Abstract
B cells are one of the key players in the pathogenesis of multiple sclerosis (MS). The peripheral B cell distributions are similar in healthy persons and MS patients. In healthy controls, B cells are rarely present in the cerebrospinal fluid (CSF) while in MS patients, a clonally expanded B cell population is detected. This consists of memory B cells, centroblasts and antibody-secreting plasma blasts and plasma cells that are responsible for intrathecal immunoglobulin G production and oligoclonal band formation in more than 90% of MS patients. Unfortunately, the targets of the autoreactive B cells and antibodies remain largely unknown. Various candidate antigens have been identified but often their involvement in the disease process is still unclear. Most studies characterizing these target antigens examined autoantibodies by analyzing sera or CSF of MS patients. An alternative approach is focusing on the clonally expanded B cells. In this way B cells directed against myelin, astroglia and axons have been denoted in MS patients. B cell immortalization, that is based on the antibody-producing potential of Epstein–Barr virus (EBV) transformed B cells, can be used to expand B cells from MS patients for the production of antibodies, that ultimately can be analysed for target identification.
Keywords: Multiple sclerosis, B cells, Antibody-secreting cells, Cerebrospinal fluid, Antibodies
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) that has been regarded as a T cell mediated disorder for long time. More research is now performed on B cells and antibodies as players in the pathogenesis of MS. Hence, oligoclonal immunoglobulin G (IgG) bands (OCB) have been found in the cerebrospinal fluid (CSF) but not in the serum of more than 90% of patients with MS, indicating an intrathecal immunoglobulin (Ig) production [1], [2]. The presence of B cells, plasma cells, complement and myelin-specific antibodies in chronic MS lesions [2], [3], [4] and the restricted Ig gene usage pattern in the variable region of the heavy chain (VH) locus in CSF B cells [5], [6], [7] suggested an antigen-driven humoral immune response in the CNS in MS. In addition, B cell follicle-like structures were characterized in the brain meninges of MS patients [8] and more recently, positive results have been achieved using the B cell depleting antibody Rituximab in clinical trials [9], [10]. These findings all contribute to the compelling evidence that the humoral immune response is implicated in the pathogenesis of MS.
B cells can exert different functions in MS pathogenesis, namely complement fixation, cell-mediated cytotoxicity, antigen presentation, T cell costimulation and cytokine or neurotrophic factor production [2], [4], [7], [11], [12], but most research focuses on autoantibody production. Numerous approaches are currently used to investigate B cell reactivity with different success rates, and despite a lot of effort it is not yet known which are the targets of the autoreactive B cells and antibodies. Since MS is characterized by a heterogeneous disease course and genetic background, novel markers for the diagnosis of new patients and a multiplex treatment strategy are needed for better control of the disease. Studying the humoral immune response thus remains a hot topic in MS research where identification of the targeted antigens and characterization of the B cell repertoire are the two main goals.
2. B cell repertoire in healthy individuals
Mature naïve B cells, which are characterized by expression of the general B cell marker CD19 and the absence of CD27 and CD38 on their surface, are released in the peripheral blood after development in the bone marrow and secondary lymphoid organs. Recognition of an antigen in lymphoid tissue triggers B cell activation, which is accompanied by an upregulation of CD80 and CD86 surface expression. After activation, B cells proliferate into plasma blasts that produce low-affinity antibody for a few days, or further differentiate into different effector B cell subtypes in germinal centers (GC) via the centrocyte and centroblast stages, which is accompanied by an upregulation of CD38 surface expression. In this case, memory B cells, plasma blasts or plasma cells are developed after extensive mutation and maturation [12], [13]. Memory B cells, expressing both CD19 and the memory surface marker CD27, are able to reactivate and differentiate into plasma cells very fast upon a new challenge with their antigen. Plasma blasts are short-lived antibody-secreting cells expressing CD19, CD27, CD38 but also the plasma cell marker CD138. Plasma cells have lost proliferating capacity and are mostly long-lived antibody-producing cells that no longer display CD19 but do express CD38, CD138 and CD27.
In healthy individuals, most B cells that populate the peripheral blood belong to the naïve B cell population, although some circulating memory B cells can be denoted. The antibody-secreting effector cells move into the target tissues to eliminate their target cells and consequently cannot be found in the blood. B cells are rarely found in the CSF of both healthy persons and patients with a non-inflammatory neurological disease (NIND) [12].
