Abstract
Mixed respiratory viral infections are double negative common and evidence that they are associated with severe disease is supported by some groups. This controversial observation can be explained by the lack of sensitivity of the assessed methods used for viral identification and by the small number of patients included in the randomized cohorts studied. Most studies showed that respiratory syncytial virus (RSV) is identified in about 70% of hospitalized infants with bronchiolitis during seasonal winter epidemics, followed by human metapneumovirus (hMPV, about 3–19%) or rhinoviruses (about 20%). Other respiratory viruses have also been reported, indicating significant causes of bronchiolitis and hospitalization during seasonal epidemics. The presence of more than one pathogen, and moreover, the association of RSV with rhinoviruses and also RSV with hMPV, may influence the natural course of bronchiolitis. A better understanding of these various interactions would help future decision-making, such as the extent to which searches for co-pathogens should be conducted in severe bronchiolitis patients already infected by RSV.
Keywords: Respiratory viruses, Mixed infections, Bronchiolitis, Cell culture, Molecular tests
1. Introduction
Respiratory virus infections represent a major public health problem because of their worldwide occurrence, ease of spread in the community and considerable morbidity and mortality. Bronchiolitis is the clinical description of the most common and most severe acute lower-respiratory tract infection during early childhood. Around 2–3% of all infants younger than 1 year are admitted to hospital with bronchiolitis, usually during winter in temperate countries.1 The peak incident age in these infants is between 2 and 6 months.2 The majority of these infants have an intense inflammation of the bronchioles leading to a clinical syndrome characterized by obstruction of expiratory airflow usually proceeded by nasal congestion and rhinorrhea. Hospital admission is required because mucus obstruction and respiratory distress interfere with feeding. In the most severely affected infants there is hypoxia and respiratory distress that may require mechanical ventilation and prolonged hospitalization.3, 4
Human respiratory syncytial virus (RSV) is the most commonly identified virus, with detection rates reaching 70–85% in hospitalized infants during seasonal winter epidemics.5, 6, 7, 8 Other respiratory viruses, including human metapneumovirus (hMPV), parainfluenza viruses (PIVs), influenza viruses (IVs), and rhinoviruses (RVs) are also significant causes of acute bronchiolitis and hospitalization during epidemics or periods of increase prevalence in the community.9, 10, 11, 12 The clinical pattern of illness seen with RSV infection is very similar to those seen with other respiratory viruses.13, 14
Several studies have acknowledged the possibility of multiple infections in RSV bronchiolitis.15, 16 The incidence of dual respiratory viral infections varies from 10% to 30% in hospitalized infants. Mixed virus infections are frequently identified in the same clinical specimen particularly for RSV with hMPV17 and more recently it was described that RSV and RVs are the viruses most frequently identified in dual infections in infants hospitalized with bronchiolitis.18 Their occurrence in bronchiolitis pathogenesis has been increasingly recognized with the establishment of new and high-sensitive molecular amplification methods, especially for viruses that are difficult to culture. Different controversial reports suggest either an association between dual infections and an increase in the disease severity18, 19, 20, 21 or the absence of an association between dual infections and an increase in the disease severity.22, 23, 24 It may be assumed that the simultaneous presence of more than one viral pathogen would be associated with epidemiologic and clinical features that differ from single infections, especially with respect to disease type and severity. Although bronchiolitis is common, little is known about what causes infants to be susceptible. The clinical importance of such presumed dual infection, particularly those pouring RSV with other viruses, remains uncertain. Clinical studies associated with mixed respiratory virus infections are needed to clarify assessment of disease severity and criteria of hospital admission.
