Abstract
Introduction
Prognosis of enteropathy-associated T cell lymphoma is poor but predictors of survival remain ill-defined. How clinical presentation, pathological features and therapies influence outcome was evaluated in 37 thoroughly characterized patients with celiac disease and T-cell lymphoma.
Patients and methods
Medical files were studied retrospectively. Lymphoma and intestinal mucosa were analysed by histopathology, multiplex PCR and intestinal intraepithelial lymphocytes phenotyping. Survival and prognostic factors were analysed using Kaplan–Meier curves with Logrank test and Cox Model.
Results
Lymphoma complicated non clonal enteropathy, celiac disease (n = 15) and type I refractory celiac disease (n = 2) in 17 patients and clonal type II refractory celiac disease in 20 patients. Twenty-five patients underwent surgery with resection of the main tumour mass in 22 cases. In univariate analysis, non clonal celiac disease, serum albumin level > 21.6 g/L at diagnosis, chemotherapy and surgical resection predicted good survival (p = 0.0007, p < 0.0001, p < 0.0001, p < 0.0001, respectively). In multivariate analysis, serum albumin level > 21.6 g/L, chemotherapy and reductive surgery were all significantly associated with increased survival (p < 0.002, p < 0.03, p < 0.03, respectively).
Conclusions
Our study underlines the prognostic value of celiac disease type in patients with T-cell lymphoma, and suggests that a combination of nutritional, chemotherapy and reductive surgery may improve survival.
Keywords: Celiac disease, Enteropathy associated T cell lymphoma (EATL), Intraepithelial lymphocytes, Refractory celiac disease, Serum albumin, Surgery
1. Introduction
Enteropathy-associated T cell lymphoma (EATL) is a rare non-Hodgkin lymphoma that has a very poor prognosis with a five-year survival estimated between 11 and 20% [1], [2], [3], [4]. In many patients, EATL is associated with celiac disease (CD), refractory or not to a gluten-free diet (GFD) [5], [6]. Yet CD is previously known in only 20–40% of cases [1], [4]. In half of cases, diagnosis of EATL is made during emergency surgery performed for obstruction, perforation or intestinal haemorrhage [3], [4]. Appropriate analysis of tissue specimens is often lacking in the emergency situation, thus hampering precise assessment of EATL phenotype and associated enteropathy and criteria to predict the outcome of this rare lymphoma lack specificity. In a recent study, a poor prognosis of EATL was associated with a large tumoral mass, elevated level of LDH and C reactive protein, but the presence of CD was the only predictor in multivariate analysis [7].
A higher risk of EATL has been reported in patients with type II refractory celiac disease (RCDII) who develop a clonal population of intraepithelial lymphocytes (IEL) with an abnormal phenotype than in patients with type I refractory celiac disease (RCDI) who have polyclonal normal IEL [8], [9]. Yet, it remains unknown whether the type of CD, clonal (RCDII) or non clonal including gluten-sensitive CD or RCDI, influences the outcome of EATL. Serum albumin was recently shown to be a valuable prognosis tool in RCD and may perhaps be useful to predict the outcome in EATL, inasmuch as enteropathy-related malnutrition is the most common obstacle to chemotherapy [10], [11]. Whenever chemotherapy is possible, chemoresistance is another factor which contributes to the bad prognosis of EATL. CHOP regimen has been the most widely used regimen but previous studies indicate an overall median survival of only 7 months [1], [2], [3], [4]. Except for a recent study which suggests a better prognosis of patients undergoing intensive chemotherapy followed by autologous stem cell transplantation (ASCT), the efficacy of other therapeutic regimen remains poorly evaluated [12].
Herein, we have taken advantage of a large series of well characterized patients with EATL and CD to assess how the nutritional status at diagnosis, the type of CD and the therapeutic regimen, including chemotherapy and surgery, might influence the outcome of EATL.
2. Patients and methods
2.1. Patients
The medical files of 47 consecutive patients diagnosed with T cell lymphoma associated with CD between 1992 and 2010 in 4 large Paris hospitals (European Hospital Georges Pompidou, Hospitals Necker Enfants-Malades, Lariboisière and Saint-Louis) were reviewed retrospectively. Ten patients were excluded due to uncharacterized CD or diagnostic of other lymphoproliferative disorders. Consequently, 37 patients were considered for the study and followed-up from January 1992 until April 2011(mean follow-up: 20 months [0–128]). The study was approved by the Ethic committee Ile-de –France II (Paris, France).
2.2. Collection of data
Clinical data recorded for each patient included age, sex, symptoms (abdominal pain, diarrhoea), gluten intake, body mass index (BMI), results of tomography scan (CT-scan), positron emission tomography scan (Pet-scan), bone marrow aspiration and biopsy.
Blood tests included haemoglobin, dosages of serum albumin, β2microglobulin, LDH, IgA (AGA) and IgG (AGG) anti-gliadin antibodies, IgA class endomysial antibodies (EMA), IgA antihuman tissue transglutaminase (tTG) antibodies and HLA-DRB1 and DQB1 genotyping [13].
Endoscopic evaluation included upper gastrointestinal endoscopy or double balloon enteroscopy with intestinal biopsies [5], [14].
For histological assessment, a minimum of four intestinal biopsies and/or surgical samples were fixed in 10% formalin, embedded in paraffin, and sections stained with H&E were reviewed by two expert pathologists (N.B. and V.V.). Villous atrophy was assessed in line with Oberhuber et al. modification of Marsh classification and graded as absent, partial or severe (subtotal/total) [14], [15], [16]. The percentage of IEL (number of IEL per 100 epithelial cells) was established on well orientated serial sections by counting at least 500 epithelial cells.