3. B cell repertoire in MS
As in healthy individuals, B cells in the peripheral blood of MS patients predominantly display a CD19+CD27− naïve phenotype [14], [15]. The CSF of MS patients and patients with other inflammatory neurological disorders (OIND) contains on average 4–5% of CD19+ B cells, opposite to the situation in healthy persons. Variability in CSF B cell numbers was seen between patients [12], [14], [16], [17], [18] but the majority were mature CD19+CD27+CD38−CD138− memory B cells. CD19+CD38highCD77+Ki67+Bcl-2− centroblasts, that normally only occur in GC in lymphatic tissues [19], were demonstrated in the CSF [12], [15], either as the result of circulating memory B cell recruitment to the CNS or intrathecal differentiation of naïve B cells. Both processes can be promoted by the ectopic GC that were identified in the brain meninges of MS patients [8] and suggest that antibody-secreting cells can be generated within the CNS.
Plasma blasts (CD19+CD38+CD138+) have also been described as an important CSF B cell population. Numbers of plasma blasts were significantly increased in MS patients and patients with a clinically isolated syndrome (CIS) when compared with OIND patients [18]. Furthermore, the number of mature memory B cells and plasma blasts in the CSF of MS patients was correlated with intrathecal IgG and IgM production, higher disease activity and lesions on magnetic resonance imaging (MRI), total CSF leukocyte numbers and intrathecal production of matrix metalloproteinase 9 (MMP-9), and the homing chemokine CxCL-13 [14], [18]. These findings all indicate that CSF B cells play a role in acute inflammation. Being the most prevalent antibody-secreting cells in the CSF, plasma blasts are held responsible for intrathecal IgG production and are consequently mentioned as main effector B cell population in acute inflammation in MS [14]. Normally, plasma blasts are short-lived antibody-secreting cells that rapidly differentiate into fully mature CD19−CD138+ plasma cells. In MS however plasma blast numbers remained virtually constant over time [14], which might demonstrate an antigen-driven chronic B cell stimulation in the CNS.
Disagreement exists concerning the contribution of CD19−CD38+CD138+ plasma cells to the B cell repertoire in the CSF compartment. While some studies pointed out very low numbers of plasma cells [14], [18], [20], another study demonstrated a high number of plasma cells in the CSF of MS patients [15], but in this case only a limited number of MS patients was used. Anyhow the distribution of plasma blasts and mature plasma cells in the CSF remains vague, although CD19+CD138+ and CD19−CD138+ cells have recently been shown to be derived from the same progenitor pool and thus represent convergent responses [20]. The absence of plasma cells in the CSF of MS patients could be explained by the fact that plasma cells migrate to the brain meninges and parenchyma so they are hard to detect in CSF. Moreover, surface marker expression profiles have not been entirely clarified for plasma cells and plasma blasts. Previously, it has even been shown that CD138 was not expressed on the surface of plasma blasts and consequently was the only surface marker allowing to differentiate between plasma cells and plasma blasts [21]. Thus the phenotypic differences between these two cell types still have to be determined in detail. Table 1 gives an overview of the different B cell subsets, their surface marker expression profiles and distribution among peripheral blood and CSF.
Table 1.
Overview B cell subsets in healthy individuals and multiple sclerosis: expression of surface markers and distribution in periphery and CSF.
| Naïve B cell | Memory B cell | Plasma blast | Plasma cell | Centroblast | References | |
|---|---|---|---|---|---|---|
| Surface markers | CD19+ CD27− CD38− | CD19+ CD27+ CD138− CD38− | CD19+ CD27high CD138+ CD38+ | CD19− CD27high CD138+ CD38+ | CD19+ CD77+ Ki67+ Bcl-2− CD38high | [3], [12], [14], [15], [16], [20] |
| Healthy persons | ||||||
| Periphery | + | (+) | − | − | − | [15], [21] |
| CSF | − | − | − | − | − | [14], [18], [22] |
| Multiple sclerosis | ||||||
| Periphery | + | (+) | − | − | − | [14], [15], [18], [22] |
| CSF | − | + | + | +/(+) | + | [12], [14], [15], [16], [17], [18], [20], [22] |
+ present; − not present; (+) present in low amounts.