2. Respiratory virus detection assays
2.1. Conventional virus culture versus molecular amplification techniques
Virus culture is the original method used to diagnose respiratory virus infections and the use of primary or immortalized cells expanded the method giving wider diagnostic abilities in the early 1960s. Virus isolation in cell cultures has long served as the “gold standard” for virus detection, and it is the method with which all other methods have been compared. The main advantage of traditional cell culture methods is the ability to isolate and identify a wide range of viruses.25, 26 This enables the identification of many viruses, including those that commonly cause respiratory infections. Growth of a virus in cell culture indicates the presence of an infectious, viable, and replication competent virus, a finding which is unattainable using other technologies such as antigen detection or nucleic acid detection.27
Viruses can also be detected directly in clinical samples using highly specific nucleic acid detection. Further, molecular amplification tests improve the sensitivity, especially for RVs and other viruses that are difficult to culture such as hMPV and human coronavirus-NL63 (hCoV-NL63).28 We recently described18 a study done during two consecutive winter seasons 2003–2004 and 2004–2005, including 180 infants with bronchiolitis hospitalized in the pediatric short-term unit or in the pediatric intensive care unit (PICU). In this study, we showed that a greater number of positive samples were detected by nucleic acids tests, than by cell culture (Table 1 ). As observed in Table 1, the diagnostic yield increase significantly for rhinoviruses with 21.2% of samples found positive by molecular amplification, whereas only 2.2% were tested positive by cell culture. We also showed that the increased sensitivity of amplification tests allowed detection of 83 additional cases (47.9%) compared to the cell culture.
Table 1.
The rate of positive viral detection by standard methods (virus antigen or virus culture) and molecular amplification in 180 infants hospitalized for bronchiolitis
| Virus | Standard methods | Molecular amplification |
|---|---|---|
| Respiratory syncytial virus | 55.9 | 66.8 |
| Influenza virus | 2.8 | 3.6 |
| Parainfluenza viruses types 1–4 | 2.2 | 5.3 |
| Adenovirus | 0 | 1.8 |
| Enterovirus | 1.7 | 4.1 |
| Rhinovirus | 2.2 | 21.2 |
Data are present as number of samples positive (percentage of evaluated samples).
Sensitive and specific respiratory virus identification is crucial for the assessment of the burden to the population caused by pediatric acute respiratory infection. Nucleic acids tests seem to be the most promising alternative to cell culture for rapid and accurate detection of respiratory virus infections, especially for newly identified viruses that are difficult to culture or have not been well replicated in vitro.29 Thus, forecasts of disease burden based on conventional cell culture methods alone are liable to underestimate true infection rates, because of the fact that cell culture require several days to detect respiratory viruses as well as several specific cell lines for each respiratory virus. Recently R-Mix, a combination of human lung carcinoma cells and mink lung cells (cells A-549 and Mv1Lu, respectively) co-cultivated in a balance single monolayer was reported to exhibit sensitivity for respiratory virus detection.30 R-Mix cells exhibit a more sensitive detection rate for respiratory viruses after 1–3 days post-infection. In the near future, as more sophisticated, yet simpler-to-use, broad-range molecular platforms become available for clinical diagnostics, virus isolation in the cell culture may once again become an advantageous diagnostic methods in light of the evidence presented above, a commendable solution would be combined culture methods using R-Mix cells and molecular amplification tests. This solution provides opportunities for assessing the occurrence and importance of mixed respiratory viral infections.
