Abstract
Rhinoviruses (RVs) are associated with exacerbations of cystic fibrosis (CF), asthma and COPD. There is growing evidence suggesting the involvement of the interferon (IFN) pathway in RV-associated morbidity in asthma and COPD. The mechanisms of RV-triggered exacerbations in CF are poorly understood. In a pilot study, we assessed the antiviral response of CF and healthy bronchial epithelial cells (BECs) to RV infection, we measured the levels of IFNs, pattern recognition receptors (PRRs) and IFN-stimulated genes (ISGs) upon infection with major and minor group RVs and poly(IC) stimulation. Major group RV infection of CF BECs resulted in a trend towards a diminished IFN response at the level of IFNs, PRRs and ISGs in comparison to healthy BECs. Contrary to major group RV, the IFN pathway induction upon minor group RV infection was significantly increased at the level of IFNs and PRRs in CF BECs compared to healthy BECs.
Keywords: Cystic fibrosis, Rhinovirus, Interferon, Bronchial epithelial cells
1. Introduction
Rhinoviruses (RVs) are small positive-sense ssRNA viruses belonging to the Picornaviridae family. RV serotypes are classified as major or minor group depending on the surface receptor used to infect target cells. More than 90% of RV serotyped strains belong to the major group and use as receptor the intercellular adhesion molecule 1 (ICAM-1), while the minor group RV strains bind to low-density lipoprotein receptor (LDLR) on target cells [1], [2]. As the causative agents of the common cold and acute respiratory tract infections in children, RVs are one of the major causes of morbidity and mortality [3]. In addition, RVs are the predominant agents associated with pulmonary exacerbations of CF lung disease as they are detected in up to 40% of all virus-associated CF exacerbations [4], [5], [6]. The mechanisms of acute virus-mediated exacerbations in CF are so far poorly understood.
We and others have reported recently that primary CF bronchial epithelial cells (BECs) have an increased susceptibility to respiratory virus infections such as RVs and parainfluenza virus [7], [8], [9]. We have confirmed these findings ex vivo in bronchoalveolar lavage (BAL) samples collected from RV-infected CF children, in which RV load was elevated in comparison to control patients [10].
RVs are also responsible for more than 50% of virus-induced asthma exacerbations [11]. Defective interferon (IFN) type I (IFN-β) and type III (IFN-λs) production of the bronchial airway epithelium upon RV infection has been identified as a contributing mechanism behind the impaired RV control in asthmatic adults and children [12], [13], [14], [15], [16]. The work of two groups studying mechanisms of viral control and IFN induction in BECs and macrophages collected from patients with chronic obstructive pulmonary disease (COPD) led to controversial results. After growing BECs at air–liquid interface (ALI), Schneider et al. showed an increase in IFN production upon RV infection. Despite that, cells showed an impaired viral control and an increased pro-inflammatory phenotype [17]. On the other hand, Mallia et al. found lower IFNs responses upon infection with RV by BAL macrophages from COPD patients compared to control subjects [18].
Since the IFN pathway is involved in the defective control of RV in infected BECs from asthmatic and COPD patients, we decided to evaluate the IFN response of CF BECs after challenging with RVs from the major and minor group (RV16 and RV1B, respectively). Also, we aimed to assess the baseline expression and induction of pattern recognition receptors (PRRs) engaged in the sensing of RV including toll-like receptor 3 (TLR3), melanoma differentiation-associated protein 5 (MDA5), and retinoic acid inducible gene I (RIG-I) [19]. Levels of IFN-stimulated genes (ISGs) such as dsRNA protein kinase R (PKR), 2′-5′-oligoadenylate synthetase 1 (OAS1), MxA (Myxovirus resistance gene A), and viperin (virus inhibitory protein, endoplasmic reticulum-associated, IFN inducible) were also measured. Finally, the inflammatory response mediated by RV infection of CF BECs has also been quantified through the measurement of CXCL8/IL-8, IL-6 and CXCL10/IP-10 cytokines release.
2. Material and methods
2.1. Study subjects
For the establishment of primary BEC cultures, we recruited healthy and CF volunteers at the University Hospitals of Bern and Zürich. The clinical characteristics of the participants used in this study have been presented elsewhere [9] and are reproduced here for clarity ease of reference (Table 1 ). The exclusion criteria were bleeding tendency, therapy with anticoagulants and/or immunosuppressive agents. For the control group steroid use within the past three months and atopy were additional exclusion criteria. The study was approved by the Ethics committees of the Cantons of Bern and Zurich, Switzerland and informed consent was obtained from all study participants and/or caregivers.
Table 1.
Demographic and clinical characteristics of study subjects.
| Control | Gender | Age (yrs) | Atopya | Steroid useb |
|---|---|---|---|---|
| 1 | f | 7 | No | No |
| 2 | f | 11 | No | No |
| 3 | m | 12 | No | No |
| 4 | f | 1 | No | No |
| 5 | m | 8 | No | No |
| 6 | m | 12 | No | No |
| 7 | f | 15 | No | No |
| 8 | m | 4 | No | No |
| 9 | m | 16 | No | No |
| 10 | m | 1 | No | No |
| 11 | m | 6 | No | No |
| 12 | f | 14 | No | No |
| 13 | f | 13 | No | No |
| 14 | m | 2 | No | No |
| CF | Gender | Age (yrs) | Atopya | Steroid useb | FEV1 % | P. aeruginosa colonizationc | Genotype |
|---|---|---|---|---|---|---|---|
| 1 | m | 8 | No | Yes | 101 | No | F508del/R347P |
| 2 | m | 4 | No | No | 108 | No | F508del/F508del |
| 3 | f | 5 | No | No | ND | Yes | F508del/2347delG |
| 4 | f | 11 | Yes | No | 67 | No | G542X/2708del13 |
| 5 | m | 9 | Yes | Yes | 106 | No | F508del/F508del |
| 6 | m | 3 | No | Yes | ND | Yes | F508del/F508del |
| 7 | f | 9 | Yes | No | 81 | No | F508del/F508del |
| 8 | m | 1 | No | No | ND | No | F508del/F508del |
| 9 | f | 15 | Yes | Yes | 38 | Yes | F508del/F508del |
| 10 | m | 6 | No | No | 127 | No | F508del/F508del |
| 11 | m | 11 | No | No | 62 | No | ND |
PA: Pseudomonas aeruginosa.
m: male; f: female.