4. Identification of B cell target antigens in MS
Multiple candidate antigens have already been identified in different studies but evidence for their role in the disease process is still lacking or their involvement is not specific to MS. Myelin oligodendrocyte glycoprotein (MOG), myelin basic protein (MBP), proteolipid protein (PLP), myelin-associated glycoprotein (MAG), phosphatidylcholine and galactocerebroside (GalC) are all myelin proteins that have been mentioned as possible autoantigens in MS [2], [3], [7], [23]. Humoral responses to axonal and neuronal antigens, such as cytoskeletal neurofilament proteins and gangliosides, have been correlated with disease progression [23]. Viruses have also been shown to participate in the abnormal humoral immune response in MS, including Epstein–Barr virus (EBV), human herpes virus type 6 (HHV-6), cytomegalovirus (CMV), measles and Chlamydia pneumoniae [7], [23]. In addition, some protective antibodies that cause remyelination and myelin repair have been found, such as antibodies directed against oligodendrocyte surface antigens or heath shock proteins [23].
Most studies investigating the specificity of the humoral immune response have focused on autoantibodies directly by analyzing reactivity of the antibodies in serum or CSF of MS patients versus healthy controls or other neurological control patients. A substantial amount of the candidate autoantigens mentioned here have been denoted in this way. Serum may not be the ideal specimen to study autoantibodies in MS because the oligoclonal immunoglobulins are only present in the CNS. As a consequence, antibodies that are unrelated to the disease may hamper the identification of disease-specific antibodies. CSF can be a useful surrogate for the brain microenvironment and would therefore be more suitable to study antibody responses in MS, although CSF may also comprise some antibodies originating from the serum. However, relevant autoreactive antibodies may bind to their targets or to Fc receptors in the CNS tissue, thereby rendering them undetectable in CSF. An alternative approach to study MS-related antigens would be to investigate the reactivity of the clonally expanded B cells in the CSF. Despite low CSF B cell numbers, several target antigens have already been identified of which some are described below.
The group of Link performed different studies on B cell reactivity using an immunospot assay. Nitrocellulose microtiter plates were coated with the antigen of interest and binding of peripheral blood mononuclear cells (PBMC) or CSF cells to this antigen was detected. When studying CSF cells and PBMC of 19 MS patients, MBP-reactive CSF B cells were found in 57% of MS patients while none were present in peripheral blood. MAG-reactive B cells were indicated both in CSF and PBMC of MS patients (55% and 11% of patients respectively) [24]. Furthermore, anti-MOG IgG secreting cells were demonstrated in the peripheral blood from 8 of 16 MS patients and in the CSF from 8 of 10 MS patients [25]. Other studies made use of recombinant antibodies that were produced from patients' CSF B cells. CD19+ or CD138+ cells from the CSF were single-cell sorted into PCR plates, the variable regions of the heavy and light Ig chains (VH and VL) were amplified and cloned into a plasmid vector which was then expressed in a bacterial expression system. The corresponding recombinant antibodies were ultimately used for reactivity screenings with immunoprecipitation, Western blot, ELISA or immunohistochemistry. Lambracht–Washington and coworkers discovered reactivity of their recombinantly produced antibodies towards MBP, 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) and glial fibrillary acidic protein (GFAP) [26]. On the other hand, von Büdingen et al. produced 9 recombinant MS antibodies following the same procedure, and in this case 8 displayed myelin reactivity and 1 was directed against astroglia [27]. Apart from myelin binding in MS brain tissue, their targets could not be identified using several techniques such as costaining with known myelin antigens on brain tissue in immunohistochemistry, Western blot with human myelin or ELISA using recombinant and native MOG and MBP. In yet another study from Zhang et al., recombinant antibodies from MS CSF were produced that demonstrated reactivity to axons with pathologic changes in MS brain tissue [28]. The targets were later identified as triosephosphate isomerase (TPI) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [29].
We have recently adapted the B cell immortalization technique to study B cell responses in MS, based on CSF and peripheral blood B cells. This technology was first used in 1977 and consisted of B cell infection with EBV, produced by the marmoset lymphocyte cell line B95-8, to obtain continually dividing B cell lines [30], [31]. It was unsuccessful due to a low efficiency of the immortalization, a slow growth rate of the B cells and sometimes low antibody production. A lot of adjustments have been made to the technology since then, such as fusion of the EBV immortalized cells with mouse myelomas or human-mouse heteromyelomas [32], [33] or the addition of cell-derived growth factors and cytokines [34], but without success. In 2004, Traggiai et al. published an improved B cell immortalization method based on a combination of EBV and a polyclonal B cell activator, the CpG oligonucleotide ODN2006, to produce antibodies with capacity to neutralize SARS coronavirus [35]. Based on this success we developed our own optimized B cell immortalization procedure to isolate immortalized B cell lines from the CSF and peripheral blood of MS patients.