Immunohistochemistry was performed on sections from paraffin embedded biopsies using antibodies directed against CD3 (rabbit polyclonal Ab; A0452), CD8 (mouse monoclonal Ab; C8/144B, M703), CD20 (C26, mouse monoclonal Ab) and CD30 (mouse monoclonal Ab, BER-H2), MIB-1 (mouse monoclonal Ab, MIB-1), BCL-2 (mouse monoclonal Ab, 124) (Dako, A/S, Glostrup, Denmark), CD4 (mouse monoclonal Ab, AB12, Thermo Fisher Scientific, Fremon CA, USA), CD5 (mouse monoclonal antibody; AC7), CD56 (mouse monoclonal Ab, 1-B6), granzyme B (mouse monoclonal Ab, 11F1) (Novocastra, Newcastle Upo Tyne, UK), TiA1 (mouse monoclonal Ab, 2G9, isotype IgG1, Immunotech, Marseille, France) and on acetone fixed frozen tissue sections using antibodies directed against CD103 (2G5.1) (Coulter Immunotech), TCRβF1 (8A3), TCRδ1 (5A6E9) (T Cell Sciences, Cambridge, MA), and a three-stage indirect immunoperoxidase technique [17], [18].
For flow-cytometry phenotyping, IEL and lamina propria lymphocytes were isolated from duodenal biopsies as described [19]. Multicolor staining of lymphocytes and analyses using a BDLSR I using CellQuest software (Becton-Dickinson) were performed as described [19].
Molecular detection of clonal TCRγ chain rearrangements was performed on DNA extracted from intestinal, and extra-intestinal frozen specimens by multiplex polymerase chain reaction (PCR) [5], [9].
2.3. Diagnosis and classification of CD
Diagnosis of CD was based on HLA-DQ2/8 typing, detection of celiac specific antibodies and of villous atrophy with increased counts of IEL on normal diet. Patients were further classified depending on their clinical and histological response to a GFD. RCD was defined by clinical relapse and/or persistent malabsorption syndrome and villous atrophy after one year of strict adherence to a GFD and was further divided into RCDI in the absence of detectable clonality in duodenal biopsies and when IEL had a normal phenotype, or conversely in RCDII, when duodenal biopsies contained clonal TCRγ chain rearrangement with abnormal IEL phenotype (defined by the presence of >50% IEL expressing intracellular CD3ɛ but no CD8 in formalin fixed sections or of over 25% CD103+ or CD45+ IEL lacking surface CD3-T cell receptor complexes by flow cytometry analysis of IEL isolated from fresh biopsies) [5], [19].
2.4. Classification, staging and treatment of EATL
EATL were classified as pleomorphic in cases of tumoral infiltration by polymorphic lymphoid cells and anaplastic-like cells when infiltration was made of large atypical cells [7], [20], [21]. EATL was subclassified into types I and II in line with pathological features [22], [23]. Staging was performed according to the Ann Arbour classification modified by Musshoff and Schmidt-Vollmer [24]. Reductive surgery refers to removal of the main primary tumour mass.
2.5. Statistical analysis
Continuous data were summarized using mean or median, as appropriate. Continuous variables were compared using the non-parametric Mann–Whitney test. Categorical variables were compared using Fisher's exact test. Overt lymphoma survival was analysed using Kaplan–Meier methodology. Cox regression models were used to assess the association between survival and covariates. Multivariate analyses were performed in a blackward fashion, entering the variable associated with the outcome at the 0.20 level in univariate analysis. To analyse the predictive value of serum albumin levels, patients were divided into two groups with values either above or below the median value calculated for all patients. Statistical analyses were performed using Stat View version 5.0 software. A P value of less than 0.05 was considered statistically significant.
3. Results
3.1. Patients’ description at diagnosis of EATL
Mean age of patients (18F, 19M) at diagnosis was 57 years [34–77]. In 12 patients (32.4%), EATL was diagnosed during emergency surgery for small bowel obstruction (n = 9) or peritonitis due to an intestinal perforated tumour (n = 3). In the other cases, abdominal pain, weight loss and/or fever led to diagnostic investigations: surgery in 8 patients, endoscopy with biopsies in 9 patients, cutaneous or percutaneous biopsy in 3 patients and image-guided needle biopsy in 4 patients. In one patient, diagnosis of EATL was made at autopsy. In 10 patients (27%), CD and EATL were diagnosed simultaneously. Further follow-up indicated that these patients were responsive to a GFD. In other cases, CD was previously known (5 CD, 2 RCDI, 20 RCDII). Mean time between diagnosis of CD and onset of EATL was 46.8 months [0–329]. Increase of β2microglobulin and LDH were noted in 85.7% and 62% of patients, respectively (Table 1 ). Mean BMI was 18, anaemia and low serum albumin level (mean 23.6 g/L; median: 21.6 g/L) were observed in 91% and 88% of patients, respectively, all these factors combined attesting severe malnutrition in the majority of patients at diagnosis of EATL (Table 1). Serum albumin levels were more decreased when EATL developed in RCDII than in non clonal forms of CD (20.5 g/L vs 27.7 g/L, p < 0.02) (Table 1).
Table 1.
Patients characteristics at diagnosis of enteropathy associated T cell lymphoma.
