–5.5 | 0.895 |
| Dairy | 21 | 2 (9.5) | 0–22.1 | 2 (9.5) | 0–22.1 | 1 (4.8) | 0–13.9 | ||||
| Unknown | 21 | – | – | – | – | – | |||||
| Region | Northern | 222 | 3 (1.4) | 0–2.9 | <0.001 | 10 (4.5) | 1.8–7.2 | <0.001 | 5 (2.3) | 0.3–4.2 | 0.059 |
| Central | 146 | 26 (17.8) | 11.6–24.0 | 29 (19.9) | 13.4–26.3 | 3 (2.1) | 0–4.4 | ||||
| Southern | 583 | 29 (5.0) | 3.2–6.7 | 53 (9.1) | 6.8–11.4 | 31 (5.3) | 3.5–7.1 | ||||
| Season | Spring (Mar–May) |
252 | 15 (6.0) | 3.0–8.9 | 0.854 | 18 (6.7) | 3.7–9.8 | 0.158 | 2 (0.8) | 0–1.9 | <0.001 |
| Summer (Jun–Aug) |
240 | 13 (5.4) | 2.6–8.3 | 22 (9.2) | 5.5–12.8 | 18 (7.5) | 3.5–9.8 | ||||
| Fall (Sep–Nov) |
250 | 18 (7.2) | 4.0–10.4 | 25 (10.0) | 6.3–13.7 | 13 (5.2) | 2.5–8.0 | ||||
| Winter (Dec–Feb) |
209 | 12 (5.7) | 2.6–8.9 | 28 (13.4) | 8.8–18.0 | 6 (2.9) | 1.2–6.4 | ||||
| Type of diarrhea | Hemorrhagic | 105 | 0 | – | 0.025 | 1 (1.0) | 0–2.8 | 0.004 | 10 (9.5) | 2.5–12.7 | 0.008 |
| Watery | 419 | 27 (6.4) | 4.1–8.8 | 49 (11.7) | 8.6–14.8 | 17 (4.1) | 2.2–6.0 | ||||
| Pasty | 427 | 31 (7.3) | 4.8–9.7 | 42 (9.8) | 7.0–12.7 | 12 (2.8) | 1.6–5.0 | ||||
| Total | 951 | 58 (6.1) | 4.6–7.6 | 92 (9.7) | 7.8–11.6 | 39 (4.1) | 2.5–5.4 | ||||
CI: Confidence interval.
ELISA revealed that 9.7% (92/951) of samples were positive for C. parvum. Statistically significant differences were observed according to region (P < 0.05) and type of diarrhea (P = 0.004), supporting the PCR results. At the farm level, ELISA revealed that C. parvum was present in 11.1% (47/425) of farms.
3.2. Comparison of PCR and ELISA results for C. parvum
Of the 951 tested samples, 95.0% (903) samples showed consistent results between PCR and ELISA in the detection of C. parvum (Table 4 ), including 5.4% (51) positive and 89.6% (852) negative samples. The samples also included 0.7% (7) samples that were PCR+/ELISA- and 4.3% (41) samples that were PCR-/ELISA+. The κ value was calculated to be 0.65, indicating good agreement. In addition, no samples positive for C. bovis/ryanae, based on the PCR results, showed positive results using ELISA.
Table 4.
Comparison of Cryptosporidium parvum detection using PCR and ELISA.
| PCR |
||||
|---|---|---|---|---|
| No. positive | No. negative | Total | ||
| ELISA | No. positive | 51 | 41 | 92 |
| No. negative | 7 | 852 | 859 | |
| Total | 58 | 893 | 951 | |
κ value = 0.65, indicating good agreement.
3.3. Sequencing and phylogenetic analysis
Of the 58 C. parvum PCR-positive samples, 9 samples were sequenced. After sequencing, 295, 758, and 467 bp of the 18S rRNA, hsp70, and gp60 sequences, respectively, were obtained. Of the 39 C. bovis/ryanae PCR-positive samples, 4 samples were sequenced, and 270 and 758 bp of the 18S rRNA and hsp70 sequences, respectively, were obtained. The obtained sequences were submitted to the GenBank database (Accession nos. KX342025–KX342059).