ND: not determined.
Defined as positive history of hay fever, eczema or asthma.
Defined as any treatment with systemic, inhaled or nasal steroids within the past 3 months.
Defined as at least one PA-positive oropharyngeal culture during the preceding 12 months.
2.2. Isolation of primary CF and control bronchial epithelial cells
BECs from 11 CF and 12 control subjects were grown from bronchial brushings performed with a 3 mm brush (ConMed, USA) as described [9].
2.3. Cell culture
Primary submerged cultures of BECs were obtained by seeding freshly brushed cells in Bronchial Epithelial Growth Medium (BEGM, Lonza, Switzerland), supplemented with Single Quots (Lonza, Switzerland) as described previously [9].
2.4. Rhinovirus infection
RV16 and RV1B viruses were amplified and titrated with Ohio HeLa cells (ECCAC, UK). BECs were infected with RVs for 1 h at a multiplicity of infection of 4 and washed three times with PBS (Life Technologies, USA). Fresh medium was added and plates were further incubated at 37 °C for 24 h until harvesting. Cells were treated in parallel with infection media (IM) and polyinosinic–polycytidylic acid (poly(IC)) at a concentration of 1 μg/ml (Invivogen, USA). Since the peak of RV replication is observed at ca. 24 h post-infection [20], [21], total RNA and supernatants were harvested at 24 h post-infection for further analysis.
2.5. Isolation of total RNA and RT-PCR
Total RNA purification was done by using the Nucleospin RNA II kit (Macherey-Nagel, Switzerland). Synthesis of cDNA was performed with Omniscript RT Kit (Qiagen, USA) following the manufacturer's protocol. RT-PCR measurements were done with the Taqman Fast universal PCR master mix and the Fast SYBR Green master mix on a 7500 Fast Real Time PCR System (all from Applied Biosystems, USA). The sequences of primers and probes are depicted in Table 2 . To analyze the mRNA expression levels of IFNs, PRRs and ISGs, the ΔΔCt method was used [22]. The mRNA expressions levels were normalized to 18S rRNA.
Table 2.
DNA sequences of primers and probes.
| 18S | FW | CGCCGCTAGAGGTGAAATTCT |
| RV | CATTCTTGGCAAATGCTTTCG | |
| P | FAM-ACCGGCGCAAGACGGACCAGA-TAMRA | |
| RV | FW | GTGAAGAGCCSCRTGTGCT |
| RV | GCTSCAGGGTTAAGGTTAGCC | |
| P | FAM-TGAGTCCTCCGGCCCCTGAATG-TAMRA | |
| IFN-λ1 | FW | GGACGCCTTGGAAGAGTCACT |
| RV | AGAAGCCTCAGGTCCCAATTC | |
| P | FAM-AGTTGCAGCTCTCCTGTCTTCCCCG-TAMRA | |
| IFNλ-2/3 | FW | CTGCCACATAGCCCAGTTCA |
| RV | CTGCCACATAGCCCAGTTCA | |
| P | FAM-TCTCCACAGGAGCTGCAGGCCTTTA-TAMRA | |
| IFN-β | FW | CACGGATACAGAACCTATGG |
| RV | ACGAACAGTGTCGCCTACTA | |
| P | FAM-TCAGACAAGATTCATCTAGCACTGGCTGGA-TAMRA | |
| TLR3 | FW | AAATTAAAGAGTTTTCTCCAGGGTGTT |
| RV | ATTCCGAATGCTTGTGTTTGC | |
| P | FAM-TTTGGCCTCTTTCTGAACAATGTCCAGC-TAMRA | |
| RIG-I | FW | CCAAGCCAAAGCAGTTTTCAA |
| RV | CACATGGATTCCCCAGTCATG | |
| P | FAM-TTGAAAAAAGAGCAAAGATATTCTGTGCCCGAC-TAMRA | |
| MDA5 | FW | GATTCAGGCACCATGGGAAGT |
| P | FAM-GGGATGCTCTTGCTGCCACATTCTCTT-TAMRA | |
| RV | AGGCCTGAGCTGGAGTTCTG | |
| PKR | FW | TCTTCATGTATGTGACACTGC |
| RV | CACACAGTCAAGGTCCTT | |
| P | - | |
| OAS1 | FW | CTGACGCTGACCTGGTTGTCT |
| RV | CCCCGGCGATTTAACTGAT | |
| P | FAM-CCTCAGTCCTCTCACCACTTTTCA-TAMRA | |
| MxA | FW | CAGCACCTGATGGCCTATCAC |
| RV | CATGAACTGGATGATCAAAGG | |
| P | FAM-AGGCCAGCAAGCGCATCTCCAG-TAMRA | |
| Viperin | FW | CACAAAGAAGTGTCCTGCTTGGT |
| RV | AAGCGCATATATTTCATCCAGAATAAG | |
| P | FAM-CCTGAATCTAACCAGAAGATGAAAGACTCC-TAMRA |
FW: forward; RV: reverse; P: probe.
2.6. ELISA
Protein levels of CXCL8/IL-8, IL-6 and CXCL10/IP-10 were measured by using the DuoSet ELISA Development kit (R&D, USA) with the following detection limits: CXCL8/IL-8 15 pg/ml, IL-6 3 pg/ml, and CXCL10/IP-10 2 pg/ml.