3. Frequency of detection of respiratory viruses in hospitalized infants
Several studies were done worldwide in winter seasons to identify respiratory viruses using both tissue culture and molecular amplification in different specimens (nasal and through swabs, nasal aspirates or bronchoalveolar lavages) from children hospitalized with respiratory symptoms. All studies clearly described the RSV as the most common causative agent in children under 3 or infants hospitalized with acute bronchiolitis.17, 18, 19, 31, 32 Approximately 70–89% of cases of bronchiolitis can be attributed to infection by RSV, and 3–25% can be attributed to infection by rhinovirus or parainfluenza viruses, and rarely, human influenza virus and adenovirus.18, 19 Numerous studies have described a prevalence of 3.3–19% for hMPV infection in children with respiratory tract infections who were admitted to the hospital.17, 18, 19, 20, 21, 22, 23 It is now recognized that hMPV is one of the several viral pathogens that can cause respiratory symptoms in the absence of other pathogens33 and is moreover the second leading cause of acute bronchiolitis in hospitalized children.13, 19, 20, 23 In our study,18 the second most common viral pathogen found in the bronchiolitis cohort was RVs present in 39 out of 180 cases representing 21.8% in infants less than 12 months of age. Rhinovirus are the most common respiratory tract viruses in older children and adults, causing about two-thirds of common colds and asthma exacerbations.34 Recent advances in virus detection methods using molecular amplification have depicted these viruses as important lower-respiratory tract pathogens.35 Our study also presented to put in evidence the important role played by these common respiratory tract viruses in hospitalized infants with bronchiolitis in the first year of life.18 The incidence of RV infections, and therefore their importance, has been underestimated in acute bronchiolitis. In accordance with other studies, we found other respiratory viruses responsible but to a lesser extent. In our total cohort corresponding to 180 infants hospitalized with acute bronchiolitis, PIVs were found in 13 cases (7.2%), hMPV in 10 cases (5.6%), hCoV-NL63 in seven cases (3.9%), and IVs in six cases (3.3%). Our results can be explained by the fact that our cohort included only infants less than 1-year old.
4. Single versus mixed respiratory virus infections
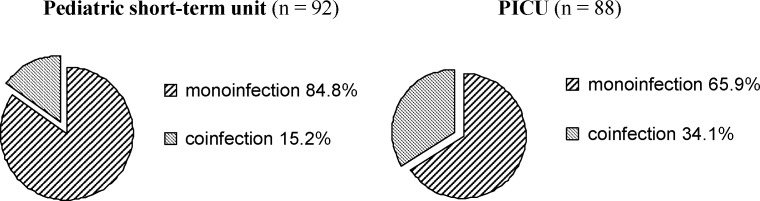
Several studies in the past have acknowledged the possibility of multiple infections in acute bronchiolitis. Dual respiratory infections occur in up to 30% of infants with a lower-respiratory tract infection,36 but the incidence varies widely,15, 16 from zero reported cases in population-based and case control studies,37, 38 up to 70% of infants with bronchiolitis.17 In addition to variations in viral infection during endemic seasons, discrepancies in the circulating rates of second pathogen can be explained by detection sensitivity, modes of sampling which could underestimate the infection rate, and the number of enrolled participants, which are often insufficient to permit a powered study. Numerous studies report dual respiratory infections principally with hMPV and RSV9, 19, 20, 21, 22, 23, 24, 37, 38 because of the same seasonal distribution of these two viruses. In our study,18 multiple virus infections were identified in 44 of the 180 hospitalized infants (23.9%) with bronchiolitis. We evaluated the frequency of dual viral infection in infants with bronchiolitis admitted to either a short-term unit (n = 92) or a PICU (n = 87) at the same hospital in France. Co-infection rates were significantly different (p = 0.0005, χ 2-test) between the two populations studied (Fig. 1 ). The mixed respiratory virus infections found in our studied cohort were dual viral infections and the main viral agents detected were RSV (36 of 44) and RVs (22 of 44), resulting in the most found dual viral respiratory infections (29.5%). High incidence of RSV coupled with RV infections is reflected in the considerable overlapping of the seasonal distribution observed for these two viruses during the winter seasons 2003–2004 and 2004–2005. In our cohort, we also found other coinfected cases, eight of 10 hMPV, five of seven hCoV-NL63, six of seven EV, and five of six IV-infected infants. Moreover, hMPV as well as hCoV-NL63, and PIV were mostly found to be RSV or RVs associated agents (Table 2 ).
Fig. 1.
The frequency of respiratory virus monoinfection and coinfection in the pediatric short-term unit (n = 92) and the pediatric intensive care unit (PICU, n = 88).
Table 2.