Low numbers of total CSF cells or PBMC (500–5000 cells per well) are infected with EBV in the presence of the CpG oligonucleotide ODN2006 and interleukin 2 (IL-2) to give optimal B cell stimulation (Fig. 1 ). ODN2006 is an unmethylated DNA sequence capable of stimulating B cells through Toll-like receptor 9 (TLR9) ligation [36]. The T cell suppressing agent cyclosporin A (CsA) and irradiated allogeneic PBMC (feeder cells) are added to the culture. In this way, B cells are transformed into continually dividing B cell lines, which can be verified by screening the culture supernatant for the presence of human IgG using the dot blotting technique. IgG-positive immortalized B cell cultures are representative for the enriched B cell populations in vivo and are maintained for antibody production. Clonality can be examined using a B cell spectratyping procedure (Identiclone™ IGH gene clonality assay, Invivoscribe) [37]. Specificity of the antibodies that are produced by the immortalized B cell lines can be determined using a variety of techniques, such as ELISA, immunohistochemistry, immunoprecipitation, Western blotting and cDNA phage display.
Fig. 1.
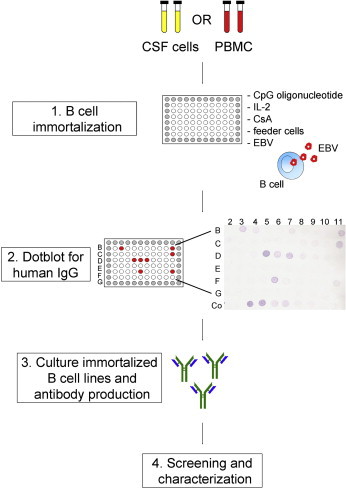
B cell immortalization procedure. Total CSF cells or PBMC are infected with EBV in the presence of the B cell stimulating factors CpG oligonucleotide and IL-2, the T cell suppressing factor CsA and irradiated allogeneic PBMC as feeder cells (1). As a source of EBV, the culture supernatant of the B95-8 cell line is used. After this immortalization phase, cultures are screened for the presence of IgG-positive B cells by dot blot for human IgG (2). As a positive control, purified human IgG is used in serial dilutions (Co). Positive cultures are maintained for the production of antibodies (3) that later on can be screened and characterized for the identification of their target antigens (4).
5. Conclusion
B cells have emerged as key players in a lot of autoimmune diseases, such as MS. The B cell repertoire in the CSF of MS patients has already been intensively studied. However, the relative contribution of the different B cell subsets to intrathecal IgG production and OCB formation still has to be defined. In addition, the site of CSF B cell activation (periphery, CNS of both) and the phenotypic differences between plasma cells and plasma blasts have to be determined in detail.
Specificity of the autoreactive B cells and antibodies remains unclear despite numerous studies using different techniques. Most of these studies focused on antibody reactivity by examining serum or CSF antibodies of MS patients. An alternative approach is to analyze reactivity of the expanded B cells in the CNS. We have adapted the B cell immortalization method for analysis of B cell responses in peripheral blood and CSF of MS patients. In a next step, the target antigens of the isolated immortalized B cells can be identified using a spectrum of techniques. B cell immortalization may also be a promising alternative for studying humoral responses in general.
Take-home messages
-
•
Most B cell research in MS focuses on autoantibody production but the targets of the autoreactive B cells and antibodies remain unknown. Various candidate antigens have been identified but their relevance in the disease process is under discussion.
-
•
The B cell population in the CSF in MS consists of clonally expanded memory B cells, centroblasts and antibody-secreting plasma blasts and plasma cells that are all generated or sustained within the central nervous system (CNS).
-
•
Disagreement exists concerning the contribution of CD19−CD138+ plasma cells to the B cell repertoire in the CSF. Phenotypic differences between plasma blasts and plasma cells have to be determined in detail to clarify their distribution.
-
•
Reactivity screening of the clonally expanded B cells in the CSF of MS patients is very appropriate to investigate their role in the disease process.
-
•
B cell immortalization is a promising approach to study humoral responses using EBV to transform B cells into antibody-producing B cell lines. Specificity of the produced antibodies can be determined using a spectrum of techniques, such as immunohistochemistry, immunoprecipitation and (cDNA) phage display.