2.7. Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software Inc., USA). The data were analyzed for normal distribution by using the Kolmogorov–Smirnov test. If normally distributed, paired data were analyzed with one-way ANOVA and the Tukey post hoc test. Paired data non-normally distributed were analyzed with the Friedman and the Dunn's post hoc tests. The analysis of unpaired data was performed with a two-way ANOVA with the Bonferroni post hoc test if normally distributed. Unpaired data non-normally distributed were analyzed with the Kruskal–Wallis test followed by a Dunn's post hoc test. A p < 0.05 was considered statistically significant. Normally distributed data are shown as mean and SEM and non-parametric data are shown as median and IQR.
3. Results
3.1. IFNs response in CF BECs to RV infection
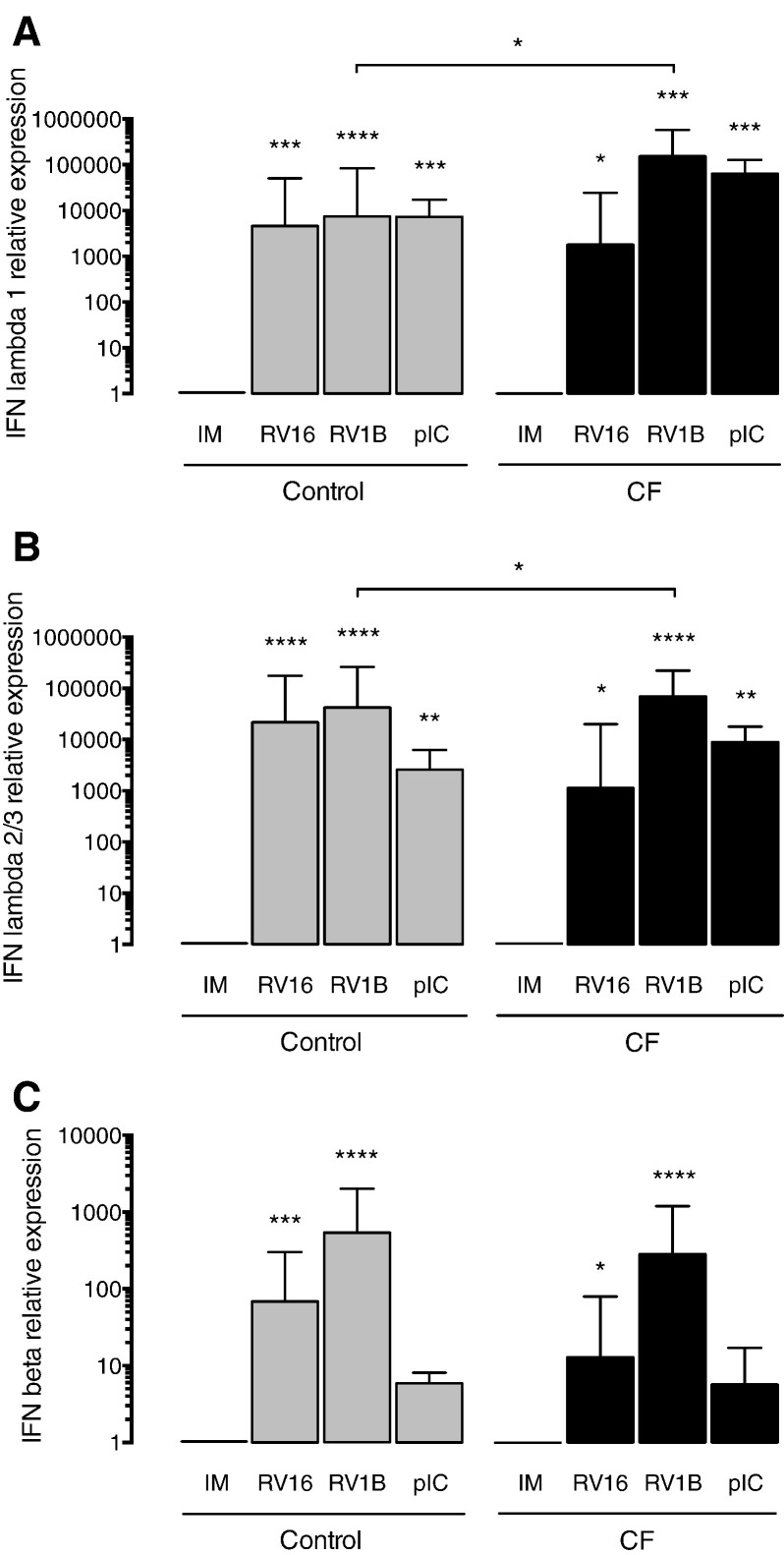
In order to determine if the antiviral responses mediated by IFNs are functional in CF BECs, we measured mRNA expression levels of IFN-λ1, IFN-λ2/3 and IFN-β in CF and control BECs infected with RV16 and RV1B. After infection of CF BECs with major group RV, we observed a non-significant trend towards lower levels of IFN-λ1 (Fig. 1A), IFN-λ2/3 (Fig. 1B), and IFN-β (Fig. 1C) in comparison to healthy BECs. In contrast, the IFN responses of CF BECs compared to healthy BECs upon infection with minor group RV were significantly increased for IFN-λ1 (Fig. 1A) and IFN-λ2/3 (Fig. 1B). We observed no difference for the experiments with poly(IC) — a strong IFN inducer — which gave similar levels of all the measured IFNs between CF BECs and healthy BECs (Fig. 1A–C).
Fig. 1.