Viral distribution in dual infection cases observed in pediatric intensive care unit (PICU) and pediatric short-term unit
| Primary viral agent | Associated virus | Short-term unit (n = 14) | PICU (n = 30) |
|---|---|---|---|
| Respiratory syncytial virus | Rhinovirus | 35.7 | 26.6 |
| hMPV | 7.1 | 13.3 | |
| hCoV-NL63 | 0 | 13.3 | |
| Influenza A/B | 14.3 | 6.6 | |
| Parainfluenza virus | 0 | 13.3 | |
| Adenovirus | 0 | 3.3 | |
| Enterovirus | 28.6 | 3.3 | |
| Rhinovirus | RSV | 35.7 | 26.6 |
| hMPV | 7.1 | 6.6 | |
| hCoV-NL63 | 7.1 | 0 | |
| Influenza A/B | 0 | 0 | |
| Parainfluenza virus | 0 | 10 | |
| Adenovirus | 0 | 3.3 | |
| Enterovirus | 0 | 3.3 | |
| Parainfluenza virus | Influenza A/B | 0 | 3.3 |
Data are presented as number of samples positive for dual infection (percentage of evaluated samples).
It may be assumed that the simultaneous presence of more than one viral pathogen would be associated with epidemiological and clinical features that differ from single infections, especially with respect to disease type and severity. We and others18, 19 used as clinical criteria for the impact of dual infection upon severity of disease such as duration of assisted ventilation, or supplemental oxygen administration and the site and duration of the hospital stay.
5. Clinical implications of dual infections
The clinical severity of bronchiolitis is defined by mild disease, as no need for hospital admission; moderate disease, as admission to the hospital; severe disease, as admission to the hospital, and a need for supplemental oxygen, including mechanical ventilation, at any time during the hospital stay.39 High-risk groups for severe infections are infants younger than 6 weeks with predisposing conditions, such as premature infants, those with chronic lung disease, congenital heart disease, and immunodeficiency disorders.40
Recent studies showed that dual viral infection was least frequent in infants with moderate disease who were admitted to the emergency or general wards, and was most commonly observed in infants with severe disease who were admitted to the PICU for mechanical ventilation.18, 19 In our study, the simultaneous presence of RVs and RSV, both at high prevalence, caused us to perform a statistical study analysis of the association of dual infection with severe disease. We used a logistic regression model to compare clinical and virological risk factors and we found a significant correlation between dual viral infection and increased disease severity in bronchiolitis. Indeed, dual infection was found to be a risk factor for admission to the PICU, independent of the host conditions. Infants with viral coinfection were almost three times more at risk for PICU admission compared to those with single viral infection. This major finding is in accordance with observations made for hMPV and RSV dual infections, reported to confer a 10-fold increase in relative risk of admission to the PICU for mechanical ventilation.19 But our results contrast with studies reporting no greater severity of illness in the case of dual infection despite high-circulating rates of the second pathogen.22, 24 Clinical parameters defining illness severity could explain such differences.
6. Conclusions
Rhinovirus must be viewed as a significant contributor agent to morbidity in infants with bronchiolitis, both in its own right and in its synergistic pathology with RSV and other respiratory viruses. Mixed respiratory virus infections induce a more severe bronchiolitis, defined by infants receiving mechanical ventilation hospitalized in the PICU, than single infections and represent a relevant risk factor independent of prematurity, age, and underlying chronic illness. It remains to be determined whether quantity of virus, viral genotype, infection with multiple species, or a combination of these factors are important in determining the severity of bronchiolitis. Currently, there are no therapeutic measures available to prevent dual infection occurring in infants except for hygienic measures and control of overcrowding which should reduce risk for all respiratory illness.