Acknowledgement
This work is supported by a grant from the Transnationale Universiteit Limburg and by Hasselt University.
References
- 1.Kabat E.A., Glusman M., Knaub V. Quantitative estimation of the albumin and gamma-globulin in normal and pathological cerebrospinal fluid by immunochemical methods. Am J Med. 1948;4:653–662. doi: 10.1016/s0002-9343(48)90389-1. [DOI] [PubMed] [Google Scholar]
- 2.Cross A.H., Trotter J.L., Lyons J. B cells and antibodies in CNS demyelinating disease. J. Neuroimmunol. 2001;112(1–2):1–14. doi: 10.1016/s0165-5728(00)00409-4. [DOI] [PubMed] [Google Scholar]
- 3.Archelos J.J., Storch M.K., Hartung H.P. The role of B cells and autoantibodies in multiple sclerosis. Ann. Neurol. 2000;47(6):694–706. [PubMed] [Google Scholar]
- 4.Ziemssen T., Ziemssen F. The role of the humoral immune system in multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE) Autoimmun. Rev. 2005;4(7):460–467. doi: 10.1016/j.autrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Baranzini S.E., Jeong M.C., Butunoi C., Murray R.S., Bernard C.C., Oksenberg J.R. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J. Immunol. 1999;163(9):5133–5144. [PubMed] [Google Scholar]
- 6.Colombo M., Dono M., Gazzola P., Roncella S., Valetto A., Chiorazzi N. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J. Immunol. 2000;164(5):2782–2789. doi: 10.4049/jimmunol.164.5.2782. [DOI] [PubMed] [Google Scholar]
- 7.Qin Y., Duquette P. B-cell immunity in MS. Int. MS J. 2003;10(4):110–120. [PubMed] [Google Scholar]
- 8.Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14(2):164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-Or A., Calabresi P.A., Arnold D., Markowitz C., Shafer S., Kasper L.H. Rituximab in relapsing–remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann. Neurol. 2008;63(3):395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 10.Hauser S.L., Waubant E., Arnold D.L., Vollmer T., Antel J., Fox R.J. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N. Engl. J. Med. 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 11.Youinou P., Hillion S., Jamin C., Pers J.O., Saraux A., Renaudineau Y. B lymphocytes on the front line of autoimmunity. Autoimmun. Rev. 2006;5(3):215–221. doi: 10.1016/j.autrev.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Meinl E., Krumbholz M., Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 2006;59(6):880–892. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- 13.Le Pottier L., Devauchelle V., Pers J.O., Jamin C., Youinou P. The mosaic of B-cell subsets (with special emphasis on primary Sjogren's syndrome) Autoimmun. Rev. 2007;6(3):149–154. doi: 10.1016/j.autrev.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Cepok S., Rosche B., Grummel V., Vogel F., Zhou D., Sayn J. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128(Pt 7):1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- 15.Corcione A., Casazza S., Ferretti E., Giunti D., Zappia E., Pistorio A. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 2004;101(30):11064–11069. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corcione A., Aloisi F., Serafini B., Capello E., Mancardi G.L., Pistoia V. B-cell differentiation in the CNS of patients with multiple sclerosis. Autoimmun. Rev. 2005;4(8):549–554. doi: 10.1016/j.autrev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie A.M., Gilden D.H., Williamson R.A., Burgoon M.P., Yu X., Helm K. Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J. Immunol. 2004;173(1):649–656. doi: 10.4049/jimmunol.173.1.649. [DOI] [PubMed] [Google Scholar]
- 18.Kuenz B., Lutterotti A., Ehling R., Gneiss C., Haemmerle M., Rainer C. Cerebrospinal fluid B cells correlate with early brain inflammation in multiple sclerosis. PLoS.ONE. 2008;3(7):e2559. doi: 10.1371/journal.pone.0002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascual V., Liu Y.J., Magalski A., de Bouteiller O., Banchereau J., Capra J.D. Analysis of somatic mutation in five B cell subsets of human tonsil. J. Exp. Med. 1994;180(1):329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winges K.M., Gilden D.H., Bennett J.L., Yu X., Ritchie A.M., Owens G.P. Analysis of multiple sclerosis cerebrospinal fluid reveals a continuum of clonally related antibody-secreting cells that are predominantly plasma blasts. J. Neuroimmunol. 2007;192(1–2):226–234. doi: 10.1016/j.jneuroim.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Jego G., Robillard N., Puthier D., Amiot M., Accard F., Pineau D. Reactive plasmacytoses are expansions of plasmablasts retaining the capacity to differentiate into plasma cells. Blood. 1999;94(2):701–712. [PubMed] [Google Scholar]