| Total | CD/RCDI (non clonal) | RCDII (clonal) | P | |
|---|---|---|---|---|
| (n = 37) | (n = 17) | (n = 20) | Non clonal vs clonal | |
| Age (mean, years) | 57 | 57 | 58 | |
| Age (median, years) | 61 | 59 | 62 | 0.78 (N.S.) |
| Sex ratio | 18F/19M | 5F/12M | 13F/7M | <0.05 |
| HLA II | ||||
| DQ2 | 100% (25/25) | 100% (7/7) | 100% (18/18) | N.S. |
| DQ2/DQ2 | 56% (14/25) | 71% (5/7) | 50% (9/18) | 0.41 (N.S.) |
| DQ2/DQ8 | 4% (1/25) | 14% (1/7) | 0% (0/18) | 0.28 (N.S.) |
| Diagnostic delay (months) Enteropathy-EATL | 47 [0–329] | 54 [0–329] | 41 [1–176] | 0.14 (N.S.) |
| Circumstances of diagnosis | ||||
| Emergency | 32% (12/37) | 41% (7/17) | 25% (5/20) | 0.29 (N.S.) |
| Weight loss > 15%* | 57% (21/37) | 59% (10/17) | 55% (11/20) | >0.99 (N.S.) |
| Fever | 60% (22/37) | 59% (10/17) | 60% (12/20) | >0.99 (N.S.) |
| Biology | ||||
| Elevated LDH | 62% (18/29) | 62% (8/13) | 63% (10/16) | >0.99 (N.S.) |
| Elevated β2microglobulin | 86% (12/14) | 75% (6/8) | 100% (6/6) | 0.47 (N.S.) |
| Nutritional status | ||||
| Mean Body Mass Index | 18 (n = 29) | 19 (n = 12) | 17 (n = 17) | 0.42 (N.S.) |
| Low albuminemia mean level | 88% (30/34) 23.6 g/l | 73% (11/15) 27.7 g/l | 100% (19/19) 20.5 g/l | <0.03 < 0.02 |
| Anaemia | 91% (31/34) | 80% (12/15) | 100% (19/19) | 0.76 (N.S.) |
Weight loss superior to 15% of initial body weight in the last 6 months.
Low albuminemia defined by a serum albumin level inferior to 35 g/L.
CD: Celiac disease; F: female; M: male; RCDI: Refractory celiac disease of type I; RCDII: Refractory celiac disease of type II.
3.2. Characteristics of EATL at diagnosis
3.2.1. Location of EATL
EATL was localized (stages Ie, IIe) in 21 patients (57%) and disseminated (IV) in 16 patients (43%). Primary location of EATL was small bowel in 28 patients and was frequently multifocal (54%). EATL presented as ulcers and/or strictures in 19 patients and as a voluminous abdominal tumour mass in 12 patients. In 4 patients, EATL developed in skin from pre-existing cutaneaous RCDII lesions and presented as nodules, ulcers or diffuse erythema. In one patient EATL presented as a voluminous inguinal lymph node and in another one as tumoral splenomegaly. Bone marrow (18%), lung and/or mediastinal lymph nodes (16%) and liver (8%) were the main metastatic locations at diagnosis. Pet-scan was performed in 13 patients (40.6%) and accurately predicted EATL location in 11 patients (86%). No difference was noted in sensitivity according to the type of CD: 75% in CD/RCDI vs 88.9% in RCD patients (N.S.). Six patients developed a haemophagocytic syndrome.
3.2.2. Pathology and phenotype of EATL
Descriptions of EATL are indicated in Table II and listed individually in Supplementary Table I. In most cases, diagnosis of EATL was based on evidence of massive usually transparietal tumour infiltration of the small intestine. Macroscopic features were characterized by ulcers in 11 patients (29.7%), infiltration and induration of intestinal wall and/or nodules in 18 patients (48.6%). Perforated tumour was noted in 6 patients at diagnosis. In 22 cases (60%), histology revealed an infiltration of lymphoma cells of pleomorphic appearance mixed with reactive cells including small lymphocytes, plasma cells, histiocytes and polynuclear cells, especially eosinophils (Fig. 1 ). The 15 other cases (40%) were classified as large cells or anaplastic-like, containing a predominant large lymphoid cells expressing CD30 with mostly high rate of proliferative KI67 marker (Fig. 1). In all cases, increased mitotic index was noted as well as expression of cytotoxic markers, TiA and/or Granzyme B. Positivity of CD103, a marker only detectable on frozen tissue section, was observed in 16 out the 18 patients tested. Expression of CD103 was absent in two cases of EATL developed on pre-existing CD103+ RCDII lesions (patients 20 and 27). In patient 20, CD103 was noted in epidermal IEL but was lost in large CD30+ dermal tumour infiltration. Expression of CD8 was observed in 8 EATL (24%), all cases associated with non clonal enteropathy (CD or RCDI). CD56 staining was negative in all tested (32 patients, 86%) cases. CD4 marker was not expressed by tumour cells but was frequently observed in reactive small lymphoid cells infiltrate.
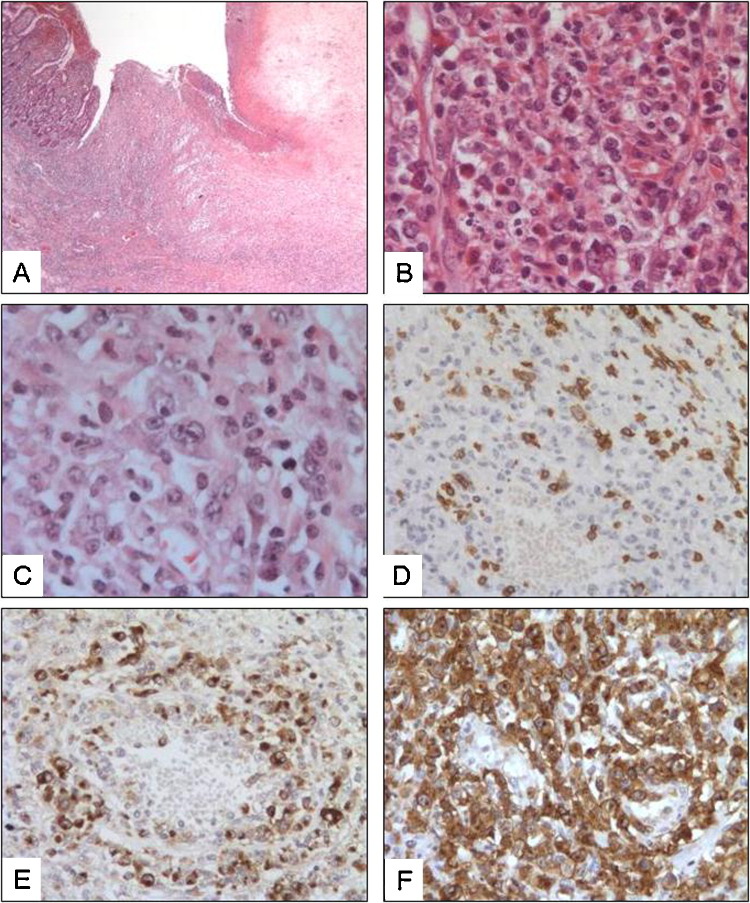
Fig. 1.