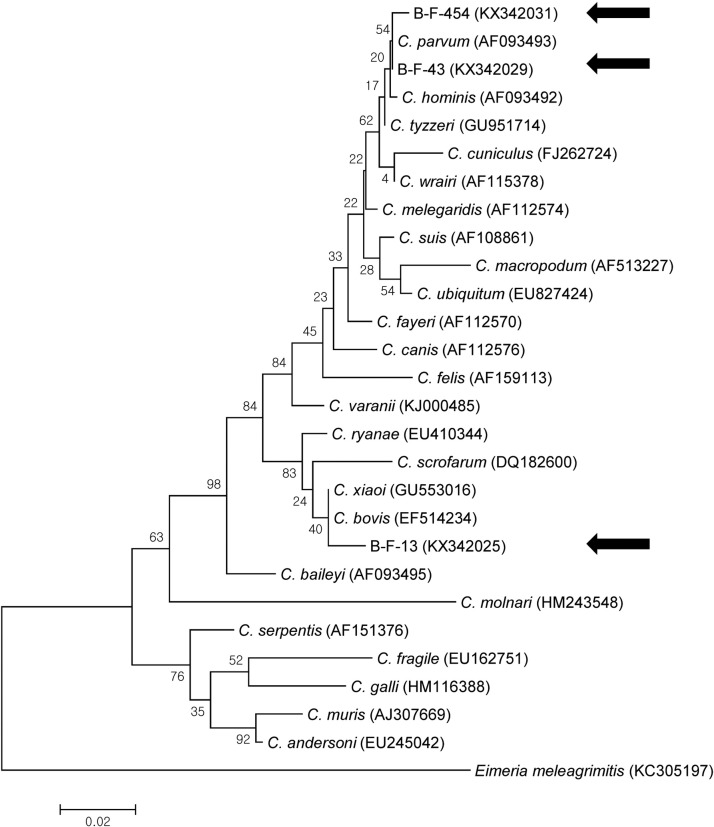
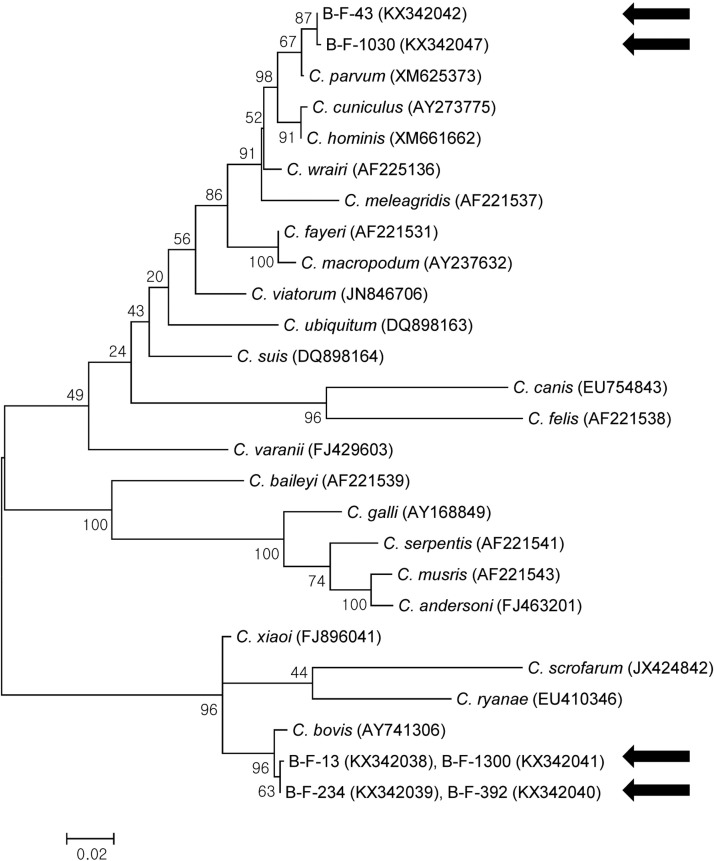
Phylogenetic analysis of 18S rRNA revealed that the sequences obtained in the present study belonged to C. parvum and C. bovis (Fig. 2 ). Although it was not possible to differentiate between C. bovis and C. ryanae using PCR based on 18S rRNA, phylogenetic analysis was used to successfully differentiate between the species. In addition, phylogenetic analysis of hsp70 yielded the same results as those obtained by analyzing 18S rRNA (Fig. 3 ).