- 22.Cepok S., Jacobsen M., Schock S., Omer B., Jaekel S., Boddeker I. Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain. 2001;124(Pt 11):2169–2176. doi: 10.1093/brain/124.11.2169. [DOI] [PubMed] [Google Scholar]
- 23.Reindl M., Khalil M., Berger T. Antibodies as biological markers for pathophysiological processes in MS. J. Neuroimmunol. 2006;180(1–2):50–62. doi: 10.1016/j.jneuroim.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Link H., Baig S., Jiang Y.P., Olsson O., Hojeberg B., Kostulas V. B cells and antibodies in MS. Res. Immunol. 1989;140(2):219–226. doi: 10.1016/0923-2494(89)90091-6. [DOI] [PubMed] [Google Scholar]
- 25.Sun J., Link H., Olsson T., Xiao B.G., Andersson G., Ekre H.P. T and B cell responses to myelin-oligodendrocyte glycoprotein in multiple sclerosis. J. Immunol. 1991;146(5):1490–1495. [PubMed] [Google Scholar]
- 26.Lambracht-Washington D., O'connor K.C., Cameron E.M., Jowdry A., Ward E.S., Frohman E. Antigen specificity of clonally expanded and receptor edited cerebrospinal fluid B cells from patients with relapsing remitting MS. J. Neuroimmunol. 2007 doi: 10.1016/j.jneuroim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Budingen H.C., Harrer M.D., Kuenzle S., Meier M., Goebels N. Clonally expanded plasma cells in the cerebrospinal fluid of MS patients produce myelin-specific antibodies. Eur. J. Immunol. 2008;38(7):2014–2023. doi: 10.1002/eji.200737784. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Da R.R., Guo W., Ren H.M., Hilgenberg L.G., Sobel R.A. Axon reactive B cells clonally expanded in the cerebrospinal fluid of patients with multiple sclerosis. J. Clin. Immunol. 2005;25(3):254–264. doi: 10.1007/s10875-005-4083-5. [DOI] [PubMed] [Google Scholar]
- 29.Kolln J., Ren H.M., Da R.R., Zhang Y., Spillner E., Olek M. Triosephosphate isomerase- and glyceraldehyde-3-phosphate dehydrogenase-reactive autoantibodies in the cerebrospinal fluid of patients with multiple sclerosis. J. Immunol. 2006;177(8):5652–5658. doi: 10.4049/jimmunol.177.8.5652. [DOI] [PubMed] [Google Scholar]
- 30.Rosen A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein–Barr virus infection of human lymphocytes in vitro. Nature. 1977;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- 31.Steinitz M., Klein G., Koskimies S., Makel O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature. 1977;269(5627):420–422. doi: 10.1038/269420a0. [DOI] [PubMed] [Google Scholar]
- 32.Kozbor D., Roder J.C., Chang T.H., Steplewski Z., Koprowski H. Human anti-tetanus toxoid monoclonal antibody secreted by EBV-transformed human B cells fused with murine myeloma. Hybridoma. 1982;1(3):323–328. doi: 10.1089/hyb.1.1982.1.323. [DOI] [PubMed] [Google Scholar]
- 33.Bron D., Feinberg M.B., Teng N.N., Kaplan H.S. Production of human monoclonal IgG antibodies against Rhesus (D) antigen. Proc. Natl. Acad. Sci. U.S.A. 1984;81(10):3214–3217. doi: 10.1073/pnas.81.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ifversen P., Zhang X.M., Ohlin M., Zeuthen J., Borrebaeck C.A. Effect of cell-derived growth factors and cytokines on the clonal outgrowth of EBV-infected B cells and established lymphoblastoid cell lines. Hum. Antibodies Hybridomas. 1993;4(3):115–123. [PubMed] [Google Scholar]
- 35.Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 2004;10(8):871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurst J., von Landenberg P. Toll-like receptors and autoimmunity. Autoimmun. Rev. 2008;7(3):204–208. doi: 10.1016/j.autrev.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 37.van Dongen J.J., Langerak A.W., Bruggemann M., Evans P.A., Hummel M., Lavender F.L. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]