IFN responses of CF and control BECs to RV infection. (A) IFN-λ1, (B) IFN-λ2/3 and (C) IFN-β mRNA levels measured 24 h post-infection by RT-PCR in CF and control BECs. IM (infection media), pIC (poly(IC)). The mRNA levels are represented as relative fold increase to mock infection (IM). Significant differences between conditions (IM, RV16, RV1B, pIC) are indicated by stars above error bars and are relative to the mock infection controls (IM). Significant differences between groups (control and CF) are indicated by horizontal lines. Stars indicate significance levels, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.2. PRRs expression upon RV infection in CF BECs
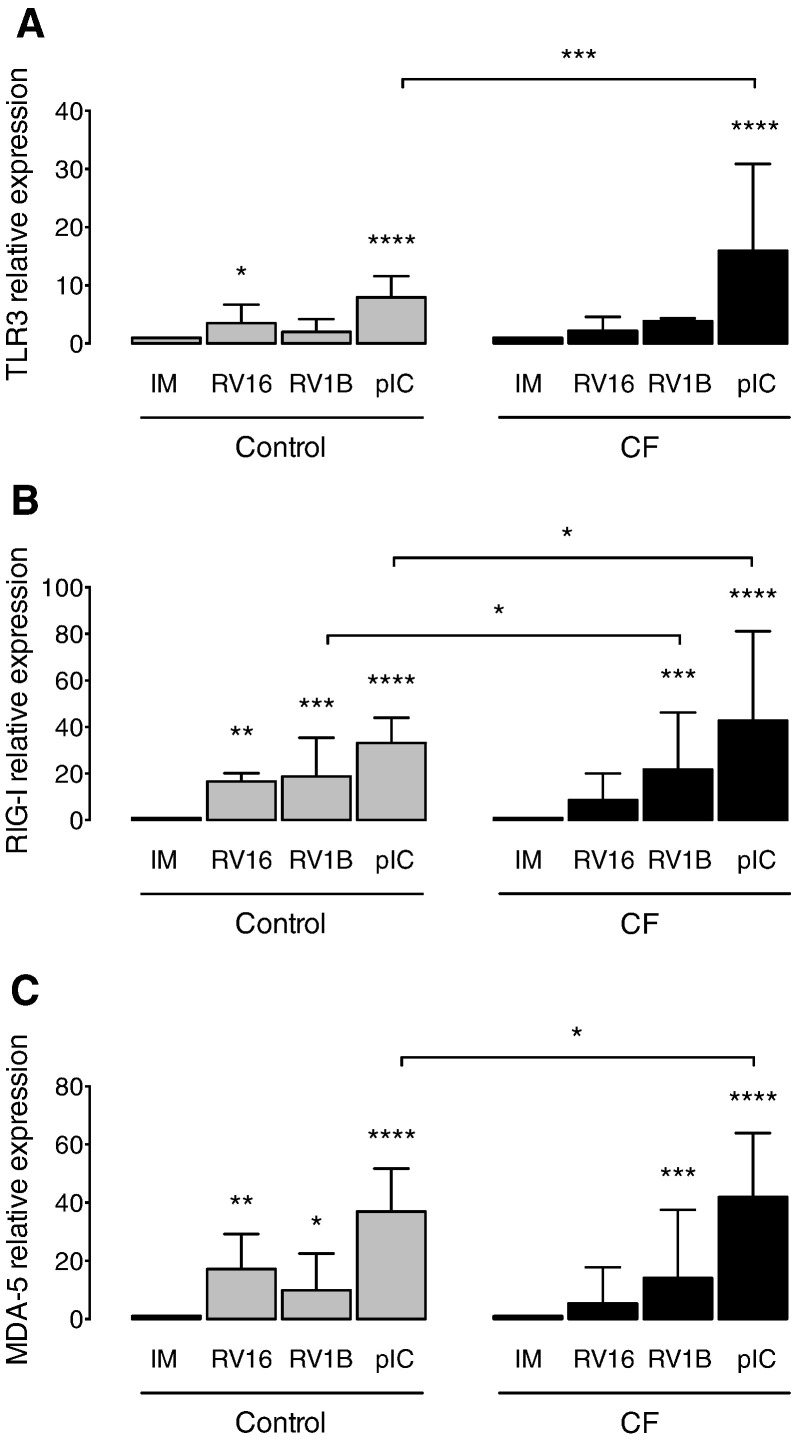
Next, we evaluated the expression of the PRRs involved in the sensing and innate immune response to RVs. The constitutive mRNA levels of the viral sensors TLR3, RIG-I and MDA5 were not different in CF compared to control cells (data not shown). We observed a non-significant trend towards a decreased expression of RIG-I (Fig. 2B) and MDA5 (Fig. 2C) upon infection with the major group virus RV16 in CF cells compared to controls. Interestingly, in CF BECs infected with RV1B, the level of RIG-I was significantly increased (Fig. 2B), and we found a trend towards an elevated expression of TLR3 (Fig. 2A) in comparison to healthy BECs. Interestingly, poly(IC) stimulation of CF BECs induced a significantly higher level of all the PRRs tested in CF BECs in comparison to healthy BECs (Fig. 2A–C).
Fig. 2.