Fig. 2.
Phylogenetic relationships of 18S rRNA of Cryptosporidium spp. identified from the diarrheal feces of young calves. A phylogenetic tree was constructed using the maximum likelihood method with 100 replicates. The sequences identified in this study are indicated using black arrows. The sequences of KX342029, KX342030, and KX342032–KX342037 are identical and those of KX342025–KX342028 are identical.
Fig. 3.
Phylogenetic relationships of hsp70 of Cryptosporidium spp. identified from the diarrheal feces of young calves. A phylogenetic tree was constructed using the maximum likelihood method with 100 replicates. The sequences identified in the present study are indicated using black arrows. The sequences of KX342042–KX342050 are identical except for KX342047.
3.4. Subtyping of C. parvum by using gp60
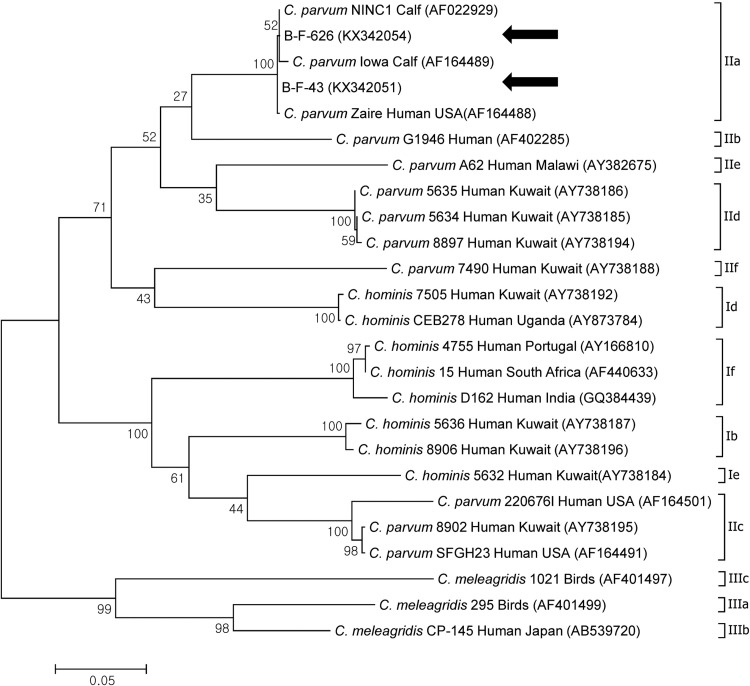
All sequences obtained from C. parvum gp60 belonged to IIa according to phylogenetic analysis (Fig. 4 ). Furthermore, 8 sequences were identified as IIaA18G3R1, and one was identified as IIaA16G3R1 according to the nomenclature system suggested by Sulaiman et al. (2005).
Fig. 4.
Phylogenetic analysis of gp60 of Cryptosporidium spp. identified from the diarrheal feces of young calves. A phylogenetic tree was constructed using the maximum likelihood method with 100 replicates. The sequences identified in the present study are indicated using black arrows. Species, isolation, host, region of identification, and GenBank accession number are included. According to gp60 sequence variation, C. hominis, C. parvum, and C. meleagridis belong to subtypes I, II, and III, respectively. The sequences of KX342051–KX342053 are identical.