References
- 1.Smyth R.L., Openshaw P.J. Bronchiolitis. Lancet. 2006;368:312–322. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- 2.Parrott R.H., Kim H.W., Arrobio J.O. Epidemiology of respiratory syncytial virus infection in Washington DC. Am J Epidemiol. 1973;98:298–300. doi: 10.1093/oxfordjournals.aje.a121565. [DOI] [PubMed] [Google Scholar]
- 3.Lebel M.H., Gauthier M., Lacroix J., Rousseau E., Buithieu M. Respiratory failure and mechanical ventilation in severe bronchiolitis. Arch Dis Child. 1989;64:1431–1437. doi: 10.1136/adc.64.10.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott E.J., Taylor G. Respiratory syncytial virus. Arch Virol. 1985;84:1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- 5.Simoes E.A. Respiratory syncytial virus infection. Lancet. 1999;354:847–852. doi: 10.1016/S0140-6736(99)80040-3. [DOI] [PubMed] [Google Scholar]
- 6.Resch B., Gusenleitner W., Muller W. The impact of respiratory syncytial virus infection: a prospective study in hospitalized infants younger than 2 years. Infection. 2002;30:193–197. doi: 10.1007/s15010-002-2122-1. [DOI] [PubMed] [Google Scholar]
- 7.Mentel R., Ilgert U., Wegner U., Zimmerman K., Bruns R., Gürtler L. Molecular and clinical characteristics of respiratory syncytial virus infections in hospitalized children. Med Microbiol Immunol. 2005;194:67–71. doi: 10.1007/s00430-003-0215-9. [DOI] [PubMed] [Google Scholar]
- 8.Henrickson K.J. Advances in the laboratory diagnosis of respiratory disease. Pediatr Infect Dis J. 2004;23:S6–S10. doi: 10.1097/01.inf.0000108187.63151.ea. [DOI] [PubMed] [Google Scholar]
- 9.Mullins J.A., Erdman D.D., Weinberg G.A., Edwards K., Hall C.B., Walker F.J. Human metapneumovirus infection among children hospitalized with a cute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viazov S., Ratjen F., Scheidhauer R., Fiedler M., Roggendorf M. High prevalence of human metapneumovirus infection in young children and genetic heterogeneity of the viral isolates. J Clin Microbiol. 2003;41:3043–3045. doi: 10.1128/JCM.41.7.3043-3045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boivin G., De Serres G., Cote S., Gilca R., Abed Y., Rochette L. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manoha C., Espinosa S., Aho S.L., Huet F., Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J Clin Virol. 2007;38:221–226. doi: 10.1016/j.jcv.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Xepapadaki P., Psarras S., Bossios A., Tsolia M., Gourgiotis D., Liapi-Adamidou G. Human metapneumovirus as a causative agent of acute bronchiolitis in infants. J Clin Virol. 2004;30:267–270. doi: 10.1016/j.jcv.2003.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright A.L., Taussig L.M., Ray C.G., Harrison H.R., Holberg C.J. The Tucson children’s respiratory study. Part II. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129:1232–1236. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 15.Ray C.G., Minnich L.L., Holberg C.J., Shehab Z.M., Wright A.L., Barton L.L. Respiratory syncytial virus-associated lower respiratory tract illnesses: possible influence of other agents. Pediatr Infect Dis J. 1993;12:15–19. [PubMed] [Google Scholar]
- 16.Drews A.L., Atmar R.L., Glezen W.P., Baxter B.D., Piedra P.A., Greenberg S.B. Dual respiratory virus infections. Clin Infect Dis. 1997;1425:1421–1429. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greensill J., McNamara P.S., Dove W., Flanagan B., Smyth R.L., Hart C.A. Human metapneumovirus in severe respiratory syncitial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–375. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richard N., Komurian-Pradel F., Javouhey E., Perret M., Rajoharison A., Bagnaud A. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J. 2008;27(3):213–217. doi: 10.1097/INF.0b013e31815b4935. [DOI] [PubMed] [Google Scholar]
- 19.Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C. Dual infection of infants by human metapneumovirus and respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005:191–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foulongne V., Guyon G., Rodière M., Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25:354–359. doi: 10.1097/01.inf.0000207480.55201.f6. [DOI] [PubMed] [Google Scholar]