Histology and immunohistochemistry in intestinal EATL. (A,B) Case 9: HE staining demonstrates trans-parietal infiltration by ulcerative and invasive EATL (A: 16×) that is made of pleomorphic small to large-size lymphoid tumour cells and is associated with apoptotic bodies, eosinophils and plasma cells (B: 400×). (C–F) Case 23: (C) HE staining shows anaplastic-like EATL that contains many large tumour cells associated with some eosinophils and small lymphocytes (400×). (D–F) Immunohistochemical staining indicates that tumour cells express CD3 (D: 200×), Granzyme B (E: 200×) and CD30 (F: 200×) and reveals their immediate proximity to blood vessels.
In three patients, diagnosis of EATL was particularly difficult, either due to early stage of transformation and/or to atypical aspect (Fig. 2 ). In one patient with RCDII (patient 32), diagnosis of EATL was made on sections of mesenteric lymph nodes. Immunohistochemistry, combined with standard microscopy, showed T zone infiltration by small CD103+CD3+CD8− lymphocytes indicating dissemination of abnormal RCDII IEL to lymph nodes but also small foci of medium to large size CD103+, CD3+, CD30+, TiA1+, Granzyme B+ lymphoid cells which strongly expressed the Ki67 proliferative marker attesting transformation in EATL. In the two other cases (CD patient 15 and RCDII patient 35), medium and large lymphoid cells CD30+ TiA1+, Granzyme B+ did not aggregate together but were spread within the two metre-long intestinal mucosa assessed by double balloon enteroscopy (Fig. 2).
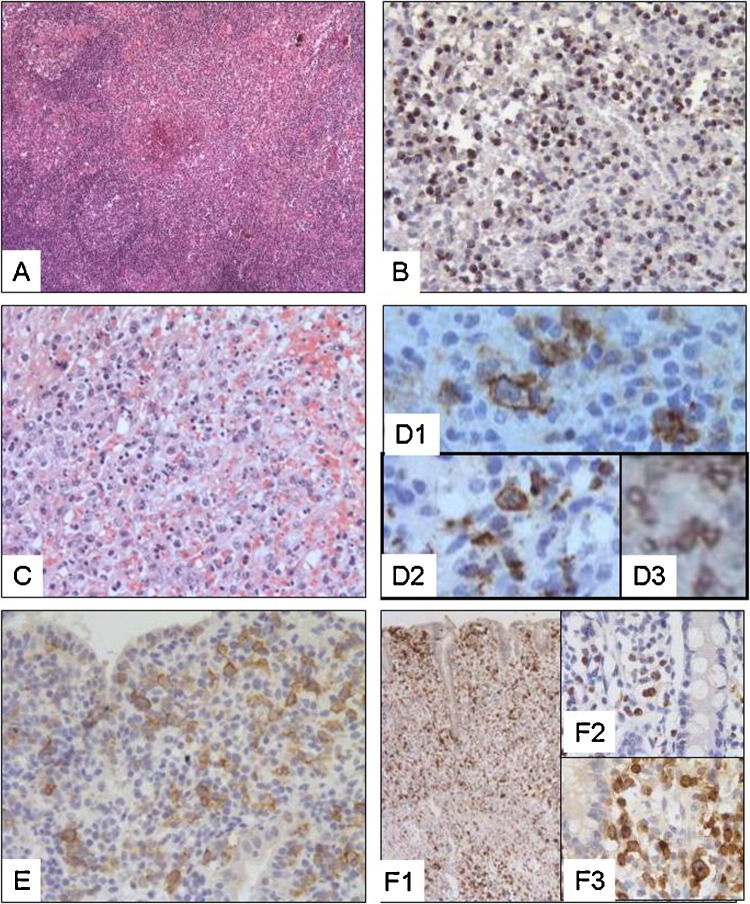
Fig. 2.
Histology and immunohistochemistry in extraintestinal and intramucosal EATL. (A,B) Case 32: (A) HE staining (50×) demonstrates lymph node infiltration by EATL. (B) Interfollicular zones are enlarged and infiltrated by lymphoid cells that stained with Granzyme B (100×). Foci of necrosis are visible (surrounded by arrows). (C,D) Case 37 (C) HE staining (200×) shows spleen infiltration by small to large-size lymphoid cells invading red pulp sinuses. Tumour cells expressed CD30 (D1: 400×), Granzyme B (D2: 400×) and CD103 (D3: 400×). (E,F) Case 15: early stage of transformation with massive infiltration of lamina propria and epithelium by medium to large-size lymphoid cells that express CD30 (E: 200×) and Granzyme B (F: 200×).
3.2.3. Characteristics of CD
All patients had CD. In 10 patients, diagnosis of CD was made simultaneously with EATL and was based on presence of positive antibodies (AGA, AGG, EMA and/or tTG), HLA-DQ2 haplotype and villous atrophy with increased IEL of normal phenotype. These patients were not on GFD at time of EATL diagnosis. Other patients were previously diagnosed with CD responsive (n = 5) or not (n = 22) to a GFD. Intestinal mucosa at distance from EATL contained IEL with normal phenotype and showed polyclonal TCR profile in the 5 patients responding to GFD, in the 10 CD patients with “de novo EATL” and in 2 CD patients refractory to GFD (RCDI). In this group of 17 patients with non clonal enteropathy, villous atrophy was partial and severe (subtotal or total) in 41% (n = 7) and 59% (n = 10) patients respectively. Except for “de novo” EATL and one known CD patient, all patients were on a strict GFD, as ascertained by dietician (mean time: 11 months [2–27]).