4. Discussion
Cryptosporidium spp. are distributed worldwide, and different species are responsible for diarrhea in pre-weaned or post-weaned calves (Santín and Zarlenga, 2009, Zhang et al., 2015, Ibrahim et al., 2016). Previous studies using different methods found variable prevalence rates of Cryptosporidium in cattle: 49.4% (39/79) in Hungary using IFA (Plutzer and Karanis, 2007), 11.9% (68/571) in the United States using PCR (Fayer et al., 2006), 75.0% (60/80) in Japan using PCR (Karanis et al., 2010), 20.0% (15/60) in New Zealand using IFA (Shrestha et al., 2014), 5.1% (150/2945) in China using PCR (Zhang et al., 2015), 21.5–22.5% in Ireland using direct fluorescence antibody testing (Mirhashemi et al., 2016), and 10.2% (49/480) in Egypt using microscopy with staining (Ibrahim et al., 2016).
Several studies in Korea have identified Cryptosporidium in cattle at rates of 41.2% (7/17) in a study using microscopy (Park et al., 2006), 14.4% (29/201) in a study using dimethyl sulfoxide modified acid-fast staining (Wee et al., 1996), and 22.2% (111/500) in a study using microscopy (Rhee et al., 1991). In the present study, the overall prevalence of Cryptosporidium detected by PCR was 9.9% (94/951), which was lower than that reported in previous studies conducted in Korea. Our results may reflect improvements in hygiene, as the previous studies were performed more than 10 years ago (some were more than 20 years ago), when the hygiene of cattle breeding systems was inadequate. As poor hygiene may result in Cryptosporidium infection in humans (Ryan et al., 2014), improvements of hygiene in cattle rearing may have resulted in the lower prevalence observed in the present study.
Our data revealed that 6.1% (58) and 9.7% (92) of the 951 diarrheal fecal samples were positive for C. parvum according to the PCR and ELISA results, respectively. In addition, the PCR and ELISA results showed the same statistically significant differences with respect to the higher prevalence in the central region and lower prevalence in the case of hemorrhagic diarrhea. Analysis of the higher prevalence in the central region revealed that all positive samples in the central region were collected from a single farm. At this farm, 39 samples were collected between July 2014 and July 2015, and 66.7% (26) and 71.8% (28) samples were positive based on PCR and ELISA results, respectively. By excluding the data from this farm, the prevalence rates in the central region were 0% (0/107) and 0.9% (1/107) by PCR and ELISA, respectively, with P-values of 0.005 and 0.002, indicating a statistically lower prevalence in the central region. Therefore, the significant difference according to region was biased by the prevalence on one farm.
In addition, a higher prevalence at the farm level than the individual level was observed, regardless of the PCR or ELISA results, indicating a wide distribution of Cryptosporidium in calves in Korea. When the data were analyzed according to the type of diarrhea, there was low prevalence of C. parvum in calves with hemorrhagic diarrhea. Although the pathogenesis of Cryptosporidium remains unclear, Cryptosporidium is not considered to cause hemorrhagic diarrhea (Cho and Yoon, 2014, Di Genova and Tonelli, 2016) and appears to have a low association with this condition.
Several studies have shown that the oocysts of Cryptosporidium are resistant to adverse environmental conditions and survive well under temperate and moist conditions (Ryan et al., 2014). The higher prevalence of Cryptosporidium in the summer compared to in the winter may be related to the high temperature in the summer (Chai et al., 2001). In the case of C. bovis/ryanae, the prevalence associated with season is consistent with the results from previous studies; however, C. parvum showed no association with seasonality according to PCR and ELISA results.
As discussed previously, Cryptosporidium is not considered to cause hemorrhagic diarrhea (Cho and Yoon, 2014). Therefore, the higher prevalence of C. bovis/ryanae in hemorrhagic diarrheal samples in the present study may have resulted from co-infection with other pathogens that cause hemorrhagic diarrhea in calves, such as Clostridium perfringens, Salmonella spp., bovine coronavirus, and Eimeria spp.
No statistically significant differences were observed for sex and type of cattle in the case of C. parvum and C. bovis/ryanae (P > 0.05). A previous study showed a higher prevalence of Cryptosporidium in males than in females (Ibrahim et al., 2016); however, in the present study, although a slightly but insignificantly higher prevalence in males was noted.