PRRs expression in CF and control BECs upon RV infection. (A) TLR3, (B) RIG-I and (C) MDA5 mRNA levels measured in cell lysates of primary CF and control BECs 24 h post-infection. IM (infection media), pIC (poly(IC)). The mRNA levels are represented as relative fold increase to mock infection (IM). Significant differences between conditions (IM, RV16, RV1B, pIC) are indicated by stars above error bars and are relative to the mock infection controls (IM). Significant differences between groups (control and CF) are indicated by horizontal lines. Stars indicate significance levels, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.3. Induction of ISGs upon infection in CF BECs
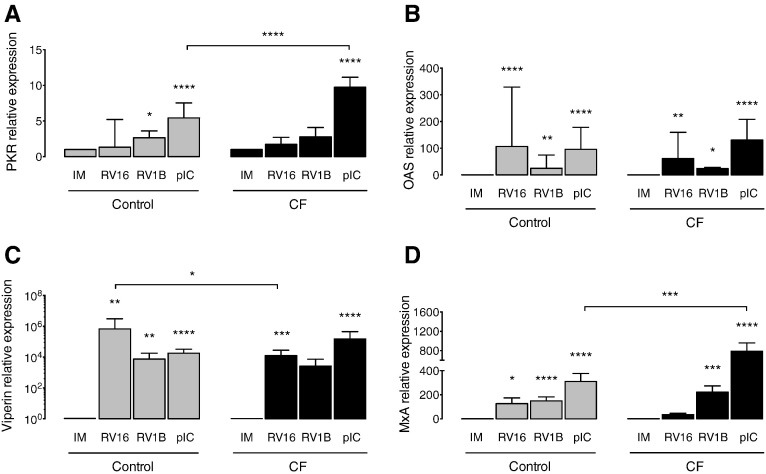
In infected cells, activation of PRRs and subsequent IFN production launches an antiviral effector mechanism leading to the expression of numerous ISGs. Thus, we analyzed the expression of selected ISGs, namely PKR, OAS1, viperin and MxA upon RVs infection and poly(IC) stimulation. We found a significantly lower expression of viperin (Fig. 3C) and a tendency to a lower expression of MxA (Fig. 3D) in CF vs. healthy BECs infected with RV16. Also, there was a significantly higher expression of PKR (Fig. 3A) and MxA (Fig. 3D) in CF BECs stimulated with poly(IC) in comparison to controls. We observed no difference in the ISGs tested between CF and healthy BECs infected with minor group RV1B (Fig. 3A–D).
Fig. 3.
ISGs levels upon RV infection of CF and control BECs. (A) PKR, (B) OAS1, (C) viperin and (D) MxA mRNA levels measured in cell lysates of primary CF and control BECs 24 h post-infection. IM (infection media), pIC (poly(IC)). The mRNA levels are represented as relative fold increase to mock infection (IM). Significant differences between conditions (IM, RV16, RV1B, pIC) are indicated by stars above error bars and are relative to the mock infection controls (IM). Significant differences between groups (control and CF) are indicated by horizontal lines. Stars indicate significance levels, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.4. Pro-inflammatory cytokine responses of CF BECs to RV infection
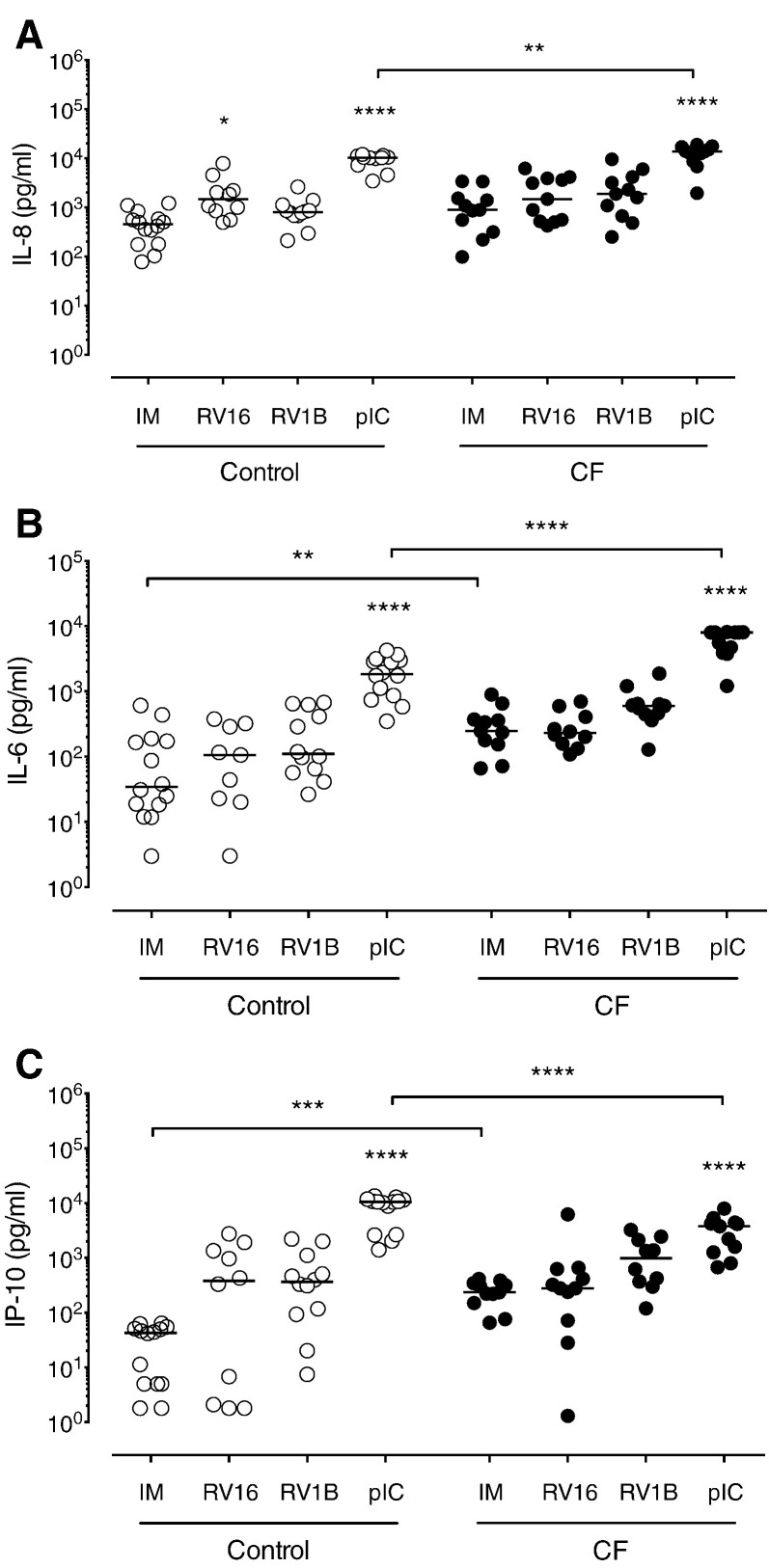
In order to evaluate the inflammatory response of CF BECs after RV infection, we measured the levels of CXCL8/IL-8, IL-6 and CXCL10/IP-10 cytokines under baseline condition and upon infection with RV16, RV1B and stimulation with poly(IC). The inflammatory cytokines produced by BECs upon RVs infection and/or poly(IC) stimulation were generally increased in CF cells. Indeed, CXCL8/IL-8 levels had the non-significant tendency to be constitutively higher and significantly increased upon poly(IC) treatment (Fig. 4A) in CF cells compared to controls. Also, in comparison to control BECs, CF BECs had significantly higher levels of IL-6 under baseline condition and after poly(IC) stimulation (Fig. 4B). The CXCL10/IP-10 levels produced by CF BECs were significantly higher at baseline in comparison to control BECs. In contrast to IL-8 and IL-6, CF BECs produced significantly less CXCL10/IP-10 in comparison to control BECs stimulated with poly(IC) (Fig. 4C).