- 21.König B., König W., Arnold R., Werchau H., Ihorst G., Forster J. Prospective study of human metapneumovirus infectionin children less than 3 years of age. J Clin Microbiol. 2004;42:4632–4635. doi: 10.1128/JCM.42.10.4632-4635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García-García M.L., Calvo C., Pérez-Breña P., De Cea J.M., Acosta B., Casas I. Prevalence and clinical characteristics of human metapneumovirus infections in hospitalized infants in spain. Pediatr Pulmonol. 2006;41:863–871. doi: 10.1002/ppul.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf D.G., Greenberg D., Kalkstein D., Shemer-Avni Y., Givon-Lavi N., Saleh N. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. April 2006;25(4):320–324. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 24.Wilkesmann A., Schildgen O., Eis-Hübinger A.M., Geikowski T., Glatzel T., Lentze M.J. Human metapneumovirus infections cause similar symptoms and clinical severity as respiratory syncytial virus infections. Eur J Pediatr. 2006;25:320324. doi: 10.1007/s00431-006-0105-4. [DOI] [PubMed] [Google Scholar]
- 25.Fenner F.J., White D.O. 4th ed. Academic Press; San Diego: 1994. Laboratory diagnosis of viral disease. Medical Virology. p. 191–218. [Google Scholar]
- 26.Kesson A.M. Respiratory virus infections. Pediatr Respir Rev. 2007;8:240–248. doi: 10.1016/j.prrv.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leland D.S., Ginocchio C.C. Role of cell culture for virus detection in the age of technology. Clin Microbiol Rev. 2007:49–78. doi: 10.1128/CMR.00002-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Pol A.C., Wolfs T.F., Jansen N.J., van Loon A.M., Rossen J.W. Diagnostic value of real-time polymerase chain reaction to detected viruses in young children admitted to the pediatric intensive care unit with lower respiratory tract infection. Crit Care. 2006;10:R6. doi: 10.1186/cc4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allender T. Human bocavirus. J Clin Virol. 2008;41:29–31. doi: 10.1016/j.jcv.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Barenfager J., Drake C., Mueller T., Troutt T., O’Brien J., Guttman K. R-Mix cells are faster, at least as sensitive and marginally more costly than conventional cell lines for detection of respiratory viruses. J Clin Virol. 2001;22:101–110. doi: 10.1016/s1386-6532(01)00171-8. [DOI] [PubMed] [Google Scholar]
- 31.Kusel M.H., Klerk N.H., Holt P.G., Kebadze T., Johnston S.L., Sly P.D. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohorth study. Pediatr Infect Dis J. 2006;25(8):680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 32.Canducci F., Debiaggi M., Sampaolo M., Marinozzi M.C., Berreè S., Terulla C. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J Med Virol. 2008;80:716–723. doi: 10.1002/jmv.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. Br Med J. 1995;310:1225–1228. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadopoulos N.G., Moustaki M., Tsolia M., Bossios A., Astra E., Prezerakou A. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 36.Parrott R.H., Vargosko A.J., Kim H.W., Cumming C., Turner H., Huebner R.J. Respiratory syncytial virus. Part II. Serological studies over a 34 month period of children with bronchiolitis, pneumonia, and minor respiratory diseases. JAMA. 1961;176:653–657. [PubMed] [Google Scholar]
- 37.Lazar I., Weibel C., Dziura J., Ferguson D., Landry M.L., Kahn J.S. Human metapneumovirus and severity of respiratory syncytial virus disease. Emerg Infect Dis. 2004;10:1318–1320. doi: 10.3201/eid1007.030983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Woensel J.B., Bos A.P., Lutter R., Rossen J.W., Schuurman R. Absence of human metapneumovirus co-infection in cases of severe respiratory syncytial virus infection. Pediatr Pulmonol. 2006;41:872–874. doi: 10.1002/ppul.20459. [DOI] [PubMed] [Google Scholar]
- 39.Smyth R.L., Fletcher J.N., Thomas H.M., Hart C.A. Immunological responses to respiratory syncytial virus infection in infancy. Arch Dis Child. 1997;76:210–214. doi: 10.1136/adc.76.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purcell K., Fergie J. Discroll Children’s Hospital respiratory syncytial virus database: risk factors, treatment and hospital course in 3308 infants and young children, 1991 to 2002. Pediatr Infect Dis J. 2004;23:418–423. doi: 10.1097/01.inf.0000126273.27123.33. [DOI] [PubMed] [Google Scholar]