Many studies have shown that the distribution of Cryptosporidium is related to age (Santín and Zarlenga, 2009, Zhang et al., 2015, Ibrahim et al., 2016). For example, C. parvum is mostly identified in pre-weaned calves; C. bovis and C. ryanae are found in post-weaned calves; and C. andersoni is present in adult cattle. Considering the target population in this study (≤3 months), the results were consistent with those of previous studies, with a higher prevalence of C. parvum than of C. bovis/ryanae and no detection of C. andersoni.
In previous studies, although diarrheic cattle showed a higher prevalence of Cryptosporidium spp., Cryptosporidium was also identified in non-diarrheic cattle (Azami, 2007, Ibrahim et al., 2016). These previous studies did not report that Cryptosporidium is an apathogenic organism, but suggested that other factors can affect Cryptosporidium infection. Age-related infection of Cryptosporidium in cattle and detection in many small children and immunocompromised individuals suggest that the immune response plays an important role in cryptosporidiosis (Ryan et al., 2014, Ibrahim et al., 2016).
Because there is no gold standard for the diagnosis of Cryptosporidium spp. (Mirhashemi et al., 2015), we used two tests, PCR and ELISA, to identify C. parvum. The κ value was used to evaluate agreement between the two different tests (Thrusfield, 2005a). In the present study, a κ value of 0.65, which indicates good agreement, was obtained. In addition, all C. bovis/ryanae PCR-positive samples yielded negative ELISA results. Our results indicated that the two tests could be applied compatibly to detect C. parvum in feces. While there was good agreement between the two tests, there were still 41 ELISA+/PCR- samples. This discrepancy may be attributed to two factors: first, the presence of PCR inhibitors such as bilirubin, bile salts, and complex polysaccharides, which are included in feces, and second, limitations in the sensitivity and specificity in each assay (Morgan et al., 1998). Thus, additional studies are required to clarify the discrepancy in detecting Cryptosporidium spp. using the two assays.
To date, several genes have been used for the molecular characterization of Cryptosporidium, including 18S rRNA, Cryptosporidium wall protein, hsp70, and gp60 (Xiao, 2010). In the present study, 18S rRNA was successfully used to identify Cryptosporidium spp.; however, because sequence identity is greater than 99% 18S rRNA among some Cryptosporidium spp. (C. bovis, C. ryanae, and C. xiaoi), it is not always possible to differentiate the species by PCR (Santín and Zarlenga, 2009, Mirhashemi et al., 2016). The sequences obtained in the present study also showed 99% 18S rRNA sequence identity for C. bovis and C. ryanae; however, phylogenetic analysis was used to differentiate these two species. In addition, we amplified hsp70 in C. bovis, and this sequence clearly differed from those in C. ryanae (EU410346) and C. xiaoi (FJ896041), with 88.9% and 97.5% identity, respectively. Because there is only one C. bovis hsp70 sequence (AY741306) in the GenBank database and the sequence contains only 384 bp, our sequence information will be useful for further investigations. We recommend targeting hsp70, rather than 18S rRNA, to identify C. bovis, C. ryanae, and C. xiaoi.
The gene gp60 has been used for subtyping C. parvum and C. hominis; some other Cryptosporidium gp60 genes have also been reported, such as C. meleagridis, C. fayeri, C. cuniculus, and C. ubiquitum (Ryan et al., 2014). The C. parvum and C. hominis subtype families can be distinguished based on variations in gp60 sequences and according to repetition of the trinucleotide region (Sulaiman et al., 2005). Significantly different clinical presentations and virulence have been reported for the C. parvum and C. hominis subtype families (Del Chierico et al., 2011, Feng et al., 2012). Of the C. parvum subtype families (from IIa to IIo), the IIa and IId families are the most dominant families responsible for zoonotic cryptosporidiosis (Ryan et al., 2014, Wang et al., 2014). IId families are considered to be dominant in Asian and African animals, while IIa families are dominant in American, European, and Oceanian animals (Wang et al., 2014).
In the present study, 8 C. parvum PCR-positive samples belonged to the IIa families according to phylogenetic analysis, and sequence analysis further identified the samples’ subtypes as IIaA18G3R1 (n = 8) and IIaA16G3R1 (n = 1). This is the first report of these subtypes in the Asian region, although they have been previously identified in Canada, the United States, England, Northern Ireland, Spain, Australia, and New Zealand, which are geographically distant from Korea (Xiao et al., 2007, Xiao, 2010, Shrestha et al., 2014). Therefore, further studies on C. parvum gp60 are required in other regions of Asia.