The other 20 patients had RCDII characterized by expansion of abnormal IEL containing intracellular CD3 but no surface CD3-T cell receptors complexes and generally no or weak CD8 and clonal rearrangement of the gamma chain of TCR detectable in duodenal biopsies (Table 1, Table 2 ). In 9 RCDII patients (patients 18, 19, 20, 22, 27, 30, 35, 36 37) frozen specimens of EATL and duodenal mucosa at distance from EATL were available and molecular analysis demonstrated the same TCRγ chain rearrangement (Supplementary Table I). CD56 was detected by flow cytometry on 40–50% of freshly isolated RCDII IEL from patients 21 and 23 but was not detected by immunohistochemistry on tissue sections of the corresponding EATL.
Table 2.
Characteristics of enteropathy associated T cell lymphoma.
| CD/RCDI | RCDII | P | |
|---|---|---|---|
| (n = 17) | (n = 20) | ||
| Staging | |||
| Ie/IIe | 9/17 (53%) | 11/20 (55%) | N.S. |
| IV | 8/17 (47%) | 9/20 (45%) | N.S. |
| Location at diagnosis | |||
| Small bowel | 16/17 (94%) | 13/20 (65%) | 0.034 |
| Mesenteric lymph nodes | 5/17 (29%) | 4/20 (20%) | N.S. |
| Spleen | 0/17 (0%) | 2/20 (10%) | N.S. |
| Liver | 1/17 (6%) | 2/20 (10%) | N.S. |
| Lung | 3/17 (18%) | 1/20 (5%) | N.S. |
| Bone Marrow | 3/17 (18%) | 4/20 (20%) | N.S. |
| Macroscopy | |||
| Ulcers | 6/17 (35%) | 4/20 (20%) | N.S. |
| Strictures | 7/17 (41%) | 6/20 (30%) | N.S. |
| Mesenteric mass | 3/17 (18%) | 2/20 (10%) | N.S. |
| Perforated tumour | 2/17 (12%) | 5/20 (25%) | N.S. |
| Cell size | |||
| Pleomorphic | 10/17 (59%) | 12/20 (60%) | N.S. |
| Large-anaplastic | 7/17 (41%) | 8/20 (40%) | N.S. |
| CD3+ | 17/17 (100%) | 20/20 (100%) | N.S. |
| CD4+ | 0/13 (0%) | 0/16 (0%) | N.S. |
| CD8+ | 8/16 (50%) | 0/18 (0%) | 0.004 |
| CD30+ | 13/16 (81%) | 15/18 (83%) | N.S. |
| GzB/TiA1+ | 16/16 (100%) | 15/15 (100%) | N.S. |
| CD56+ | 0/13 (0%) | 0/19 (0%) | N.S. |
| CD103+ | 7/7 (100%) | 9/11 (82%) | N.S. |
CD: celiac disease; RCDI: refractory celiac disease of type I; RCDII: refractory celiac disease of type II.
Pleomorphic: infiltration by polymorphic lymphoid cells (small and/or medium and/or large lymphoid cells); large-anaplastic: infiltration by large cells with an immunoblastic or anaplastic appearance.
EBER in situ hybridization performed in EATL of 10 patients: negative in all cases.
ALK tested in 6 patients and negative in all cases.
3.3. Treatments
Twenty-five patients (67.6%) were operated mainly in emergency (56%) with tumour resection in 22 patients (59%). Surgical procedure is described for each patient in Table 2. Thirty-one patients (86%) received at least one cycle of chemotherapy and 14 patients (38%) were treated by several lines. Five patients could not receive any chemotherapy due to uncontrolled gastrointestinal haemorrhage, post-operative cardiopulmonary arrest, or severe acute respiratory syndrome. Number of patients treated by several lines of chemotherapy (≥2) was lower in patients with EATL and RCDII (26%) than in patients with EATL and gluten free diet-responsive CD or RCDI (56%), although the difference did not reach statistical significance. Chemotherapy was based on anthracycline-containing regimen (n = 20; 54%) mainly CHOP or on cytarabin and/or etopeside-containing treatment (n = 20; 54%) (Table 3 and Supplementary Table II). Allogeneic (allo-SCT) and autologous (ASCT) stem cell transplantation after intensification were performed in three and two patients respectively. Two out the three patients treated by intensification and allo-SCT were still alive at the latest follow-up (patients 2 and 14). One patient was treated by palliative radiotherapy for relapsing skin lesions of EATL.
Table 3.
Treatments of enteropathy associated T cell lymphoma EATL.
| Treatment | Numbers of patients treated |
|---|---|
| Surgery | 25/37 (68%) |
| Emergency surgery | 16/37 (43%) |
| Diagnostic surgery | 8/37 (22%) |
| Elective surgery | 3/37 (8%) |
| Reductive surgery | 22/37 (59%) |
| Chemotherapy | 31/37 (86%) |
| Main regimens | |
| ACVBP | n = 2 |
| AVmCP | n = 1 |
| BAD | n = 1 |
| CDE | n = 2 |
| CEEP | n = 1 |
| CHOP | n = 10 |
| CHEP | n = 1 |
| Cytarabin-Etopeside | n = 4 |
| CYVE | n = 1 |
| ESHAP-Mini-ESHAC | n = 4 |
| Etopeside-Doxorubicine | n = 3 |
| IVE-MTX | n = 1 |
| MINE | n = 1 |
| Nutritional support | |
| Parenteral nutrition | 16/37 (43%) |
| Radiation | 2/37 (5%) |
ASCT: autologous stem cell transplantation; Allo-SCT: allogeneic stem cell transplantation; γγ: mesenteric lymph nodes; L.S.: lost sight; O.S.: overall survival (months); PN: parenteral nutrition; R.S.: reductive surgery.