Fig. 4.
Pro-inflammatory cytokine response of CF and control BECs upon RV infection. (A) IL-6, (B) IL-8 and (C) IP-10 protein levels were assessed in CF and control BECs by ELISA at 24 h post-infection. IM (infection media), pIC (poly(IC)). Significant differences between conditions (IM, RV16, RV1B, pIC) are indicated by stars above and are relative to the mock infection control (IM). Significant differences between groups (control and CF) are indicated by horizontal lines. Stars indicate significance levels, *p < 0.05, **p < 0.01, ****p < 0.0001.
4. Discussion
RV infections have been associated with pulmonary exacerbations of asthma, COPD and CF lung disease. There is growing evidence that an impaired antiviral response mediated by the IFN pathway may contribute to the increased susceptibility towards RV infection of asthmatic [12], [13], [16] and COPD [17], [18], [23] patients. In order to determine if the IFN pathway is also playing a role in the RV-associated morbidity of the CF lung disease, we used primary cultures of CF and healthy BECs. The expression levels of IFN-λ1 and IFN-λ2/3 in CF BECs infected with RV1B were higher than the levels found in healthy BECs. Also, primary CF and healthy BECs stimulated with poly(IC) gave similar IFN levels. However, we observed a trend towards lower RV16-induced levels of IFN-λ1, IFN-λ2/3 and IFN-β in CF BECs in comparison to healthy BECs. This observation is in line with the results from Chattoraj et al. who reported a decreased IFN response in CF BECs in comparison to healthy BECs after infection with major group RV. Interestingly, the IFN response of CF BECs was even lower after co-infection with Pseudomonas aeruginosa and major group RV [24]. Also, the same authors suggest that RV infection of CF patients is exacerbating lower airway symptoms by promoting the outgrowth of P. aeruginosa [25]. Interestingly, Stelzer-Braid et al. reported recently that most of CF pulmonary exacerbations triggered by RVs are not associated with the presence of bacterial pathogens as assessed by multiplex RT-PCR [26]. Thus, the exact contribution and the mechanism of P. aeruginosa in RV-induced exacerbation of CF deserve further investigation.
The level of PRRs engaged in the sensing of RVs [19], [27] were measured upon infection of major and minor group RVs and after poly(IC) stimulation. In line with the decreased IFN responses of CF BECs infected with major group RV, we observed a non-significant trend towards a decreased induction of TLR3, RIG-I, and MDA5 in CF BECs in comparison to healthy BECs after infection with major group RV. Interestingly, we found a higher induction of TLR3, RIG-I, and MDA5 in CF vs. healthy BECs upon treatment with the IFN inducer poly(IC). At the level of ISGs, we observed a decreased induction of viperin and MxA in CF BECs in comparison to healthy BECs after infection with major group RV. This observation is in accordance with our finding of impaired IFNs and PRRs expressions upon RV16 infection in CF BECs compared to controls. The baseline and RV infection-induced ISGs expressions were similar in CF and healthy BECs challenged with minor group RV. Consistent with the increased PRRs levels induced by poly(IC) treatment, poly(IC) treatment of CF BECs also led to a higher expression of some of the ISGs tested such as PKR and MxA, suggesting a dysfunction in the signaling pathways involving dsRNA PRRs such as TLR3. To our knowledge, this is the first report describing a hyperresponsiveness of CF cells to PRRs stimulation.
While the infection of CF BECs with major and minor group RV exert opposite IFN responses in comparison to healthy BECs, the elevated inflammatory response triggered by infection was similar for major and minor group RVs. The inflammatory cytokines produced by CF BECs upon poly(IC) stimulation were altered as demonstrated by the elevated levels of the pro-inflammatory cytokines CXCL8/IL-8 and IL-6. Also, we found an increased constitutive release of CXCL8/IL-8, IL-6 and CXCL10/IP-10 by CF BECs compared to healthy BECs. This observation is in accordance with previous reports attributing an intrinsic inflammatory phenotype to CF airway cells [7], [28], and is compatible with our ex vivo data showing elevated inflammatory cytokine concentrations in BALs of stable CF patients [10]. In line with the elevated PRRs and ISGs of CF BECs stimulated with poly(IC), poly(IC) stimulation led to a hyper-inflammatory response of CF BECs in comparison to healthy BECs as evidenced by the elevated levels of CXCL8/IL-8 and IL-6. However, since both cytokines are constitutively elevated in CF BECs, it is unclear yet if the increased inflammatory response of CF BECs to poly(IC) is solely due to poly(IC) stimulation or as an additive effect to the already elevated baseline levels of CXCL8/IL-8 and IL-6. Additional experiments are required with other inflammatory stimuli in order to address this question. In contrast to IL-8 and IL-6, CXCL10/IP-10 levels upon stimulation with poly(IC) were significantly decreased in CF cells in comparison to control cells. This observation is possibly explained by the fact that the regulation of the CXCL10/IP-10 gene transcription is under the control of the IFN pathway [29], [30].