In Korea, the number of cattle produced annually is increasing, while the number of stock breeding farms is decreasing; therefore, the cattle population per farm is increasing (Oh et al., 2016). This makes the farm environment more vulnerable to Cryptosporidium spp., which are transmitted via the fecal-oral route with direct contact (Zhang et al., 2015). Because a very small number of oocysts can initiate C. parvum infection (Okhuysen et al., 1999), and there are a limited number of medicines available to treat cryptosporidiosis (Ryan et al., 2014), prevention is the most important method for reducing economic losses in the livestock industry.
This is the first nationwide epidemiological study on the prevalence of Cryptosporidium in the diarrheal feces of calves in Korea. Our study showed that Cryptosporidium spp. play an important role in diarrhea in young calves and that C. parvum is the dominant Cryptosporidium species. In addition, gp60 analysis revealed that C. parvum in Korea belonged to the IIaA18G3R1 and IIaA16G3R1 families, which have not been previously reported in Asia. Considering the zoonotic significance of C. parvum IIa subtype and the dense rearing system in Korea, prevention and continuous monitoring of Cryptosporidium are required.
Conflicts of interest
The authors have no conflict of interest.
Acknowledgement
This study was supported by a grant from the Animal and Plant Quarantine Agency of the Ministry of Agriculture, Food and Rural Affairs of the Republic of Korea.
References
- Azami M. Prevalence of Cryptosporidium infection in cattle in Isfahan, Iran. J. Eukaryot. Microbiol. 2007;54:100–102. doi: 10.1111/j.1550-7408.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- Baldursson S., Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks-an update 2004–2010. Water Res. 2011;45:6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Kim N.Y., Guk S.M., Park Y.K., Seo M., Han E.T., Lee S.H. High prevalence and seasonality of cryptosporidiosis in a small rural village occupied predominantly by aged people in the Republic of Korea. Am. J. Trop. Med. Hyg. 2001;65:518–522. doi: 10.4269/ajtmh.2001.65.518. [DOI] [PubMed] [Google Scholar]
- Cheun H.I., Choi T.K., Chung G.T., Cho S.H., Lee Y.H., Kimata I., Kim T.S. Genotypic characterization of Cryptosporidium oocysts isolated from healthy people in three different counties of Korea. J. Vet. Med. Sci. 2007;69:1099–1101. doi: 10.1292/jvms.69.1099. [DOI] [PubMed] [Google Scholar]
- Cho Y.I., Yoon K.J. An overview of calf diarrhea-infectious etiology, diagnosis, and intervention. J. Vet. Sci. 2014;15:1–17. doi: 10.4142/jvs.2014.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M.H., Kim A.K., Im K.I. Detection of Cryptosporidium oocysts from out-patients of the Severance Hospital, Korea. Korean J. Parasitol. 1993;31:193–199. doi: 10.3347/kjp.1993.31.3.193. [DOI] [PubMed] [Google Scholar]
- Del Chierico F., Onori M., Di Bella S., Bordi E., Petrosillo N., Menichella D., Caccio S., Callea F., Putignani L. Cases of cryptosporidiosis co-infections in AIDS patients: a correlation between clinical presentation and GP60 subgenotype lineages from aged formalin-fixed stool samples. Ann. Trop. Med. Parasitol. 2011;105:339–349. doi: 10.1179/1364859411Y.0000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Agriculture . USDA-APHIS-VS-CEAH; Fort Collins, CO: 2008. Dairy Part II: Changes in the US Dairy Cattle Industry, 1991–2007; pp. 57–61. [Google Scholar]