Chemotherapy regimens: ACVBP: doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone; AVmCP: adriamycin, VM 26, cyclophosphamide and prednisone; BAD: bortezomib, cytarabine, dexamethasone; CDE: cyclophosphamide, doxorubicin, etoposide; CEEP: cyclophosphamide, epirubicin, vindesine and prednisone; CHEP: cyclophosphamide, adriamycin, etoposide and prednisolone; CHOP: cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisolone; CYVE: cytarabine, etopeside, thiotepa, cyclophosphamide. ESHAP: etoposide, methylprednisolone, cytarabine, cisplatin; Mini-ESHAC: etoposide, methylprednisolone, cytarabine, carboplatin; FMC: fludarabine, mitoxantrone, cyclophosphamide; IVE: high-dose ifosfamide, vincristine and etoposide/methotrexate; MINE: Mesna, ifosfamide, novantrone and etopeside.
3.4. Outcome
3.4.1. Prognostic factors
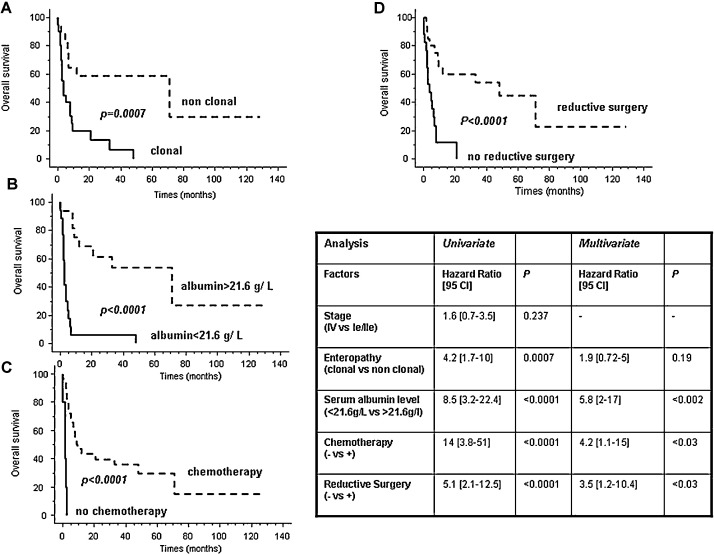
Twenty-seven patients died during the follow-up. The median overall survival was 7 months. The two and five-year overall survival rates were 34.5% and 25.8%, respectively. Concerning the type of CD, the five-year survival rate was 58.8% when EATL complicated non clonal CD (GFD responsive CD or RCDI) but 0% when EATL was associated with clonal RCDII (Fig. 3 ). The same striking difference in prognosis was observed when patients were differentiated by serum albumin levels: the five year survival rate was 53.5% in patients with serum albumin level superior to 21.6 g/L at diagnosis and null in patients with level ≤ 21.6 g/L (Fig. 3). In univariate analysis, the type of CD, serum albumin levels, realization of at least one cycle of chemotherapy and more surprisingly surgical tumour resection were predictors for overall survival (Fig. 3). Neither disease stage (localized versus disseminated) nor type of lymphoma (pleomorphic versus anaplastic) did predict overall survival. In multivariate analysis, serum albumin level > 21.6 g/L, treatment with at least one cycle of chemotherapy and reductive surgery remained good prognostic factors (Fig. 3).
Fig. 3.
EATL survival. (A) Kaplan–Meier curve of EATL survival according to the type of associated enteropathy. The dashed and solid curves represent the overall survival in patients with EATL associated with non clonal enteropathy (CD/RCDI) and EATL developed on clonal enteropathy (RCDII), respectively. (B) Kaplan–Meier curve of EATL survival according to the serum albumin level at diagnosis. The dashed and solid curves represent the overall survival in patients with serum albumin level > 21.6 g/L and patients with serum albumin level ≤ 21.6 g/L at diagnosis, respectively. (C) Kaplan–Meier curve of survival according to the realization of chemotherapy. The dashed and solid curves represent the overall survival in patients treated with chemotherapy and patients not treated by chemotherapy, respectively. (D) Kaplan–Meier curve of survival according to the realization of tumour resection surgery. The dashed and solid curves represent the overall survival in patients having tumour reductive surgery and patients without tumour reductive surgery, respectively.
3.4.2. Causes of death
The main cause of mortality was tumour progression combined with malnutrition (n = 11; 29.7%), infections (n = 10; 27%) facilitated by chemotherapy-induced neutropenia (n = 9; 24.3%), uncontrolled gastro-intestinal haemorrhage (n = 5; 13.5%), peritonitis (n = 5; 13.5%) or lethal thrombosis (n = 4; 10.8%).
4. Discussion
Our series reporting 37 cases of EATL associated with CD confirms the bad prognosis and shows a five year survival rate of 25.8% close to those previously reported [3], [4]. Our data however reveal new factors impacting survival: the CD type, serum albumin and tumour reduction surgery.
EATL was recently sub-classified into two types according pathological and genetic features [23], [24]: type I EATL generally associated with CD and CD3+CD56-CD8− phenotype and type II EATL generally not associated with CD with a CD3+ CD56+ CD8+/− phenotype [22]. In the present study, all EATL were associated with CD, composed of pleomorphic cells and/or contained some large or anaplastic-like lymphoma cells strongly positive for CD30. None of them expressed CD56. They may thus be classified as EATL type I [23], [25]. Whether the lack of CD56 is characteristic for type I EATL is unclear. A recent study reported that up to 30% of type I EATL had detectable expression of this natural killer marker on paraffin sections [7]. Interestingly, CD56 was detected by flow cytometry in 40–50% of abnormal IEL in two RCDII patients but not in the corresponding lymphomas. It is thus not excluded that some lymphoma cells express low level of CD56 not detectable by immunohistochemistry. Confirming previous studies, we observed that most CD-associated EATL expressed the IEL-specific integrin CD103 [5], [26], [27]. As previously suggested, this characteristic as well as the clonal filiation between RCDII IEL and EATL demonstrated herein in 9 cases by analysis of TCRγ chain rearrangement points to their origin from the IEL compartment [5], [26]. CD103 was however not detected in two cases of EATL that developed in skin and mesenteric lymph nodes of RCDII patients respectively. Since the same clonal TCRγ rearrangement was detected in RCDII IEL and in EATL in both patients, loss of CD103 may result either from the lack of an inductive signal away from the gut or more likely from dedifferentiation during transformation.