In healthy cells, the responses to major and minor group RVs have been evaluated by a few groups. A study by Wang et al. gave similar antiviral responses of healthy BECs to RV1B and RV39 [27]. In line with our data, it was reported by two other groups that minor group RV is a higher inducer of IFN-λs and IFN-β in comparison to major group RV [20], [31]. Recently, Schuler et al. found that major and minor group RVs induced a distinct antiviral signaling and inflammatory cytokine responses in primary healthy macrophages [32].
While RVs are the most frequently detected viruses during virus-induced CF exacerbation, there is a growing number of studies reporting that several viral pathogens can trigger CF exacerbation in children and adults including influenza virus, respiratory syncytial virus, parainfluenza virus, coronavirus, adenovirus and metapneumovirus [4], [33], [34], [35], [36]. All these viruses are inducing distinct pathogenesis in the lung and it is therefore conceivable that the mechanisms of virus-induced CF exacerbation are at least in part virus-specific. In line with this hypothesis, a recent study by Ramirez et al. demonstrated that CF exacerbations triggered by RV or influenza virus led to a distinct antiviral response [37]. Therefore, it would be interesting to test our in vitro system with other clinically relevant respiratory viruses such as influenza and respiratory syncytial virus.
In summary, we report a differential IFN pathway response of CF BECs after infection with major and minor group RVs. Major group RV infection is leading to a diminished IFN pathway response at the level of IFNs, PRRs, and ISGs. However, IFN pathway induction in CF BECs upon minor group RV infection was higher than in healthy BECs. Also, CF BECs have an intrinsic inflammatory phenotype as assessed by the constitutive elevated release of CXCL8/IL-8 and IL-6 and CXCL10/IP-10 cytokines. Finally, since our study is based on a small number of subjects, our data need further confirmation in a large study population.
Author's contribution
Conception and design: MPA, TG, MRE, SLJ. Acquisition of data: AS, ABS, CC, AJ, AM. Analysis and interpretation: MPA, TG, MRE, SLJ, NR. Drafting the manuscript for important intellectual content: all authors. Final approval of the manuscript: all authors.
Conflict of interest
SLJ has received grant funding and/or personal fees from AstraZeneca, Boehringer Ingelheim, Centocor, Chiesi, Genentech, GlaxoSmithKline, Merck, Novartis Sanofi Pasteur and Synairgen and is a share-holder in Synairgen.
Acknowledgments
The authors would like to thank all the participants in this study. SLJ and MRE are supported by a chair from Asthma UK (CH11SJ), MRC Centre grant G1000758 and Predicta FP7 Collaborative Project grant 260895. This study has been supported by a financial grant from the Mukoviszidose Institut gGmbH, Bonn project S05/12, the research and development arm of the German Cystic Fibrosis Association Mukoviszidose e.V.
References
- 1.Hofer F., Gruenberger M., Kowalski H., Machat H., Huettinger M., Kuechler E. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci U S A. 1994;91(5):1839–1842. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greve J.M., Davis G., Meyer A.M., Forte C.P., Yost S.C., Marlor C.W. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 3.Monto A.S., Malosh R.E., Petrie J.G., Thompson M.G., Ohmit S.E. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over 3 surveillance seasons. J Infect Dis. 2014;210(11):1792–1799. doi: 10.1093/infdis/jiu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wat D., Gelder C., Hibbitts S., Cafferty F., Bowler I., Pierrepoint M. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7(4):320–328. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Almeida M.B., Zerbinati R.M., Tateno A.F., Oliveira C.M., Romao R.M., Rodrigues J.C. Rhinovirus C and respiratory exacerbations in children with cystic fibrosis. Emerg Infect Dis. 2010;16(6):996–999. doi: 10.3201/eid1606.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collinson J., Nicholson K.G., Cancio E., Ashman J., Ireland D.C., Hammersley V. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax. 1996;51(11):1115–1122. doi: 10.1136/thx.51.11.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutanto E.N., Kicic A., Foo C.J., Stevens P.T., Mullane D., Knight D.A. Innate inflammatory responses of pediatric cystic fibrosis airway epithelial cells: effects of nonviral and viral stimulation. Am J Respir Cell Mol Biol. 2011;44(6):761–767. doi: 10.1165/rcmb.2010-0368OC. [DOI] [PubMed] [Google Scholar]
- 8.Zheng S., De B.P., Choudhary S., Comhair S.A., Goggans T., Slee R. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity. 2003;18(5):619–630. doi: 10.1016/s1074-7613(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 9.Schogler A., Kopf B.S., Edwards M.R., Johnston S.L., Casaulta C., Kieninger E. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur Respir J. 2015;45(2):428–439. doi: 10.1183/09031936.00102014. [DOI] [PubMed] [Google Scholar]
- 10.Kieninger E., Singer F., Tapparel C., Alves M.P., Latzin P., Tan H.L. High rhinovirus burden in lower airways of children with cystic fibrosis. Chest. 2013;143(3):782–790. doi: 10.1378/chest.12-0954. [DOI] [PubMed] [Google Scholar]
- 11.Johnston N.W., Johnston S.L., Duncan J.M., Greene J.M., Kebadze T., Keith P.K. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115(1):132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wark P.A., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A., Bartlett N.W. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 14.Forbes R.L., Gibson P.G., Murphy V.E., Wark P.A. Impaired type I and III interferon response to rhinovirus infection during pregnancy and asthma. Thorax. 2012;67(3):209–214. doi: 10.1136/thoraxjnl-2011-200708. [DOI] [PubMed] [Google Scholar]