- Di Genova B.M., Tonelli R.R. Infection strategies of intestinal parasite pathogens and host cell responses. Front. Microbiol. 2016;7:256. doi: 10.3389/fmicb.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R., Santín M., Trout J.M., Greiner E. Prevalence of species and genotypes of Cryptosporidium found in 1–2-year-old dairy cattle in the eastern United States. Vet. Parasitol. 2006;135:105–112. doi: 10.1016/j.vetpar.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Feng Y., Wang L., Duan L., Gomez-Puerta L.A., Zhang L., Zhao X., Hu J., Zhang N., Xiao L. Extended outbreak of cryptosporidiosis in a pediatric hospital, China. Emerg. Infect. Dis. 2012;18:312–314. doi: 10.3201/eid1802.110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M., Abdel-Ghany A., Abdel-Latef G., Abdel-Aziz S., Aboelhadid S. Epidemiology and public health significance of Cryptosporidium isolated from cattle buffaloes, and humans in Egypt. Parasitol. Res. 2016;115:2439–2448. doi: 10.1007/s00436-016-4996-3. [DOI] [PubMed] [Google Scholar]
- Jung B., Lee S., Kwak D. Evidence of Neospora caninum exposure among native Korean goats (Capra hircus coreanae) Vet. Med. 2014;59:637–640. [Google Scholar]
- Karanis P., Kourenti C., Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health. 2007;5:1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- Karanis P., Eiji T., Palomino L., Boonrod K., Plutzer J., Ongerth J., Igarashi I. First description of Cryptosporidium bovis in Japan and diagnosis and genotyping of Cryptosporidium spp. in diarrheic pre-weaned calves in Hokkaido. Vet. Parasitol. 2010;169:387–390. doi: 10.1016/j.vetpar.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Mirhashemi M.E., Zintl A., Grant T., Lucy F.E., Mulcahy G., De Waal T. Comparison of diagnostic techniques for the detection of Cryptosporidium oocysts in animal samples. Exp. Parasitol. 2015;151:14–20. doi: 10.1016/j.exppara.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirhashemi M.E., Zintl A., Grant T., Lucy F., Mulcahy G., De Waal T. Molecular epidemiology of Cryptosporidium species in livestock in Ireland. Vet. Parasitol. 2016;216:18–22. doi: 10.1016/j.vetpar.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo I.P., Yun H.J., Choi S.H., Lee Y.O., Nam S. Cryptosporidiosis in chicken. Korean J. Vet. Res. 1988;28:175–177. (in Korean with English abstract) [Google Scholar]
- Moon S., Kwak W., Lee S., Kim W., Oh J., Youn S. Epidemiological characteristics of the first water-borne outbreak of cryptosporidiosis in Seoul, Korea. J. Korean Med. Sci. 2013;28:983–989. doi: 10.3346/jkms.2013.28.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan U.M., Pallant L., Dwyer B.W., Forbes D.A., Rich G., Thompson R.C. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: clinical trial. J. Clin. Microbiol. 1998;36:995–998. doi: 10.1128/jcm.36.4.995-998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D.A., Oberst R.D. Cryptosporidiosis: a global challenge. Ann. N. Y. Acad. Sci. 2000;916:102–111. doi: 10.1111/j.1749-6632.2000.tb05279.x. [DOI] [PubMed] [Google Scholar]
- Oh J., Lee S.H., Lee S.J., Kim Y.H., Park S.C., Rhee M.H., Kwon O.D., Kim T.H., Kwak D. Detection of antibodies against Toxoplasma gondii in cattle raised in Gyeongbuk province, Korea. J. Food Prot. 2016;79:821–824. doi: 10.4315/0362-028X.JFP-15-512. [DOI] [PubMed] [Google Scholar]