Four EATL developed from skin localization of RCDII and, in one patient, cutaneous and intestinal lymphomas developed simultaneously. These data point out to the epitheliotropism of RCDII and EATL cells that may be favoured by CD103 binding to E-cadherin expressed by skin and gut epithelial cells [28]. In some cases, pathological diagnosis of EATL was particularly arduous due to the lack of massive infiltration and tumour cells had to be tracked by their abnormal cytology and CD30 expression in mesenteric lymph nodes or along the intestinal wall. This was notably the case in patient 32 in whom regular follow-up by Pet-scan allowed to detect enlarged mesenteric lymph nodes, in which immuno-histochemistry demonstrated the presence of RCDII IEL but also of small clusters of large lymphoma cells. Finally, it may be noted that Pet-scan was particularly useful for EATL diagnosis [29] and allowed localization of the tumour in 86% of cases whatever the type of CD.
This study stresses the interest to precisely define the type of CD in patients with EATL. Thus, univariate analysis revealed a striking difference in the prognosis of EATL depending on its association with clonal or non clonal CD. The five-year survival rate was almost 60% for EATL associated with gluten-free diet responsive CD or RCDI but 0% in cases associated with RCDII. One explanation may lie on the fact that clonal RCDII IEL are already engaged in malignant transformation [9], [30]. RCDII IEL are however non proliferative cells and thus difficult to eradicate by regular chemotherapy. They may represent a reservoir of cells susceptible to more aggressive transformation [9]. A second non exclusive hypothesis is the severe malnutrition often associated with RCDII. Yet low serum albumin levels indicative of protein-losing enteropathy, a condition significantly associated with RCDII [9] and severe malnutrition, also had a very poor prognosis value. Furthermore the prognostic value of low serum albumin levels persisted but not that of RCDII in multivariate analysis (Fig. 3), suggesting that malnutrition is central in the poor prognosis associated with RCDII. Altogether these data stress the need to provide nutritional support and to treat the enteropathy as early as possible to improve tolerance to chemotherapy and increase overall survival.
Indeed, aggressive treatment appears to increase survival. Despite the heterogeneity in chemotherapy regimens, it was possible to observe a higher survival rate in patients who had surgical resection and chemotherapy. The benefit of tumour reduction surgery might be surprising as generally not considered as the first choice treatment of lymphoma [31]. Yet our observation of a very significant positive impact of debulking surgery on overall survival rate independently of nutritional status should encourage a more systematic recourse to elective surgery, as already advocated in some cases of metastatic solid tumours [32], [33]. Five patients received an intensive regimen of chemotherapy followed by ASCT or allo-SCT and had a median survival of 10–50 months. These data are in keeping with a recent Scottish study showing that high dosage IVE/MTX chemotherapy followed by ASCT regimen resulted in better survival than anthracycline-based chemotherapy [12]. In the later study, patients treated by both chemotherapy and surgery also had a better prognosis. Surgery may avoid complications of chemotherapy such as haemorrhage or peritonitis due to tumour necrosis. Timing for surgery needs however to be defined.
In conclusion, our data confirm the poor outcome of EATL but highlight how a multidisciplinary approach may improve the prognosis by identifying underlying enteropathy, delineating the nutritional status and designing therapeutic strategies combining elective surgery, intensive chemotherapy and nutritional support.
Conflicts of interest
The authors declare no competing financial interests.
Footnotes
This work was supported by CELAC, Institut National du Cancer (INCA).