- 15.Sykes A., Edwards M.R., Macintyre J., del Rosario A., Bakhsoliani E., Trujillo-Torralbo M.B. Rhinovirus 16-induced IFN-alpha and IFN-beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012;129(6):1506–1514. doi: 10.1016/j.jaci.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Edwards M.R., Regamey N., Vareille M., Kieninger E., Gupta A., Shoemark A. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6(4):797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider D., Ganesan S., Comstock A.T., Meldrum C.A., Mahidhara R., Goldsmith A.M. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(3):332–340. doi: 10.1164/rccm.200911-1673OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallia P., Message S.D., Gielen V., Contoli M., Gray K., Kebadze T. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183(6):734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slater L., Bartlett N.W., Haas J.J., Zhu J., Message S.D., Walton R.P. Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sykes A., Macintyre J., Edwards M.R., Del Rosario A., Haas J., Gielen V. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax. 2014;69(3):240–246. doi: 10.1136/thoraxjnl-2012-202909. [DOI] [PubMed] [Google Scholar]
- 21.Nakagome K., Bochkov Y.A., Ashraf S., Brockman-Schneider R.A., Evans M.D., Pasic T.R. Effects of rhinovirus species on viral replication and cytokine production. J Allergy Clin Immunol. 2014;134(2):332–341. doi: 10.1016/j.jaci.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Baines K.J., Hsu A.C., Tooze M., Gunawardhana L.P., Gibson P.G., Wark P.A. Novel immune genes associated with excessive inflammatory and antiviral responses to rhinovirus in COPD. Respir Res. 2013;14:15. doi: 10.1186/1465-9921-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chattoraj S.S., Ganesan S., Faris A., Comstock A., Lee W.M., Sajjan U.S. Pseudomonas aeruginosa suppresses interferon response to rhinovirus infection in cystic fibrosis but not in normal bronchial epithelial cells. Infect Immun. 2011;79(10):4131–4145. doi: 10.1128/IAI.05120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chattoraj S.S., Ganesan S., Jones A.M., Helm J.M., Comstock A.T., Bright-Thomas R. Rhinovirus infection liberates planktonic bacteria from biofilm and increases chemokine responses in cystic fibrosis airway epithelial cells. Thorax. 2011;66(4):333–339. doi: 10.1136/thx.2010.151431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stelzer-Braid S., Johal H., Skilbeck K., Steller A., Alsubie H., Tovey E. Detection of viral and bacterial respiratory pathogens in patients with cystic fibrosis. J Virol Methods. 2012;186(1–2):109–112. doi: 10.1016/j.jviromet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q., Nagarkar D.R., Bowman E.R., Schneider D., Gosangi B., Lei J. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183(11):6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribeiro C.M., Paradiso A.M., Carew M.A., Shears S.B., Boucher R.C. Cystic fibrosis airway epithelial Ca2 + i signaling: the mechanism for the larger agonist-mediated Ca2 + i signals in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280(11):10202–10209. doi: 10.1074/jbc.M410617200. [DOI] [PubMed] [Google Scholar]
- 29.Zaheer R.S., Proud D. Human rhinovirus-induced epithelial production of CXCL10 is dependent upon IFN regulatory factor-1. Am J Respir Cell Mol Biol. 2010;43(4):413–421. doi: 10.1165/rcmb.2009-0203OC. [DOI] [PubMed] [Google Scholar]
- 30.Taima K., Imaizumi T., Yamashita K., Ishikawa A., Fujita T., Yoshida H. Expression of IP-10/CXCL10 is upregulated by double-stranded RNA in BEAS-2B bronchial epithelial cells. Respiration. 2006;73(3):360–364. doi: 10.1159/000091646. [DOI] [PubMed] [Google Scholar]
- 31.Wark P.A., Grissell T., Davies B., See H., Gibson P.G. Diversity in the bronchial epithelial cell response to infection with different rhinovirus strains. Respirology. 2009;14(2):180–186. doi: 10.1111/j.1440-1843.2009.01480.x. [DOI] [PubMed] [Google Scholar]
- 32.Schuler B.A., Schreiber M.T., Li L., Mokry M., Kingdon M.L., Raugi D.N. Major and minor group rhinoviruses elicit differential signaling and cytokine responses as a function of receptor-mediated signal transduction. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flight W.G., Bright-Thomas R.J., Tilston P., Mutton K.J., Guiver M., Morris J. Incidence and clinical impact of respiratory viruses in adults with cystic fibrosis. Thorax. 2014;69(3):247–253. doi: 10.1136/thoraxjnl-2013-204000. [DOI] [PubMed] [Google Scholar]
- 34.Wark P.A., Tooze M., Cheese L., Whitehead B., Gibson P.G., Wark K.F. Viral infections trigger exacerbations of cystic fibrosis in adults and children. Eur Respir J. 2012;40(2):510–512. doi: 10.1183/09031936.00202311. [DOI] [PubMed] [Google Scholar]
- 35.Hoek R.A., Paats M.S., Pas S.D., Bakker M., Hoogsteden H.C., Boucher C.A. Incidence of viral respiratory pathogens causing exacerbations in adult cystic fibrosis patients. Scand J Infect Dis. 2013;45(1):65–69. doi: 10.3109/00365548.2012.708942. [DOI] [PubMed] [Google Scholar]
- 36.Goffard A., Lambert V., Salleron J., Herwegh S., Engelmann I., Pinel C. Virus and cystic fibrosis: rhinoviruses are associated with exacerbations in adult patients. J Clin Virol. 2014;60(2):147–153. doi: 10.1016/j.jcv.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramirez I.A., Caverly L.L., Kalikin L.M., Goldsmith A.M., Lewis T.C., Burke D.T. Differential responses to rhinovirus- and influenza-associated pulmonary exacerbations in patients with cystic fibrosis. Ann Am Thorac Soc. 2014;11(4):554–561. doi: 10.1513/AnnalsATS.201310-346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]