- Okhuysen P.C., Chappell C.L., Crabb J.H., Sterling C.R., DuPont H.L. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- Park J.H., Guk S.M., Han E.T., Shin E.H., Kim J.L., Chai J.Y. Genotype analysis of Cryptosporidium spp. prevalent in a rural village in Hwasun-gun, Republic of Korea. Korean J. Parasitol. 2006;44:27–33. doi: 10.3347/kjp.2006.44.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M.M., Matos O., Gatei W., Das P., Stantic-Pavlinic M., Bern C., Sulaiman I.M., Glaberman S., Lal A.A., Xiao L. A comparison of Cryptosporidium subgenotypes from several geographic regions. J. Eukaryot. Microbiol. 2001;48:28–31. doi: 10.1111/j.1550-7408.2001.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Plutzer J., Karanis P. Genotype and subtype analyses of Cryptosporidium isolates from cattle in Hungary. Vet. Parasitol. 2007;146:357–362. doi: 10.1016/j.vetpar.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Rhee J.K., Seu Y.S., Park B.K. Isolation and identification of Cryptosporidium from various animals in Korea. Korean J. Parasitol. 1991;29:139–148. doi: 10.3347/kjp.1991.29.2.139. [DOI] [PubMed] [Google Scholar]
- Ryan U., Fayer R., Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014;141:1667–1685. doi: 10.1017/S0031182014001085. [DOI] [PubMed] [Google Scholar]
- Santín M., Zarlenga D.S. A multiplex polymerase chain reaction assay to simultaneously distinguish Cryptosporidium species of veterinary and public health concern in cattle. Vet. Parasitol. 2009;166:32–37. doi: 10.1016/j.vetpar.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Satoh M., Kimata I., Iseki M., Nakai Y. Gene analysis of Cryptosporidium parvum HNJ-1 strain isolated in Japan. Parasitol. Res. 2005;97:452–457. doi: 10.1007/s00436-005-1474-8. [DOI] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R.D., Grinberg A., Dukkipati V.S.R., Pleydell E.J., Prattley D.J., French N.P. Infections with multiple Cryptosporidium species and new genetic variants in young dairy calves on a farm located within a drinking water catchment area in New Zealand. Vet. Parasitol. 2014;202:287–291. doi: 10.1016/j.vetpar.2014.03.034. [DOI] [PubMed] [Google Scholar]
- Smith H.V., Nichols R.A., Mallon M., Macleod A., Tait A., Reilly W.J., Browning L.M., Gray D., Reid S.W., Wastling J.M. Natural Cryptosporidium hominis infections in Scottish cattle. Vet. Rec. 2005;156:710–711. doi: 10.1136/vr.156.22.710. [DOI] [PubMed] [Google Scholar]
- Sulaiman I.M., Hira P.R., Zhou L., Al-Ali F.M., Al-Shelahi F.A., Shweiki H.M., Iqbal J., Khalid N., Xiao L. Unique endemicity of cryptosporidiosis in children in Kuwait. J. Clin. Microbiol. 2005;43:2805–2809. doi: 10.1128/JCM.43.6.2805-2809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrusfield M. Diagnostic testing. In: Thrusfield M., editor. Veterinary Epidemiology. Blackwell Publishing; Oxford, UK: 2005. pp. 305–330. [Google Scholar]
- Thrusfield M. Surveys. In: Thrusfield M., editor. Veterinary Epidemiology. Blackwell Publishing; Oxford, UK: 2005. pp. 228–246. [Google Scholar]
- Wang R., Zhang L., Axén C., Bjorkman C., Jian F., Amer S., Liu A., Feng Y., Li G., Lv C. Cryptosporidium parvum IId family: clonal population and dispersal from Western Asia to other geographical regions. Sci. Rep. 2014;4:4208. doi: 10.1038/srep04208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S.H., Joo H.D., Kang Y.B. Evaluation for detection of Cryptosporidium oocysts in diarrheal feces of calves. Korean J. Parasitol. 1996;34:121–126. doi: 10.3347/kjp.1996.34.2.121. [DOI] [PubMed] [Google Scholar]
- Xiao L., Zhou L., Santin M., Yang W., Fayer R. Distribution of Cryptosporidium parvum subtypes in calves in eastern United States. Parasitol. Res. 2007;100:701–706. doi: 10.1007/s00436-006-0337-2. [DOI] [PubMed] [Google Scholar]
- Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp. Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang X.X., Tan Q.D., Zhou D.H., Ni X.T., Liu G.X., Yang Y.C., Zhu X.Q. Prevalence and molecular characterization of Cryptosporidium spp. in dairy cattle, northwest China. Parasitol. Res. 2015;114:2781–2787. doi: 10.1007/s00436-015-4537-5. [DOI] [PubMed] [Google Scholar]