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dld.2012.12.001.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Novakovic B.J., Novakovic S., Frkovic-Grazio S. A single-center report on clinical features and treatment response in patients with intestinal T cell non-Hodgkin's lymphomas. Oncology Reports. 2006;16:191–195. [PubMed] [Google Scholar]
- 2.Egan L.J., Walsh S.V., Stevens F.M. Celiac-associated lymphoma. A single institution experience of 30 cases in the combination chemotherapy era. Journal of Clinical Gastroenterology. 1995;21:123–129. [PubMed] [Google Scholar]
- 3.Daum S., Ullrich R., Heise W. Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German study group on intestinal non-Hodgkin's lymphoma. Journal of Clinical Oncology. 2003;21:2740–2746. doi: 10.1200/JCO.2003.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Gale J., Simmonds P.D., Mead G.M. Enteropathy-type intestinal t-cell lymphoma: clinical features and treatment of 31 patients in a single center. Journal of Clinical Oncology. 2000;18:795–803. doi: 10.1200/JCO.2000.18.4.795. [DOI] [PubMed] [Google Scholar]
- 5.Cellier C., Delabesse E., Helmer C. Refractory sprue, coeliac disease, and enteropathy-associated t-cell lymphoma. French coeliac disease study group. Lancet. 2000;356:203–208. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 6.Ekstrom Smedby K., Vajdic C.M., Falster M. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the interlymph consortium. Blood. 2008;111:4029–4038. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delabie J., Holte H., Vose J.M. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118:148–155. doi: 10.1182/blood-2011-02-335216. [DOI] [PubMed] [Google Scholar]
- 8.Al-Toma A., Verbeek W.H., Hadithi M. Survival in refractory coeliac disease and enteropathy associated T cell lymphoma: retrospective evaluation of single centre experience. Gut. 2007;56:1373–1378. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malamut G., Afchain P., Verkarre V. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90. doi: 10.1053/j.gastro.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 10.Rubio-Tapia A., Kelly D.G., Lahr B.D. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99–107. doi: 10.1053/j.gastro.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandre J., Gross-Goupil M., Falissard B. Evaluation of the nutritional and inflammatory status in cancer patients for the risk assessment of severe haematological toxicity following chemotherapy. Annals of Oncology. 2003;14:36–41. doi: 10.1093/annonc/mdg013. [DOI] [PubMed] [Google Scholar]
- 12.Sieniawski M., Angamuthu N., Boyd K. Evaluation of enteropathy-associated t-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood. 2010;115:3664–3670. doi: 10.1182/blood-2009-07-231324. [DOI] [PubMed] [Google Scholar]
- 13.Jabado N., Le Deist F., Cant A. Bone marrow transplantation from genetically HLA-nonidentical donors in children with fatal inherited disorders excluding severe combined immunodeficiencies: use of two monoclonal antibodies to prevent graft rejection. Pediatrics. 1996;98/53:420–428. [PubMed] [Google Scholar]
- 14.Cellier C., Cuillerier E., Patey-Mariaud de Serre N. Push enteroscopy in celiac sprue and refractory sprue. Gastrointestinal Endoscopy. 1999;50:613–617. doi: 10.1016/s0016-5107(99)80007-8. [DOI] [PubMed] [Google Scholar]
- 15.Oberhuber G., Granditsch G., Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. European Journal of Gastroenterology and Hepatology. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Marsh M. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunologic approach to the spectrum of gluten sensitivity (celiac sprue) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 17.Verkarre V., Asnafi V., Lecomte T. Refractory coeliac sprue is a diffuse gastrointestinal disease. Gut. 2003;52:205–211. doi: 10.1136/gut.52.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutlu T., Brousse N., Rambaud C. Numbers of T cell receptor (TCR) alpha beta+ but not of TCR gamma delta+ intraepithelial lymphocytes correlate with the grade of villous atrophy in coeliac patients on a long term normal diet. Gut. 1993;34:208–214. doi: 10.1136/gut.34.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cellier C., Patey N., Mauvieux L. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998;114:471–481. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- 20.Chott A., Dragosics B., Radaszkiewicz T. Peripheral T-cell lymphomas of the intestine. American Journal of Pathology. 1992;141:1361–2171. [PMC free article] [PubMed] [Google Scholar]
- 21.Domizio P., Owen R.A., Shepherd N.A. Primary lymphoma of the small intestine. A clinicopathological study of 119 cases. American Journal of Surgical Pathology. 1993;17:429–442. doi: 10.1097/00000478-199305000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Deleeuw R.J., Zettl A., Klinker E. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007;132:1902–1911. doi: 10.1053/j.gastro.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 23.Isaacson P.G., Chott A., Ott G. Enteropathy-associated t-cell lymphoma. In: Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., Vardiman J.W., editors. World Health Organisation classification of tumors Pathology and genetics of tumors of haematipoietic and lymphoid tissues. IARC Press; Lyon: 2008. pp. 289–291. [Google Scholar]
- 24.Musshoff K., Schmidt-Vollmer H. Proceedings: Prognosis of non-Hodgkin's lymphomas with special emphasis on the staging classification. Zeitschrift fur Krebsforschung und Klinische Onkologie. Cancer Research and Clinical Oncology. 1975;8:323–341. doi: 10.1007/BF00573019. [DOI] [PubMed] [Google Scholar]
- 25.Chott A., Haedicke W., Mosberger I. Most CD56+ intestinal lymphomas are CD8+CD5-T-cell lymphomas of monomorphic small to medium size histology. American Journal of Pathology. 1998;153:1483–1490. doi: 10.1016/S0002-9440(10)65736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer J., Cerf-Bensussan N., Jarry A. Enteropathy-associated T cell lymphoma (malignant histiocytosis of the intestine) is recognized by a monoclonal antibody (HML-1) that defines a membrane molecule on human mucosal lymphocytes. American Journal of Pathology. 1988;132:1–5. [PMC free article] [PubMed] [Google Scholar]
- 27.Cerf-Bensussan N., Begue B., Gagnon J. The human intraepithelial lymphocyte marker hml-1 is an integrin consisting of a beta 7 subunit associated with a distinctive alpha chain. European Journal of Immunology. 1992;22:885. doi: 10.1002/eji.1830220341. [DOI] [PubMed] [Google Scholar]
- 28.Cepek K.L., Shaw S.K., Parker C.M. Adhesion between epithelial cells and t lymphocytes mediated by e-cadherin and the alpha e beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann M., Vogelsang H., Kletter K. 18f-fluoro-deoxy-glucose positron emission tomography (18f-fdg-pet) for assessment of enteropathy-type T cell lymphoma. Gut. 2003;52:347–351. doi: 10.1136/gut.52.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verkarre V., Romana S.P., Cellier C. Recurrent partial trisomy 1q22-q44 in clonal intraepithelial lymphocytes in refractory celiac sprue. Gastroenterology. 2003;125:40–46. doi: 10.1016/s0016-5085(03)00692-9. [DOI] [PubMed] [Google Scholar]
- 31.Ansell S.M., Armitage J. Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clinic Proceedings. 2005;80:1087–1097. doi: 10.4065/80.8.1087. [DOI] [PubMed] [Google Scholar]
- 32.Bristow R.E., Tomacruz R.S., Armstrong D.K. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. Journal of Clinical Oncology. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 33.Rini B.I. Metastatic renal cell carcinoma: many treatment options, one patient. Journal of Clinical Oncology. 2009;27:3225–3234. doi: 10.1200/JCO.2008.19.9836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.